Cardiolipin for Enhanced Cellular Uptake and Cytotoxicity of Thermosensitive Liposome-Encapsulated Daunorubicin toward Breast Cancer Cell Lines
Abstract
1. Introduction
2. Results and Discussion
2.1. Formulation Preparation
2.2. Characterization of NP Formulations
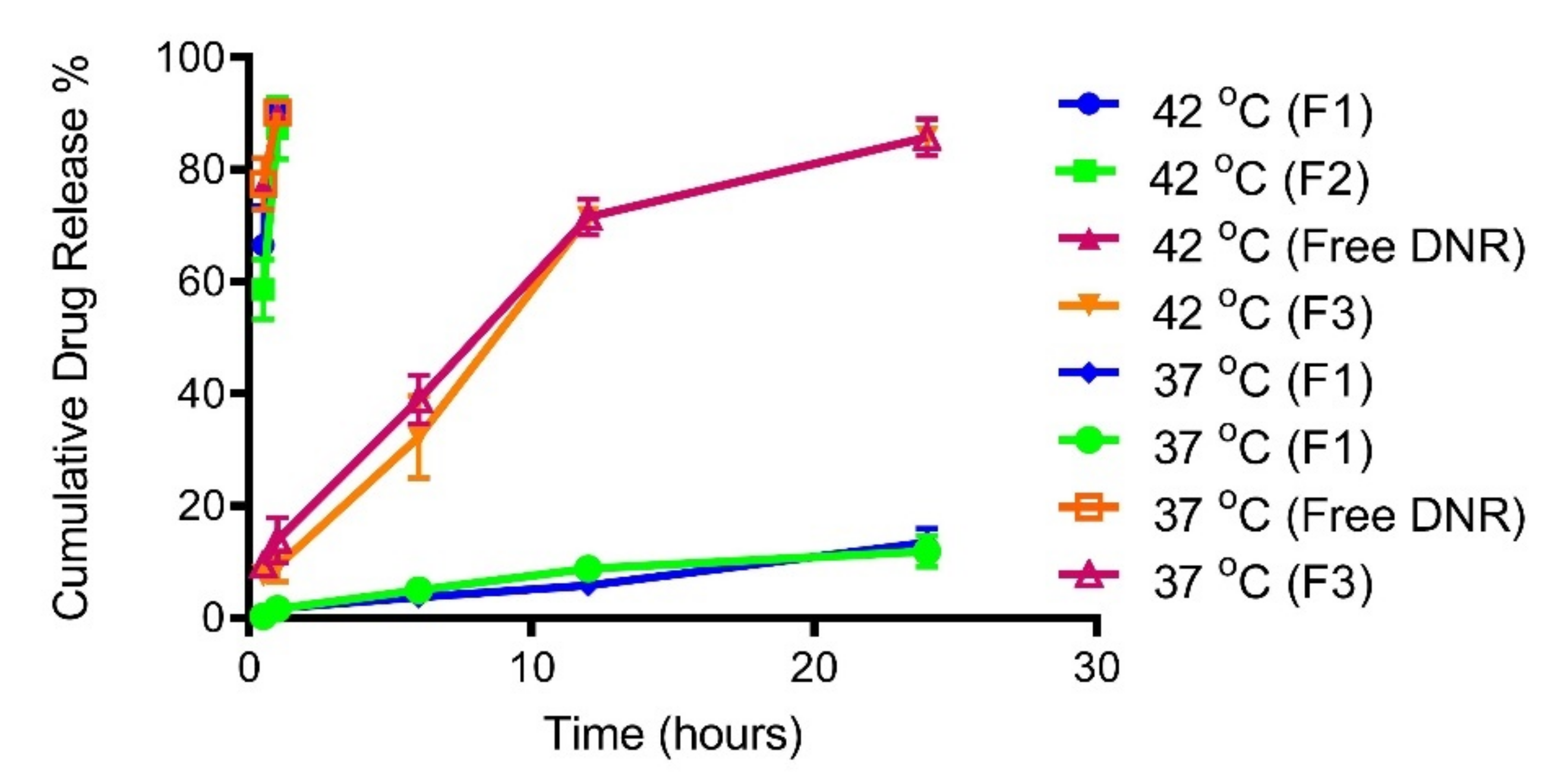
2.3. Temperature Triggered Release In Vitro
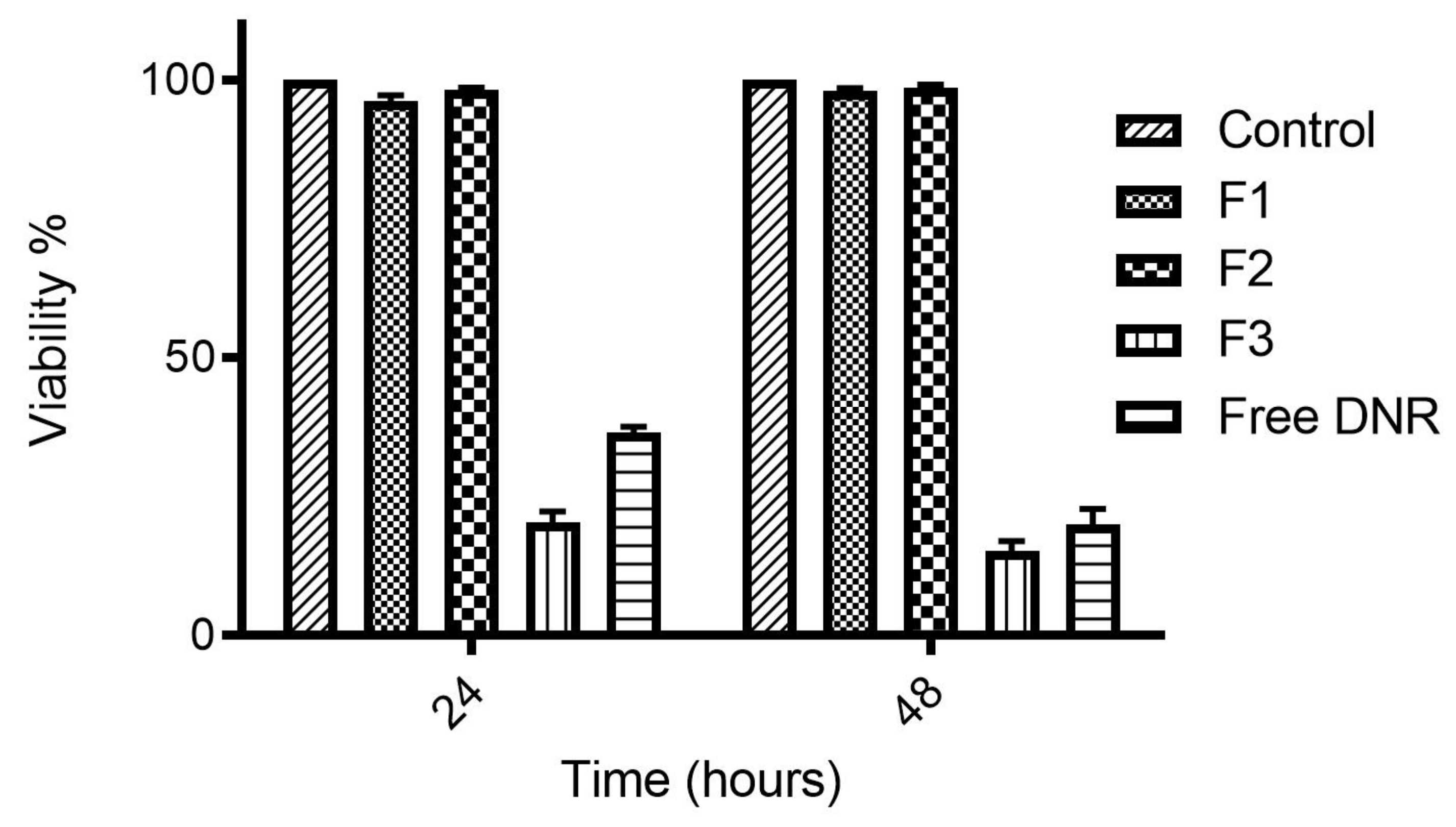
2.4. Cytotoxicity of DNR-Loaded TSLs
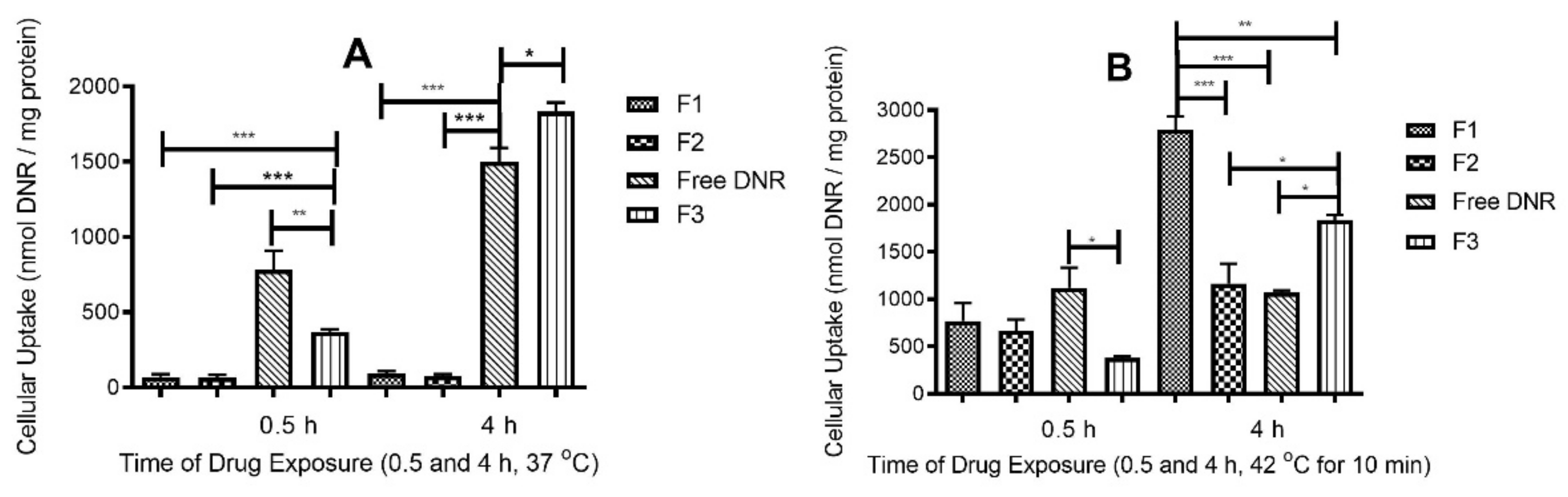
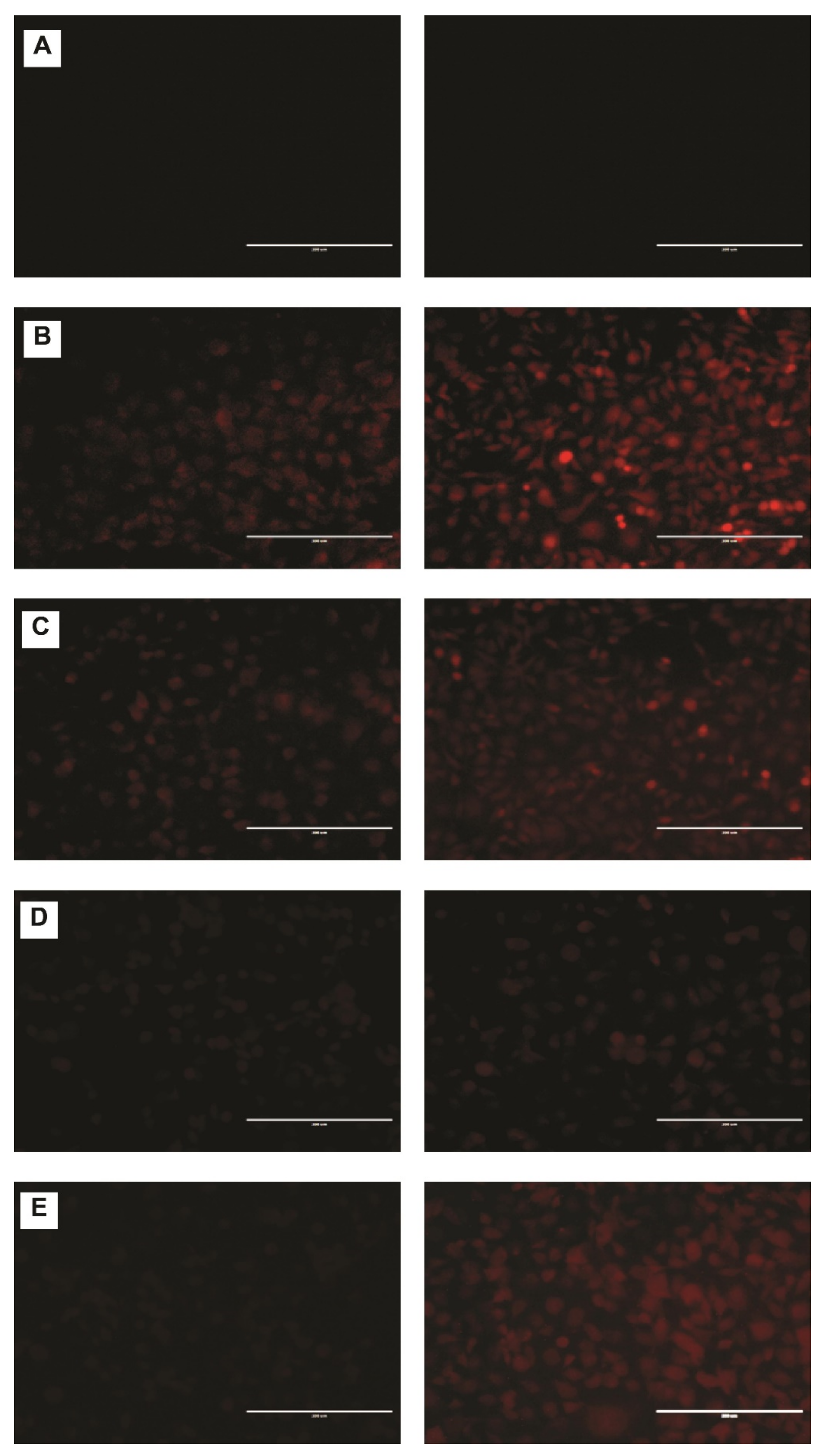
2.5. DNR Accumulation into MDA-MB-231 Cell Lines
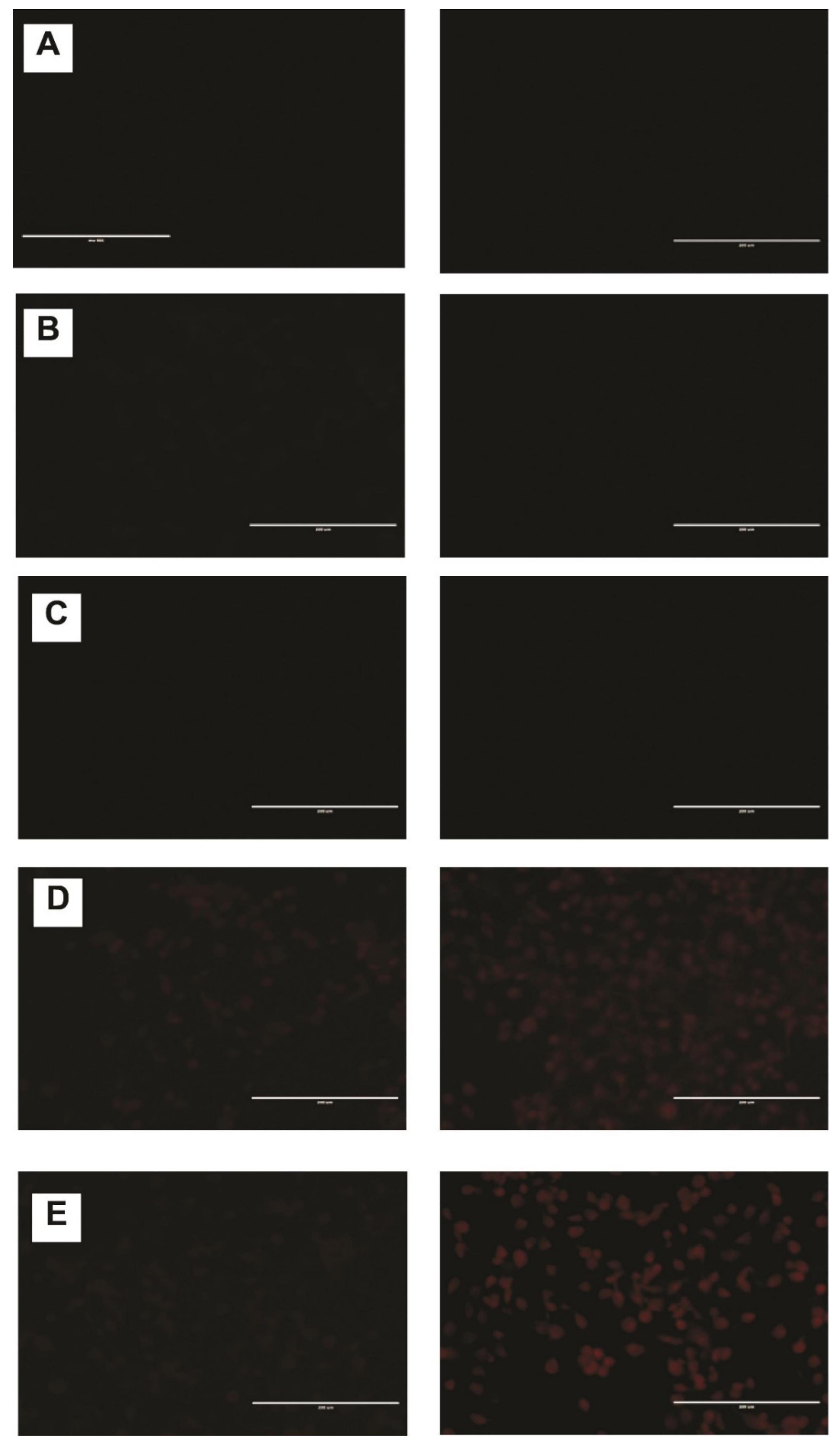
2.6. DNR Internalized Efficiently from CL-TSLs in MDA-MB-231 Cancer Cells
2.7. Short-Term Stability Studies
3. Materials and Methods
3.1. Materials
3.2. Liposomes Preparation
3.3. Drug Encapsulation in Liposomes (Active Loading)
3.4. Encapsulation Efficiency (EE%) and Drug Loading (DL%) Measurement
3.5. Determination of Particle Size of Liposomal Formulations
3.6. Determination of Zeta Potential of Liposomal Formulations
3.7. Determination of Osmolality of Liposomal Formulations
3.8. In Vitro Release Studies
3.9. Stability Studies
3.10. Cell Culture
3.10.1. Measurement of Cytotoxicity by MTT Assay
3.10.2. Cellular Daunorubicin Uptake
3.10.3. Fluorescence Microscopy
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yun, H.; Shi, R.; Yang, Q.; Zhang, X.; Wang, Y.; Zhou, X.; Mu, K. Over expression of hRad9 protein correlates with reduced chemosensitivity in breast cancer with administration of neoadjuvant chemotherapy. Sci. Rep. 2014, 4, 7548. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R.; Wessner, C.; Stanczak, M.; Liu, J.-B.; Li, J.; Nam, K.; Forsberg, F.; Eisenbrey, J.R. Monitoring progression of ductal carcinoma in situ using photoacoustics and contrast-enhanced ultrasound. Transl. Oncol. 2019, 12, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, R.E.; Baker, J.A.; Helvie, M.A. Breast cancer deaths averted over 3 decades. Cancer 2019, 125, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Cain, E.H.; Saha, A.; Harowicz, M.R.; Marks, J.R.; Marcom, P.K.; Mazurowski, M.A. Multivariate machine learning models for prediction of pathologic response to neoadjuvant therapy in breast cancer using MRI features: A study using an independent validation set. Breast Cancer Res. Treat. 