Enhanced Hydrogen Evolution Reactivity of T’-Phase Tungsten Dichalcogenides (WS2, WSe2, and WTe2) Materials: A DFT Study
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Defect Structures and H Adsorption
3.2. Electronic Structures of the Defective Configurations
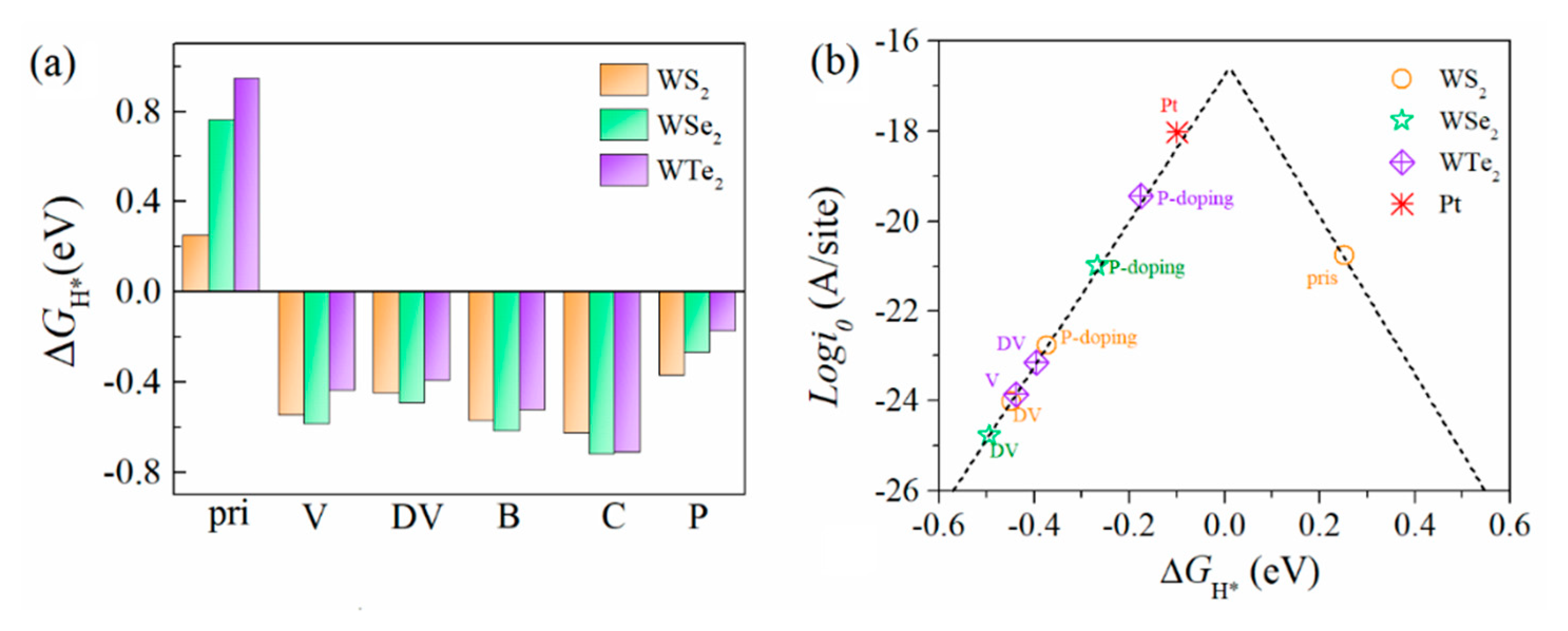
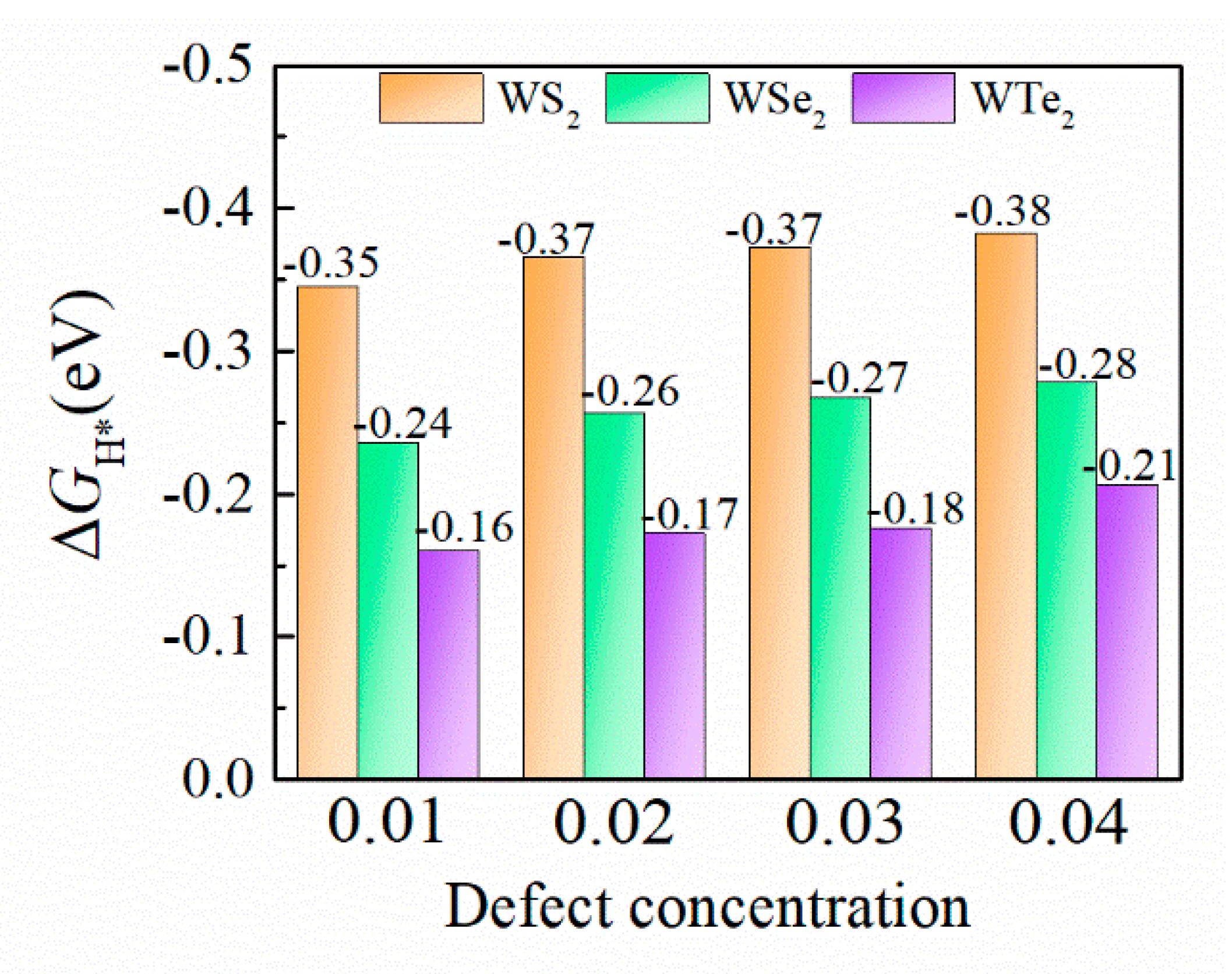
3.3. HER Activity of the Defective Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, M.; Ding, Y.; Yu, L.; Du, X.; Zhao, Y. In situ grown pristine cobalt sulfide as bifunctional photocatalyst for hydrogen and oxygen evolution. Adv. Funct. Mater. 2017, 27, 1605846. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D transition-metal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ye, Y.; Liu, T.; Wang, X.; Zhang, B.; Wang, M.; Han, H.; Li, C. Unraveling a single-step simultaneous two-electron transfer process from semiconductor to molecular catalyst in a CoPy/CdS hybrid system for photocatalytic H2 evolution under strong alkaline conditions. J. Am. Chem. Soc. 2016, 138, 10726–10729. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y.; Zhao, H.; Su, J.; Zhang, H.; Wang, Y. Investigation of anion doping effect to boost overall water splitting. J. Catal. 2020, 381, 84–95. [Google Scholar] [CrossRef]
- Michalsky, R.; Zhang, Y.-J.; Peterson, A.A. Trends in the Hydrogen Evolution Activity of Metal Carbide Catalysts. ACS Catal. 2014, 4, 1274–1278. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef]
- Tang, T.; Wang, Z.; Guan, J. A review of defect engineering in two-dimensional materials for electrocatalytic hydrogen evolution reaction. Chin. J. Catal. 2022, 43, 636–678. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Zhang, X.; You, T. Defect- and S-rich ultrathin MoS2 nanosheet embedded N-doped carbon nanofibers for efficient hydrogen. J. Mater. Chem. A. 2015, 3, 15927–15934. