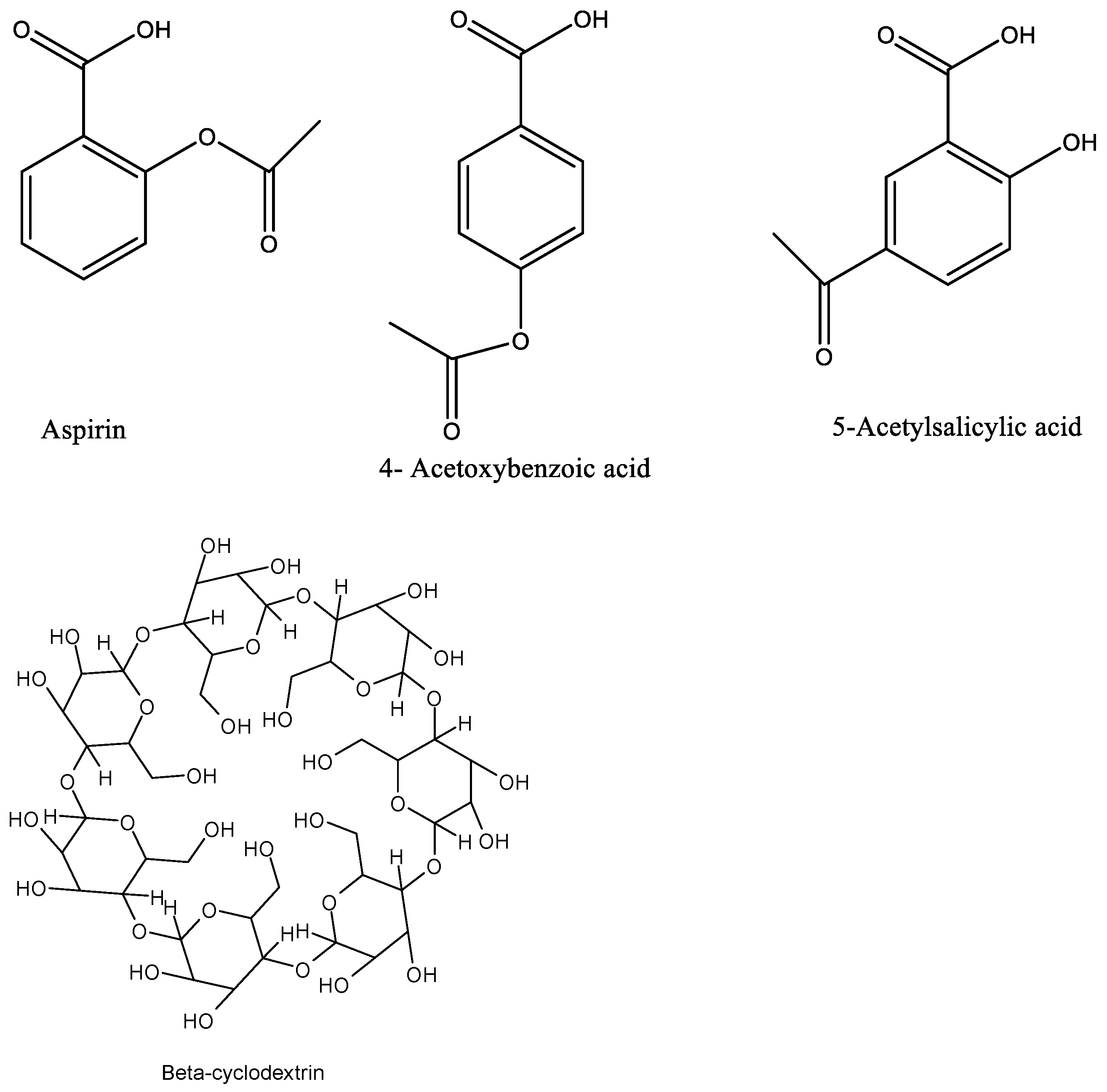
2.2. Conductance Measurements
Conductance Measurements of Acetylsalicylic Acid, 4-Acetoxybenzoic Acid and 5-Acetylsalicylic Acid in Water at 298.15 K
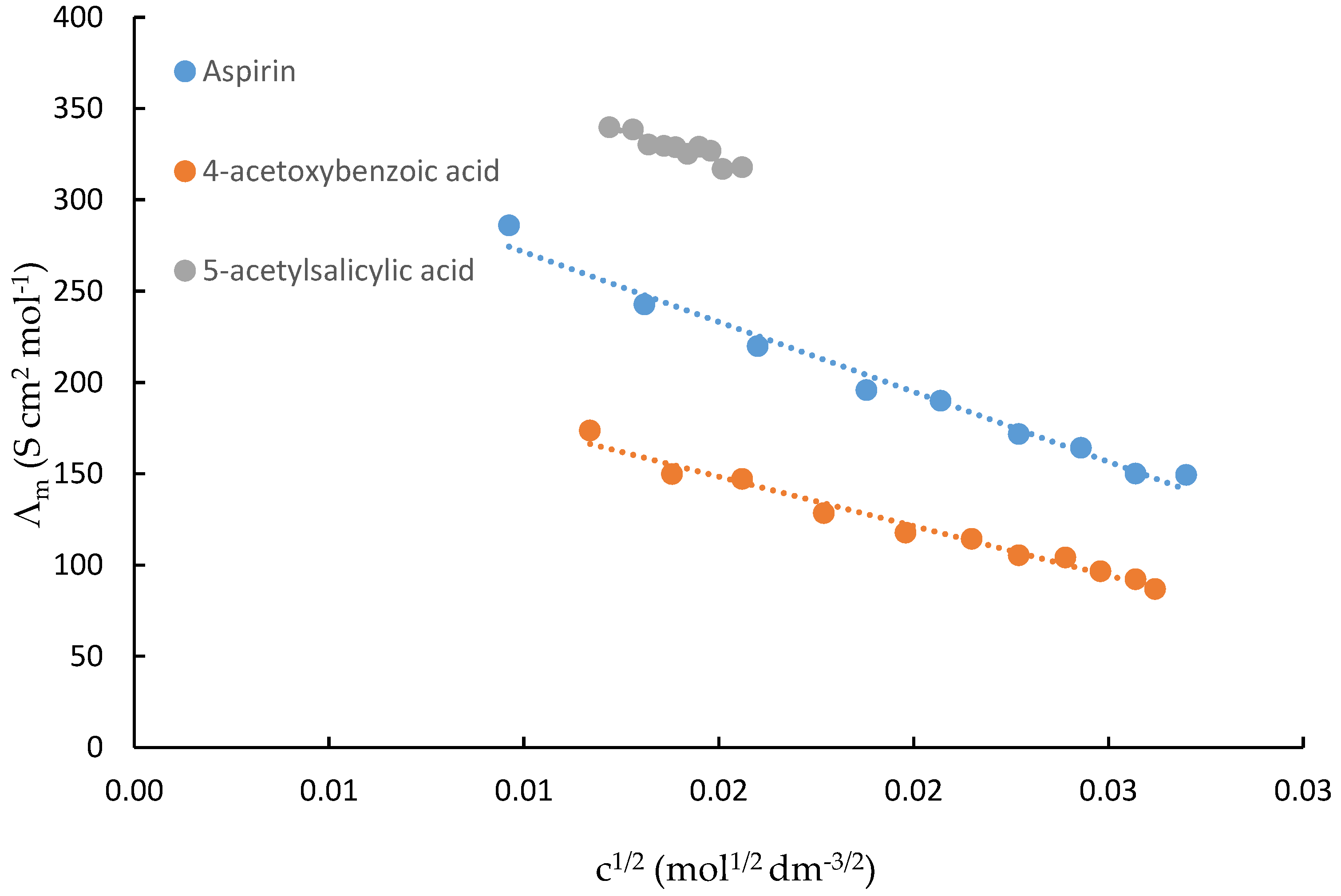
Plots of the molar conductance (Λ
m) against the c
1/2 values of acetylsalicylic acid, 4-acetoxybenzoic acid and 5-acetylsalicylic acid in water are shown in
Figure 2. A computer program was used to calculate the molar conductance at an infinite dilution, Λ
0m, and the association or ion-pair formation constant (K
ass) for the process represented by Equation (1), and the molar conductance, Λm (S cm
2 mol
−1), was calculated by the Fuoss–Hsia equation using the expansion suggested by Fernandez Prini as described elsewhere [
24].