2019, 173, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Mahvi, D.A.; Liu, R.; Grinstaff, M.W.; Colson, Y.L.; Raut, C.P. Local cancer recurrence: The realities, challenges, and opportunities for new therapies. CA Cancer J. Clin. 2018, 68, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Singh, M.S.; Upadhyay, P.; Bhaskar, S. Nanoparticle mediated co-delivery of paclitaxel and a TLR-4 agonist results in tumor regression and enhanced immune response in the tumor microenvironment of a mouse model. Int. J. Pharm. 2013, 445, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer 2018, 18, 41. [Google Scholar] [CrossRef]
- Hernandez-Aya, L.F.; Gonzalez-Angulo, A.M. Adjuvant systemic therapies in breast cancer. Surg. Clin. 2013, 93, 473–491. [Google Scholar] [CrossRef]
- Corti, A.; Pastorino, F.; Curnis, F.; Arap, W.; Ponzoni, M.; Pasqualini, R. Targeted drug delivery and penetration into solid tumors. Med. Res. Rev. 2012, 32, 1078–1091. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Weiss, R.B. The anthracyclines: Will we ever find a better doxorubicin? In Seminars in oncology 1992, 19, 670–686. [Google Scholar]
- Zucchi, R.; Danesi, R. Cardiac toxicity of antineoplastic anthracyclines. Curr. Med. Chem. Anti Cancer Agents 2003, 3, 151–171. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Allen, T.M. Long-circulating (sterically stabilized) liposomes for targeted drug delivery. Trends Pharmacol. Sci. 1994, 15, 215–220. [Google Scholar] [CrossRef]
- Matsuo, H.; Wakasugi, M.; Takanaga, H.; Ohtani, H.; Naito, M.; Tsuruo, T.; Sawada, Y. Possibility of the reversal of multidrug resistance and the avoidance of side effects by liposomes modified with MRK-16, a monoclonal antibody to P-glycoprotein. J. Control. Release 2001, 77, 77–86. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297. [Google Scholar]
- Huang, S.K.; Stauffer, P.R.; Hong, K.; Guo, J.W.; Phillips, T.L.; Huang, A.; Papahadjopoulos, D. Liposomes and hyperthermia in mice: Increased tumor uptake and therapeutic efficacy of doxorubicin in sterically stabilized liposomes. Cancer Res. 1994, 54, 2186–2191. [Google Scholar]