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Y.; Zhu, D.; Vasileff, A.; Ling, T.; Qiao, S.-Z. Identification of pH-dependent synergy on Ru/MoS2 interface: A comparation of alkaline and acidic hydrogen evolution. Nanoscale 2017, 9, 16616–16621. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Vikraman, D.; Akbar, K.; Hussain, S.; Yoo, G.; Jang, J.-Y.; Chun, S.-H.; Jung, J.; Park, H.J. Direct synthesis of thickness-tunable MoS2 quantum dot thin layers: Optical, structural and electrical properties and their application to hydrogen evolution. Nano Energy 2017, 35, 101–114. [Google Scholar] [CrossRef]
- Ye, S.; Ding, C.; Chen, R.; Fan, F.; Fu, P.; Yin, H.; Wang, X.; Wang, Z.; Du, P.; Li, C. Mimicking the key functions of photosystem II in artificial photosynthesis for photoelectrocatalytic water splitting. J. Am. Chem. Soc. 2018, 140, 3250–3256. [Google Scholar] [CrossRef]
- Huang, H.; Fan, X.; Singh, D.J.; Zheng, W. Recent progress of TMD nanomaterials: Phase transitions and applications. Nanoscale 2020, 12, 1247–1268. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef]
- Karunadasa, H.I.; Montalvo, E.; Sun, Y.; Majda, M.; Long, J.R.; Chang, C.J. A molecular MoS2 edge site mimic for catalytic hydrogen generation. Science 2012, 335, 698–702. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Guo, H.; Chen, W.; Yuan, J.; Martinez, U.; Gupta, G.; Mohite, A.; Ajayan, P.M.; Lou, J. Unveiling active sites for the hydrogen evolution reaction on monolayer MoS2. Adv. Mater. 2017, 29, 1701955. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Tedstone, A.A.; Lewis, D.J.; O’Brien, P. Synthesis, properties, and applications of transition metal-doped layered transition metal dichalcogenides. Chem. Mater. 