Table 2 lists the values for Λ
0m, the ion-pair formation constant, and K
ip (Equation (2)) as well as the dissociation constant, K
a, and the pK
a values of acetylsalicylic acid, 4-acetoxybenzoic acid and 5-acetylsalicylic acid in water at 298.15K.
The ion-pair formation constant for the process described by Equation (1) is expressed in terms of activities,
a, as shown in in Equation (2):
In Equation (2), concentrations are in mol dm
−3, and γ ±
i2 is the mean molar ionic coefficient calculated by the use of the Debye–Hückel equation, where γ
HA is the activity coefficient of the un-ionized species. In very dilute solutions, γ
HA may be considered equal to unity. As far as the standard Gibbs energy of the solution in the standard state of 1 mol dm
−3, ∆
sG
0, is concerned, the application of Equation (3) requires knowledge of the ion-pair formation constant in solution. The availability of K
ip and solubilities in water allows the calculation the ionic molar concentration, c
i, and the molar concentration of ion pairs, c
ip, as previously shown [
24]. The data refer to the standard state (1 mol dm
−3).
Based on data from Pierre and Jencks [
25], it was estimated that, at the end of the conductance measurements of acetylsalicylic acid, 5% of it was hydrolyzed. It is assumed that their percentage would not have significantly affected the obtained Λ
0m, K
ip, K
a and pK
a values. In the case of 4-acetoxybenzoic acid, this percentage was estimated to be even lower. The availability of Λ
0m leads to the calculation of the ionic conductances at an infinite dilution for the anion (λ
A−) since the value for the proton (λ
H+ = 349.8 S cm
2 mol
−1) [
26] is known. For this purpose, the law of the independent migration of ions (Equation (4)) is used:
In this equation, υ+ is the number of cations and υ− is the number of anions.
Thus, values of 42.2, 51.2 and 39.2 S cm
2 mol
−1 were calculated, respectively, for the ionic conductivities at an infinite dilution in water at 298.15 K for acetylsalicylate, acetoxybenzoate and 5-acetylsalicylate anions. As far as the salicylate anions are concerned, their λ
A values, as expected, were higher than that for the unsubstituted anion (λ
A = 36.14 S cm
2 mol
−1) [
27]. A similar pattern was found for the substituted benzoate anion relative to the unsubstituted one (λ
A = 32.2 S cm
2 mol
−1) [
28]. On the other hand, the 4-acetoxybenzoic acid anion had a higher increase in the λ
− value than the other two anions, which may be due to the lower solvation of this anion relative to the others.
As far as the pKa values are concerned, the data in
Table 2 show that, among the considered systems, 4-acetoxybenzoic acid had the highest pK
a value (4.34, which is in excellent agreement with the value of 4.38 [
29] reported in water at this temperature and determined by conductimetry). Hence, it is the weakest acid, and therefore, its conjugate base has a greater proton affinity than acetylsalicylic acid and 5-acetylsalicylic acid. For acetylsalicylic acid, the pKa value shown in
Table 2 is in fair agreement with the values of 3.483 [
30], 3.49 [
30,
31] and 3.50 [
32,
33,
34,
35] reported in water at this temperature.
5-acetylsalicylic acid showed the lowest pKa value (3.04) among this series. Consequently, it was more acidic than acetylsalicylic acid and 4-acetoxybenzoic acid. This can be explained on structural terms, given that the acetyl group at position 5 of the benzene ring is an electron-withdrawing group (deactivating group), stabilizing this anion in water and making it a stronger acid.