- Li, L.; ten Hagen, T.L.; Hossann, M.; Süss, R.; van Rhoon, G.C.; Eggermont, A.M.; Haemmerich, D.; Koning, G.A. Mild hyperthermia triggered doxorubicin release from optimized stealth thermosensitive liposomes improves intratumoral drug delivery and efficacy. J. Control. Release 2013, 168, 142–150. [Google Scholar] [CrossRef]
- Liu, J.; Conboy, J.C. Phase transition of a single lipid bilayer measured by sum-frequency vibrational spectroscopy. J. Am. Chem. Soc. 2004, 126, 8894–8895. [Google Scholar] [CrossRef]
- Attwood, S.J.; Choi, Y.; Leonenko, Z. Preparation of DOPC and DPPC supported planar lipid bilayers for atomic force microscopy and atomic force spectroscopy. Int. J. Mol. Sci. 2013, 14, 3514–3539. [Google Scholar] [CrossRef]
- Leonenko, Z.; Finot, E.; Ma, H.; Dahms, T.; Cramb, D. Investigation of temperature-induced phase transitions in DOPC and DPPC phospholipid bilayers using temperature-controlled scanning force microscopy. Biophys. J. 2004, 86, 3783–3793. [Google Scholar] [CrossRef] [PubMed]
- Ikon, N.; Su, B.; Hsu, F.-F.; Forte, T.M.; Ryan, R.O. Exogenous cardiolipin localizes to mitochondria and prevents TAZ knockdown-induced apoptosis in myeloid progenitor cells. Biochem. Biophys. Res. Commun. 2015, 464, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Castedo, M.; Macho, A.; Zamzami, N.; Hirsch, T.; Marchetti, P.; Uriel, J.; Kroemer, G. Mitochondrial perturbations define lymphocytes undergoing apoptotic depletion in vivo. Eur. J. Immunol. 1995, 25, 3277–3284. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, V.; Capozzi, A.; Recalchi, S.; Signore, M.; Mattei, V.; Garofalo, T.; Misasi, R.; Degli Esposti, M.; Sorice, M. Altered Traffic of Cardiolipin during Apoptosis: Exposure on the Cell Surface as a Trigger for “Antiphospholipid Antibodies”. J. Immunol. Res. 2015, 2015, 847985. [Google Scholar] [CrossRef]
- Sennato, S.; Bordi, F.; Cametti, C.; Coluzza, C.; Desideri, A.; Rufini, S. Evidence of domain formation in cardiolipin− glycerophospholipid mixed monolayers. A thermodynamic and AFM study. J. Phys. Chem. B 2005, 109, 15950–15957. [Google Scholar] [CrossRef]
- Kuwana, T.; Mackey, M.R.; Perkins, G.; Ellisman, M.H.; Latterich, M.; Schneiter, R.; Green, D.R.; Newmeyer, D.D. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 2002, 111, 331–342. [Google Scholar] [CrossRef]