2016, 28, 1965–1974. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, Y.; Liu, G.; Zheng, W.; Feng, W.; Gao, F.; Cao, W.; Fu, Y.; Hu, W.; Hu, P. Tuning electrochemical catalytic activity of defective 2D terrace MoSe2 heterogeneous catalyst via cobalt doping. J. Mater. Chem. A 2017, 5, 11357–11363. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, S.; Zhang, F.; Zheng, W.; Zhao, L.; Feng, Q. Metal-semiconductor 1T/2H-MoS2 by a heteroatom-doping strategy for enhanced electrocatalytic hydrogen evolution. Catal. Commun. 2021, 156, 106325. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Y.; Cheng, Z.; Tan, Y.; Yuan, T.; Shen, Z. Distance synergy of single Ag atoms doped MoS2 for hydrogen evolution electrocatalysis. Appl. Surf. Sci. 2021, 547, 149113. [Google Scholar] [CrossRef]
- Wu, W.; Niu, C.; Wei, C.; Jia, Y.; Li, C.; Xu, Q. Activation of MoS2 basal planes for hydrogen evolution by zinc. Angew. Chem. Int. Ed. 2019, 58, 2029–2033. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Yang, W.; Liu, Y.; Yu, Y. Engineering sulfur vacancies in basal plane of MoS2 for enhanced hydrogen evolution reaction. J. Catal. 2020, 391, 91–97. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, Q.; Li, J.-J.; Xia, Y.-X.; Li, H.-X.; Li, Y.-J. Engineering Isolated S Vacancies over 2D MoS2 Basal Planes for Catalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2022, 5, 3521–3530. [Google Scholar] [CrossRef]
- Li, J.; Kang, J.; Cai, Q.; Hong, W.; Jian, C.; Liu, W.; Banerjee, K. Boosting hydrogen evolution performance of MoS2 by band structure engineering. Adv. Mater. Interfaces 2017, 4, 1700303. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Akbar, K.; Adaikalam, K.; Lee, S.H.; Chun, S.-H.; Jung, J.; Kim, H.-S.; Park, H.J. Facile synthesis of molybdenum diselenide layers for high-performance hydrogen evolution electrocatalysts. ACS Omega 2018, 3, 5799–5807. [Google Scholar] [CrossRef]