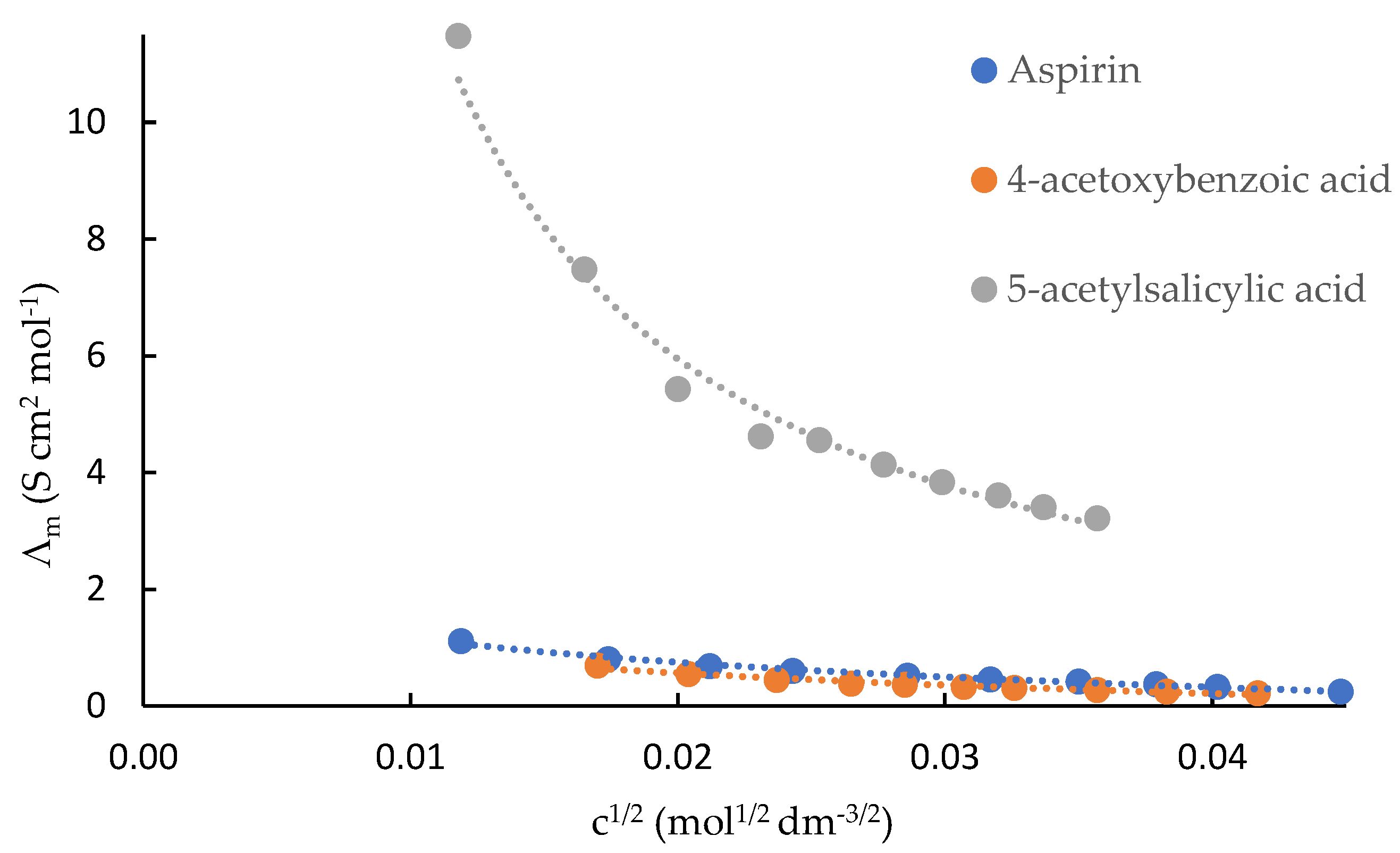
Plots of molar conductances (Λ
m) against the c
1/2 of acetylsalicylic acid, 4-acetoxybenzoic acid and 5-acetylsalicylic acid in DMF are shown in
Figure 3. The Shed turbo basic program was unable to derive accurate data of these compounds in this solvent. Hence, K
ip values were not calculated. For the three compounds in DMF, ion pairs predominated, and this is clearly reflected in their extremely low Λ
m values.
