Abstract
Nitrosamines occur widespread in food, drinking water, cosmetics, as well as tobacco smoke and can arise endogenously. More recently, nitrosamines have been detected as impurities in various drugs. This is of particular concern as nitrosamines are alkylating agents that are genotoxic and carcinogenic. We first summarize the current knowledge on the different sources and chemical nature of alkylating agents with a focus on relevant nitrosamines. Subsequently, we present the major DNA alkylation adducts induced by nitrosamines upon their metabolic activation by CYP450 monooxygenases. We then describe the DNA repair pathways engaged by the various DNA alkylation adducts, which include base excision repair, direct damage reversal by MGMT and ALKBH, as well as nucleotide excision repair. Their roles in the protection against the genotoxic and carcinogenic effects of nitrosamines are highlighted. Finally, we address DNA translesion synthesis as a DNA damage tolerance mechanism relevant to DNA alkylation adducts.
Keywords:
N-nitroso compounds; N-nitrosamines; DNA alkylation; DNA damage; DNA repair; MGMT; AAG; ALKBH; BER; NER; TLS 1. Sources of DNA Alkylation Damage
DNA alkylation lesions are one of the most common types of DNA damage and can be induced by endogenous compounds, environmental agents, and alkylating drugs used in anticancer therapy. These alkylating agents are mutagenic, toxic, clastogenic, and teratogenic and represent well-known human carcinogens [1]. Depending on the given alkylating agent, different positions in the DNA can be attacked via nucleophilic substitution (SN-reaction). Alkylating agents can act via a SN1 or a SN2 reaction. During the SN2 (second-order nucleophilic substitution) reaction, the addition of the nucleophile and the elimination of the leaving group occur simultaneously. In opposition to that, during the SN1 (first-order nucleophilic substitution) reaction, the addition of the nucleophile and the elimination of the leaving group occur in two separated steps. The SN1 reaction is more important when the targeted carbon atom within the alkylating agent is surrounded by interfering bulky groups. Generally, alkylating agents can form adducts at all O- and N-atoms of purines and pyrimidines (Figure 1), as well as at phosphotriesters of the DNA backbone. While alkylating agents of the SN1-type alkylate O- and N-atoms, SN2 reagents mainly alkylate N-atoms [2].
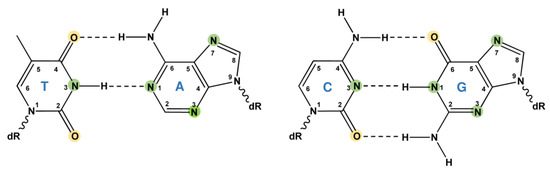
Figure 1.
Alkylation of DNA bases. Alkylating agents of the SN1-type alkylate O- and N-atoms, whereas SN2 reagents mainly alkylate N-atoms. O-Atoms are depicted in yellow, whereas N-atoms are shown in green. dR: deoxyribose moiety.
O-alkylations are highly mutagenic and cytotoxic [3]. Although only amounting to less than 8% of total alkylations [2], O6-alkylguanine represents the most critical type of DNA alkylation damage. O6-methylguanine (O6-MeG) and O6-ethylguanine (O6-EtG) can mispair with thymine, leading to GC → AT transition mutations following two rounds of replication [4]. O4-methylthymine (O4-MeT) also represents a pre-mutagenic DNA lesion, but is only induced in minor amounts. N-alkylations are predominantly cytotoxic and less mutagenic, although recent in vivo data indicate mutagenicity for the replication-blocking lesion N3-methyladenine (N3-MeA) [5].
Endogenous alkylation (methylation) can occur by the intracellular methyl group donor S-adenosyl-l-methionine (SAM) [6] via nitrosation [7]. Whereas SAM acts by a SN2 mechanism and generates N7-Methylguanine (N7-MeG) and N3-MeA [6,8], enzymatic nitrosation of glycine and glycine derivatives as well as of bile acids predominantly forms O6-alkylating agents [7,9,10]. In addition, it is well established that bacteria in the stomach and gut catalyze the nitrosation of various secondary amines derived from ingested food, thereby forming nitrosamines (see below).
Most alkylating agents found in the environment belong to the class of nitrosamines. The class of nitrosamides is frequently used in basic research or as drugs for the treatment of various tumor entities. In addition, compounds such as alkylsulfonates and nitrogen mustards also act via DNA alkylation.
1.1. Nitrosamines (N-Nitrosamines)
Nitrosamines represent compounds with the chemical structure R1R2N−N=O (R = aryl or alkyl group). They are pro-carcinogens, which require metabolic activation to form alkylating agents. Nitrosamines undergo enzymatic α-hydroxylation by CYP450 monooxygenases to form dealkylated primary nitrosamines, which further decompose to diazonium ions. Rearrangement and subsequent elimination of nitrogen result in the formation of carbenium ions, the final DNA alkylating species (for details on nitrosamines chemistry, see [11]). Around 300 structurally different nitrosamines are known [12], whereof more than 20 were assessed by the International Agency for Research on Cancer (IARC) according to their carcinogenicity in humans [13]. Nitrosamines are ubiquitously present in the environment (water, air, food) and arise, for example, in tobacco smoke and during industrial processes. Important nitrosamines found in food, personal care products, and drugs are listed in Table 1.
1.1.1. N-Nitrosamines as Contaminants in Food
The first nitrosamine identified in the environment was N-nitrosodimethylamine (NDMA), representing the most prevalent N-nitroso compound (NOC) in the diet [14]. Nitrosamines were detected in smoked fish, bacon, sausages, and cheese, but also in beverages such as beer and drinking water [14]. Apart from NDMA, several other nitrosamines were detected in food (Table 1 and Figure 2), including N-nitrosodiethylamine (NDEA), N-nitrosopiperazine (NPIP), and N-nitrosopyrrolidine (NPYR) [15]. The curing process, the temperature, and the amounts of secondary amines are the major factors that influence nitrosamine formation in food [16]. Furthermore, NOCs can be generated endogenously in the gastrointestinal tract, particularly in the stomach and the large intestine [17]. The intake of dietary heme or heme-containing red meat was shown to increase endogenous NOC formation in both rodent models and human volunteers [18,19,20,21]. NOC levels were determined in feces as apparent total nitroso compounds (ATNCs), which include N-nitrosamines, S-nitrosothiols, and nitrosyl-iron as major constituents [19,21].

Table 1.
Important nitrosamines, formed DNA alkylation adducts, and their sources.
Table 1.
Important nitrosamines, formed DNA alkylation adducts, and their sources.
| Nitrosamines | Abbreviation | Major DNA Alkylation Adducts | Sources |
|---|---|---|---|
| N-nitrosodimethylamine | NDMA | N7-MeG, N3-MeA O6-MeG, O2-MeT, O4-MeT | Food, drugs, tobacco smoke |
| N-nitrosodiethylamine | NDEA | N7-EtG, N3-EtA, O6-EtG, O2-EtT, O4-EtT | Food, drugs |
| N-nitrosopiperidine | NPIP | 7-(2-oxopropyl)-N1,N2-etheno-G, N2-(3,4,5,6-tetrahydro-2H-pyran-2-yl)-2‘-G | Food |
| N-nitrosopyrrolidine | NPYR | N7,8-ButanoG, N7-(4-Oxobutyl)-G, O4-(4-OH-Butyl)-T, and others | Food |
| N-nitrosodiethanolamine | NDELA | O6-OHEtG and others; glyoxal adducts | Cosmetics |
| N-nitroso-N-methyl-4-aminobutanoic acid | NMBA | unknown | Drugs |
| N-nitrosodiisopropylamine | NDIPA | unknown | Drugs |
| N-nitrosoethylisopropylamine | NEIPA | unknown | Drugs |
| N-nitrosomethylphenylamine | NMPA | unknown | Drugs |
| N-nitrosovarenicline | - | unknown | Drugs |
| N-nitrososalbutamol | - | unknown | Drugs |
| 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; (nicotine-derived nitrosamine ketone) | NNK | N7-MeG, N3-MeA, N3-MeG, O6-MeG, O4-MeG, O6-pobG | Tobacco smoke |
| 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; (nicotine-derived nitrosamine alcohol) | NNAL | N7-MeG, N3-MeA, N3-MeG, O6-MeG, O4-MeG, O6-pobG | Tobacco smoke |
| N‘-nitrosonornicotine | NNN | O6-pobG | Tobacco smoke |
| N’-nitrosoanabasine | NAB | unknown | Tobacco smoke |
| N’-nitrosoanatabine | NAT | unknown | Tobacco smoke |
As mentioned above, N-nitrosamines have to undergo metabolic activation to cause DNA damage. NDMA is primarily metabolized by CYP2E1, but also by CYP2A6, via α-hydroxylation [22]. This gives rise to methyldiazonium-ions that react with DNA under the formation of N7-methylguanine (N7-MeG), N3-methyladenine (N3-MeA), and O6-methylguanine (O6-MeG) as the most abundant lesions (Figure 3A) [23]. O2-methylthymine (O2-MeT) and O4-Methylthymine (O4-Me) were only detected at very low levels [23]. NDEA was shown to be activated mainly by CYP2A6 via α-hydroxylation [24], which leads to the formation of the respective ethylated DNA bases, i.e., N7-EtG, N3-EtA, O6-EtG, O2-EtT, and O4-EtT, as prevailing adducts [25]. As a minor pathway, ß-hydroxylation of NDEA can occur (Figure 3B). This gives rise to a 2-hydroxyethyldiazonium ion and DNA adducts such as N7-HOEtG, which is, however, found only in trace amounts [26]. It is further noteworthy that more than 50% of the ethyl adducts were detected at the hydrogen-linked phosphotriester oxygen [25].
The metabolic activation and DNA adduct formation of NPIP and NPYR as well as of other food-relevant nitrosamines have been recently reviewed elsewhere [27].
1.1.2. N-Nitrosamines as Impurities in Cosmetics
N-nitrosodiethanolamine (NDELA) was found as the predominant nitrosamine impurity in cosmetics [28] and was reported to be a substrate for CYP2E1-mediated toxification [29]. The metabolism of NDELA can occur via both α- and ß-hydroxylation pathways. The ß-hydroxylation of NDELA results in the formation of N-nitroso-2-hydroxymorpholine, which subsequently gives rise to glyoxal, whereas α-hydroxylation yields 2-hydroxyethyldiazonium ions [30]. Therefore, both hydroxyethyl and glyoxal DNA adducts are formed [31].
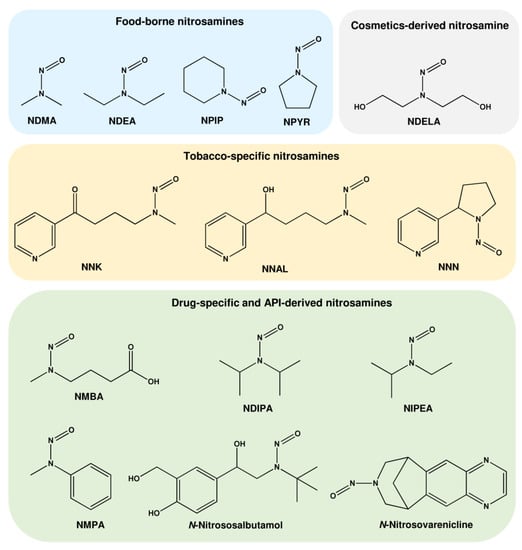
Figure 2.
Chemical structure of important nitrosamines found in food, cosmetics, tobacco smoke, and drugs. NDMA: N-nitrosodimethylamine; NDEA: N-nitrosodiethylamine; NPIP: N-nitrosopiperidine; NPYR: N-nitrosopyrrolidine; NDELA: N-nitrosodiethanolamine; NNK: nicotine-derived nitrosamine ketone; NNAL: nicotine-derived nitrosamine alcohol; NNN: N’-nitrosonornicotine; NMBA: N-nitroso-N-methyl-4-aminobutanoic acid; NDIPA: N-nitrosodiisopropylamine; NIPEA: N-nitrosoethylisopropylamine; NMPA: N-nitrosomethylphenylamine.
1.1.3. N-Nitrosamines Formed by Smoking
Another source of N-nitrosamines is cigarette smoke, which contains tobacco-specific nitrosamines [32]. Among them, the nitrosation products of nicotine, namely N-nitrosonornicotine (NNN) and nicotine-derived nitrosamine ketone (NNK), have been shown to contribute to cancer risk [33,34,35]. Additionally, nitrosation of anabasine and anatabine generates N-nitrosoanabasine (NAB) and N-nitrosoanatabine (NAT). Reduction of NNK and NNA forms nicotine-derived nitrosamine alcohol (NNAL) and iso-NNAL, whereas oxidation of NNA generates iso-NNAC. NNK undergoes metabolic activation by hepatic CYP2A6 and respiratory CYP2A13, with the latter being much more efficient [36,37]. NNAL was also reported to be metabolized by lung CYP2A13 [38]. Metabolism of NNK produces either carbenium ions or pyridyloxobutylating (pob)-agents, which lead to the formation of DNA methylation adducts (N7-MeG, N3-MeA, N3-MeG, O6-MeG, O4-MeG) and various pob-adducts, with O(6)-[4-oxo-4-(3-pyridyl)butyl]guanine (O6-pobG) being among them [39]. Thus, O6-pobG represents the second frequent pyridyloxobutylation product after the corresponding N7 alkylguanine adduct 7-[4-(3-pyridyl)-4-oxobut-1-yl]-guanine (N7-pobG) in NNK-exposed rats [40]. NNN was shown to be metabolized by both CYP2A6 and CYP3A4 [41]. In opposition to NNK, NNN only induces pob-adducts, with O6-pobG being among them [42,43]. Both DNA methylation and DNA pyridyloxobutylation are important events in NNK- and NNN-induced carcinogenesis in the rat [44], whereas in the mouse lung, DNA methylation seems to be of higher relevance [45]. For further reading on the metabolism and DNA adduct formation of tobacco-specific nitrosamines, we recommend a recently published comprehensive review [39].
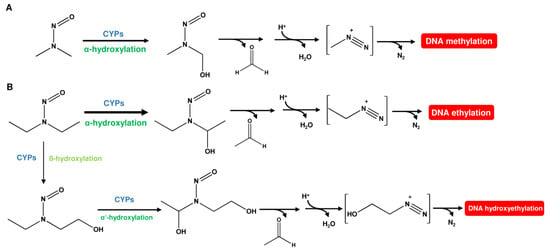
Figure 3.
Metabolic activation pathways for NDMA and NDEA. (A) N-nitrosodimethylamine (NDMA) undergoes hydroxylation by CPYs to form N-nitroso(hydroxymethyl)methylamine. This phase I metabolite rearranges under the release of formaldehyde and water to an unstable methyldiazonium ion, which then methylates the DNA. (B) N-nitrosodiethylamine (NDEA) is primarily hydroxylated at the α-carbon by CYPs. The hydroxylated metabolite then rearranges under the release of acetaldehyde and water to an unstable ethyldiazonium ion, which causes DNA ethylation. NDEA can also undergo ß-hydroxylation as a minor pathway, resulting in the formation of an unstable hydroxyethyldiazonium ion that can hydroxyethylate the DNA.
1.1.4. N-Nitrosamines as Impurities in Drugs
A couple of years ago, nitrosamines were detected in various batches of sartanes [46], which are angiotensine receptor blockers and frequently prescribed antihypertensive drugs. The sartanes (valsartan and others) contained up to 20 µg of NDMA per tablet [47], which raised a major concern, leading to one of the largest drug recalls across Europe and the US. Metformin, a drug used for the therapy of type 2 diabetes, was shown to contain NDMA in late 2019 [48]. A detailed analysis of more than 1000 samples consisting of metformin active pharmaceutical ingredient (API) and drug products revealed that roughly 18% of all samples exceeded the limit of 32 ppb of NDMA, which is based on the acceptable intake (AI) of NDMA (96 ng) multiplied by the maximum daily dose of the API [49]. The AI is defined according to the ICH guideline M7(R1) for genotoxic and potentially carcinogenic impurities in pharmaceuticals and represents the daily, lifelong intake level corresponding to a theoretical cancer risk of 10−5 [50]. Interestingly, most of the API samples had no NDMA impurity, while a substantial amount of the finished dosage form was contaminated with NDMA at levels above the AI. Nitrosamines were also found in other drugs including ranitidine, a histamine receptor antagonist, and the antibiotic rifampicin [51]. In addition to short nitrosamines such as NDMA and NDEA, more complex nitrosamines have been identified in APIs and their products, e.g., N-nitroso-N-methyl-4-aminobutanoic acid (NMBA), N-nitrosoethylisopropylamine (NEIPA or NIPEA), and N-nitrosomethylphenylamine (NMPA) [52]. Furthermore, nitrosamines derived from the API were detected. Varenicline, a partial nicotinic acetylcholine receptor agonist and a drug used as an aid to smoking cessation, contained the impurity N-nitroso-varenicline and was, thus, recalled [53]. Very recently, the drug Ventolin with the API salbutamol was recalled due to the impurity N-nitrososalbutamol in three batches [54]. Whether API-derived nitrosamines also occur in other drugs is currently a matter of intensive research.
1.2. Nitrosamides (N-nitrosamides)
Nitrosamides represent compounds with the chemical structure R1C(=X)N(-R2)-N=O (R: hydrogen atoms or organic residues) and can be divided into the N-nitrosamides (R1-C(=O)N(-R2)-N=O) and the derivates N-nitrosoureas (R1R2N(=O)N(-R3)-N=O), N-nitrosoguanidines (R1R2N(=NH)N(-R3)–N=O), and N-nitrosocarbamates (R1-O-C(=O)N(–R2)–N=O). Opposite to nitrosamines, nitrosamides do not require metabolic activation, but decompose spontaneously in aqueous medium, forming diazonium ions and finally carbenium ions as alkylating species. The most relevant class among the nitrosamides are the N-nitrosoureas, which comprise highly mutagenic compounds used in basic research as well as multiple compounds used in anticancer therapy (Table 2 and Figure 4).

Table 2.
Important nitrosamides, DNA alkylation adducts, and their sources.
N-Methyl-N-nitrosourea (MNU) and its glucose derivate streptozotocin (N-(methylnitrosocarbamoyl)-α-D-glucosamine) represent the first generation of methylating anticancer drugs, forming N7-MeG, N3-MeA, N3-MeG, and O6-MeG [55]. Chloroethylating agents such as carmustine and lomustine are used as anticancer drugs for the treatment of glioblastoma, astrocytoma, malignant melanoma, gastrointestinal and pancreatic cancer, and Hodgkin’s and non-Hodgkin’s lymphoma [56]. These drugs chloroethylate, among others, the O6-position of guanine, forming O6-chloroethylguanine (O6-ClEtG). This adduct is unstable and undergoes intramolecular rearrangement, forming the N1,O6-ethenoguanine adduct and subsequently a N1-guanine-N3-cytosine interstrand DNA crosslink [57]. Apart from the mentioned N-nitrosoureas, MNNG (N-Methyl-N’-nitro-N-nitrosoguanidine) was used as an SN1-methylating drug in multiple studies.
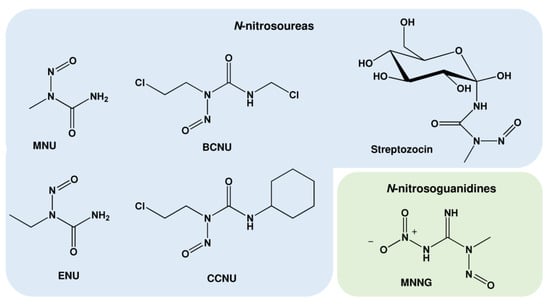
Figure 4.