- Lutter, M.; Fang, M.; Luo, X.; Nishijima, M.; Xie, X.-S.; Wang, X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat. Cell Biol. 2000, 2, 754–756. [Google Scholar] [CrossRef]
- García-Sáez, A.J.; Ries, J.; Orzáez, M.; Pérez-Payà, E.; Schwille, P. Membrane promotes tBID interaction with BCLXL. Nat. Struct. Mol. Biol. 2009, 16, 1178–1185. [Google Scholar] [CrossRef]
- Unsay, J.D.; Cosentino, K.; Subburaj, Y.; García-Sáez, A.J. Cardiolipin effects on membrane structure and dynamics. Langmuir 2013, 29, 15878–15887. [Google Scholar] [CrossRef]
- Gubernator, J.; Chwastek, G.; Korycińska, M.; Stasiuk, M.; Grynkiewicz, G.; Lewrick, F.; Süss, R.; Kozubek, A. The encapsulation of idarubicin within liposomes using the novel EDTA ion gradient method ensures improved drug retention in vitro and in vivo. J. Control. Release 2010, 146, 68–75. [Google Scholar] [CrossRef]
- Wei, X.; Shamrakov, D.; Nudelman, S.; Peretz-Damari, S.; Nativ-Roth, E.; Regev, O.; Barenholz, Y. Cardinal role of intraliposome doxorubicin-sulfate nanorod crystal in doxil properties and performance. ACS Omega 2018, 3, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Plourde, K.; Derbali, R.M.; Desrosiers, A.; Dubath, C.; Vallée-Bélisle, A.; Leblond, J. Aptamer-based liposomes improve specific drug loading and release. J. Control. Release 2017, 251, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.; Vreeland, W.; DeVoe, D.L. Microfluidic remote loading for rapid single-step liposomal drug preparation. Lab Chip 2014, 14, 3359–3367. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.W.; Prince, R.C.; Crofts, A.R. The response of fluorescent amines to pH gradients across liposome membranes. Biochim. Et Biophys. Acta Biomembr. 1972, 274, 323–335. [Google Scholar] [CrossRef]
- Nicholas, A.R.; Scott, M.J.; Kennedy, N.I.; Jones, M.N. Effect of grafted polyethylene glycol (PEG) on the size, encapsulation efficiency and permeability of vesicles. Biochim. Biophys. Acta Biomembr. 2000, 1463, 167–178. [Google Scholar] [CrossRef]
- Mayer, L.D.; Tai, L.C.; Bally, M.B.; Mitilenes, G.N.; Ginsberg, R.S.; Cullis, P.R. Characterization of liposomal systems containing doxorubicin entrapped in response to pH gradients. Biochim. Biophys. Acta Biomembr. 1990, 1025, 143–151. [Google Scholar] [CrossRef]