Given that most enthalpy data reported in the literature were obtained by the use of the van ’t Hoff equation, which has well-established limitations [
22,
36,
37], we proceeded with the determination of solution enthalpies by calorimetry, as discussed below.
2.3. Standard Enthalpies of Solution of Acetylsalicylic Acid, 4-Acetoxybenzoic Acid and 5-Acetylsalicylic Acid in Water and DMF at 298.15 K
The enthalpies of solution of acetylsalicylic acid in water at various concentrations are reported in
Table 3. No measurements could be carried out at higher concentrations since the dissolution rate of this compound in water was relatively slow. Therefore, the higher reported concentration is the one at which acetylsalicylic acid is fully dissolved in water. Since there were no systematic changes in the Δ
sH values with changes in the concentration of solute, the standard Δ
sH
0 value reported in
Table 3 is the average value. The enthalpies of solution of 4-acetoxybenzoic acid and 5-acetylsalicylic acid in water at 298.15 K are also reported in
Table 3. In the dissolution of a solute in water or any other solvent, there are two processes involved, as described in Equation (5):
In this equation, Δ
clH
0 is the notation used to indicate the crystal lattice process (endothermic), while Δ
solvH
0 is the solvation process (exothermic). The results shown in
Table 3 for acetylsalicylic acid and related compounds clearly indicate that their dissolution is endothermic (enthalpies of solution are positive), and therefore, the contribution of the crystal lattice enthalpy to the Δ
sH
0 values predominates. Thus, the endothermic character of the reaction follows the sequence:
5-acetylsalicylic acid > 4-acetoxybenzoic acid > acetylsalicylic acid
As expected, the Δ
sH
0 value for acetylsalicylic acid differs significantly from the apparent value reported by Apelblat and coworkers [
16], which was derived from the van ’t Hoff equation, of 22.9 kJ mol
−1.
2.4. Determination of the Gibbs Free Energy and Entropies of Solution of Acetylsalicylic Acid, 4-Acetoxybenzoic Acid and 5-Acetylsalicylic Acid in Water at 298.15 K
The values of the thermodynamic solubility product, K
sp, and Δ
sG
0 values for acetylsalicylic acid and related compounds were calculated from Equations (6) and (8), respectively, after correcting for ion-pair formation in water.
In Equation (6), aH+ and aA− represent the activities of the species H+ and A− in solution respectively. The activity of the solid is unity by convention. The activity, a, on the molar scale (mol dm−3) is related to the ionic molar concentration, ci, by the mean molar ionic activity coefficient, γ ± i.
The availability of Gibbs energy (Equation (3)) and enthalpy values allows the calculation of the entropy of solution, ΔsS. For this purpose, the following equation was used.
Data are reported in
Table 4. Quite clearly, the contributions of the enthalpy and entropy to the Gibbs energy of the process lead to the conclusion that the dissolution of these compounds in water is unfavored.
As far as the enthalpies of solution of acetylsalicylic acid, 4-acetoxybenzoic acid and 5-acetylsalicylic acid in DMF at various concentrations are concerned, the results are reported in
Table 5. All observed heats were corrected for the heat from breaking the empty ampoules in DMF (Q = −0.0518 J). Since there were no systematic changes in the Δ
sH values with changes in the concentration of the solute, the reported standard Δ
sH
0 values are the average values of the data reported in
Table 5. This table also includes the standard deviations of the data.
Again, the results shown in
Table 5 for acetylsalicylic acid and related compounds clearly indicate that their dissolution is endothermic (the enthalpies of solution are positive), and therefore, the contribution of the crystal lattice enthalpy to the Δ
sH values predominates. Thus, the endothermic character of the reaction follows the sequence:
Acetylsalicylic acid > 5-acetylsalicylic acid > 4-acetoxybenzoic acid
To our knowledge, there are no reports in the literature on the enthalpy of solution of these compounds in DMF.