Chemical structure of important nitrosamides. MNU: N-methyl-N-nitrosourea; ENU: N-ethyl-N-nitrosourea; BCNU: 1,3-Bis(2-chloroethyl)-1-nitrosourea; CCNU: 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea; MNNG: N-methyl-N’-nitro-N-nitrosoguanidine.
1.3. Further Alkylating Agents
As mentioned above, MNU was initially used as an anticancer drug. However, due to its unstable nature, it was replaced by the newly designed compounds procarbazine (PCB, PCZ, N-Methylhydrazine, Natulan®, Matulane®) and dacarbazine (DIC, Imidazole carboxamide, dimethyl-triazeno-imidazole-carboxamide, DTIC®-Dome), which generate similar reactive alkylating species (Table 3 and Figure 5) [55]. However, metabolic activation by cytochrome P450 is necessary, which might be an obstacle for anticancer therapy. The latest-generation drug is temozolomide (TMZ, Temodal®, Temodar®), which does not require metabolic activation and is used in the clinic, preferentially for the treatment of malignant gliomas [58]. TMZ decomposes spontaneously into methyltriazenoimidazole carboxamide (MITC), finally giving rise to methyl carbenium ions [59] similar to those generated by NDMA. Additional alkylating agents not belonging to the group of nitrosamides and nitrosamines are alkylsulfonates such as ethyl methanesulfonate (EMS), methyl methanesulfonate (MMS), and Busulfan, the methanesulfonate diester of 1,4-butanediol. These alkylsulfonates act in an SN2 reaction and, therefore, produce mainly N7-MeG and N3-MeA [60].
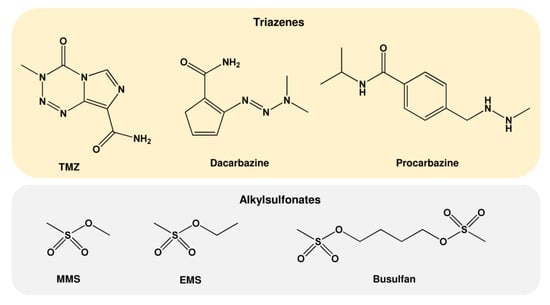
Figure 5.
Chemical structure of further alkylating agents. TMZ: temozolomide; MMS: methyl methanesulfonate; EMS: ethyl methanesulfonate.

Table 3.
Further alkylating agents, DNA alkylation adducts, and their sources.
Table 3.
Further alkylating agents, DNA alkylation adducts, and their sources.
| Further Alkylating Agents | Abbreviations | Major DNA Alkylation Adducts | Sources |
|---|---|---|---|
| Triazenes | |||
| N-Isopropyl-4-(2-methylhydrazinomethyl)benzamid | Procarbazine | N7-MeG, N3-MeA N3-MeG, O6-MeG | Anticancer drug |
| 5-(3,3-Dimethyl-1-triazenyl)imidazol-4-carboxamid | Dacarbazine | N7-MeG, N3-MeA N3-MeG, O6-MeG | Anticancer drug |
| 4-Methyl-5-oxo-2,3,4,6,8-pentazabicyclo [4.3.0]nona-2,7,9-trien-9-carboxamid | TMZ | N7-MeG, N3-MeA N3-MeG, O6-MeG | Anticancer drug |
| Alkylsulfonates Ethyl methanesulfonate Methyl methanesulfonate 4-methylsulfonyloxybutyl methanesulfonate | EMS MMS Busulfan | N7-MeG, N3-MeA N7-EtG, N3-EtA N7-MeG, N3-MeA | Basic research Basic research Anticancer drug |
2. Repair of DNA Alkylation Damage
The different DNA adducts produced by alkylating agents can be repaired by multiple DNA repair mechanisms (Figure 6). Generally, straight-chain alkyl lesions at the N3-A, N3-G, and N7-G position are removed by base excision repair (BER) initiated by the alkyladenine glycosylase (AAG). The AlkB homolog (ALKBH) demethylases directly revert N1-MeA, N1-MeG, N3-MeT, and N3-MeC, while the O6-methylguanine-DNA methyltransferase MGMT removes alkyl adducts from the O4-G and O6-G position. Among them, MGMT can also remove O6-ClEtG. However, after the formation of crosslinks, MGMT is not effective and the crosslink has to be resolved by the interstrand crosslink repair mechanism. Besides these mechanisms, the nucleotide excision repair pathway (NER) is involved in the repair of bulky alky adducts, and replication-blocking DNA alkylation lesions can be bypassed by translesion synthesis (TLS).
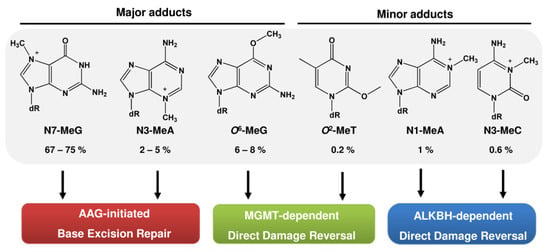
Figure 6.
DNA methylation adducts and involved DNA repair pathways. Given is the relative percentage of the total methylated DNA adducts in rat liver following exposure to NDMA for 2 h [23]. AAG: alkyladenine glycosylase; MGMT: O6-methylguanine-DNA methyltransferase; ALKBH: AlkB homolog.
2.1. Repair by MGMT
Among the adducts induced by alkylating compounds, alkylations at the O6-position and O4-position are removed by the DNA repair protein MGMT. The MGMT gene is located at chromosomal position 10q26.3 and encodes an mRNA of 866 nucleotides and a protein containing 207 amino acids with a molecular weight of 24 kDa [61,62]. MGMT can remove methyl groups from O4-MeG and O6-MeG, but not from methylphosphotriesters [63,64]. However, the repair of O6-MeG is between 105 and 103 times faster than that of O4-MeG [65]. Besides methyl adducts, longer alkyl adducts can also be repaired by MGMT, such as ethyl-, n-propyl-, n-butyl-, 2-chloroethyl-, 2-hydroxyethyl-, iso-propyl-, and iso-butyl adducts. However, in this case, the repair efficiency decreases with increasing size of the alkyl group [66,67]. Importantly, O6-pobG can also be repaired by MGMT [68,69,70,71] and, if not repaired, induces G→A and G→T mutations [72].
Although being only a minor alkylation product with less than 8% of total alkylations, O6-MeG represents the most carcinogenic lesion. Thus, it was shown that the neurotropic carcinogenic activity of MNU depends on the lack of repair of O6-MeG [73]. In addition, transgenic mice overexpressing MGMT in their skin are protected against tumor formation upon exposure to MNU and nimustine (ACNU) [74,75,76]. Data obtained in different mouse models also revealed that MGMT protects against methylation-induced liver cancer [77], lung cancer [78,79], thymic lymphomas [80,81], and colon cancer [82,83].
A landmark study demonstrated that MGMT causes a threshold in NOC-induced colon cancer formation at low methylation dose levels [84]. Wild-type DNA-repair-proficient mice showed a non-linear tumor formation, whereas MGMT-deficient mice developed tumors in a linear, dose-dependent manner [84]. Intriguingly, these findings correlated very well with the NOC-induced O6-MeG levels and γH2AX formation in colorectal tissue [84,85]. Transgenic mice with high expression levels of the bacterial MGMT homolog Ada showed decreased liver tumor formation after treatment with the nitrosamines NDMA and NDEA [77]. Consistent with this finding, MGMT knockout mice revealed a higher frequency of GC → AT transition mutations in the liver and lung upon treatment with the tobacco-specific nitrosamine NNK [86]. This correlated very well with increased levels of O6-MeG and O6-pobG in both organs of MGMT-deficient mice after NNK treatment [86].
Moreover, O6-alkylating agents are also highly toxic. Thus, repair by MGMT almost completely prevents cell killing at lower concentrations. In line with this, MGMT confers resistance to methylating and chloroethylating anticancer drugs during anticancer therapy in humans [87,88,89]. At high concentrations, other repair pathways, e.g., base excision repair, may become saturated and the N-alkylations then mediate cytotoxicity.
Mechanistically, MGMT acts by direct damage reversal. The alkyl group is transferred from the O6-group of guanine onto a cysteine residue (Cys145) in the active center of MGMT in a one-step reaction, thereby restoring guanine [63,64]. Alkylated MGMT is thereafter ubiquitinated and degraded by the proteasome [90] (Figure 7A). Therefore, the repair capacity of O6-alkylation damage is determined by the cellular MGMT level. However, as O6-MeG does not interfere with replication, it is not toxic by itself and replication is required for toxicity [91]. If not repaired by MGMT, O6-MeG mispairs with thymine (T) during the first round of replication [92] (Figure 7B).
Within the second round of replication, the generated O6-MeG-T mispair is converted into an A→T transversion in one of the daughter cells, whereas the O6-MeG-T mispair is retained in the second. For toxicity, the O6-MeG-T mispair has to be processed by the mismatch repair (MMR) system [93]. However, MMR acts on the newly synthesized strand, where it removes the thymine, leaving the O6-MeG behind. As consequence, thymine is re-incorporated opposite O6-MeG at high frequency. Subsequent additional rounds of repair by MMR and reinsertion of T destabilizes the DNA in a futile repair cycle. Destabilization is caused by the large size of the DNA stretches removed by MMR [94]. The generated single-stranded DNA is quite unstable and can break, e.g., when encountering the replication machinery, leading to the formation of toxic DNA double-strand breaks (DSBs) [95]. We should note that it has been estimated that only ~1% of the endogenously formed SSBs can be converted into DSBs [96]. Concerning O6-MeG, it has been shown that treatment of A172 cells with 20 μM TMZ induces 14,000 O6-MeG adducts, which are converted into 32 DSBs determined as γH2AX foci, representing a conversion rate of 0.23% [97]. If not repaired by homologous recombination (HR) [98] in the post-treatment cell cycle [99], DSBs trigger downstream pathways, such as cell death and senescence (Figure 7B). As already mentioned, O6-MeG is not highly toxic, which is also observed in glioma cells exposed to TMZ. In this case, unrepaired O6-MeG and the subsequent DSBs trigger mostly senescence and little cell death [100,101].
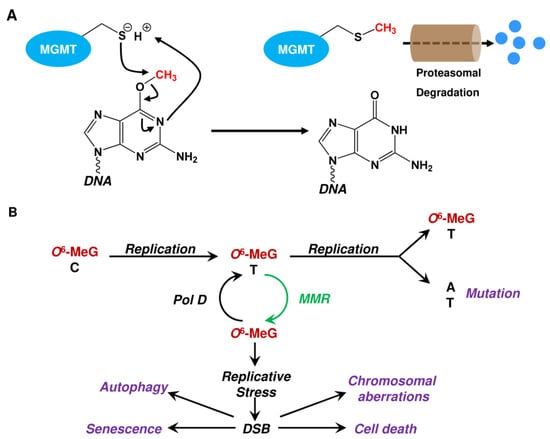
Figure 7.
(A) Repair of O6-methylguanine adducts by MGMT. (B) Biological consequences of unrepaired O6-methylguanine (O6-MeG). Pol D: polymerase delta; MMR: mismatch repair; DSB: DNA double-strand breaks.
O6-ClG adducts can also be repaired by MGMT. If not repaired, O6-ClG undergoes intramolecular rearrangement, forming the N1-O6-ethenoguanine adduct and subsequently a N1-guanine-N3-cytosine interstrand DNA crosslink [57]. If not removed, these interstrand crosslinks block DNA replication and lead to DSBs, with subsequent cell death induction [102].
Strong differences in MGMT activity and expression have been observed in humans [103]. The differential expression and activity of MGMT are most likely caused by multiple polymorphisms found in the MGMT gene and its promoter. However, an association between these polymorphisms and cancer risk has not been proven (for further reading, see [104]). Moreover, the MGMT expression can be regulated epigenetically and transcriptionally. In rodent cells, MGMT expression is regulated at the transcriptional level and its expression can be increased upon exposure of cells to alkylating agents. Thus, the basal MGMT expression depends on the transcription factors p53 and SP1 [105,106,107,108]. In this case, p53 has been shown to bind and sequester SP1 by preventing its binding to the MGMT promoter [109]. MGMT induction was also reported in rodent cells upon genotoxic stress such as UVC, ionizing radiation, and alkylating agents as well as corticosteroids [110,111,112,113,114,115] and in human HeLaS3 cells upon treatment with different activators of protein kinase C (PKC) such as phorbol-12-myristate-13-acetate (TPA) and 1,2-diacylglycerol (DAG) [115]. In human cells, transcriptional regulation of MGMT is also controlled by SP1 and upregulated by glucocorticoids, but not by genotoxic stress such as TMZ and radiation [116]. In humans, MGMT expression is regulated via epigenetic mechanisms. Thus, methylation of CpG islands localized between -249 and -103 as well as between +107 and +196 within the promoter induces transcriptional silencing [117,118,119,120,121]. As a consequence, the MGMT activity differs between different organs, being highest in the liver and lowest in the brain, myeloid tissue, and hematopoietic stem cells (for review, see [122]) and also between different individuals. Using peripheral blood mononuclear cells (PBMCs), high inter-individual but only moderate intra-individual variations were observed [123]. Concerning MGMT expression during development, it was shown that fetal liver has a lower MGMT activity than the corresponding adult tissue, whereas in most other paired tissues, the activities are in the same range [124]. Moreover, reduced MGMT expression was observed during cytokine-stimulated in vitro maturation of peripheral blood monocytes into dendritic cells [125]. Of note, MGMT promoter methylation with subsequent abrogated expression and activity is frequently observed in different tumors such as brain tumors [126]. Therefore, MGMT is a predictive marker for the effectiveness of methylating anticancer drugs, and clinical trials are underway analyzing the influence of MGMT inhibition on the therapeutic success (for further reading, see [127,128]).
Apart from its epigenetic and transcriptional regulation, MGMT is influenced on the protein and activity level by natural compounds and drugs. On the one hand, antioxidants such as curcumin, cysteine prodrugs such as Oltipraz and N-acetylcystein, and coffee diterpenes were shown to increase MGMT levels and activity in cultured cells and in rat liver [129,130]. On the other hand, the disulfide compounds disulfiram and α-lipoic acid were identified as direct MGMT inhibitors that trigger MGMT depletion in cancer cells [131,132]. Furthermore, the nitric oxide donor S-nitroso-N-acetylpenicillamine was reported to downregulate MGMT expression in glioma [133], while the anti-estrogen tamoxifen was shown to promote MGMT degradation in colorectal cancer cells [134]. Changes in MGMT level and activity have a tremendous impact on the susceptibility of cells, tissues, and organs toward SN1 alkylating agents such as nitrosamines and anticancer drugs as pointed out above.
Overall, MGMT represents the most important pathway in the repair of DNA alkylation damage at the O4 and O6 position of guanine, which is typically induced by nitrosamines.
2.2. Repair by the ALKBH Family
Apart from MGMT, a second direct reversal mechanism exists, which targets N-alkyl lesions and consists of the ALKBH demethylase family. These demethylases were first described in E. coli as part of the adaptive response [135]. During this response, upregulation of the MGMT-like repair protein Ada and the oxygenase AlkB was described. AlkB was shown to repair DNA alkylation damage such as N1-MeA and N3-MeC in an oxygen, alpha-ketoglutarate (α-KG), and Fe(II)-dependent reaction, by coupling oxidative decarboxylation of α-KG to hydroxylation of methylated DNA bases [136,137] (Figure 8A). N1-MeA and N3-MeC represent replication-blocking lesions. Thus, in AlkB-deficient cells, only ~12% of them are bypassed. Furthermore, N3-MeC is strongly mutagenic, inducing C → T and C → A mutations, whereas N1-MeA caused little mutagenicity [138]. Apart from N1-MeA and N3-MeC, AlkB also repairs N1-MeG and N3-MeT with lower efficiency [138]. Both lesions are also highly mutagenic. N1-MeG induces G→T, G→A, and G→C mutations, whereas N3-MeT induces T→A and T→C mutations. Whereas AlkB preferentially repairs N1-MeA and N3-MeC in single-stranded DNA (ssDNA), N1-MeG and N3-MeT are preferentially repaired in double-stranded DNA (dsDNA) [139]. Finally, exocyclic DNA adducts such as 1,N6-ethenoadenine (εA) and 3,N4-ethenocytosine (εC) are also substrates of AlkB [140,141,142].
In human cells, nine AlkB homologs (ALKBH1 to ALKBH8 and FTO) are known [143], but only ALKBH2 and ALKBH3 act as α-KG- and Fe(II)-dependent dioxygenases at N1-MeA and N3-MeC [144,145]. The ALKBH2 gene is localized at chromosome position 14q24.11, harbors 4 exons, contains a coding sequence of 786 bp, and encodes a protein consisting of 261 AS with a molecular weight of 33.1 kDa. ALKBH3 is located at position 11p11.2, harbors 10 exons, contains a coding sequence of 7861 bp, and encodes a protein consisting of 286 AS with a molecular weight of 37.9 kDa. As mentioned above, ALKBH2 and ALKBH3 have also been shown to repair εA [140,146], which is thought to occur via epoxide formation at the etheno bond followed by hydrolysis to a glycol derivative, giving rise to glyoxal and the restored adenine base (Figure 8B) [140]. ALKBH2 was also demonstrated to repair εC [147,148]. However, these etheno-adducts are not typical alkylation products, but rather produced by vinyl chloride or lipid peroxidation. Furthermore, N1-MeG and N3-MeT can be repaired, however at lower rates [138,149,150]. ALKBH2 and ALKBH3 are not only involved in DNA repair, but also in epigenetic regulation. Similar to the α-ketoglutarate (α-KG)/Fe(II)-dependent dioxygenase TET (ten-eleven translocation), ALKBH2 and ALKBH3 can oxidize 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC) [151].
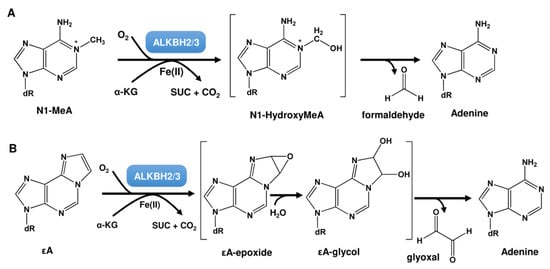
Figure 8.
ALKBH-mediated repair of (A) N1-MeA and (B) εA. α-KG: alpha-ketoglutarate; SUC: succinate; dR: deoxyribose moiety.