- Johnston, M.J.; Edwards, K.; Karlsson, G.; Cullis, P.R. Influence of drug-to-lipid ratio on drug release properties and liposome integrity in liposomal doxorubicin formulations. J. Liposome Res. 2008, 18, 145–157. [Google Scholar] [CrossRef]
- Lewis, R.N.; McElhaney, R.N. The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes. Biochim. Biophys. Acta Biomembr. 2009, 1788, 2069–2079. [Google Scholar] [CrossRef]
- Nag, O.K.; Yadav, V.R.; Hedrick, A.; Awasthi, V. Post-modification of preformed liposomes with novel non-phospholipid poly (ethylene glycol)-conjugated hexadecylcarbamoylmethyl hexadecanoic acid for enhanced circulation persistence in vivo. Int. J. Pharm. 2013, 446, 119–129. [Google Scholar] [CrossRef]
- Gabizon, A.; Papahadjopoulos, D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc. Natl. Acad. Sci. USA 1988, 85, 6949–6953. [Google Scholar] [CrossRef]
- Sun, X.; Yan, X.; Jacobson, O.; Sun, W.; Wang, Z.; Tong, X.; Xia, Y.; Ling, D.; Chen, X. Improved tumor uptake by optimizing liposome based RES blockade strategy. Theranostics 2017, 7, 319. [Google Scholar] [CrossRef]
- Koukourakis, M.; Koukouraki, S.; Fezoulidis, I.a.; Kelekis, N.; Kyrias, G.; Archimandritis, S.; Karkavitsas, N. High intratumoural accumulation of stealth® liposomal doxorubicin (Caelyx®) in glioblastomas and in metastatic brain tumours. Br. J. Cancer 2000, 83, 1281–1286. [Google Scholar] [CrossRef]
- Solomon, R.; Gabizon, A.A. Clinical pharmacology of liposomal anthracyclines: Focus on pegylated liposomal doxorubicin. Clin. Lymphoma Myeloma 2008, 8, 21–32. [Google Scholar] [CrossRef]
- Bandak, S.; Goren, D.; Horowitz, A.; Tzemach, D.; Gabizon, A. Pharmacological studies of cisplatin encapsulated in long-circulating liposomes in mouse tumor models. Anti Cancer Drugs 1999, 10, 911–920. [Google Scholar] [CrossRef]
- Yatvin, M.B.; Weinstein, J.N.; Dennis, W.H.; Blumenthal, R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science 1978, 202, 1290–1293. [Google Scholar] [CrossRef]
- Blok, M.; Van Deenen, L.; De Gier, J. Effect of the gel to liquid crystalline phase transition on the osmotic behaviour of phosphatidycholine liposomes. Biochim. Biophys. Acta Biomembr. 1976, 433, 1–12. [Google Scholar] [CrossRef]