As no solubility results were obtained for acetylsalicylic acid and related compounds in DMF due to their solvation in this solvent, standard Gibbs energies of solution could not be obtained. Their derivation requires that the composition of these compounds in the solid and in the saturated solution is the same.
As mentioned earlier, ΔsH0 is made by the contribution of the crystal lattice enthalpy and the solvation enthalpy. In order to remove the contribution of the crystal lattice process, it is a common practice to calculate the transfer thermodynamic parameters according to the following process:
The combination of Δ
sH
0 for acetylsalicylic acid and related compounds in water and in DMF, yields the transfer enthalpy, Δ
tH
0, of these compounds from the reference solvent to DMF (Equation (8)).
These data provide information regarding the differences in the enthalpic stabilities of these compounds in two solvents and are calculated on the assumption that these compounds are fully dissociated in DMF at infinite dilution. The data reported in
Table 6 show that these compounds are enthalpically more stable in DMF than in water.
2.5. Interaction of Acetylsalicylic Acid with β-Cyclodextrin in Water and DMF at 298.15 K
The stability constants for the complexation process involving acetylsalicylic acid (nonionized, pH 1.74–1.76, and ionized, pH 5.95–6.09) and β-cyclodextrin in water at 298 K were reported in the literature [
15]. In this work, we attempted to obtain the thermodynamic parameters of complexation by titration calorimetry at the above pHs using a four-channel heat conduction microcalorimeter. In both cases, no heats were detected. This was not an indication that complexation does not take place; it simply means that calorimetry is not a suitable reporter of the molecular events taking place, particularly if the process is entropically controlled. It is relevant to mention that there are two different processes taking place at pHs of 1.75 and 6. Thus, at the lower pH the process is defined by Equation (9). As previously discussed by Danil de Namor and coworkers [
22], this is referred as an association process, K
ass:
At pH 6, the process is represented by Equation (10):
The thermodynamic data for the processes represented by Equations (9) and (10) are reported in
Table 7.
The lower interaction of the ionic relative to the neutral guest with β-CD in water reflected in the K
ass and K
s values reported in
Table 7 must be due to the lower hydrophobic character of the charged relative to the neutral guest, although the higher hydration of the ion in the former relative to the latter is likely to have a negative effect on the penetration of the guest into the hydrophobic cavity of the receptor. Indeed, the more favorable entropy of the neutral relative to the ionic guest is typical for processes in which a higher dehydration or desolvation takes place upon complexation. It is well-established that β-cyclodextrin hosts eight water molecules in its cavity [
21], and the higher contribution of the entropy to the more favorable Gibbs energy of the association process provides a clear indication that, in the formation of the adduct, the neutral guest penetrates more into the cavity of the receptor than the ionic guest, and therefore the amount of water released from the cavity is higher in the association than in the complexing process. In both cases, the interaction of the receptor with these guests is entropy-controlled, given that the enthalpic contribution to the Gibbs energy is nil.
As far as the interaction of acetylsalicylic acid with β-cyclodextrin in DMF is concerned, the conductance measurements discussed above showed that, in a dipolar aprotic solvent such as DMF, acetylsalicylic acid is undissociated, and therefore ion pairs predominate in solution. On the other hand the previously reported thermodynamic parameters for the transfer of β-cyclodextrin from water to DMF [
21] clearly indicated that this receptor is better solvated in DMF than in water (Δ
tG
0 β-CD (H
2O→DMF)= −4.26 KJmol
−1). The transfer process is enthalpically controlled, with a high loss in entropy. The most remarkable result found previously was the increase in enthalpic stability with an increase in moving from α to β and to γ cyclodextrin, suggesting that these receptors host the solvent in their cavities. This is in accord with the results shown in
Table 7 for the association process of β CD and neutral acetylsalicylic acid in DMF, where the host–guest interaction is greater than that for the same process in water as a result of a more favorable contribution of the entropy resulting from the release of DMF from the ligand cavity. Hence, this release requires energy; therefore, the process is slightly endothermic.