Mouse embryonic fibroblasts (MEFs) derived from Alkbh2 and Alkbh3 knockout mice were ∼2-fold more sensitive to MMS-induced cytotoxicity as compared to wild-type cells [152]. While the spontaneous mutation frequency was not enhanced in both knockout MEFs, Alkbh2-deficient cells showed an increased mutant frequency after MMS treatment [152]. Another study showed that Alkbh2-deficient mice accumulate N1-MeA adducts in the genome [153]. This was not the case in Alkbh3-deficient mice, indicating that the reversal of N1-MeA is mainly mediated by ALKBH2 in mammalian cells. However, Alkbh2/Alkbh3 double-knockout mice are more susceptible than Alkbh2-deficient mice to alkylation-induced, inflammation-driven colon carcinogenesis and toxicity, strongly suggesting that both ALKBH2 and ALKBH3 are required for the repair of DNA alkylation damage [154]. This is supported by the finding that ALKBH2 and ALKBH3 show different substrate specificity. Thus, AKBH2 acts preferentially on dsDNA, whereas ALKBH3 acts on ssDNA [144]. In line with this, ALKBH2 but not ALKBH3 can interact with PCNA during replication [155,156]. In opposition to that, ALKBH3 interacts with the ssDNA-binding proteins recombinase A (RecA) and RAD51C [157]. This interaction is supposed to recruit ALKBH3 to alkylation lesions at the 3’-tailed DNA generated during homologous recombination. Novel findings indicate that the recruitment of ALKBH3 to the DNA is associated with transcriptional-coupled repair [158,159]. Thus, upon stalling of the RNA polymerase II, RNF113A ubiquitinates several transcription-associated proteins, leading to recruitment of the ASCC (activating signal co-integrator complex) via the ubiquitin-binding subunit ASCC2 [160]. In a subsequent step, the ASCC helicase subunit ASCC3 unwinds the DNA, thereby generating ssDNA, allowing ALKBH3 to target the alkylation lesion also in the context of dsDNA [161]. Importantly, deficiency in all ASCC components sensitizes cells to alkylating agents [158,159,160,161].
As mentioned above, ALKBH2/3 can revert N1-MeA and N3-MeC lesions. These lesions are not the major lesions induced by clinically used alkylating agents. Especially N1-MeA is thought to be non-cytotoxic because of the efficient repair by ALKBH proteins. Therefore, it is surprising that ALKBH2 has been reported to confer resistance against TMZ in human glioblastoma cells [162]. Moreover, a positive correlation between promoter methylation of ALKBH3 and cellular N3-MeC levels was shown in breast cancer cell lines, suggesting a role in alkylation-based chemotherapy [163]. In glioblastoma, the ALKBH-dependent repair might also be associated with the superior prognosis of IDH1 (Isocitrate dehydrogenase 1) mutant tumors. Mutant IDH1 can convert α-KG into the oncometabolite D-2-hydroxyglutarate, which can directly inhibit ALKBH-dependent repair [164] and sensitizes cells to alkylating agents such as MMS and MNNG [165] and even CCNU [166].
Taken together, repair by the ALKBH family plays only a role at minor DNA lesions such as N1-MeA, N3-MeC, N1-MeG, and N3-MeT.
2.3. Repair by Base Excision Repair (BER) Initiated by AAG and Role of PARP-1
BER is a highly conserved pathway, which is responsible for the removal of N-methylated DNA adducts, such as N7-MeG and N3-MeA (Figure 9). Apart from that, BER is involved in the repair of oxidative DNA damage, e.g., 8-Oxoguanine, and DNA damage resulting from spontaneous deamination, such as the conversion of cytosine to uracil [167]. The N-methylated DNA lesions are detected by the enzyme AAG, which is also called N-methylpurine-DNA glycosylase (MPG) [168,169]. Further substrates of AAG include hypoxanthine (Hx), εA, and N1-MeG [170]. Both εA and N1-MeG are also substrates for ALKBH-mediated direct repair as mentioned above [140,150]. Whether larger N-alkyl adducts, such as N7-EtG, are removed by AAG has not been tested so far. However, it is known that N3-EtA and N7-EtG are prone to undergo spontaneous depurination, resulting in the formation of an apurinic site (AP site) [171]. This process also occurs with N7-MeG adducts, albeit with slower kinetics [172].
AAG null mouse embryonic stem (ES) cells are hypersensitive to alkylation-induced cell killing and chromosomal damage as shown after treatment with MMS and BCNU [173]. In line with that, an increased number of mutations was found in splenic lymphocytes of AAG-deficient mice exposed to MMS [174]. Furthermore, MEFs derived from AAG-/- mice are hypersensitive to the alkylating agent MeOSO2(CH2)2-lexitropsin [168]. This is a methylsulfonate ester covalently attached to N-methylpyrrolecarboxamide dipeptide, which displays DNA minor groove binding and, thus, predominantly induces N3-MeA [175]. Exposure of AAG-/- ES cells to MeOSO2(CH2)2-lexitropsin caused chromosomal aberrations, p53 accumulation, and apoptosis [176]. AAG-deficient ES cells were further reported to show higher initial N3-MeA levels upon MNU treatment and a strongly attenuated repair as compared to wild-type ES cells, highlighting the role of AAG-mediated repair [177]. On the other hand, overexpression of AAG was demonstrated to render cells hypersensitive to DNA alkylation damage by MMS, resulting in increased chromosomal instability [178]. This study provided the first evidence that imbalanced AAG-mediated BER is detrimental to cells. The hypersensitivity phenotype was also observed after mitochondrial overexpression of AAG and MMS treatment [179]. Overexpression of AAG was further reported to increase the sensitivity of cells toward TMZ [180], which is potentiated by the BER inhibitor methoxyamine and Poly(ADP-ribose) polymerase (PARP) inhibitors [181].
Increased colon cancer formation was observed in AAG-/- mice initiated with the NOC-related compound azoxymethane (AOM) in combination with the tumor promoter dextran sodium sulfate (DSS) that triggers colitis [82,182]. Interestingly, dose–response studies with AOM revealed a non-linear colon cancer formation in AAG-deficient mice with a threshold similar to that of wild-type animals, but with increased tumor formation at higher AOM doses [84]. A very recent study investigated the role of AAG in liver carcinogenesis induced by NDMA [5], which causes N7-MeG, N3-MeA, and O6-MeG as the most abundant lesions. As N7-MeG is of minor relevance due to its spontaneous depurination and O6-MeG is removed by MGMT, the observed effects were primarily attributed to the induced N3-MeA and its replication-blocking properties. The lack of AAG increased mutation rates in liver and promoted liver cancer formation, whereas AAG overexpression resulted in fewer mutations, but more liver tissue damage and lethality [5]. The latter phenotype is very likely due to the fast removal of N3-MeA in mice with AAG overexpression, which leads to an accumulation of SSBs as repair intermediates. These SSBs can then collide with the replication fork, thereby causing replication fork collapse and DSB formation [183]. These findings illustrate the importance of balanced AAG levels to prevent mutagenicity on the one hand and tissue damage on the other hand after exposure to alkylating agents such as nitrosamines. Intriguingly, AAG levels in PBMCs were reported to vary up to 10-fold between human individuals [184,185].
On the biochemical level, AAG is a monofunctional enzyme that harbors only a DNA glycosylase activity, whereas bifunctional DNA glycosylases such as OGG1 (8-oxoguanine DNA glycosylase 1) possess an additional AP lyase activity [167]. AAG catalyzes the hydrolysis of the N-glycosidic bond between the damaged DNA base (e.g., N7-MeG or N3-MeA) and the deoxyribose moiety [168,186] (Figure 9). This process may be stimulated by UV-DDB, as shown very recently for the AAG substrates εA and Hx in vitro [187]. The release of the damaged base generates an AP site. Subsequently, AP endonuclease (APE1) catalyzes the incision of the phosphodiester backbone at the AP site. This reaction generates a DNA nick with a 5′-deoxyribose-5-phosphate (5′-dRP) and a free 3′-OH group [188]. The 5`dRP moiety is eliminated by DNA polymerase ß (Pol ß) via its intrinsic lyase activity, which produces a 5′-phosphate terminus [189]. BER is then typically completed via the so-called “short-patch” pathway, in which Pol ß catalyzes the incorporation of a new nucleotide using the 3′-OH group as a primer [190]. Finally, the remaining nick is sealed by DNA ligase III in a complex with the scaffold protein X-ray repair cross-complementing protein 1 (XRCC1), which stabilizes DNA ligase III [191,192]. Furthermore, XRCC1 interacts with Pol ß, which was shown to be required for efficient BER [193]. It should be mentioned that DNA ligase I may substitute for DNA ligase III in short-patch BER, which occurs in an XRCC1-independent manner [194].
If the 5′-dRP moiety is chemically modified and is, therefore, no substrate for Pol ß, BER proceeds via the “long-patch” pathway. Long-patch BER is also the prevailing pathway during the S-phase of the cell cycle and at low ATP levels [167,195,196]. This pathway involves additional factors including Pol δ and ε, PCNA, flap endonuclease-1 (FEN1), and DNA ligase I [197]. First, Pol δ and ε catalyze strand displacement synthesis with a stretch of 2-13 nucleotides, thereby generating a so-called 5′-flap structure [198]. In the next step, this flap structure is removed by FEN1 [195] and the remaining nick is sealed by DNA ligase I [199].
Furthermore, the nuclear protein PARP-1 is an important factor that accelerates BER, although not being essential for this process [200,201]. PARP-1 is a DNA damage sensor and activated by DNA strand breaks, including SSB intermediates arising during BER [202,203]. Upon activation, PARP-1 catalyzes the synthesis of poly(ADP-ribose) (PAR) with consumption of NAD+ [204]. The formed biopolymer is covalently attached to PARP-1 itself and to other acceptor proteins, such as histones, chromatin remodelers, and DNA repair factors [205,206]. This results in direct or indirect modulation of the local chromatin structure, thereby facilitating the BER process [201]. Furthermore, PARP-1 recruits XRCC1 and other repair proteins through their PAR-binding motif, allowing for a non-covalent interaction with high affinity [207,208,209]. PAR binding to XRCC1 is indispensable for XRCC1 function in BER and SSB repair [210]. On the other hand, XRCC1 in its complex with Pol ß and DNA ligase III has recently been shown to curtail excessive PARP-1 activation at formed SSB intermediates, which can result in PARP-1-mediated cytotoxicity [211]. The relevance of PARP-1 for DNA alkylation damage was demonstrated in PARP-1 null mice and MEFs derived thereof, which are hypersensitive towards both MNU and MMS with increased levels of DNA damage [212,213,214]. This was also illustrated in colorectal cancer cells with PARP-1 deficiency, which display increased DNA strand break levels after treatment with TMZ [215]. Loss of PARP-1 in vivo caused increased levels of deletion mutations following exposure to N-nitrosobis(2-hydroxypropyl)amine (BHP) [216]. In line with this finding, PARP-1-deficient mice were shown to be susceptible to BHP-induced liver cancer [217]. Furthermore, PARP-1-/- mice exposed to the NOC-related compound AOM develop a higher number of colonic tumors and liver nodules [218]. Colon tumor induction upon AOM/DSS challenge was potentiated in MGMT and PARP-1 double knockout mice [215], demonstrating the important roles of these proteins in the protection against NOC-induced genomic instability and cancer. However, this study further revealed that PARP-1 promotes inflammation-driven tumor progression, highlighting the opposing functions of PARP-1 during tumorigenesis [215].
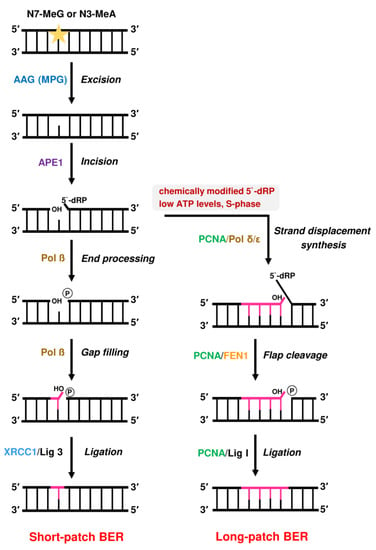
Figure 9.
Base excision repair (BER) of DNA alkylation adducts such as N7-MeG or N3-MeA.
Overactivation of PARP-1 in response to severe DNA damage results in PARP-1-mediated cell death [185,219]. This is associated with NAD+ depletion and rapid ATP loss, which is caused by PARP-1-dependent suppression of glycolysis via PARylation of hexokinase I [220,221]. In line with this notion, genetic or pharmacological abrogation of PARP-1 protected against MMS-induced, AAG-dependent tissue damage and neuronal degeneration [185,219]. PARP-1 overactivation can directly trigger cell death via its product PAR, which is called PARthanatos [222]. This PARP-1-dependent cell death pathway is observed particularly in neuronal cells, e.g., following oxidative injury or glutamate excitotoxicity, and is characterized by a PAR-triggered release of apoptosis-inducing factor (AIF) from mitochondria [222,223,224,225]. AIF was shown to translocate to the nucleus, where it interacts with macrophage migration inhibitory factor, resulting in neuronal cell death [226]. More recently, PARP-1 was also demonstrated to play a central role in PARthanatos observed in cancer cell lines following treatment with the alkylating agents MNNG and MMS, which was dependent on the MGMT level [227]. Overall, these studies illustrate the close connection of the DNA repair proteins AAG, MGMT, and PARP-1 in genome protection and cell death.
Taken together, BER is the main DNA repair pathway for DNA alkylation adducts formed at the N-position of nucleotides by both SN1 (e.g., nitrosamines and nitrosoureas) and SN2 alkylating agents (e.g., MMS or EMS).
2.4. Repair by Nucleotide Excision Repair (NER)
The mechanism of NER has been described in detail in multiple review articles [228,229,230] and is summarized below. NER can repair intrastrand-crosslinks such as UV-light-induced photolesions ((6–4) photoproducts (6-4PPs) and cyclobutene pyrimidine dimers (CPDs)) as well as bulky chemical adducts, induced mostly by natural compounds such as the mycotoxin aflatoxin and the main product of incomplete combustion of organic material, benzo(a)pyrene (B[a]P). Moreover, NER is involved in the repair of interstrand-crosslinks, which can be induced by anticancer agents such as chloronitrosoureas and cisplatin. The repair of interstrand-crosslinks is highly complex and utilizes, besides the NER pathway, homologous recombination, translesion polymerases, and the Fanconia Anemia components (for details on interstrand-crosslink repair, see [231,232]). Factors involved in NER belong to different complementation groups such as xeroderma pigmentosum (XP), Cockayne’s syndrome (CS), and trichothiodystrophy (TTD), which are associated hereditary disorders of the same name.
NER can be divided in two distinct pathways. The global genomic repair (GGR) removes lesions from the non-transcribed regions of the genome and the non-transcribed strand of transcribed regions, whereas the transcription coupled repair (TCR) removes RNA-polymerase-blocking lesions from the transcribed strand of expressed genes (Figure 10).
During GGR, lesions strongly distorting the DNA structure are recognized by the XPC–HR23B complex and thereafter verified by the RPA–XPA complex. Lesions that do not strongly distort the DNA helix are recognized by the complex DDB1–DDB2 (XPE). As an example, XPC–HR23B recognizes (6–4)PPs [233], whereas the DDB1–DDB2 complex recognizes CPDs [234]. After recognition of the lesion, the transcription factor TFIIH unwinds the DNA around the lesion [235]. TFIIH consists of two helicase subunits, XPB and XPD, and several accessory components (GTF2H1, GTF2H2, GTF2H3, GTF2H4, CDK7, CCNH, and MNAT1). After unwinding and formation of an open complex, the XPF–ERCC1 complex performs an incision 5′ of the DNA lesion. Thereafter, DNA synthesis by PCNA and the DNA polymerases (δ, ε, and/or κ) leads to the formation of a flap, which is removed by XPG-mediated 3′ incision and the nick is sealed by DNA ligase I, or the Ligase-III-XRCC1 complex [236]. However, if the damage load is too high, DNA synthesis is inhibited and the 3′ incision by XPG does not occur. In this case, XPG can be replaced by EXO1, which further excises the DNA, generating long single-stranded DNA stretches, activating the DNA damage response [237].
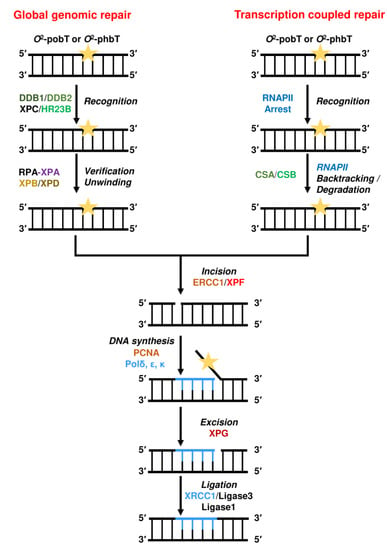
Figure 10.
Nucleotide excision repair (NER) of bulky DNA alkylation adducts. Depicted are both global genomic repair and transcription-coupled repair.
During TCR, the DNA lesion blocks the RNAPII, leading to assembly of CSA, CSB, and the transcription factor TFIIH at the site of the lesion. CSB uses its DNA translocase activity to remove the RNAPII complex from the lesion [238]. However, it is still unclear whether this is mediated by degradation or backtracking of the RNAPII. Nevertheless, after TFIIH is displaced from the lesion, the lesion becomes accessible and is excised by the exonucleases XPF–ERCC1 and XPG. Again, the DNA polymerases (δ, ε, and/or κ) together with DNA ligase I or the Ligase-III-XRCC1 complex restore the DNA.
Based on the NER mechanism, it seems obvious that especially bulky adducts might be subject to repair. However, as mentioned above, even bulky adducts such as pyridyloxobutyl (pob) DNA adducts are preferentially repaired by MGMT. Thus, multiple studies have shown that MGMT can remove the pob group from O6-pobG [239,240,241,242]. However, despite the efficient repair of O6-pobdG by MGMT, the mutation rate following exposure to NNKOAc was not affected in CHO cells [241]. NNKOAc (4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanol) is an activated form of NNAL, which is used experimentally because it forms exclusively pob adducts in the presence of cellular esterases. This study indicated that other large adducts, which are not repaired by MGMT and tolerated by translesion synthesis (TLS; see section below), may contribute to mutagenesis.
Besides O6-pobG, tobacco-specific nitrosamines also generate other pyridylbutylating products and the corresponding pyridylhydroxybutylating (phb) products [240,243,244,245,246]. These are the O-adducts O2-[4-(3-pyridyl)-4-oxobut-1-yl]-cytosine (O2-pobC), O2-[4-(3-pyridyl)-4-oxobut-1-yl]-2′-deoxythymidine (O2-pobT), O4-[4-(3-pyridyl)-4-oxobut-1-yl]-thymidine (O4-pobT), O4-[4-(3-pyridyl)-4-hydroxylbut-1-yl]-thymidine (O4-phbT), O2-[4-(3-pyridyl)-4-hydroxylbut-1-yl]-thymidine (O2-phbT), and O6-[4-(3-pyridyl)-4-hydroxylbut-1-yl]-2’-deoxyguanosine (O6-phbG) and the N-adducts N7-pobG, N7-phbG, N6-(4-oxo-4-(3-pyridyl)-1-butyl)-2′-deoxyadenosine (N6-pobA), and N6-(4-hydroxy-4-(3-pyridyl)-1-butyl)-2′-deoxyadenosine (N6-phbA). Furthermore, pyridylbutylating and pyridylhydroxybutylating products on the phosphate backbone were detected [247,248].
Using an in vitro NER assay with pyridyloxobutylated plasmid DNA as a substrate, it was shown that nuclear extracts from human lymphoid cell lines deficient in XPA and XPC were less active at repairing pyridyloxobutyl adducts than extracts from normal cells. Moreover, the NER-deficient cells were hypersensitive to NNKOAc [249]. However, as, in the used plasmids, 7-pobG, O2-pobC, O2-pobT, and O6-pobG comprised 31.2%, 22.7%, 25.5%, and 20.6% of the total POB adducts, respectively, an association of these effects with a specific adduct was not possible. Additionally, LC-MS/MS-based measurement of these four pob-adducts was compared in MGMT-deficient, MGMT/BER-deficient, and MGMT/NER (XPD)-deficient CHO cells upon NNKOAc treatment [241]. The results showed a reduced repair of only O2-pobT in NER-deficient cells. The reduced repair was associated with increased AT→TA transversions, suggesting that these mutations are caused by O2-pobdT. The differential repair of pob-adducts may also be responsible for differences in the susceptibility to NNK-induced carcinogenesis in different organs of the mouse. Thus, binding of XPA and XPB to pob adducts was increased in liver extracts following NNK treatment, whereas it was decreased in lung extracts [250].