- Ta, T.; Porter, T.M. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J. Control. Release 2013, 169, 112–125. [Google Scholar] [CrossRef]
- Kneidl, B.; Peller, M.; Winter, G.; Lindner, L.H.; Hossann, M. Thermosensitive liposomal drug delivery systems: State of the art review. Int. J. Nanomed. 2014, 9, 4387. [Google Scholar]
- Gaber, M.H.; Wu, N.Z.; Hong, K.; Huang, S.K.; Dewhirst, M.W.; Papahadjopoulos, D. Thermosensitive liposomes: Extravasation and release of contents in tumor microvascular networks. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 1177–1187. [Google Scholar] [CrossRef]
- Gaber, M.H.; Hong, K.; Huang, S.K.; Papahadjopoulos, D. Thermosensitive sterically stabilized liposomes: Formulation and in vitro studies on mechanism of doxorubicin release by bovine serum and human plasma. Pharm. Res. 1995, 12, 1407–1416. [Google Scholar] [CrossRef]
- Needham, D.; Anyarambhatla, G.; Kong, G.; Dewhirst, M.W. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res. 2000, 60, 1197–1201. [Google Scholar]
- Landon, C.D.; Park, J.-Y.; Needham, D.; Dewhirst, M.W. Nanoscale drug delivery and hyperthermia: The materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed. J. 2011, 3, 38. [Google Scholar] [CrossRef]
- Guo, J.; Lu, W.-L. Effects of stealth liposomal daunorubicin plus tamoxifen on the breast cancer and cancer stem cells. J. Pharm. Pharm. Sci. 2010, 13, 136–151. [Google Scholar] [CrossRef]
- Klimtová, I.; Šimůnek, T.; Mazurová, Y.; Hrdina, R.; Gerš, V.; Adamcová, M. Comparative study of chronic toxic effects of daunorubicin and doxorubicin in rabbits. Hum. Exp. Toxicol. 2002, 21, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Lammers, T.; Hennink, W.; Storm, G. Tumour-targeted nanomedicines: Principles and practice. Br. J. Cancer 2008, 99, 392–397. [Google Scholar] [CrossRef]
- Pisco, A.O.; Jackson, D.A.; Huang, S. Reduced intracellular drug accumulation in drug-resistant leukemia cells is not only solely due to MDR-mediated efflux but also to decreased uptake. Front. Oncol. 2014, 4, 306. [Google Scholar] [CrossRef]
- Bassett, J.B.; Anderson, R.U.; Tacker, J.R. Use of temperature-sensitive liposomes in the selective delivery of methotrexate and cis-platinum analogues to murine bladder tumor. J. Urol. 1986, 135, 612–615. [Google Scholar] [CrossRef]