- Qi, K.; Cui, X.; Gu, L.; Yu, S.; Fan, X.; Luo, M.; Xu, S.; Li, N.; Zheng, L.; Zhang, Q. Single-atom cobalt array bound to distorted 1T MoS2 with ensemble effect for hydrogen evolution catalysis. Nat. Commun. 2019, 10, 5231. [Google Scholar] [CrossRef]
- Shang, B.; Cui, X.; Jiao, L.; Qi, K.; Wang, Y.; Fan, J.; Yue, Y.; Wang, H.; Bao, Q.; Fan, X.; et al. Lattice -Mismatch-Induced UltrasTable 1T-Phase MoS2–Pd/Au for Plasmon-Enhanced Hydrogen Evolution. Nano Lett. 2019, 19, 2758–2764. [Google Scholar] [CrossRef]
- Wei, S.; Cui, X.; Xu, Y.; Shang, B.; Zhang, Q.; Gu, L.; Fan, X.; Zheng, L.; Hou, C.; Huang, H. Iridium-triggered phase transition of MoS2 nanosheets boosts overall water splitting in alkaline media. ACS Energy Lett. 2018, 4, 368–374. [Google Scholar] [CrossRef]
- Tsai, C.; Li, H.; Park, S.; Park, J.; Han, H.S.; Nørskov, J.K.; Zheng, X.; Abild-Pedersen, F. Electrochemical generation of sulfur vacancies in the basal plane of MoS2 for hydrogen evolution. Nat. Commun. 2017, 8, 15113. [Google Scholar] [CrossRef]
- Ling, F.; Liu, X.; Jing, H.; Chen, Y.; Zeng, W.; Zhang, Y.; Kang, W.; Liu, J.; Fang, L.; Zhou, M. Optimizing edges and defects of supported MoS2 catalysts for hydrogen evolution via an external electric field. Phys. Chem. Chem. Phys. 2018, 20, 26083–26090. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Fan, X.; Singh, D.J.; Zheng, W. Modulation of hydrogen evolution catalytic activity of basal plane in monolayer platinum and palladium dichalcogenides. ACS Omega 2018, 3, 10058–10065. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Akbar, K.; Karuppasamy, K.; Chun, S.-H.; Jung, J.; Kim, H.-S. Design of Basal Plane Edges in Metal-Doped Nanostripes-Structured MoSe2 Atomic Layers To Enhance Hydrogen Evolution Reaction Activity. ACS Sustain. Chem. Eng. 2019, 7, 458–469. [Google Scholar] [CrossRef]