Similar results were also observed for pyridylhydroxybutylated adducts. A recent study showed that, among the different PHB adducts induced by NNALOAc (4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol), NER counteracts the formation of O2-phbdT and O4-phbdT, whereas MGMT repairs predominantly O6-phbdG and, with lesser efficiency, O4-phbdT [251].
NER also seems to be involved in the repair of smaller adducts. Initial results indicating a removal of O6-n-butyl-guanine (O6-BuG) by NER were obtained in bacteria [252,253]. Further experiments in DNA repair-deficient eukaryotic cells also showed that the repair of O6-BuG (induced by N-n-butyl-N-nitrosourea (BNU)) was not correlated with MGMT activity, but rather with NER efficiency [254,255,256]. In line with this, XPA-deficient cells showed a decreased bypass efficiency of branched-chain lesions (O6-iPr-dG and O6-iBu-dG, O6-sBu-dG) but not on their straight-chain counterparts (O6-nPr-dG and O6-nBu-dG) [257].
Using MGMT or NER-deficient cells, it was shown that O6-EtG was repaired predominantly by MGMT and more slowly by NER [258]. In opposition to that, O2-EtG and O4-EtG were neither repaired by MGMT nor NER. Moreover, MGMT and NER participate in the repair of O6-carboxymethylguanine (O6-CMeG) [259,260]. This DNA alkylation adduct was found to be associated with red meat intake [18] and is induced by azaserine as well as N-nitrosoglycine [261,262]. Interestingly, a crosstalk between NER and BER on alkylated DNA bases has been observed recently. Here, UV-DDB (DDB1/DDB2 complex) was shown to recognize ϵA and stimulated AAG activity [187].
Overall, in contrast to the involvement of MGMT, ALKBH proteins, and BER, only limited data suggest an important role for NER in the repair of and protection against DNA alkylation damage.
2.5. Bypass by Translesion Synthesis (TLS)
With the exception of small methyl or ethyl adducts, larger and more complex O6-alkyl-dG lesions strongly block DNA replication. If not repaired, these lesions can cause DSBs and cell death. To avoid this, replication-blocking lesions can be tolerated by a specific bypass mechanism, the translesion synthesis (TLS) (Figure 11). As an example, knockdown of the TLS polymerase eta (Pol η) results in sensitivity to the chloroalkylating anticancer drugs fotemustine and CCNU in melanoma and glioblastoma cells, respectively [263], and knockout of Rev3L leads to hypersensitivity against TMZ and fotemustine [264]. Moreover, RAD18 is required for bypassing TMZ-induced O6MeG MMR intermediates in the S-phase [265].
TLS utilizes specified polymerases, namely Y-family polymerases (Pol η, Pol ι, Pol κ, and REV1), B-family polymerase (Pol ζ), and A-family polymerases (Pol θ and Pol ν), which can insert nucleotides opposite DNA lesions [266]. In this process, replicative polymerases such as polymerase delta (Pol δ) and polymerase epsilon (Pol ε) are blocked by the lesion. This leads to RAD18/UBE2A-dependent ubiquitination of PCNA at residue K164, and exchange of the replicative polymerase with one of the Y-family polymerases Pol η, Pol ι, or Pol κ. These polymerases can synthesize across the damaged DNA; however, due to their low processivity, they can insert only a few nucleotides [266]. In most cases, only one or two nucleotides are inserted and the Y-family polymerases are thereafter exchanged with the B-family polymerase zeta (Pol ζ, consisting of the subunits Rev3 and Rev7), which (together with Rev1) can extend the newly synthesized DNA. Pol θ can perform both insertion and extension. Finally, the TLS-polymerases are exchanged with the replicative polymerase and normal replication restarts [266]. However, the Y-family polymerases show a weak selectivity and exert no proofreading 3′→5′ exonuclease activity. Therefore, depending on the type of DNA lesion and TLS-polymerase, wrong nucleotides can be inserted and mutations may arise (Figure 11).
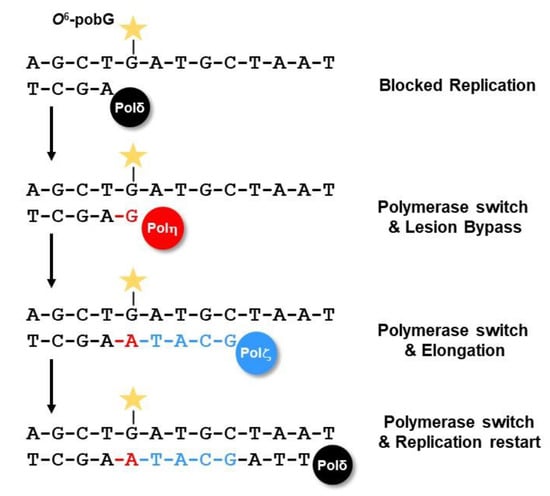
Figure 11.
DNA translesion synthesis.
2.5.1. Bypass of O6-alkyl-dG Lesions
Among all O6-alkyl-dG lesions, only O6-MeG and O6-EtG do not strongly block replication and can be bypassed by replicative polymerases [267]. However, it has been shown that also O6-MeG can slow down Pol α [268] and Pol δ [269] and that Pol η efficiently bypasses O6-MeG [269]. In this process, all polymerases can insert cytosine or thymine opposite to O6-MeG, with Pol η acting more accurately, i.e., inserting cytosine. Similar results are obtained for O6-EtG, which can slow down Pol α and Pol η, leading to error-prone synthesis by both polymerases [270].
In contrast, larger and more complex O6-alkyl-dG lesions strongly block replicative polymerases. Whereas O6-MeG did not impede DNA replication, the efficiency of the bypass decreased with the size of the alkyl group [257]. Moreover, the G→A mutation frequency also decreases with the adduct size. In both processes, branched-chain lesions (O6-iPr-dG and O6-iBu-dG, O6-sBu-dG) exerted stronger effects than their straight-chain counterparts (O6-nPr-dG and O6-nBu-dG). Concerning TLS, REV1 is involved in bypassing all of these lesions except O6-MeG. Whereas straight-chain lesions were mainly bypassed by Pol η and extension was performed by Pol ζ, the branched-chain lesions were predominantly bypassed by Pol ι and Pol κ.
Another O6-alkyl-dG lesion bypassed by TLS is O6-CMeG. Thus, O6-CmeG can be bypassed by Pol η in an error-prone manner and by Pol κ, which performs an error-free insertion [271]. The subsequent extension step was carried out by Pol η, Pol κ, and Pol ζ.
Data indicate that bulky pyridylbutylating products are also subject to TLS if not repaired by MGMT or NER. Thus, a strong blockage of replicative polymerases was also observed for O6-pobG, which can be bypassed by Pol η and induces G→A transitions [272]. Furthermore, using recombinant REV1, a one-base incorporation opposite O6-MeG and O6-BzG but not O6-pobG was observed [273]. In this case, REV1 preferentially incorporated dCTP opposite O6-MeG and O6-BzG.
2.5.2. Bypass of O2/O4-alkyl-dT Lesions
In opposition to O6-MeG, O2-MeT and O4-MeT have been shown to strongly block DNA replication. Moreover, O4-alkylthymidines have been shown to primarily induce T→C mutations [274], and the exonuclease-free Klenow fragment of E. coli DNA polymerase I was shown to incorporate both dATP and dTTP opposite O2-ethylthymidine [275]. Using different recombinant polymerases, TLS of O2/O4-alkyl-dT lesions was further analyzed. In the case of O2-alkyl-dT lesions, straight-chain lesions (O2-methylthymidine (O2-MeT), O2-ethylthymidine (O2-EtT), O2-propylthymidine (O2-PrT), O2-butylthymidine (O2-BuT)) and corresponding branched-chain lesions (O2-iPrT, O2-nBuT, O2-iBuT, O2-sBuT) were bypassed by Pol η and, to a lesser degree, Pol κ, but not by Pol ι [276]. Moreover, Pol η was more efficient in incorporating the correct nucleotide opposite O2-alkyldT lesions with a branched-chain alkyl group than the corresponding lesions with a straight-chain alkyl group [276]. In the case of O4-alkyldT, all straight-chain lesions besides O4-MeT and all branched-chain lesions were shown to moderately block DNA replication and to be bypassed by Pol η or Pol ζ, but not by Pol κ or Pol ι [277]. A replication block was also observed for O2-pobT and O4-pobT, which can be bypassed by Pol η and Pol ζ and predominantly induce T→A transversion and T→C transition [278]. Compared to O6-pobG, this block was more pronounced.
2.5.3. Bypass of N-alkyl-dG Lesions
As mentioned above, among methylated nucleotides, N1-MeA and N3-MeC are the most critical replication-blocking lesions. Therefore, it is obvious that these lesions are also subject to TLS. It has been shown that multiple TLS polymerases are involved in the bypass of N1-MeA [279,280]. (i) Pol ι inserts a nucleotide opposite N1-MeA and Pol θ performs the extension. (ii) Pol η mediates both insertion and extension. (iii) Pol λ inserts a nucleotide opposite N1-MeA and Pol ζ performs the extension. In all cases, TLS is predominantly error-free. Similar results were obtained for N3-MeA. Using 3-deaza-3-methyladenine (3-dMeA), a stable analog of N3-MeA, three different mechanisms were described [281]. (i) Pol ι inserts a nucleotide opposite N3-MeA and Pol κ extends synthesis. (ii) Pol θ can perform both insertion and extension. (iii) Pol ζ extends synthesis by an as-yet-unidentified polymerase.
Using different recombinant polymerases, Choi and Guengerich analyzed TLS of oligonucleotides containing guanine differentially methylated at the N2-position (N2-methylguanine (N2-MeG), N2-ethylguanine (N2-EtG), N2,N2-dimethylguanine (N2,N2-diMeG), N2-isobutylguanine (N2-IbG), N2-benzylguanine (N2-BzG), N2-naphtylguanine (N2-NaphG), N2-9-anthracenylguanine (N2-AnthG), or N2-6-benzo[a]pyrenylguanine (N2-BPG)) [272,273,282]. The data indicate that Pol η effectively bypassed N2-MeG, N2-EtG N2-IbG, N2-BzG, and N2-NaphG, but not N2-AnthG and N2-BPG, whereas Pol δ only bypassed N2-MeG and N2-EtG [282]. Pol ι bypassed N2-MeG as well as N2-EtG, and partially bypassed N2-IbG, N2-BzG, and N2-NaphG, but was blocked by N2-AnthG and N2-BPG [283]. Misinsertion of T increased with the adduct size. Besides Pol η and Pol ι, Pol κ also mediated the bypass of N2-MeG, N2-EtG, N2,N2-diMeG, N2-IbG, N2-BzG, N2-NaphG, N2-AnthG, and N2-BPG. Overall, Pol κ is more efficient than Pol η or Pol ι in incorporating dCTP opposite large N2-G adducts (N2-AnthG and N2-BPG) [284]. Finally, REV1 was shown to perform one base incorporation opposite N2-MeG, N2-EtG, N2,N2-diMeG, N2-IbG, N2-BzG, N2-NaphG, N2-AnthG, and N2-BPG [273]. REV1 preferentially incorporated dCTP opposite G and all of the modified G adducts, with a relatively low misinsertion frequency [273]. Another N2-adduct is 1,N2-Etheno(ε)guanine (εG). Whereas Pol δ was completely blocked by this adduct, Pols ι and κ showed similar rates of incorporation of dTTP and dCTP in vitro. Pol η was most active, and showed the highest error frequency [285]. Finally, εA has also been shown to be subject to TLS. Thus, in human cells, Pol ι can insert a nucleotide opposite εA and Pol ζ performs the extension [286]. Alternatively, Pol θ can perform both insertion and extension. In both cases, TLS is predominantly error-free.
3. Conclusions
Nitrosamines are genotoxic and carcinogenic compounds that occur not only in food, cosmetics, and tobacco smoke, but have also recently been found in drugs as by-products of the API synthesis or even as an API derivative. The DNA adduct formation pathways are well described for food-borne nitrosamines such as NDMA, the cosmetics-related nitrosamine NDELA, as well as tobacco-specific nitrosamines such as NNN and NNK. However, many other nitrosamines, particularly those found in drugs, are insufficiently studied so far with respect to DNA damage induction, or have hitherto even been unknown, thus leaving a data gap. More studies are required to understand the structure–genotoxicity relationship of more complex nitrosamines, such as nitrosamines with larger, branched alkyl residues and API-derived nitrosamines.
The DNA repair pathways involved in the removal of small DNA alkylation adducts, such as N7-MeG, N3-MeA, or O6-MeG, are comprehensively understood and mainly involve BER as well as direct damage reversal by MGMT, while ALKBH-mediated repair plays a minor role. The repair of larger O6-alky adducts caused by tobacco-specific nitrosamines, e.g., O6-pobG, is also well studied, requiring both MGMT and the NER pathway. In contrast to that, the repair of other DNA alkylation adducts induced, for example, by NDEA or NDELA is hardly characterized so far. Another knowledge gap concerns the repair of adducts generated by complex nitrosamines, such as those found as drug impurities. It is, therefore, necessary to investigate relevant DNA repair pathways in much more detail for those compounds. Such experimental data are eagerly awaited and will be instrumental for in silico approaches to predict the genotoxic (and carcinogenic) potency of poorly characterized or hitherto unknown nitrosamines. Finally, this will be helpful for the risk assessment of nitrosamines to derive AIs for drugs.
Author Contributions
Conceptualization, J.F. and M.C.; writing—original draft preparation, J.F. and M.C.; writing—review and editing, J.F. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the German Research Foundation DFG (FA1034/3-1 and FA1034/3-3, CH 665/8-1, SFB 1361/2 TP 05). The document has been produced by making use of the results under the specific contract no. 01 FWC EMA/2020/46/TDA/L1.02 between Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. and the European Medicines Agency. The opinions expressed are those of the contractor only and do not represent the European Medicines Agency. This document expresses the opinion of the authors of the paper and may not be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or one of its committees or working parties.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AAG | alkyladenine glycosylase |
| ALKBH | AlkB homolog |
| AP | apurinic/apyrimidinic |
| APE1 | AP endonuclease 1 |
| BER | base excision repair |
| CPDs | cyclobutane pyrimidine dimers |
| CS | Cockayne syndrome |
| CYP450 | cytochrome P450 |
| dRP | deoxyribose-5-phosphate |
| DSB | DNA double-strand break |
| ERCC1 | excision repair cross-complementing 1 |
| EXO1 | exonuclease 1 |
| FEN1 | flap endonuclease 1 |
| GG-NER | global genome NER |
| HR | homologous recombination |
| IR | ionizing radiation |
| MGMT | O6-methylguanine-DNA methyltransferase |
| MMR | mismatch repair |
| MPG | N-methylpurine-DNA glycosylase (alias AAG) |
| NER | nucleotide excision repair |
| OGG1 | 8-oxoguanine DNA glycosylase |
| PAR | poly(ADP-ribose) |
| PARP-1 | poly(ADP-ribose) polymerase-1 |
| PCNA | proliferating cellular nuclear antigen |
| Pol | DNA polymerase |
| RFC | replication factor C |
| RNAP | RNA polymerase |
| RPA | replication protein A |
| SSB | DNA single-strand break |
| TC-NER | transcription-coupled NER |
| TFIIH | transcription factor II H |
| TLS | translesion synthesis |
| TTD | trichothiodystrophy |
| XP | xeroderma pigmentosum |
| XRCC1 | X-ray repair cross-complementing protein 1 |
| 6-4PP | pyrimidine-(6,4)-pyrimidone photoproduct |
References
- Druckrey, H.; Preussmann, R.; Ivankovic, S.; Schmahl, D. Organotropic carcinogenic effects of 65 various N-nitroso- compounds on BD rats. Z. Fur Krebsforsch. 1967, 69, 103–201. [Google Scholar] [CrossRef]
- Beranek, D.T. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat. Res. 1990, 231, 11–30. [Google Scholar] [CrossRef]
- Kaina, B.; Christmann, M.; Naumann, S.; Roos, W.P. MGMT: Key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair 2007, 6, 1079–1099. [Google Scholar] [CrossRef] [PubMed]
- Loechler, E.L.; Green, C.L.; Essigmann, J.M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc. Natl. Acad. Sci. USA 1984, 81, 6271–6275. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.E.; Corrigan, J.J.; Armijo, A.L.; Nazari, I.S.; Kohale, I.N.; Torous, D.K.; Avlasevich, S.L.; Croy, R.G.; Wadduwage, D.N.; Carrasco, S.E.; et al. Excision of mutagenic replication-blocking lesions suppresses cancer but promotes cytotoxicity and lethality in nitrosamine-exposed mice. Cell Rep. 2021, 34, 108864. [Google Scholar] [CrossRef] [PubMed]
- Rydberg, B.; Lindahl, T. Nonenzymatic methylation of DNA by the intracellular methyl group donor S-adenosyl-L-methionine is a potentially mutagenic reaction. EMBO J. 1982, 1, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Taverna, P.; Sedgwick, B. Generation of an endogenous DNA-methylating agent by nitrosation in E. coli. J. Bacteriol. 1996, 178, 5105–5111. [Google Scholar] [CrossRef]
- Barrows, L.R.; Magee, P.N. Nonenzymatic methylation of DNA by S-adenosylmethionine in vitro. Carcinogenesis 1982, 3, 349–351. [Google Scholar] [CrossRef]
- Shuker, D.E.; Margison, G.P. Nitrosated glycine derivatives as a potential source of O6-methylguanine in DNA. Cancer Res 1997, 57, 366–369. [Google Scholar]
- Busby, W.F., Jr.; Shuker, D.E.; Charnley, G.; Newberne, P.M.; Tannenbaum, S.R.; Wogan, G.N. Carcinogenicity in rats of the nitrosated bile acid conjugates N-nitrosoglycocholic acid and N-nitrosotaurocholic acid. Cancer Res. 1985, 45, 1367–1371. [Google Scholar]
- Beard, J.C.; Swager, T.M. An Organic Chemist’s Guide to N-Nitrosamines: Their Structure, Reactivity, and Role as Contaminants. J. Org. Chem. 2021, 86, 2037–2057. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Approaches to cancer prevention based on an understanding of N-nitrosamine carcinogenesis. Proc. Soc. Exp. Biol. Medicine. Soc. Exp. Biol. Med. 1997, 216, 181–191. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 15 February 2023).