- Speelmans, G.; Staffhorst, R.W.; de Kruijff, B.; de Wolf, F.A. Transport studies of doxorubicin in model membranes indicate a difference in passive diffusion across and binding at the outer and inner leaflet of the plasma membrane. Biochemistry 1994, 33, 13761–13768. [Google Scholar] [CrossRef]
- Lei, T.; Srinivasan, S.; Tang, Y.; Manchanda, R.; Nagesetti, A.; Fernandez-Fernandez, A.; McGoron, A.J. Comparing cellular uptake and cytotoxicity of targeted drug carriers in cancer cell lines with different drug resistance mechanisms. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, R.; Yang, J.; Shi, Y.; Ma, G.; Zhang, Z.; Zhang, X. Enhanced retention and anti-tumor efficacy of liposomes by changing their cellular uptake and pharmacokinetics behavior. Biomaterials 2015, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wan, B.; Yang, Y.; Ren, X.; Guo, L.-H. Length effects on the dynamic process of cellular uptake and exocytosis of single-walled carbon nanotubes in murine macrophage cells. Sci. Rep. 2017, 7, 1518. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hirsh, D.J.; Cabral-Lilly, D.; Zirkel, A.; Gruner, S.M.; Janoff, A.S.; Perkins, W.R. Doxorubicin physical state in solution and inside liposomes loaded via a pH gradient. Biochim. Biophys. Acta Biomembr. 1998, 1415, 23–40. [Google Scholar] [CrossRef]
- Briuglia, M.-L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Bartlett, G.R. Phosphorus assay in column chromatography. J. Biol. Chem. 1959, 234, 466–468. [Google Scholar] [CrossRef]
- Smith, P.E.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]


| Formulation | EE % | DL (%) | Particle Size (nm) | PI | Zeta Potential (mV) | Osmolality (Osm/L) |
|---|---|---|---|---|---|---|
| F1 | 98.0 ± 0.8 | 16.2 ± 0.4 | 115 ± 1.3 | 0.12 ± 0.03 | −27.7 ± 1.9 | 309 ± 3.6 |
| F2 | 96.0 ± 3.6 | 15.7 ± 0.7 | 124 ± 1.7 | 0.11 ± 0.01 | −9.7 ± 2.8 | 300 ± 14.9 |
| F3 | 94.0 ± 3.7 | 17.0 ± 0.9 | 85.0 ± 3.4 | 0.15 ± 0.03 | −2.5 ± 2.1 | 280 ± 14.5 |
| Time Points | Initial Analysis | After 1 Month Analysis | ||||
|---|---|---|---|---|---|---|
| Formulation | F1 | F2 | F3 | F1 | F2 | F3 |
| Particle Size (nm) | 115 ± 1.3 | 124 ± 1.7 | 85.0 ± 3.4 | 119 ± 2.5 | 125 ± 1.4 | 88.0 ± 2.4 |