- Kang, M.; Lin, C.; Yang, H.; Guo, Y.; Liu, L.; Xue, T.; Liu, Y.; Gong, Y.; Zhao, Z.; Zhai, T. Proximity enhanced hydrogen evolution reactivity of substitutional doped monolayer WS2. ACS Appl. Mater. Interfaces 2021, 13, 19406–19413. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Liu, T.; Chen, T.; Shen, X.; Ding, T.; Cao, L.; Wang, L.; Luo, Q.; Yao, T. In situ investigation on doping effect in Co-doped tungsten diselenide nanosheets for hydrogen evolution reaction. J. Phys. Chem. C 2021, 125, 6229–6236. [Google Scholar] [CrossRef]
- Kadam, S.R.; Enyashin, A.N.; Houben, L.; Bar-Ziv, R.; Bar-Sadan, M. Ni–WSe2 nanostructures as efficient catalysts for electrochemical hydrogen evolution reaction (HER) in acidic and alkaline media. J. Mater. Chem. A 2020, 8, 1403–1416. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Zhang, Z.; Hu, S.; Ma, M.; Zhang, Z.; Zhou, W.; Liu, H. Addressable surface engineering for N-doped WS2 nanosheet arrays with abundant active sites and the optimal local electronic structure for enhanced hydrogen evolution reaction. Nanoscale 2020, 12, 22541–22550. [Google Scholar] [CrossRef]
- Sarma, P.V.; Vineesh, T.V.; Kumar, R.; Sreepal, V.; Prasannachandran, R.; Singh, A.K.; Shaijumon, M.M. Nanostructured Tungsten Oxysulfide as an Efficient Electrocatalyst for Hydrogen Evolution Reaction. ACS Catal. 2020, 10, 6753–6762. [Google Scholar] [CrossRef]
- Sun, L.; Gao, M.; Jing, Z.; Cheng, Z.; Zheng, D.; Xu, H.; Zhou, Q.; Lin, J. 1 T-Phase Enriched P doped WS2 nanosphere for highly efficient electrochemical hydrogen evolution reaction. Chem. Eng. J. 2022, 429, 132187. [Google Scholar] [CrossRef]