- Lijinsky, W. N-Nitroso compounds in the diet. Mutat. Res. 1999, 443, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Gushgari, A.J.; Halden, R.U. Critical review of major sources of human exposure to N-nitrosamines. Chemosphere 2018, 210, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Behsnilian, D.; Butz, P.; Greiner, R.; Lautenschlaeger, R. Process-induced undesirable compounds: Chances of non-thermal approaches. Meat Sci. 2014, 98, 392–403. [Google Scholar] [CrossRef]
- Fahrer, J.; Kaina, B. O6-methylguanine-DNA methyltransferase in the defense against N-nitroso compounds and colorectal cancer. Carcinogenesis 2013, 34, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.H.; Bailey, N.; Bandaletova, T.; Bowman, R.; Cross, A.J.; Pollock, J.; Shuker, D.E.; Bingham, S.A. Red meat enhances the colonic formation of the DNA adduct O6-carboxymethyl guanine: Implications for colorectal cancer risk. Cancer Res. 2006, 66, 1859–1865. [Google Scholar] [CrossRef]
- Joosen, A.M.; Kuhnle, G.G.; Aspinall, S.M.; Barrow, T.M.; Lecommandeur, E.; Azqueta, A.; Collins, A.R.; Bingham, S.A. Effect of processed and red meat on endogenous nitrosation and DNA damage. Carcinogenesis 2009, 30, 1402–1407. [Google Scholar] [CrossRef]
- Santarelli, R.L.; Vendeuvre, J.L.; Naud, N.; Tache, S.; Gueraud, F.; Viau, M.; Genot, C.; Corpet, D.E.; Pierre, F.H. Meat processing and colon carcinogenesis: Cooked, nitrite-treated, and oxidized high-heme cured meat promotes mucin-depleted foci in rats. Cancer Prev. Res. 2010, 3, 852–864. [Google Scholar] [CrossRef]
- Seiwert, N.; Adam, J.; Steinberg, P.; Wirtz, S.; Schwerdtle, T.; Adams-Quack, P.; Hovelmeyer, N.; Kaina, B.; Foersch, S.; Fahrer, J. Chronic intestinal inflammation drives colorectal tumor formation triggered by dietary heme iron in vivo. Arch. Toxicol. 2021, 95, 2507–2522. [Google Scholar] [CrossRef]
- Yamazaki, H.; Inui, Y.; Yun, C.H.; Guengerich, F.P.; Shimada, T. Cytochrome P450 2E1 and 2A6 enzymes as major catalysts for metabolic activation of N-nitrosodialkylamines and tobacco-related nitrosamines in human liver microsomes. Carcinogenesis 1992, 13, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Den Engelse, L.; Menkveld, G.J.; De Brij, R.J.; Tates, A.D. Formation and stability of alkylated pyrimidines and purines (including imidazole ring-opened 7-alkylguanine) and alkylphosphotriesters in liver DNA of adult rats treated with ethylnitrosourea or dimethylnitrosamine. Carcinogenesis 1986, 7, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Camus, A.M.; Geneste, O.; Honkakoski, P.; Bereziat, J.C.; Henderson, C.J.; Wolf, C.R.; Bartsch, H.; Lang, M.A. High variability of nitrosamine metabolism among individuals: Role of cytochromes P450 2A6 and 2E1 in the dealkylation of N-nitrosodimethylamine and N-nitrosodiethylamine in mice and humans. Mol. Carcinog. 1993, 7, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Verna, L.; Whysner, J.; Williams, G.M. N-nitrosodiethylamine mechanistic data and risk assessment: Bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol. Ther. 1996, 71, 57–81. [Google Scholar] [CrossRef] [PubMed]
- von Hofe, E.; Kleihues, P.; Keefer, L.K. Extent of DNA 2-hydroxyethylation by N-nitrosomethylethylamine and N-nitrosodiethylamine in vivo. Carcinogenesis 1986, 7, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hecht, S.S. Metabolic Activation and DNA Interactions of Carcinogenic N-Nitrosamines to Which Humans Are Commonly Exposed. Int. J. Mol. Sci. 2022, 23, 4559. [Google Scholar] [CrossRef]
- Schothorst, R.C.; Somers, H.H. Determination of N-nitrosodiethanolamine in cosmetic products by LC-MS-MS. Anal. Bioanal. Chem. 2005, 381, 681–685. [Google Scholar] [CrossRef]
- Liu, Y.; Glatt, H. Mutagenicity of N-nitrosodiethanolamine in a V79-derived cell line expressing two human biotransformation enzymes. Mutat. Res. 2008, 643, 64–69. [Google Scholar] [CrossRef]
- Loeppky, R.N.; Goelzer, P. Microsome-mediated oxidation of N-nitrosodiethanolamine (NDELA), a bident carcinogen. Chem. Res. Toxicol. 2002, 15, 457–469. [Google Scholar] [CrossRef]
- Loeppky, R.N.; Ye, Q.; Goelzer, P.; Chen, Y. DNA adducts from N-nitrosodiethanolamine and related beta-oxidized nitrosamines in vivo: (32)P-postlabeling methods for glyoxal- and O(6)-hydroxyethyldeoxyguanosine adducts. Chem. Res. Toxicol. 2002, 15, 470–482. [Google Scholar] [CrossRef]
- Hecht, S.S. Progress and challenges in selected areas of tobacco carcinogenesis. Chem. Res. Toxicol. 2008, 21, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S.; Chen, C.B.; Hirota, N.; Ornaf, R.M.; Tso, T.C.; Hoffmann, D. Tobacco-specific nitrosamines: Formation from nicotine in vitro and during tobacco curing and carcinogenicity in strain A mice. J. Natl. Cancer Inst. 1978, 60, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S.; Rivenson, A.; Braley, J.; DiBello, J.; Adams, J.D.; Hoffmann, D. Induction of oral cavity tumors in F344 rats by tobacco-specific nitrosamines and snuff. Cancer Res. 1986, 46, 4162–4166. [Google Scholar] [PubMed]
- Hecht, S.S.; Hoffmann, D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis 1988, 9, 875–884. [Google Scholar] [CrossRef] [PubMed]
- DeVore, N.M.; Scott, E.E. Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone binding and access channel in human cytochrome P450 2A6 and 2A13 enzymes. J. Biol. Chem. 2012, 287, 26576–26585. [Google Scholar] [CrossRef]
- Wong, H.L.; Zhang, X.; Zhang, Q.Y.; Gu, J.; Ding, X.; Hecht, S.S.; Murphy, S.E. Metabolic activation of the tobacco carcinogen 4-(methylnitrosamino)-(3-pyridyl)-1-butanone by cytochrome P450 2A13 in human fetal nasal microsomes. Chem. Res. Toxicol. 2005, 18, 913–918. [Google Scholar] [CrossRef]
- Jalas, J.R.; Ding, X.; Murphy, S.E. Comparative metabolism of the tobacco-specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol by rat cytochrome P450 2A3 and human cytochrome P450 2A13. Drug Metab. Dispos. Biol. Fate Chem. 2003, 31, 1199–1202. [Google Scholar] [CrossRef]
- Li, Y.; Hecht, S.S. Metabolism and DNA Adduct Formation of Tobacco-Specific N-Nitrosamines. Int. J. Mol. Sci. 2022, 23, 5109. [Google Scholar] [CrossRef]
- Lao, Y.; Villalta, P.W.; Sturla, S.J.; Wang, M.; Hecht, S.S. Quantitation of pyridyloxobutyl DNA adducts of tobacco-specific nitrosamines in rat tissue DNA by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem. Res. Toxicol. 2006, 19, 674–682. [Google Scholar] [CrossRef]
- Patten, C.J.; Smith, T.J.; Friesen, M.J.; Tynes, R.E.; Yang, C.S.; Murphy, S.E. Evidence for cytochrome P450 2A6 and 3A4 as major catalysts for N’-nitrosonornicotine alpha-hydroxylation by human liver microsomes. Carcinogenesis 1997, 18, 1623–1630. [Google Scholar] [CrossRef]
- Hecht, S.S. DNA adduct formation from tobacco-specific N-nitrosamines. Mutat. Res. 1999, 424, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S.; Trushin, N.; Castonguay, A.; Rivenson, A. Comparative tumorigenicity and DNA methylation in F344 rats by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N-nitrosodimethylamine. Cancer Res. 1986, 46, 498–502. [Google Scholar] [PubMed]
- Trushin, N.; Rivenson, A.; Hecht, S.S. Evidence supporting the role of DNA pyridyloxobutylation in rat nasal carcinogenesis by tobacco-specific nitrosamines. Cancer Res. 1994, 54, 1205–1211. [Google Scholar] [PubMed]
- Peterson, L.A.; Hecht, S.S. O6-methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res. 1991, 51, 5557–5564. [Google Scholar] [PubMed]
- Shen, L.; Kondo, Y.; Rosner, G.L.; Xiao, L.; Hernandez, N.S.; Vilaythong, J.; Houlihan, P.S.; Krouse, R.S.; Prasad, A.R.; Einspahr, J.G.; et al. MGMT promoter methylation and field defect in sporadic colorectal cancer. J. Natl. Cancer Inst. 2005, 97, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- FDA. Laboratory Analysis of Valsartan Products. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-analysis-valsartan-products (accessed on 21 December 2022).
- EMA. EMA Update on Metformin Diabetes Medicines. Available online: https://www.ema.europa.eu/en/news/ema-update-metformin-diabetes-medicines (accessed on 21 December 2022).
- Keire, D.A.; Bream, R.; Wollein, U.; Schmaler-Ripcke, J.; Burchardt, A.; Conti, M.; Zmyslowski, A.; Keizers, P.; Morin, J.; Poh, J.; et al. International Regulatory Collaboration on the Analysis of Nitrosamines in Metformin-Containing Medicines. AAPS J. 2022, 24, 56. [Google Scholar] [CrossRef] [PubMed]
- EMA. Guideline ICH M7 (R1)—Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk; EMA: London, UK, 2015. [Google Scholar]
- Tuesuwan, B.; Vongsutilers, V. Nitrosamine Contamination in Pharmaceuticals: Threat, Impact, and Control. J. Pharm. Sci. 2021, 110, 3118–3128. [Google Scholar] [CrossRef]
- FDA. Control of Nitrosamine Impurities in Human Drugs. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/control-nitrosamine-impurities-human-drugs (accessed on 21 December 2022).
- FDA. Pfizer Expands Voluntary Nationwide Recall to include All Lots of CHANTIX® (Varenicline) Tablets Due to N-Nitroso Varenicline Content. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/pfizer-expands-voluntary-nationwide-recall-include-all-lots-chantixr-varenicline-tablets-due-n (accessed on 21 December 2022).
- HSA. Recall of Ventolin 2mg Tablets. Available online: https://www.hsa.gov.sg/announcements/product-recall/recall-of-ventolin-2mg-tablets (accessed on 21 December 2022).
- Skipper, H.E.; Schabel, F.M., Jr.; Trader, M.W.; Thomson, J.R. Experimental evaluation of potential anticancer agents. VI. Anatomical distribution of leukemic cells and failure of chemotherapy. Cancer Res. 1961, 21, 1154–1164. [Google Scholar]
- Brulikova, L.; Hlavac, J.; Hradil, P. DNA interstrand cross-linking agents and their chemotherapeutic potential. Curr. Med. Chem. 2012, 19, 364–385. [Google Scholar] [CrossRef]
- Ludlum, D.B. DNA alkylation by the haloethylnitrosoureas: Nature of modifications produced and their enzymatic repair or removal. Mutat. Res. 1990, 233, 117–126. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Stevens, M.F.; Bradshaw, T.D. Temozolomide: Mechanisms of action, repair and resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Glaab, W.E.; Tindall, K.R.; Skopek, T.R. Specificity of mutations induced by methyl methanesulfonate in mismatch repair-deficient human cancer cell lines. Mutat. Res. 1999, 427, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Tano, K.; Shiota, S.; Collier, J.; Foote, R.S.; Mitra, S. Isolation and structural characterization of a cDNA clone encoding the human DNA repair protein for O6-alkylguanine. Proc. Natl. Acad. Sci. USA 1990, 87, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Rydberg, B.; Spurr, N.; Karran, P. cDNA cloning and chromosomal assignment of the human O6-methylguanine-DNA methyltransferase. cDNA expression in E. coli and gene expression in human cells. J. Biol. Chem. 1990, 265, 9563–9569. [Google Scholar] [CrossRef] [PubMed]
- Sassanfar, M.; Dosanjh, M.K.; Essigmann, J.M.; Samson, L. Relative efficiencies of the bacterial, yeast, and human DNA methyltransferases for the repair of O6-methylguanine and O4-methylthymine. Suggestive evidence for O4-methylthymine repair by eukaryotic methyltransferases. J. Biol. Chem. 1991, 266, 2767–2771. [Google Scholar] [CrossRef]
- Koike, G.; Maki, H.; Takeya, H.; Hayakawa, H.; Sekiguchi, M. Purification, structure, and biochemical properties of human O6-methylguanine-DNA methyltransferase. J. Biol. Chem. 1990, 265, 14754–14762. [Google Scholar] [CrossRef]
- Zak, P.; Kleibl, K.; Laval, F. Repair of O6-methylguanine and O4-methylthymine by the human and rat O6-methylguanine-DNA methyltransferases. J. Biol. Chem. 1994, 269, 730–733. [Google Scholar] [CrossRef]
- Pegg, A.E. Mammalian O6-alkylguanine-DNA alkyltransferase: Regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990, 50, 6119–6129. [Google Scholar] [PubMed]
- Pegg, A.E. Repair of O(6)-alkylguanine by alkyltransferases. Mutat. Res. 2000, 462, 83–100. [Google Scholar] [CrossRef]
- Peterson, L.A.; Liu, X.K.; Hecht, S.S. Pyridyloxobutyl DNA adducts inhibit the repair of O6-methylguanine. Cancer Res. 1993, 53, 2780–2785. [Google Scholar]
- Wang, L.; Spratt, T.E.; Liu, X.K.; Hecht, S.S.; Pegg, A.E.; Peterson, L.A. Pyridyloxobutyl adduct O6-[4-oxo-4-(3-pyridyl)butyl]guanine is present in 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone-treated DNA and is a substrate for O6-alkylguanine-DNA alkyltransferase. Chem. Res. Toxicol. 1997, 10, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Spratt, T.E.; Pegg, A.E.; Peterson, L.A. Synthesis of DNA oligonucleotides containing site-specifically incorporated O6-[4-oxo-4-(3-pyridyl)butyl]guanine and their reaction with O6-alkylguanine-DNA alkyltransferase. Chem. Res. Toxicol. 1999, 12, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Thomson, N.M.; Kenney, P.M.; Peterson, L.A. The pyridyloxobutyl DNA adduct, O6-[4-oxo-4-(3-pyridyl)butyl]guanine, is detected in tissues from 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-treated A/J mice. Chem. Res. Toxicol. 2003, 16, 1–6. [Google Scholar] [CrossRef]
- Pauly, G.T.; Peterson, L.A.; Moschel, R.C. Mutagenesis by O(6)-[4-oxo-4-(3-pyridyl)butyl]guanine in E. coli and human cells. Chem. Res. Toxicol. 2002, 15, 165–169. [Google Scholar] [CrossRef]
- Kleihues, P.; Margison, G.P. Carcinogenicity of N-methyl-N-nitrosourea: Possible role of excision repair of O6-methylguanine from DNA. J. Natl. Cancer Inst. 1974, 53, 1839–1841. [Google Scholar]
- Becker, K.; Dosch, J.; Gregel, C.M.; Martin, B.A.; Kaina, B. Targeted expression of human O(6)-methylguanine-DNA methyltransferase (MGMT) in transgenic mice protects against tumor initiation in two-stage skin carcinogenesis. Cancer Res. 1996, 56, 3244–3249. [Google Scholar]
- Becker, K.; Gregel, C.; Fricke, C.; Komitowski, D.; Dosch, J.; Kaina, B. DNA repair protein MGMT protects against N-methyl-N-nitrosourea-induced conversion of benign into malignant tumors. Carcinogenesis 2003, 24, 541–546. [Google Scholar] [CrossRef]
- Becker, K.; Gregel, C.M.; Kaina, B. The DNA repair protein O6-methylguanine-DNA methyltransferase protects against skin tumor formation induced by antineoplastic chloroethylnitrosourea. Cancer Res. 1997, 57, 3335–3338. [Google Scholar]
- Nakatsuru, Y.; Matsukuma, S.; Nemoto, N.; Sugano, H.; Sekiguchi, M.; Ishikawa, T. O6-methylguanine-DNA methyltransferase protects against nitrosamine-induced hepatocarcinogenesis. Proc. Natl. Acad. Sci. USA 1993, 90, 6468–6472. [Google Scholar] [CrossRef]
- Sakumi, K.; Shiraishi, A.; Shimizu, S.; Tsuzuki, T.; Ishikawa, T.; Sekiguchi, M. Methylnitrosourea-induced tumorigenesis in MGMT gene knockout mice. Cancer Res. 1997, 57, 2415–2418. [Google Scholar] [PubMed]
- Liu, L.; Qin, X.; Gerson, S.L. Reduced lung tumorigenesis in human methylguanine DNA--methyltransferase transgenic mice achieved by expression of transgene within the target cell. Carcinogenesis 1999, 20, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Dumenco, L.L.; Allay, E.; Norton, K.; Gerson, S.L. The prevention of thymic lymphomas in transgenic mice by human O6-alkylguanine-DNA alkyltransferase. Science 1993, 259, 219–222. [Google Scholar] [CrossRef]
- Liu, L.; Allay, E.; Dumenco, L.L.; Gerson, S.L. Rapid repair of O6-methylguanine-DNA adducts protects transgenic mice from N-methylnitrosourea-induced thymic lymphomas. Cancer Res. 1994, 54, 4648–4652. [Google Scholar] [PubMed]
- Wirtz, S.; Nagel, G.; Eshkind, L.; Neurath, M.F.; Samson, L.D.; Kaina, B. Both base excision repair and O6-methylguanine-DNA methyltransferase protect against methylation-induced colon carcinogenesis. Carcinogenesis 2010, 31, 2111–2117. [Google Scholar] [CrossRef]
- Bugni, J.M.; Meira, L.B.; Samson, L.D. Alkylation-induced colon tumorigenesis in mice deficient in the Mgmt and Msh6 proteins. Oncogene 2009, 28, 734–741. [Google Scholar] [CrossRef]
- Fahrer, J.; Frisch, J.; Nagel, G.; Kraus, A.; Dörsam, B.; Thomas, A.D.; Reissig, S.; Waisman, A.; Kaina, B. DNA repair by MGMT, but not AAG, causes a threshold in alkylation-induced colorectal carcinogenesis. Carcinogenesis 2015, 36, 1235–1244. [Google Scholar] [CrossRef]
- Kraus, A.; McKeague, M.; Seiwert, N.; Nagel, G.; Geisen, S.M.; Ziegler, N.; Trantakis, I.A.; Kaina, B.; Thomas, A.D.; Sturla, S.J.; et al. Immunological and mass spectrometry-based approaches to determine thresholds of the mutagenic DNA adduct O(6)-methylguanine in vivo. Arch. Toxicol. 2019, 93, 559–572. [Google Scholar] [CrossRef]
- Sandercock, L.E.; Hahn, J.N.; Li, L.; Luchman, H.A.; Giesbrecht, J.L.; Peterson, L.A.; Jirik, F.R. Mgmt deficiency alters the in vivo mutational spectrum of tissues exposed to the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Carcinogenesis 2008, 29, 866–874. [Google Scholar] [CrossRef]