| PI | 0.12 ± 0.03 | 0.11 ± 0.01 | 0.15 ± 0.03 | 0.22 ±0.01 | 0.17 ± 0.06 | 0.16 ± 0.07 |
| EE% | 98.0 ± 0.8 | 96.0 ± 3.6 | 94.0 ± 3.7 | 95.0 ± 2.5 | 93.0 ± 1.4 | 92.0 ± 2.1 |
| DL% | 16.2 ± 0.4 | 15.7 ± 0.7 | 17.0 ± 0.9 | 15.8 ± 0.4 | 15.4 ± 0.2 | 15.9 ± 0.7 |
| Zeta Potential (mV) | −27.7 ± 1.9 | −9.7 ± 2.8 | −2.5 ± 2.1 | −24.4 ± 1.4 | −7.4 ± 2.9 | −4.0 ± 2.1 |
| Osmolarity (Osm/L) | 309 ± 3.6 | 300 ± 14.9 | 280 ± 14.5 | 311 ± 6.7 | 314 ± 2.9 | 249 ± 11.2 |
| Ingredients | F1 | F2 | F3 * | F4 # |
|---|---|---|---|---|
| DPPC | 57 | 57 | - | 57 |
| MSPC | 40 | 40 | - | 40 |
| DSPC | - | - | 10 | - |
| Cholesterol | 30 | 30 | 5 | 30 |
| DSPE-mPEG (2000) | 3 | 3 | - | 3 |
| CL | 20 | - | - | 20 |
| DNR | 1 | 1 | 1 | - |
| DNR: lipid ratio | 1:5 | 1:5 | 1:10:5 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrbyawi, H.; Boddu, S.H.S.; Poudel, I.; Annaji, M.; Mita, N.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Cardiolipin for Enhanced Cellular Uptake and Cytotoxicity of Thermosensitive Liposome-Encapsulated Daunorubicin toward Breast Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 11763. https://doi.org/10.3390/ijms231911763
Alrbyawi H, Boddu SHS, Poudel I, Annaji M, Mita N, Arnold RD, Tiwari AK, Babu RJ. Cardiolipin for Enhanced Cellular Uptake and Cytotoxicity of Thermosensitive Liposome-Encapsulated Daunorubicin toward Breast Cancer Cell Lines. International Journal of Molecular Sciences. 2022; 23(19):11763. https://doi.org/10.3390/ijms231911763
Chicago/Turabian StyleAlrbyawi, Hamad, Sai H. S. Boddu, Ishwor Poudel, Manjusha Annaji, Nur Mita, Robert D. Arnold, Amit K. Tiwari, and R. Jayachandra Babu. 2022. "Cardiolipin for Enhanced Cellular Uptake and Cytotoxicity of Thermosensitive Liposome-Encapsulated Daunorubicin toward Breast Cancer Cell Lines" International Journal of Molecular Sciences 23, no. 19: 11763. https://doi.org/10.3390/ijms231911763
APA StyleAlrbyawi, H., Boddu, S. H. S., Poudel, I., Annaji, M., Mita, N., Arnold, R. D., Tiwari, A. K., & Babu, R. J. (2022). Cardiolipin for Enhanced Cellular Uptake and Cytotoxicity of Thermosensitive Liposome-Encapsulated Daunorubicin toward Breast Cancer Cell Lines. International Journal of Molecular Sciences, 23(19), 11763. https://doi.org/10.3390/ijms231911763