- Wang, F.; Niu, S.; Liang, X.; Wang, G.; Chen, M. Phosphorus incorporation activates the basal plane of tungsten disulfide for efficient hydrogen evolution catalysis. Nano Res. 2022, 15, 2855–2861. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, J.; Ma, J.; Liu, P.; Gao, D.; Tao, K.; Xue, D. N-doped WS2 nanosheets: A high-performance electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 11234–11238. [Google Scholar] [CrossRef]
- Bai, S.; Yang, M.; Jiang, J.; He, X.; Zou, J.; Xiong, Z.; Liao, G.; Liu, S. Recent advances of MXenes as electrocatalyst for hydrogen evolution reaction. npj 2D Mater. Appl. 2021, 5, 78. [Google Scholar] [CrossRef]
- Posada-Pérez, S.; Viñes, R.; Valero, R.; Rodriguez, J.; Illas, F. Adsorption and dissociation of molecular hydrogen on orthorhombic β-Mo2C and cubic δ-MoC(001) surfaces. Surf. Sci. 2017, 656, 24–32. [Google Scholar] [CrossRef]
- Morales-García, Á.; Calle-Vallejo, F.; Illas, F. MXenes: New horizons in catalysis. ACS Catal. 2020, 10, 13487–13503. [Google Scholar] [CrossRef]
- Hou, P.; Tian, Y.; Jin, D.; Liu, X.; Du, F.; Meng, X. Advances in theoretical calculations of MXenes as HER and OER (water splitting) electrocatalysts. J. Phys. D Appl. Phys. 2022, 55, 464002. [Google Scholar] [CrossRef]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.; Jin, S. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Yamaguchi, H.; Li, J.; Silva, R.; Alves, D.C.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 2013, 12, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.S.; Sai, N.; Lu, P.; Coker, E.N.; Liu, S.; Artyushkova, K.; Luk, T.S.; Kaehr, B.; Brinker, C.J. Understanding catalysis in a multiphasic two-dimensional transition metal dichalcogenide. Nat. Commun. 2015, 6, 8311. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Jiao, Y.; Ma, F.; Jiao, Y.; Waclawik, E.; Du, A. Charge mediated semiconducting-to-metallic phase transition in molybdenum disulfide monolayer and hydrogen evolution reaction in new 1T′ phase. J. Phys. Chem. C 2015, 119, 13124–13128. [Google Scholar] [CrossRef]
- Yue, X.; Yang, J.; Li, W.; Jing, Y.; Dong, L.; Zhang, Y.; Li, X. Electron Irradiation Induces the Conversion from 2H-WSe2 to 1T-WSe2 and Promotes the Performance of Electrocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2022, 10, 2420–2428. [Google Scholar] [CrossRef]
- Ji, W.; Chu, X.; Zhang, S.; Wang, D.; Ma, Y. Active basal plane in ZT-phased MX2 (M = Mo, W; X = S, Se, Te) catalysts for the hydrogen evolution reaction: A theoretical study. Int. J. Hydrogen Energy 2018, 43, 19432–19437. [Google Scholar] [CrossRef]
- Lee, J.; Kang, S.; Yim, K.; Kim, K.Y.; Jang, H.W.; Kang, Y.; Han, S. Hydrogen evolution reaction at anion vacancy of two-dimensional transition-metal dichalcogenides: Ab initio computational screening. J. Phys. Chem. Lett. 2018, 9, 2049–2055. [Google Scholar] [CrossRef]
- Voiry, D.; Salehi, M.; Silva, R.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G.; Chhowalla, M. Conducting MoS2 nanosheets as catalysts for hydrogen evolution reaction. Nano Lett. 2013, 13, 6222–6227. [Google Scholar] [CrossRef] [PubMed]
- Eshete, Y.A.; Ling, N.; Kim, S.; Kim, D.; Hwang, G.; Cho, S.; Yang, H. Vertical heterophase for electrical, electrochemical, and mechanical manipulations of layered MoTe2. Adv. Funct. Mater. 2019, 29, 1904504. [Google Scholar] [CrossRef]
- Seok, J.; Lee, J.-H.; Bae, D.; Ji, B.; Son, Y.-W.; Lee, Y.H.; Yang, H.; Cho, S. Hybrid catalyst with monoclinic MoTe2 and platinum for efficient hydrogen evolution. APL Mater. 2019, 7, 071118. [Google Scholar] [CrossRef]