- Gerson, S.L. Clinical relevance of MGMT in the treatment of cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 2388–2399. [Google Scholar] [CrossRef]
- Hegi, M.E.; Liu, L.; Herman, J.G.; Stupp, R.; Wick, W.; Weller, M.; Mehta, M.P.; Gilbert, M.R. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Kaina, B.; Christmann, M. DNA repair in resistance to alkylating anticancer drugs. Int. J. Clin. Pharmacol. Ther. 2002, 40, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Xu-Welliver, M.; Pegg, A.E. Degradation of the alkylated form of the DNA repair protein, O(6)-alkylguanine-DNA alkyltransferase. Carcinogenesis 2002, 23, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Kawate, H.; Sakumi, K.; Tsuzuki, T.; Nakatsuru, Y.; Ishikawa, T.; Takahashi, S.; Takano, H.; Noda, T.; Sekiguchi, M. Separation of killing and tumorigenic effects of an alkylating agent in mice defective in two of the DNA repair genes. Proc. Natl. Acad. Sci. USA 1998, 95, 5116–5120. [Google Scholar] [CrossRef]
- Eadie, J.S.; Conrad, M.; Toorchen, D.; Topal, M.D. Mechanism of mutagenesis by O6-methylguanine. Nature 1984, 308, 201–203. [Google Scholar] [CrossRef]
- Duckett, D.R.; Drummond, J.T.; Murchie, A.I.H.; Reardon, J.T.; Sancar, A.; Lilley, D.M.J.; Modrich, P. Human MutSalpha recognizes damaged DNA base pairs containing O6-methylguanine, O4-methylthymine, or the cisplatin-d(GpG) adduct. Proc. Natl. Acad. Sci. USA 1996, 93, 6443–6447. [Google Scholar] [CrossRef]
- Mojas, N.; Lopes, M.; Jiricny, J. Mismatch repair-dependent processing of methylation damage gives rise to persistent single-stranded gaps in newly replicated DNA. Genes Dev. 2007, 21, 3342–3355. [Google Scholar] [CrossRef]
- Ochs, K.; Kaina, B. Apoptosis induced by DNA damage O6-methylguanine is Bcl-2 and caspase-9/3 regulated and Fas/caspase-8 independent. Cancer Res. 2000, 60, 5815–5824. [Google Scholar]
- Vilenchik, M.M.; Knudson, A.G. Endogenous DNA double-strand breaks: Production, fidelity of repair, and induction of cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 12871–12876. [Google Scholar] [CrossRef]
- Stratenwerth, B.; Geisen, S.M.; He, Y.; Beltzig, L.; Sturla, S.J.; Kaina, B. Molecular Dosimetry of Temozolomide: Quantification of Critical Lesions, Correlation to Cell Death Responses, and Threshold Doses. Mol. Cancer Ther. 2021, 20, 1789–1799. [Google Scholar] [CrossRef]
- Roos, W.P.; Nikolova, T.; Quiros, S.; Naumann, S.C.; Kiedron, O.; Zdzienicka, M.Z.; Kaina, B. Brca2/Xrcc2 dependent HR, but not NHEJ, is required for protection against O(6)-methylguanine triggered apoptosis, DSBs and chromosomal aberrations by a process leading to SCEs. DNA Repair 2009, 8, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Quiros, S.; Roos, W.P.; Kaina, B. Processing of O6-methylguanine into DNA double-strand breaks requires two rounds of replication whereas apoptosis is also induced in subsequent cell cycles. Cell Cycle 2010, 9, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Aasland, D.; Gotzinger, L.; Hauck, L.; Berte, N.; Meyer, J.; Effenberger, M.; Schneider, S.; Reuber, E.E.; Roos, W.P.; Tomicic, M.T.; et al. Temozolomide Induces Senescence and Repression of DNA Repair Pathways in Glioblastoma Cells via Activation of ATR-CHK1, p21, and NF-kappaB. Cancer Res. 2019, 79, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, C.; Tatsch, L.; Brandstetter Vilar, J.; Rasenberger, B.; Beltzig, L.; Kaina, B.; Tomicic, M.T.; Christmann, M. Targeting c-IAP1, c-IAP2, and Bcl-2 Eliminates Senescent Glioblastoma Cells Following Temozolomide Treatment. Cancers 2021, 13, 3585. [Google Scholar] [CrossRef] [PubMed]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013, 332, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Margison, G.P.; Povey, A.C.; Kaina, B.; Santibanez Koref, M.F. Variability and regulation of O(6)-alkylguanine-DNA alkyltransferase. Carcinogenesis 2003, 24, 625–635. [Google Scholar] [CrossRef]
- Povey, A.C.; Margison, G.P.; Santibanez-Koref, M.F. Lung cancer risk and variation in MGMT activity and sequence. DNA Repair 2007, 6, 1134–1144. [Google Scholar] [CrossRef]
- Grombacher, T.; Eichhorn, U.; Kaina, B. p53 is involved in regulation of the DNA repair gene O6-methylguanine-DNA methyltransferase (MGMT) by DNA damaging agents. Oncogene 1998, 17, 845–851. [Google Scholar] [CrossRef]
- Harris, L.C.; Remack, J.S.; Houghton, P.J.; Brent, T.P. Wild-type p53 suppresses transcription of the human O6-methylguanine-DNA methyltransferase gene. Cancer Res. 1996, 56, 2029–2032. [Google Scholar]
- Blough, M.D.; Zlatescu, M.C.; Cairncross, J.G. O6-methylguanine-DNA methyltransferase regulation by p53 in astrocytic cells. Cancer Res. 2007, 67, 580–584. [Google Scholar] [CrossRef]
- Srivenugopal, K.S.; Shou, J.; Mullapudi, S.R.; Lang, F.F., Jr.; Rao, J.S.; Ali-Osman, F. Enforced expression of wild-type p53 curtails the transcription of the O(6)-methylguanine-DNA methyltransferase gene in human tumor cells and enhances their sensitivity to alkylating agents. Clin. Cancer Res. 2001, 7, 1398–1409. [Google Scholar] [PubMed]
- Bocangel, D.; Sengupta, S.; Mitra, S.; Bhakat, K.K. p53-Mediated down-regulation of the human DNA repair gene O6-methylguanine-DNA methyltransferase (MGMT) via interaction with Sp1 transcription factor. Anticancer. Res. 2009, 29, 3741–3750. [Google Scholar] [PubMed]
- Christmann, M.; Kaina, B. Transcriptional regulation of human DNA repair genes following genotoxic stress: Trigger mechanisms, inducible responses and genotoxic adaptation. Nucleic Acids Res. 2013, 41, 8403–8420. [Google Scholar] [CrossRef]
- Fritz, G.; Tano, K.; Mitra, S.; Kaina, B. Inducibility of the DNA repair gene encoding O6-methylguanine-DNA methyltransferase in mammalian cells by DNA-damaging treatments. Mol. Cell. Biol. 1991, 11, 4660–4668. [Google Scholar]
- Grombacher, T.; Kaina, B. Constitutive expression and inducibility of O6-methylguanine-DNA methyltransferase and N-methylpurine-DNA glycosylase in rat liver cells exhibiting different status of differentiation. Biochim. Et Biophys. Acta 1995, 1270, 63–72. [Google Scholar] [CrossRef]
- Grombacher, T.; Mitra, S.; Kaina, B. Induction of the alkyltransferase (MGMT) gene by DNA damaging agents and the glucocorticoid dexamethasone and comparison with the response of base excision repair genes. Carcinogenesis 1996, 17, 2329–2336. [Google Scholar] [CrossRef]
- Rafferty, J.A.; Clarke, A.R.; Sellappan, D.; Koref, M.S.; Frayling, I.M.; Margison, G.P. Induction of murine O6-alkylguanine-DNA-alkyltransferase in response to ionising radiation is p53 gene dose dependent. Oncogene 1996, 12, 693–697. [Google Scholar]
- Boldogh, I.; Ramana, C.V.; Chen, Z.; Biswas, T.; Hazra, T.K.; Grosch, S.; Grombacher, T.; Mitra, S.; Kaina, B. Regulation of expression of the DNA repair gene O6-methylguanine-DNA methyltransferase via protein kinase C-mediated signaling. Cancer Res. 1998, 58, 3950–3956. [Google Scholar]
- Aasland, D.; Reich, T.R.; Tomicic, M.T.; Switzeny, O.J.; Kaina, B.; Christmann, M. Repair gene O(6)-methylguanine-DNA methyltransferase is controlled by SP1 and up-regulated by glucocorticoids, but not by temozolomide and radiation. J. Neurochem. 2018, 144, 139–151. [Google Scholar] [CrossRef]
- Costello, J.F.; Futscher, B.W.; Kroes, R.A.; Pieper, R.O. Methylation-related chromatin structure is associated with exclusion of transcription factors from and suppressed expression of the O-6-methylguanine DNA methyltransferase gene in human glioma cell lines. Mol. Cell. Biol. 1994, 14, 6515–6521. [Google Scholar]
- Costello, J.F.; Futscher, B.W.; Tano, K.; Graunke, D.M.; Pieper, R.O. Graded methylation in the promoter and body of the O6-methylguanine DNA methyltransferase (MGMT) gene correlates with MGMT expression in human glioma cells. J. Biol. Chem. 1994, 269, 17228–17237. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.C.; Potter, P.M.; Tano, K.; Shiota, S.; Mitra, S.; Brent, T.P. Characterization of the promoter region of the human O6-methylguanine-DNA methyltransferase gene. Nucleic Acids Res. 1991, 19, 6163–6167. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; von Wronski, M.A.; Brent, T.P. Localization of methylation sites in the human O6-methylguanine-DNA methyltransferase promoter: Correlation with gene suppression. Carcinogenesis 1995, 16, 1385–1390. [Google Scholar] [CrossRef]
- Qian, X.C.; Brent, T.P. Methylation hot spots in the 5’ flanking region denote silencing of the O6-methylguanine-DNA methyltransferase gene. Cancer Res. 1997, 57, 3672–3677. [Google Scholar] [PubMed]
- Christmann, M.; Verbeek, B.; Roos, W.P.; Kaina, B. O(6)-Methylguanine-DNA methyltransferase (MGMT) in normal tissues and tumors: Enzyme activity, promoter methylation and immunohistochemistry. Biochim. Et Biophys. Acta 2011, 1816, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Janssen, K.; Eichhorn-Grombacher, U.; Schlink, K.; Nitzsche, S.; Oesch, F.; Kaina, B. Long-time expression of DNA repair enzymes MGMT and APE in human peripheral blood mononuclear cells. Arch. Toxicol. 2001, 75, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Krokan, H.; Haugen, A.; Myrnes, B.; Guddal, P.H. Repair of premutagenic DNA lesions in human fetal tissues: Evidence for low levels of O6-methylguanine-DNA methyltransferase and uracil-DNA glycosylase activity in some tissues. Carcinogenesis 1983, 4, 1559–1564. [Google Scholar] [CrossRef]
- Briegert, M.; Enk, A.H.; Kaina, B. Change in expression of MGMT during maturation of human monocytes into dendritic cells. DNA Repair 2007, 6, 1255–1263. [Google Scholar] [CrossRef]
- Christmann, M.; Nagel, G.; Horn, S.; Krahn, U.; Wiewrodt, D.; Sommer, C.; Kaina, B. MGMT activity, promoter methylation and immunohistochemistry of pretreatment and recurrent malignant gliomas: A comparative study on astrocytoma and glioblastoma. Int. J. Cancer. J. Int. Du Cancer 2010, 127, 2106–2118. [Google Scholar] [CrossRef]
- Kaina, B.; Christmann, M. DNA repair in personalized brain cancer therapy with temozolomide and nitrosoureas. DNA Repair 2019, 78, 128–141. [Google Scholar] [CrossRef]
- Vilar, J.B.; Christmann, M.; Tomicic, M.T. Alterations in Molecular Profiles Affecting Glioblastoma Resistance to Radiochemotherapy: Where Does the Good Go? Cancers 2022, 14, 2416. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Velu, C.S.; Smith, Q.R.; Bhat, G.J.; Srivenugopal, K.S. Increased expression of the MGMT repair protein mediated by cysteine prodrugs and chemopreventative natural products in human lymphocytes and tumor cell lines. Carcinogenesis 2007, 28, 378–389. [Google Scholar] [CrossRef]
- Huber, W.W.; Scharf, G.; Nagel, G.; Prustomersky, S.; Schulte-Hermann, R.; Kaina, B. Coffee and its chemopreventive components Kahweol and Cafestol increase the activity of O6-methylguanine-DNA methyltransferase in rat liver--comparison with phase II xenobiotic metabolism. Mutat. Res. 2003, 522, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, A.; Zhang, R.; Ali-Osman, F.; Bobustuc, G.C.; Srivenugopal, K.S. Disulfiram is a direct and potent inhibitor of human O6-methylguanine-DNA methyltransferase (MGMT) in brain tumor cells and mouse brain and markedly increases the alkylating DNA damage. Carcinogenesis 2014, 35, 692–702. [Google Scholar] [CrossRef]
- Göder, A.; Nagel, G.; Kraus, A.; Dörsam, B.; Seiwert, N.; Kaina, B.; Fahrer, J. Lipoic acid inhibits the DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT) and triggers its depletion in colorectal cancer cells with concomitant autophagy induction. Carcinogenesis 2015, 36, 817–831. [Google Scholar] [CrossRef]
- Tsai, C.K.; Huang, L.C.; Wu, Y.P.; Kan, I.Y.; Hueng, D.Y. SNAP reverses temozolomide resistance in human glioblastoma multiforme cells through down-regulation of MGMT. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 14171–14184. [Google Scholar] [CrossRef]
- Kuo, C.C.; Liu, J.F.; Shiah, H.S.; Ma, L.C.; Chang, J.Y. Tamoxifen accelerates proteasomal degradation of O6-methylguanine DNA methyltransferase in human cancer cells. Int. J. Cancer. J. Int. Du Cancer 2007, 121, 2293–2300. [Google Scholar] [CrossRef]
- Samson, L.; Cairns, J. A new pathway for DNA repair in E. coli. Nature 1977, 267, 281–283. [Google Scholar] [CrossRef]
- Falnes, P.O.; Johansen, R.F.; Seeberg, E. AlkB-mediated oxidative demethylation reverses DNA damage in E. coli. Nature 2002, 419, 178–182. [Google Scholar] [CrossRef]
- Trewick, S.C.; Henshaw, T.F.; Hausinger, R.P.; Lindahl, T.; Sedgwick, B. Oxidative demethylation by E. coli AlkB directly reverts DNA base damage. Nature 2002, 419, 174–178. [Google Scholar] [CrossRef]
- Delaney, J.C.; Essigmann, J.M. Mutagenesis, genotoxicity, and repair of 1-methyladenine, 3-alkylcytosines, 1-methylguanine, and 3-methylthymine in alkB E. coli. Proc. Natl. Acad. Sci. USA 2004, 101, 14051–14056. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tang, Q.; Bian, K.; Humulock, Z.T.; Yang, X.; Jost, M.; Drennan, C.L.; Essigmann, J.M.; Li, D. Adaptive Response Enzyme AlkB Preferentially Repairs 1-Methylguanine and 3-Methylthymine Adducts in Double-Stranded DNA. Chem. Res. Toxicol. 2016, 29, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.C.; Smeester, L.; Wong, C.; Frick, L.E.; Taghizadeh, K.; Wishnok, J.S.; Drennan, C.L.; Samson, L.D.; Essigmann, J.M. AlkB reverses etheno DNA lesions caused by lipid oxidation in vitro and in vivo. Nat. Struct. Mol. Biol. 2005, 12, 855–860. [Google Scholar] [CrossRef]
- Mishina, Y.; Yang, C.G.; He, C. Direct repair of the exocyclic DNA adduct 1,N6-ethenoadenine by the DNA repair AlkB proteins. J. Am. Chem. Soc. 2005, 127, 14594–14595. [Google Scholar] [CrossRef] [PubMed]
- Frick, L.E.; Delaney, J.C.; Wong, C.; Drennan, C.L.; Essigmann, J.M. Alleviation of 1,N6-ethanoadenine genotoxicity by the E. coli adaptive response protein AlkB. Proc. Natl. Acad. Sci. USA 2007, 104, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, B.; Bates, P.A.; Paik, J.; Jacobs, S.C.; Lindahl, T. Repair of alkylated DNA: Recent advances. DNA Repair 2007, 6, 429–442. [Google Scholar] [CrossRef]
- Aas, P.A.; Otterlei, M.; Falnes, P.O.; Vagbo, C.B.; Skorpen, F.; Akbari, M.; Sundheim, O.; Bjoras, M.; Slupphaug, G.; Seeberg, E.; et al. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature 2003, 421, 859–863. [Google Scholar] [CrossRef]
- Duncan, T.; Trewick, S.C.; Koivisto, P.; Bates, P.A.; Lindahl, T.; Sedgwick, B. Reversal of DNA alkylation damage by two human dioxygenases. Proc. Natl. Acad. Sci. USA 2002, 99, 16660–16665. [Google Scholar] [CrossRef]
- Qi, R.; Bian, K.; Chen, F.; Tang, Q.; Zhou, X.; Li, D. Sequence Dependent Repair of 1,N(6)-Ethenoadenine by DNA Repair Enzymes ALKBH2, ALKBH3, and AlkB. Molecules 2021, 26, 5285. [Google Scholar] [CrossRef]
- Fu, D.; Samson, L.D. Direct repair of 3,N(4)-ethenocytosine by the human ALKBH2 dioxygenase is blocked by the AAG/MPG glycosylase. DNA Repair 2012, 11, 46–52. [Google Scholar] [CrossRef]
- Zdzalik, D.; Domanska, A.; Prorok, P.; Kosicki, K.; van den Born, E.; Falnes, P.O.; Rizzo, C.J.; Guengerich, F.P.; Tudek, B. Differential repair of etheno-DNA adducts by bacterial and human AlkB proteins. DNA Repair 2015, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, P.; Robins, P.; Lindahl, T.; Sedgwick, B. Demethylation of 3-methylthymine in DNA by bacterial and human DNA dioxygenases. J. Biol. Chem. 2004, 279, 40470–40474. [Google Scholar] [CrossRef] [PubMed]
- Falnes, P.O. Repair of 3-methylthymine and 1-methylguanine lesions by bacterial and human AlkB proteins. Nucleic Acids Res. 2004, 32, 6260–6267. [Google Scholar] [CrossRef] [PubMed]
- Bian, K.; Lenz, S.A.P.; Tang, Q.; Chen, F.; Qi, R.; Jost, M.; Drennan, C.L.; Essigmann, J.M.; Wetmore, S.D.; Li, D. DNA repair enzymes ALKBH2, ALKBH3, and AlkB oxidize 5-methylcytosine to 5-hydroxymethylcytosine, 5-formylcytosine and 5-carboxylcytosine in vitro. Nucleic Acids Res. 2019, 47, 5522–5529. [Google Scholar] [CrossRef]
- Nay, S.L.; Lee, D.H.; Bates, S.E.; O’Connor, T.R. Alkbh2 protects against lethality and mutation in primary mouse embryonic fibroblasts. DNA Repair 2012, 11, 502–510. [Google Scholar] [CrossRef]
- Ringvoll, J.; Nordstrand, L.M.; Vagbo, C.B.; Talstad, V.; Reite, K.; Aas, P.A.; Lauritzen, K.H.; Liabakk, N.B.; Bjork, A.; Doughty, R.W.; et al. Repair deficient mice reveal mABH2 as the primary oxidative demethylase for repairing 1meA and 3meC lesions in DNA. EMBO J. 2006, 25, 2189–2198. [Google Scholar] [CrossRef]
- Calvo, J.A.; Meira, L.B.; Lee, C.Y.; Moroski-Erkul, C.A.; Abolhassani, N.; Taghizadeh, K.; Eichinger, L.W.; Muthupalani, S.; Nordstrand, L.M.; Klungland, A.; et al. DNA repair is indispensable for survival after acute inflammation. J. Clin. Investig. 2012, 122, 2680–2689. [Google Scholar] [CrossRef]
- Gilljam, K.M.; Feyzi, E.; Aas, P.A.; Sousa, M.M.; Muller, R.; Vagbo, C.B.; Catterall, T.C.; Liabakk, N.B.; Slupphaug, G.; Drablos, F.; et al. Identification of a novel, widespread, and functionally important PCNA-binding motif. J. Cell Biol. 2009, 186, 645–654. [Google Scholar] [CrossRef]
- Fu, D.; Samson, L.D.; Hubscher, U.; van Loon, B. The interaction between ALKBH2 DNA repair enzyme and PCNA is direct, mediated by the hydrophobic pocket of PCNA and perturbed in naturally-occurring ALKBH2 variants. DNA Repair 2015, 35, 13–18. [Google Scholar] [CrossRef]
- Mohan, M.; Akula, D.; Dhillon, A.; Goyal, A.; Anindya, R. Human RAD51 paralogue RAD51C fosters repair of alkylated DNA by interacting with the ALKBH3 demethylase. Nucleic Acids Res. 2019, 47, 11729–11745. [Google Scholar] [CrossRef]
- Brickner, J.R.; Townley, B.A.; Mosammaparast, N. Intersections between transcription-coupled repair and alkylation damage reversal. DNA Repair 2019, 81, 102663. [Google Scholar] [CrossRef] [PubMed]
- Soll, J.M.; Brickner, J.R.; Mudge, M.C.; Mosammaparast, N. RNA ligase-like domain in activating signal cointegrator 1 complex subunit 1 (ASCC1) regulates ASCC complex function during alkylation damage. J. Biol. Chem. 2018, 293, 13524–13533. [Google Scholar] [CrossRef]
- Brickner, J.R.; Soll, J.M.; Lombardi, P.M.; Vagbo, C.B.; Mudge, M.C.; Oyeniran, C.; Rabe, R.; Jackson, J.; Sullender, M.E.; Blazosky, E.; et al. A ubiquitin-dependent signalling axis specific for ALKBH-mediated DNA dealkylation repair. Nature 2017, 551, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Dango, S.; Mosammaparast, N.; Sowa, M.E.; Xiong, L.J.; Wu, F.; Park, K.; Rubin, M.; Gygi, S.; Harper, J.W.; Shi, Y. DNA unwinding by ASCC3 helicase is coupled to ALKBH3-dependent DNA alkylation repair and cancer cell proliferation. Mol. Cell 2011, 44, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, T.C.; Prestegarden, L.; Grudic, A.; Hegi, M.E.; Tysnes, B.B.; Bjerkvig, R. The DNA repair protein ALKBH2 mediates temozolomide resistance in human glioblastoma cells. Neuro-Oncol. 2013, 15, 269–278. [Google Scholar] [CrossRef]
- Stefansson, O.A.; Hermanowicz, S.; van der Horst, J.; Hilmarsdottir, H.; Staszczak, Z.; Jonasson, J.G.; Tryggvadottir, L.; Gudjonsson, T.; Sigurdsson, S. CpG promoter methylation of the ALKBH3 alkylation repair gene in breast cancer. BMC Cancer 2017, 17, 469. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Bian, K.; Tang, Q.; Fedeles, B.I.; Singh, V.; Humulock, Z.T.; Essigmann, J.M.; Li, D. Oncometabolites d- and l-2-Hydroxyglutarate Inhibit the AlkB Family DNA Repair Enzymes under Physiological Conditions. Chem. Res. Toxicol. 2017, 30, 1102–1110. [Google Scholar] [CrossRef]
- Wang, P.; Wu, J.; Ma, S.; Zhang, L.; Yao, J.; Hoadley, K.A.; Wilkerson, M.D.; Perou, C.M.; Guan, K.L.; Ye, D.; et al. Oncometabolite D-2-Hydroxyglutarate Inhibits ALKBH DNA Repair Enzymes and Sensitizes IDH Mutant Cells to Alkylating Agents. Cell Rep. 2015, 13, 2353–2361. [Google Scholar] [CrossRef]
- Tran, T.Q.; Ishak Gabra, M.B.; Lowman, X.H.; Yang, Y.; Reid, M.A.; Pan, M.; O’Connor, T.R.; Kong, M. Glutamine deficiency induces DNA alkylation damage and sensitizes cancer cells to alkylating agents through inhibition of ALKBH enzymes. PLoS Biol. 2017, 15, e2002810. [Google Scholar] [CrossRef]
- Krokan, H.E.; Bjoras, M. Base excision repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012583. [Google Scholar] [CrossRef]
- Engelward, B.P.; Weeda, G.; Wyatt, M.D.; Broekhof, J.L.; de Wit, J.; Donker, I.; Allan, J.M.; Gold, B.; Hoeijmakers, J.H.; Samson, L.D. Base excision repair deficient mice lacking the Aag alkyladenine DNA glycosylase. Proc. Natl. Acad. Sci. USA 1997, 94, 13087–13092. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Ellenberger, T. Dissecting the broad substrate specificity of human 3-methyladenine-DNA glycosylase. J. Biol. Chem. 2004, 279, 9750–9757. [Google Scholar] [CrossRef]
- Lee, C.Y.; Delaney, J.C.; Kartalou, M.; Lingaraju, G.M.; Maor-Shoshani, A.; Essigmann, J.M.; Samson, L.D. Recognition and processing of a new repertoire of DNA substrates by human 3-methyladenine DNA glycosylase (AAG). Biochemistry 2009, 48, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Lin, C.R. Noninvasive measurement of smoking-associated N(3)-ethyladenine and N(7)-ethylguanine in human salivary DNA by stable isotope dilution nanoflow liquid chromatography-nanospray ionization tandem mass spectrometry. Toxicol. Lett. 2014, 225, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Gates, K.S.; Nooner, T.; Dutta, S. Biologically relevant chemical reactions of N7-alkylguanine residues in DNA. Chem. Res. Toxicol. 2004, 17, 839–856. [Google Scholar] [CrossRef]
- Engelward, B.P.; Dreslin, A.; Christensen, J.; Huszar, D.; Kurahara, C.; Samson, L. Repair-deficient 3-methyladenine DNA glycosylase homozygous mutant mouse cells have increased sensitivity to alkylation-induced chromosome damage and cell killing. EMBO J. 1996, 15, 945–952. [Google Scholar] [CrossRef]
- Elder, R.H.; Jansen, J.G.; Weeks, R.J.; Willington, M.A.; Deans, B.; Watson, A.J.; Mynett, K.J.; Bailey, J.A.; Cooper, D.P.; Rafferty, J.A.; et al. Alkylpurine-DNA-N-glycosylase knockout mice show increased susceptibility to induction of mutations by methyl methanesulfonate. Mol. Cell. Biol. 1998, 18, 5828–5837. [Google Scholar] [CrossRef]
- Encell, L.; Shuker, D.E.; Foiles, P.G.; Gold, B. The in vitro methylation of DNA by a minor groove binding methyl sulfonate ester. Chem. Res. Toxicol. 1996, 9, 563–567. [Google Scholar] [CrossRef]
- Engelward, B.P.; Allan, J.M.; Dreslin, A.J.; Kelly, J.D.; Wu, M.M.; Gold, B.; Samson, L.D. A chemical and genetic approach together define the biological consequences of 3-methyladenine lesions in the mammalian genome. J. Biol. Chem. 1998, 273, 5412–5418. [Google Scholar] [CrossRef]
- Smith, S.A.; Engelward, B.P. In vivo repair of methylation damage in Aag 3-methyladenine DNA glycosylase null mouse cells. Nucleic Acids Res. 2000, 28, 3294–3300. [Google Scholar] [CrossRef]
- Coquerelle, T.; Dosch, J.; Kaina, B. Overexpression of N-methylpurine-DNA glycosylase in Chinese hamster ovary cells renders them more sensitive to the production of chromosomal aberrations by methylating agents--a case of imbalanced DNA repair. Mutat. Res. 1995, 336, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Fishel, M.L.; Seo, Y.R.; Smith, M.L.; Kelley, M.R. Imbalancing the DNA base excision repair pathway in the mitochondria; targeting and overexpressing N-methylpurine DNA glycosylase in mitochondria leads to enhanced cell killing. Cancer Res. 2003, 63, 608–615. [Google Scholar] [PubMed]
- Trivedi, R.N.; Almeida, K.H.; Fornsaglio, J.L.; Schamus, S.; Sobol, R.W. The role of base excision repair in the sensitivity and resistance to temozolomide-mediated cell death. Cancer Res. 2005, 65, 6394–6400. [Google Scholar] [CrossRef]
- Tang, J.B.; Svilar, D.; Trivedi, R.N.; Wang, X.H.; Goellner, E.M.; Moore, B.; Hamilton, R.L.; Banze, L.A.; Brown, A.R.; Sobol, R.W. N-methylpurine DNA glycosylase and DNA polymerase beta modulate BER inhibitor potentiation of glioma cells to temozolomide. Neuro-Oncol. 2011, 13, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Meira, L.B.; Bugni, J.M.; Green, S.L.; Lee, C.W.; Pang, B.; Borenshtein, D.; Rickman, B.H.; Rogers, A.B.; Moroski-Erkul, C.A.; McFaline, J.L.; et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Investig. 2008, 118, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Ensminger, M.; Iloff, L.; Ebel, C.; Nikolova, T.; Kaina, B.; Löbrich, M. DNA breaks and chromosomal aberrations arise when replication meets base excision repair. J. Cell Biol. 2014, 206, 29–43. [Google Scholar] [CrossRef]
- Crosbie, P.A.; Watson, A.J.; Agius, R.; Barber, P.V.; Margison, G.P.; Povey, A.C. Elevated N3-methylpurine-DNA glycosylase DNA repair activity is associated with lung cancer. Mutat. Res. 2012, 732, 43–46. [Google Scholar] [CrossRef]
- Calvo, J.A.; Moroski-Erkul, C.A.; Lake, A.; Eichinger, L.W.; Shah, D.; Jhun, I.; Limsirichai, P.; Bronson, R.T.; Christiani, D.C.; Meira, L.B.; et al. Aag DNA glycosylase promotes alkylation-induced tissue damage mediated by Parp1. PLoS Genet. 2013, 9, e1003413. [Google Scholar] [CrossRef]
- Chakravarti, D.; Ibeanu, G.C.; Tano, K.; Mitra, S. Cloning and expression in E. coli of a human cDNA encoding the DNA repair protein N-methylpurine-DNA glycosylase. J. Biol. Chem. 1991, 266, 15710–15715. [Google Scholar] [CrossRef]
- Jang, S.; Kumar, N.; Schaich, M.A.; Zhong, Z.; van Loon, B.; Watkins, S.C.; Van Houten, B. Cooperative interaction between AAG and UV-DDB in the removal of modified bases. Nucleic Acids Res. 2022, 50, 12856–12871. [Google Scholar] [CrossRef]
- Demple, B.; Sung, J.S. Molecular and biological roles of Ape1 protein in mammalian base excision repair. DNA Repair 2005, 4, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Beard, W.A.; Wilson, S.H. Structure and mechanism of DNA polymerase Beta. Chem. Rev. 2006, 106, 361–382. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.K.; Berg, B.J.; Prasad, R.; Molina, J.T.; Beard, W.A.; Tomkinson, A.E.; Wilson, S.H. Mammalian abasic site base excision repair. Identification of the reaction sequence and rate-determining steps. J. Biol. Chem. 1998, 273, 21203–21209. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, E.; Taylor, R.; Cevasco, M.; Abbondandolo, A.; Caldecott, K.; Frosina, G. Involvement of XRCC1 and DNA ligase III gene products in DNA base excision repair. J. Biol. Chem. 1997, 272, 23970–23975. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.M.; Wickstead, B.; Cronin, S.; Caldecott, K.W. Role of a BRCT domain in the interaction of DNA ligase III-alpha with the DNA repair protein XRCC1. Curr. Biol. CB 1998, 8, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Dianova, I.I.; Sleeth, K.M.; Allinson, S.L.; Parsons, J.L.; Breslin, C.; Caldecott, K.W.; Dianov, G.L. XRCC1-DNA polymerase beta interaction is required for efficient base excision repair. Nucleic Acids Res. 2004, 32, 2550–2555. [Google Scholar] [CrossRef]
- Sleeth, K.M.; Robson, R.L.; Dianov, G.L. Exchangeability of mammalian DNA ligases between base excision repair pathways. Biochemistry 2004, 43, 12924–12930. [Google Scholar] [CrossRef]
- Klungland, A.; Lindahl, T. Second pathway for completion of human DNA base excision-repair: Reconstitution with purified proteins and requirement for DNase IV (FEN1). EMBO J. 1997, 16, 3341–3348. [Google Scholar] [CrossRef]
- Petermann, E.; Ziegler, M.; Oei, S.L. ATP-dependent selection between single nucleotide and long patch base excision repair. DNA Repair 2003, 2, 1101–1114. [Google Scholar] [CrossRef]
- Svilar, D.; Goellner, E.M.; Almeida, K.H.; Sobol, R.W. Base excision repair and lesion-dependent subpathways for repair of oxidative DNA damage. Antioxid. Redox Signal. 2011, 14, 2491–2507. [Google Scholar] [CrossRef]
- Stucki, M.; Pascucci, B.; Parlanti, E.; Fortini, P.; Wilson, S.H.; Hubscher, U.; Dogliotti, E. Mammalian base excision repair by DNA polymerases delta and epsilon. Oncogene 1998, 17, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.S.; McKenna, A.E.; Motycka, T.A.; Matsumoto, Y.; Tomkinson, A.E. Interaction between PCNA and DNA ligase I is critical for joining of Okazaki fragments and long-patch base-excision repair. Curr. Biol. CB 2000, 10, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Ström, C.E.; Johansson, F.; Uhlen, M.; Szigyarto, C.A.; Erixon, K.; Helleday, T. Poly (ADP-ribose) polymerase (PARP) is not involved in base excision repair but PARP inhibition traps a single-strand intermediate. Nucleic Acids Res. 2011, 39, 3166–3175. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Schreiber, V.; Dantzer, F. The diverse roles and clinical relevance of PARPs in DNA damage repair: Current state of the art. Biochem. Pharmacol. 2012, 84, 137–146. [Google Scholar] [CrossRef]
- Langelier, M.F.; Planck, J.L.; Roy, S.; Pascal, J.M. Crystal structures of poly(ADP-ribose) polymerase-1 (PARP-1) zinc fingers bound to DNA: Structural and functional insights into DNA-dependent PARP-1 activity. J. Biol. Chem. 2011, 286, 10690–10701. [Google Scholar] [CrossRef]
- Eustermann, S.; Wu, W.F.; Langelier, M.F.; Yang, J.C.; Easton, L.E.; Riccio, A.A.; Pascal, J.M.; Neuhaus, D. Structural Basis of Detection and Signaling of DNA Single-Strand Breaks by Human PARP-1. Mol. Cell 2015, 60, 742–754. [Google Scholar] [CrossRef]
- Mangerich, A.; Bürkle, A. Pleiotropic cellular functions of PARP1 in longevity and aging: Genome maintenance meets inflammation. Oxidative Med. Cell. Longev. 2012, 2012, 321653. [Google Scholar] [CrossRef]
- Jungmichel, S.; Rosenthal, F.; Altmeyer, M.; Lukas, J.; Hottiger, M.O.; Nielsen, M.L. Proteome-wide identification of poly(ADP-Ribosyl)ation targets in different genotoxic stress responses. Mol. Cell 2013, 52, 272–285. [Google Scholar] [CrossRef]
- Gupte, R.; Liu, Z.; Kraus, W.L. PARPs and ADP-ribosylation: Recent advances linking molecular functions to biological outcomes. Genes Dev. 2017, 31, 101–126. [Google Scholar] [CrossRef]
- Pleschke, J.M.; Kleczkowska, H.E.; Strohm, M.; Althaus, F.R. Poly(ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J. Biol. Chem. 2000, 275, 40974–40980. [Google Scholar] [CrossRef]
- Teloni, F.; Altmeyer, M. Readers of poly(ADP-ribose): Designed to be fit for purpose. Nucleic Acids Res. 2016, 44, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Fahrer, J.; Kranaster, R.; Altmeyer, M.; Marx, A.; Bürkle, A. Quantitative analysis of the binding affinity of poly(ADP-ribose) to specific binding proteins as a function of chain length. Nucleic Acids Res. 2007, 35, e143. [Google Scholar] [CrossRef] [PubMed]
- Breslin, C.; Hornyak, P.; Ridley, A.; Rulten, S.L.; Hanzlikova, H.; Oliver, A.W.; Caldecott, K.W. The XRCC1 phosphate-binding pocket binds poly (ADP-ribose) and is required for XRCC1 function. Nucleic Acids Res. 2015, 43, 6934–6944. [Google Scholar] [CrossRef]
- Demin, A.A.; Hirota, K.; Tsuda, M.; Adamowicz, M.; Hailstone, R.; Brazina, J.; Gittens, W.; Kalasova, I.; Shao, Z.; Zha, S.; et al. XRCC1 prevents toxic PARP1 trapping during DNA base excision repair. Mol. Cell 2021, 81, 3018–3030.e5. [Google Scholar] [CrossRef] [PubMed]
- de Murcia, J.M.; Niedergang, C.; Trucco, C.; Ricoul, M.; Dutrillaux, B.; Mark, M.; Oliver, F.J.; Masson, M.; Dierich, A.; LeMeur, M.; et al. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc. Natl. Acad. Sci. USA 1997, 94, 7303–7307. [Google Scholar] [CrossRef]
- Trucco, C.; Oliver, F.J.; de Murcia, G.; Menissier-de Murcia, J. DNA repair defect in poly(ADP-ribose) polymerase-deficient cell lines. Nucleic Acids Res. 1998, 26, 2644–2649. [Google Scholar] [CrossRef]
- Masutani, M.; Nozaki, T.; Nakamoto, K.; Nakagama, H.; Suzuki, H.; Kusuoka, O.; Tsutsumi, M.; Sugimura, T. The response of Parp knockout mice against DNA damaging agents. Mutat. Res. 2000, 462, 159–166. [Google Scholar] [CrossRef]
- Dörsam, B.; Seiwert, N.; Foersch, S.; Stroh, S.; Nagel, G.; Begaliew, D.; Diehl, E.; Kraus, A.; McKeague, M.; Minneker, V.; et al. PARP-1 protects against colorectal tumor induction, but promotes inflammation-driven colorectal tumor progression. Proc. Natl. Acad. Sci. USA 2018, 115, E4061–E4070. [Google Scholar] [CrossRef]
- Shibata, A.; Kamada, N.; Masumura, K.; Nohmi, T.; Kobayashi, S.; Teraoka, H.; Nakagama, H.; Sugimura, T.; Suzuki, H.; Masutani, M. Parp-1 deficiency causes an increase of deletion mutations and insertions/rearrangements in vivo after treatment with an alkylating agent. Oncogene 2005, 24, 1328–1337. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Masutani, M.; Nozaki, T.; Kusuoka, O.; Tsujiuchi, T.; Nakagama, H.; Suzuki, H.; Konishi, Y.; Sugimura, T. Increased susceptibility of poly(ADP-ribose) polymerase-1 knockout mice to nitrosamine carcinogenicity. Carcinogenesis 2001, 22, 1–3. [Google Scholar] [CrossRef]
- Nozaki, T.; Fujihara, H.; Watanabe, M.; Tsutsumi, M.; Nakamoto, K.; Kusuoka, O.; Kamada, N.; Suzuki, H.; Nakagama, H.; Sugimura, T.; et al. Parp-1 deficiency implicated in colon and liver tumorigenesis induced by azoxymethane. Cancer Sci. 2003, 94, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Corrigan, J.J.; Fake, K.R.; Calvo, J.A.; Samson, L.D. PARP inhibitors protect against sex- and AAG-dependent alkylation-induced neural degeneration. Oncotarget 2017, 8, 68707–68720. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Umanah, G.K.; Chang, C.; Stevens, D.A.; Karuppagounder, S.S.; Gagne, J.P.; Poirier, G.G.; Dawson, V.L.; Dawson, T.M. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc. Natl. Acad. Sci. USA 2014, 111, 10209–10214. [Google Scholar] [CrossRef] [PubMed]
- Fouquerel, E.; Goellner, E.M.; Yu, Z.; Gagne, J.P.; Barbi de Moura, M.; Feinstein, T.; Wheeler, D.; Redpath, P.; Li, J.; Romero, G.; et al. ARTD1/PARP1 negatively regulates glycolysis by inhibiting hexokinase 1 independent of NAD+ depletion. Cell Rep. 2014, 8, 1819–1831. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Ke, Y.; Zeng, X.; Gao, J.; Ba, X.; Wang, R. The key players of parthanatos: Opportunities for targeting multiple levels in the therapy of parthanatos-based pathogenesis. Cell. Mol. Life Sci. CMLS 2022, 79, 60. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Kim, N.S.; Yu, S.W.; Wang, H.; Koh, D.W.; Sasaki, M.; Klaus, J.A.; Otsuka, T.; Zhang, Z.; Koehler, R.C.; et al. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc. Natl. Acad. Sci. USA 2006, 103, 18308–18313. [Google Scholar] [CrossRef]
- Yu, S.W.; Andrabi, S.A.; Wang, H.; Kim, N.S.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc. Natl. Acad. Sci. USA 2006, 103, 18314–18319. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, N.S.; Haince, J.F.; Kang, H.C.; David, K.K.; Andrabi, S.A.; Poirier, G.G.; Dawson, V.L.; Dawson, T.M. Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos). Sci. Signal. 2011, 4, ra20. [Google Scholar] [CrossRef]
- Wang, Y.; An, R.; Umanah, G.K.; Park, H.; Nambiar, K.; Eacker, S.M.; Kim, B.; Bao, L.; Harraz, M.M.; Chang, C.; et al. A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science 2016, 354, aad6872. [Google Scholar] [CrossRef]
- Yang, M.; Wang, C.; Zhou, M.; Bao, L.; Wang, Y.; Kumar, A.; Xing, C.; Luo, W.; Wang, Y. KDM6B promotes PARthanatos via suppression of O6-methylguanine DNA methyltransferase repair and sustained checkpoint response. Nucleic Acids Res. 2022, 50, 6313–6331. [Google Scholar] [CrossRef]
- Christmann, M.; Tomicic, M.T.; Roos, W.P.; Kaina, B. Mechanisms of human DNA repair: An update. Toxicology 2003, 193, 3–34. [Google Scholar] [CrossRef] [PubMed]
- Spivak, G. Nucleotide excision repair in humans. DNA Repair 2015, 36, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Scharer, O.D. Nucleotide excision repair in eukaryotes. Cold Spring Harb. Perspect. Biol. 2013, 5, a012609. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, T.; Roos, W.P.; Kramer, O.H.; Strik, H.M.; Kaina, B. Chloroethylating nitrosoureas in cancer therapy: DNA damage, repair and cell death signaling. Biochim. Et Biophys. Acta. Rev. Cancer 2017, 1868, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Muniandy, P.A.; Liu, J.; Majumdar, A.; Liu, S.T.; Seidman, M.M. DNA interstrand crosslink repair in mammalian cells: Step by step. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 23–49. [Google Scholar] [CrossRef]
- Kusumoto, R.; Masutani, C.; Sugasawa, K.; Iwai, S.; Araki, M.; Uchida, A.; Mizukoshi, T.; Hanaoka, F. Diversity of the damage recognition step in the global genomic nucleotide excision repair in vitro. Mutat. Res. 2001, 485, 219–227. [Google Scholar] [CrossRef]
- Tang, J.; Chu, G. Xeroderma pigmentosum complementation group E and UV-damaged DNA-binding protein. DNA Repair 2002, 1, 601–616. [Google Scholar] [CrossRef]
- Evans, E.; Moggs, J.G.; Hwang, J.R.; Egly, J.M.; Wood, R.D. Mechanism of open complex and dual incision formation by human nucleotide excision repair factors. EMBO J. 1997, 16, 6559–6573. [Google Scholar] [CrossRef]
- Krasikova, Y.; Rechkunova, N.; Lavrik, O. Nucleotide Excision Repair: From Molecular Defects to Neurological Abnormalities. Int. J. Mol. Sci. 2021, 22, 6220. [Google Scholar] [CrossRef]
- Giannattasio, M.; Follonier, C.; Tourriere, H.; Puddu, F.; Lazzaro, F.; Pasero, P.; Lopes, M.; Plevani, P.; Muzi-Falconi, M. Exo1 competes with repair synthesis, converts NER intermediates to long ssDNA gaps, and promotes checkpoint activation. Mol. Cell 2010, 40, 50–62. [Google Scholar] [CrossRef]
- Svejstrup, J.Q. Rescue of arrested RNA polymerase II complexes. J. Cell Sci. 2003, 116, 447–451. [Google Scholar] [CrossRef]
- Mijal, R.S.; Thomson, N.M.; Fleischer, N.L.; Pauly, G.T.; Moschel, R.C.; Kanugula, S.; Fang, Q.; Pegg, A.E.; Peterson, L.A. The repair of the tobacco specific nitrosamine derived adduct O6-[4-Oxo-4-(3-pyridyl)butyl]guanine by O6-alkylguanine-DNA alkyltransferase variants. Chem. Res. Toxicol. 2004, 17, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Urban, A.M.; Upadhyaya, P.; Cao, Q.; Peterson, L.A. Formation and repair of pyridyloxobutyl DNA adducts and their relationship to tumor yield in A/J mice. Chem. Res. Toxicol. 2012, 25, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Perdigao, J.; Pegg, A.E.; Lao, Y.; Hecht, S.S.; Lindgren, B.R.; Reardon, J.T.; Sancar, A.; Wattenberg, E.V.; Peterson, L.A. The influence of repair pathways on the cytotoxicity and mutagenicity induced by the pyridyloxobutylation pathway of tobacco-specific nitrosamines. Chem. Res. Toxicol. 2009, 22, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Kotandeniya, D.; Murphy, D.; Yan, S.; Park, S.; Seneviratne, U.; Koopmeiners, J.S.; Pegg, A.; Kanugula, S.; Kassie, F.; Tretyakova, N. Kinetics of O(6)-pyridyloxobutyl-2’-deoxyguanosine repair by human O(6)-alkylguanine DNA alkyltransferase. Biochemistry 2013, 52, 4075–4088. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Wang, Y. Liquid Chromatography-Tandem Mass Spectrometry for the Quantification of Tobacco-Specific Nitrosamine-Induced DNA Adducts in Mammalian Cells. Anal. Chem. 2017, 89, 9124–9130. [Google Scholar] [CrossRef] [PubMed]
- Balbo, S.; Johnson, C.S.; Kovi, R.C.; James-Yi, S.A.; O’Sullivan, M.G.; Wang, M.; Le, C.T.; Khariwala, S.S.; Upadhyaya, P.; Hecht, S.S. Carcinogenicity and DNA adduct formation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in F-344 rats. Carcinogenesis 2014, 35, 2798–2806. [Google Scholar] [CrossRef]
- Carlson, E.S.; Upadhyaya, P.; Villalta, P.W.; Ma, B.; Hecht, S.S. Analysis and Identification of 2’-Deoxyadenosine-Derived Adducts in Lung and Liver DNA of F-344 Rats Treated with the Tobacco-Specific Carcinogen 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone and Enantiomers of its Metabolite 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem. Res. Toxicol. 2018, 31, 358–370. [Google Scholar] [CrossRef]
- Stepanov, I.; Hecht, S.S. Mitochondrial DNA adducts in the lung and liver of F344 rats chronically treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and (S)-4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem. Res. Toxicol. 2009, 22, 406–414. [Google Scholar] [CrossRef]
- Ma, B.; Zarth, A.T.; Carlson, E.S.; Villalta, P.W.; Stepanov, I.; Hecht, S.S. Pyridylhydroxybutyl and pyridyloxobutyl DNA phosphate adduct formation in rats treated chronically with enantiomers of the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Mutagenesis 2017, 32, 561–570. [Google Scholar] [CrossRef]
- Ma, B.; Villalta, P.W.; Zarth, A.T.; Kotandeniya, D.; Upadhyaya, P.; Stepanov, I.; Hecht, S.S. Comprehensive High-Resolution Mass Spectrometric Analysis of DNA Phosphate Adducts Formed by the Tobacco-Specific Lung Carcinogen 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone. Chem. Res. Toxicol. 2015, 28, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.J.; Bedard, L.L.; Massey, T.E. Repair of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA pyridyloxobutylation by nucleotide excision repair. Cancer Lett. 2008, 260, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.J.; Massey, T.E. In vivo treatment with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induces organ-specific alterations in in vitro repair of DNA pyridyloxobutylation. Mutat. Res. 2009, 663, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Leng, J.; Tan, Y.; Price, N.E.; Wang, Y. Quantification of DNA Lesions Induced by 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol in Mammalian Cells. Chem. Res. Toxicol. 2019, 32, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.W.; Sledziewska-Gojska, E.; Hirani-Hojatti, S. In vivo effect of DNA repair on the transition frequency produced from a single O6-methyl- or O6-n-butyl-guanine in a T:G base pair. Mol. Gen. Genet. 1988, 213, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.W.; Sledziewska-Gojska, E.; Hirani-Hojatti, S.; Borowy-Borowski, H. uvrA and recA mutations inhibit a site-specific transition produced by a single O6-methylguanine in gene G of bacteriophage phi X174. Proc. Natl. Acad. Sci. USA 1985, 82, 7173–7177. [Google Scholar] [CrossRef]
- Boyle, J.M.; Margison, G.P.; Saffhill, R. Evidence for the excision repair of O6-n-butyldeoxyguanosine in human cells. Carcinogenesis 1986, 7, 1987–1990. [Google Scholar] [CrossRef]
- Boyle, J.M.; Saffhill, R.; Margison, G.P.; Fox, M. A comparison of cell survival, mutation and persistence of putative promutagenic lesions in Chinese hamster cells exposed to BNU or MNU. Carcinogenesis 1986, 7, 1981–1985. [Google Scholar] [CrossRef]
- Bol, S.A.; van Steeg, H.; van Oostrom, C.T.; Tates, A.D.; Vrieling, H.; de Groot, A.J.; Mullenders, L.H.; van Zeeland, A.A.; Jansen, J.G. Nucleotide excision repair modulates the cytotoxic and mutagenic effects of N-n-butyl-N-nitrosourea in cultured mammalian cells as well as in mouse splenocytes in vivo. Mutagenesis 1999, 14, 317–322. [Google Scholar] [CrossRef]
- Du, H.; Wang, P.; Li, L.; Wang, Y. Repair and translesion synthesis of O(6)-alkylguanine DNA lesions in human cells. J. Biol. Chem. 2019, 294, 11144–11153. [Google Scholar] [CrossRef]
- Bronstein, S.M.; Skopek, T.R.; Swenberg, J.A. Efficient repair of O6-ethylguanine, but not O4-ethylthymine or O2-ethylthymine, is dependent upon O6-alkylguanine-DNA alkyltransferase and nucleotide excision repair activities in human cells. Cancer Res. 1992, 52, 2008–2011. [Google Scholar] [PubMed]
- Kostka, T.; Empl, M.T.; Seiwert, N.; Geisen, S.M.; Hoffmann, P.; Adam, J.; Seeger, B.; Shay, J.W.; Christmann, M.; Sturla, S.J.; et al. Repair of O6-carboxymethylguanine adducts by O6-methylguanine-DNA methyltransferase in human colon epithelial cells. Carcinogenesis 2021, 42, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, C.M.N.; Escher, N.A.; Kim, H.S.; Geisen, S.M.; Fontana, G.A.; Yeo, J.E.; Scharer, O.D.; Sturla, S.J. A combination of direct reversion and nucleotide excision repair counters the mutagenic effects of DNA carboxymethylation. DNA Repair 2022, 110, 103262. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.L.; Fairhurst, N.; Challis, B.C.; Shuker, D.E. Synthesis, characterization, and immunochemical detection of O6-(carboxymethyl)-2’-deoxyguanosine: A DNA adduct formed by nitrosated glycine derivatives. Chem. Res. Toxicol. 1997, 10, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Geisen, S.M.; Aloisi, C.M.N.; Huber, S.M.; Sandell, E.S.; Escher, N.A.; Sturla, S.J. Direct Alkylation of Deoxyguanosine by Azaserine Leads to O(6)-Carboxymethyldeoxyguanosine. Chem. Res. Toxicol. 2021, 34, 1518–1529. [Google Scholar] [CrossRef]
- Tomicic, M.T.; Aasland, D.; Naumann, S.C.; Meise, R.; Barckhausen, C.; Kaina, B.; Christmann, M. Translesion polymerase eta is upregulated by cancer therapeutics and confers anticancer drug resistance. Cancer Res. 2014, 74, 5585–5596. [Google Scholar] [CrossRef]
- Roos, W.P.; Tsaalbi-Shtylik, A.; Tsaryk, R.; Guvercin, F.; de Wind, N.; Kaina, B. The translesion polymerase Rev3L in the tolerance of alkylating anticancer drugs. Mol. Pharmacol. 2009, 76, 927–934. [Google Scholar] [CrossRef]
- Hanisch, D.; Krumm, A.; Diehl, T.; Stork, C.M.; Dejung, M.; Butter, F.; Kim, E.; Brenner, W.; Fritz, G.; Hofmann, T.G.; et al. Class I HDAC overexpression promotes temozolomide resistance in glioma cells by regulating RAD18 expression. Cell Death Dis. 2022, 13, 293. [Google Scholar] [CrossRef]
- Kaszubowski, J.D.; Trakselis, M.A. Beyond the Lesion: Back to High Fidelity DNA Synthesis. Front. Mol. Biosci. 2021, 8, 811540. [Google Scholar] [CrossRef]
- Groth, P.; Auslander, S.; Majumder, M.M.; Schultz, N.; Johansson, F.; Petermann, E.; Helleday, T. Methylated DNA causes a physical block to replication forks independently of damage signalling, O(6)-methylguanine or DNA single-strand breaks and results in DNA damage. J. Mol. Biol. 2010, 402, 70–82. [Google Scholar] [CrossRef]
- Voigt, J.M.; Topal, M.D. O6-methylguanine-induced replication blocks. Carcinogenesis 1995, 16, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Haracska, L.; Prakash, S.; Prakash, L. Replication past O(6)-methylguanine by yeast and human DNA polymerase eta. Mol. Cell. Biol. 2000, 20, 8001–8007. [Google Scholar] [CrossRef] [PubMed]
- Perrino, F.W.; Blans, P.; Harvey, S.; Gelhaus, S.L.; McGrath, C.; Akman, S.A.; Jenkins, G.S.; LaCourse, W.R.; Fishbein, J.C. The N2-ethylguanine and the O6-ethyl- and O6-methylguanine lesions in DNA: Contrasting responses from the “bypass” DNA polymerase eta and the replicative DNA polymerase alpha. Chem. Res. Toxicol. 2003, 16, 1616–1623. [Google Scholar] [CrossRef]
- Räz, M.H.; Dexter, H.R.; Millington, C.L.; van Loon, B.; Williams, D.M.; Sturla, S.J. Bypass of Mutagenic O(6)-Carboxymethylguanine DNA Adducts by Human Y- and B-Family Polymerases. Chem. Res. Toxicol. 2016, 29, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Chowdhury, G.; Zang, H.; Angel, K.C.; Vu, C.C.; Peterson, L.A.; Guengerich, F.P. Translesion synthesis across O6-alkylguanine DNA adducts by recombinant human DNA polymerases. J. Biol. Chem. 2006, 281, 38244–38256. [Google Scholar] [CrossRef]
- Choi, J.Y.; Guengerich, F.P. Kinetic analysis of translesion synthesis opposite bulky N2- and O6-alkylguanine DNA adducts by human DNA polymerase REV1. J. Biol. Chem. 2008, 283, 23645–23655. [Google Scholar] [CrossRef]
- Shrivastav, N.; Li, D.; Essigmann, J.M. Chemical biology of mutagenesis and DNA repair: Cellular responses to DNA alkylation. Carcinogenesis 2010, 31, 59–70. [Google Scholar] [CrossRef]
- Grevatt, P.C.; Solomon, J.J.; Bhanot, O.S. In vitro mispairing specificity of O2-ethylthymidine. Biochemistry 1992, 31, 4181–4188. [Google Scholar] [CrossRef]
- Williams, N.L.; Wang, P.; Wang, Y. Replicative Bypass of O(2)-Alkylthymidine Lesions in Vitro. Chem. Res. Toxicol. 2016, 29, 1755–1761. [Google Scholar] [CrossRef]
- Wu, J.; Li, L.; Wang, P.; You, C.; Williams, N.L.; Wang, Y. Translesion synthesis of O4-alkylthymidine lesions in human cells. Nucleic Acids Res. 2016, 44, 9256–9265. [Google Scholar] [CrossRef]
- Du, H.; Leng, J.; Wang, P.; Li, L.; Wang, Y. Impact of tobacco-specific nitrosamine-derived DNA adducts on the efficiency and fidelity of DNA replication in human cells. J. Biol. Chem. 2018, 293, 11100–11108. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Yoon, J.H.; Roy Choudhury, J.; Prakash, L.; Prakash, S. Genetic Control of Replication through N1-methyladenine in Human Cells. J. Biol. Chem. 2015, 290, 29794–29800. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Basu, D.; Choudhury, J.R.; Prakash, S.; Prakash, L. DNA polymerase lambda promotes error-free replication through Watson-Crick impairing N1-methyl-deoxyadenosine adduct in conjunction with DNA polymerase zeta. J. Biol. Chem. 2021, 297, 100868. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Roy Choudhury, J.; Park, J.; Prakash, S.; Prakash, L. Translesion synthesis DNA polymerases promote error-free replication through the minor-groove DNA adduct 3-deaza-3-methyladenine. J. Biol. Chem. 2017, 292, 18682–18688. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Guengerich, F.P. Adduct size limits efficient and error-free bypass across bulky N2-guanine DNA lesions by human DNA polymerase eta. J. Mol. Biol. 2005, 352, 72–90. [Google Scholar] [CrossRef]
- Choi, J.Y.; Guengerich, F.P. Kinetic evidence for inefficient and error-prone bypass across bulky N2-guanine DNA adducts by human DNA polymerase iota. J. Biol. Chem. 2006, 281, 12315–12324. [Google Scholar] [CrossRef]
- Choi, J.Y.; Angel, K.C.; Guengerich, F.P. Translesion synthesis across bulky N2-alkyl guanine DNA adducts by human DNA polymerase kappa. J. Biol. Chem. 2006, 281, 21062–21072. [Google Scholar] [CrossRef]
- Choi, J.Y.; Zang, H.; Angel, K.C.; Kozekov, I.D.; Goodenough, A.K.; Rizzo, C.J.; Guengerich, F.P. Translesion synthesis across 1,N2-ethenoguanine by human DNA polymerases. Chem. Res. Toxicol. 2006, 19, 879–886. [Google Scholar] [CrossRef]
- Yoon, J.H.; Johnson, R.E.; Prakash, L.; Prakash, S. DNA polymerase theta accomplishes translesion synthesis opposite 1,N(6)-ethenodeoxyadenosine with a remarkably high fidelity in human cells. Genes Dev. 2019, 33, 282–287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).