- Wei, C.; Wu, W.; Li, H.; Lin, X.; Wu, T.; Zhang, Y.; Xu, Q.; Zhang, L.; Zhu, Y.; Yang, X. Atomic plane-vacancy engineering of transition-metal dichalcogenides with enhanced hydrogen evolution capability. ACS Appl. Mater. Interfaces 2019, 11, 25264–25270. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Ji, B.; Bae, D.; Lee, J.-H.; Park, H.J.; Kim, Y.-M.; Son, Y.-W.; Yang, H.; Cho, S. Role of anionic vacancy for active hydrogen evolution in WTe2. Appl. Surf. Sci. 2020, 515, 145972. [Google Scholar] [CrossRef]
- Song, J.; Kwon, S.; Hossain, M.D.; Chen, S.; Li, Z.; Goddard, W.A. Reaction Mechanism and Strategy for Optimizing the Hydrogen Evolution Reaction on Single-Layer 1T′ WSe2 and WTe2 Based on Grand Canonical Potential Kinetics. ACS Appl. Mater. Interfaces 2021, 13, 55611–55620. [Google Scholar] [CrossRef]
- Ling, N.; Zheng, S.; Lee, Y.; Zhao, M.; Kim, E.; Cho, S.; Yang, H. Active hydrogen evolution on the plasma-treated edges of WTe2. APL Mater. 2021, 9, 061108. [Google Scholar] [CrossRef]
- Sokolikova, M.S.; Sherrell, P.C.; Palczynski, P.; Bemmer, V.L.; Mattevi, C. Direct solution-phase synthesis of 1T’ WSe2 nanosheets. Nat. Commun. 2019, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Fan, X.; Singh, D.J.; Chen, H.; Jiang, Q.; Zheng, W. Controlling phase transition for single-layer MTe2 (M = Mo and W): Modulation of the potential barrier under strain. Phys. Chem. Chem. Phys. 2016, 18, 4086–4094. [Google Scholar] [CrossRef]
- Xiao, B.; Huang, Q.; Wu, J.; Song, E.; Jiang, Q. Tetragonal transition metal selenide for hydrogen evolution. Appl. Surf. Sci. 2022, 591, 153249. [Google Scholar] [CrossRef]
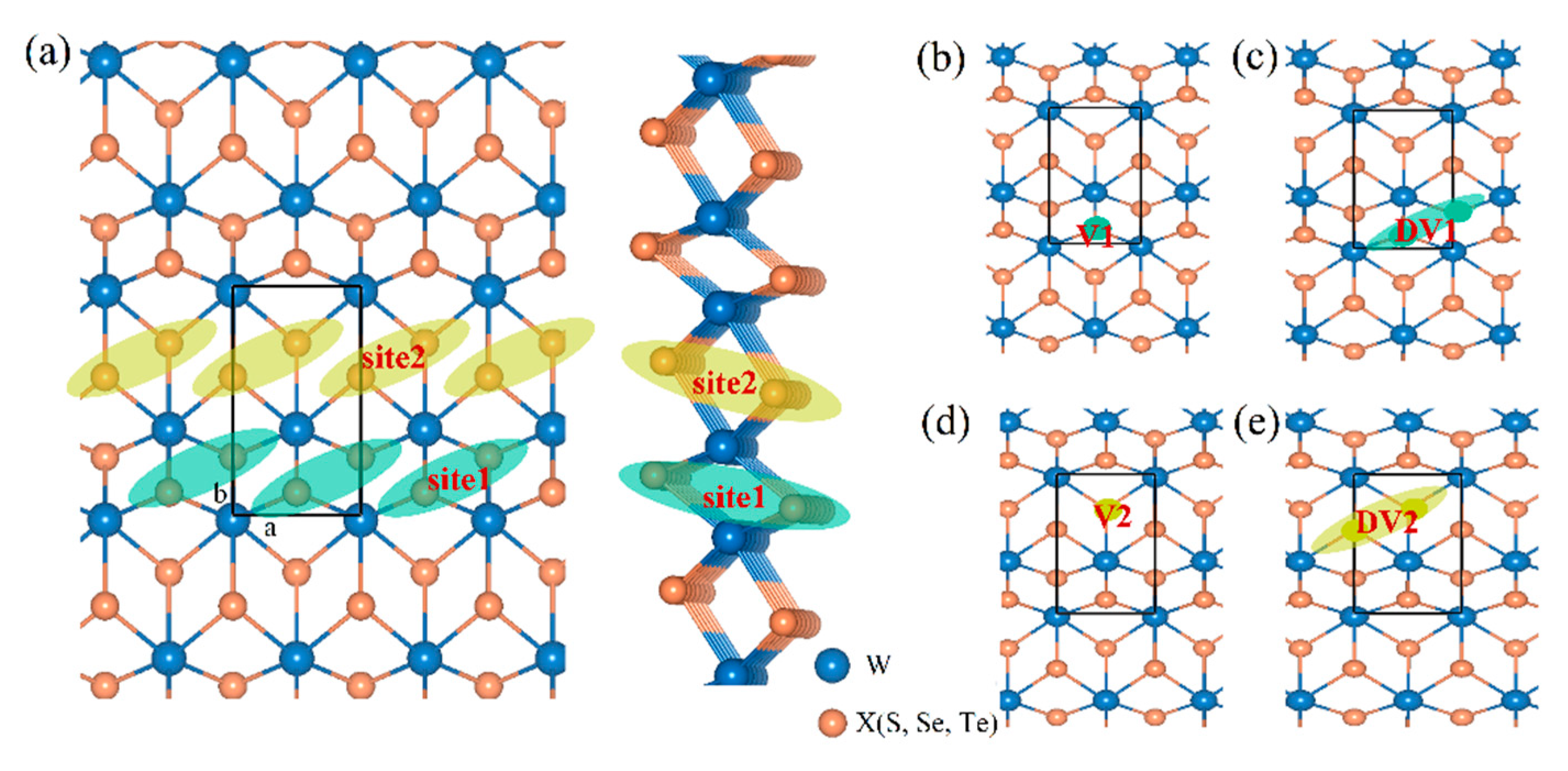
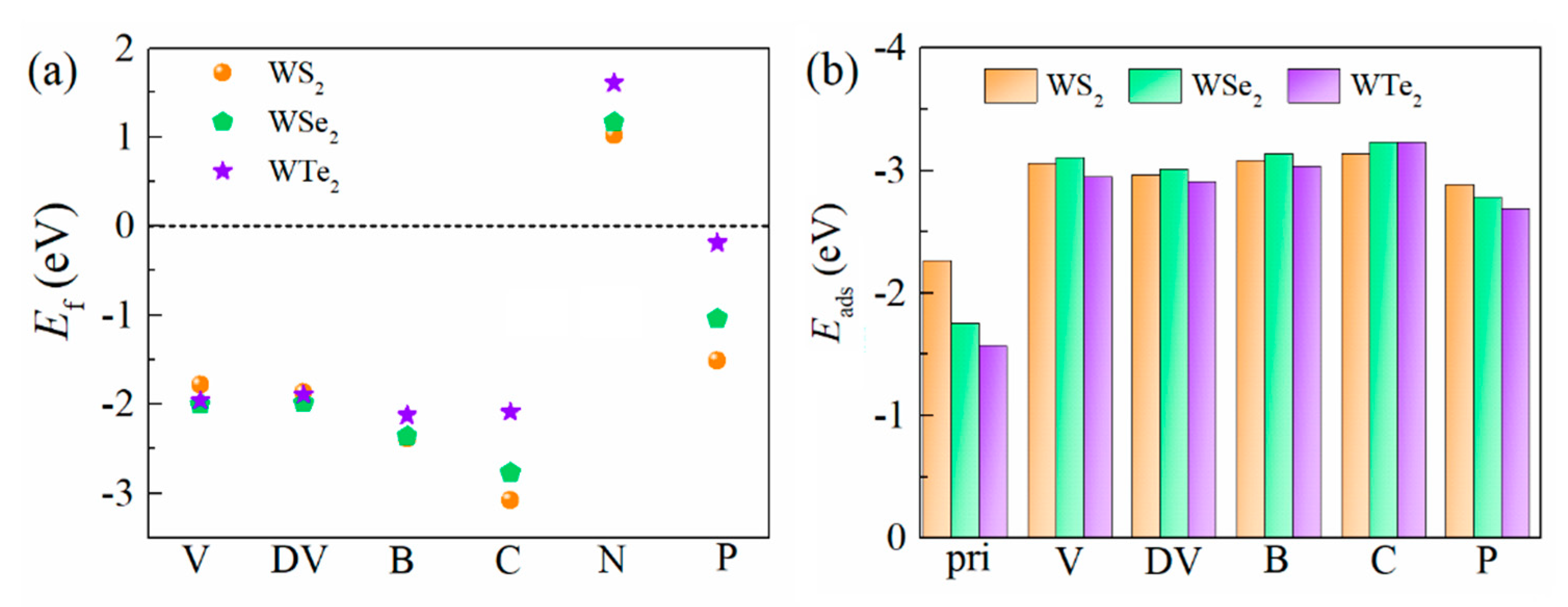
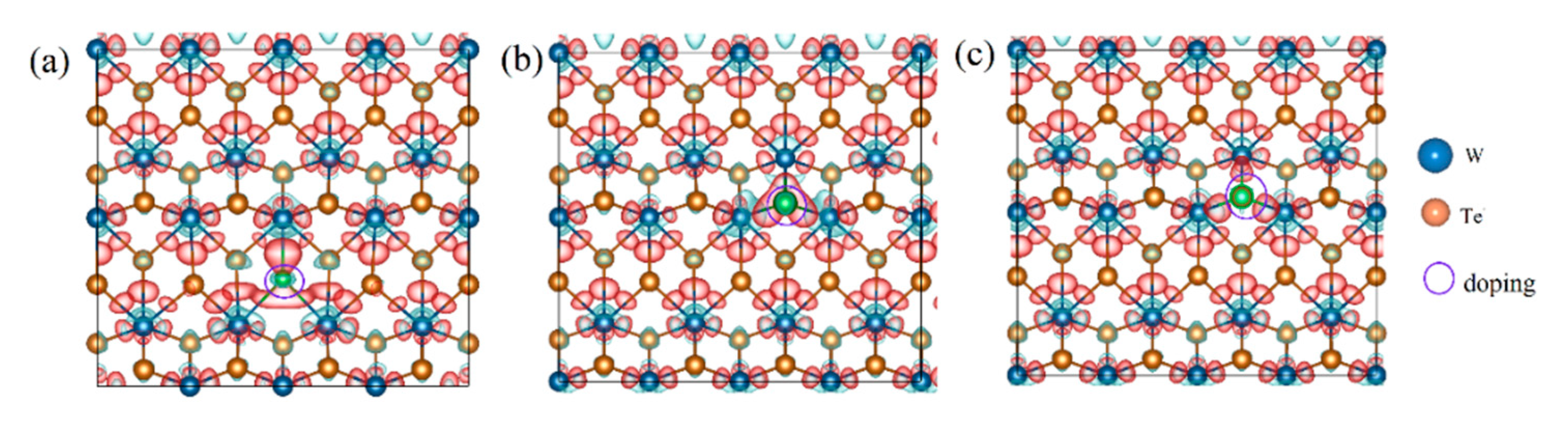

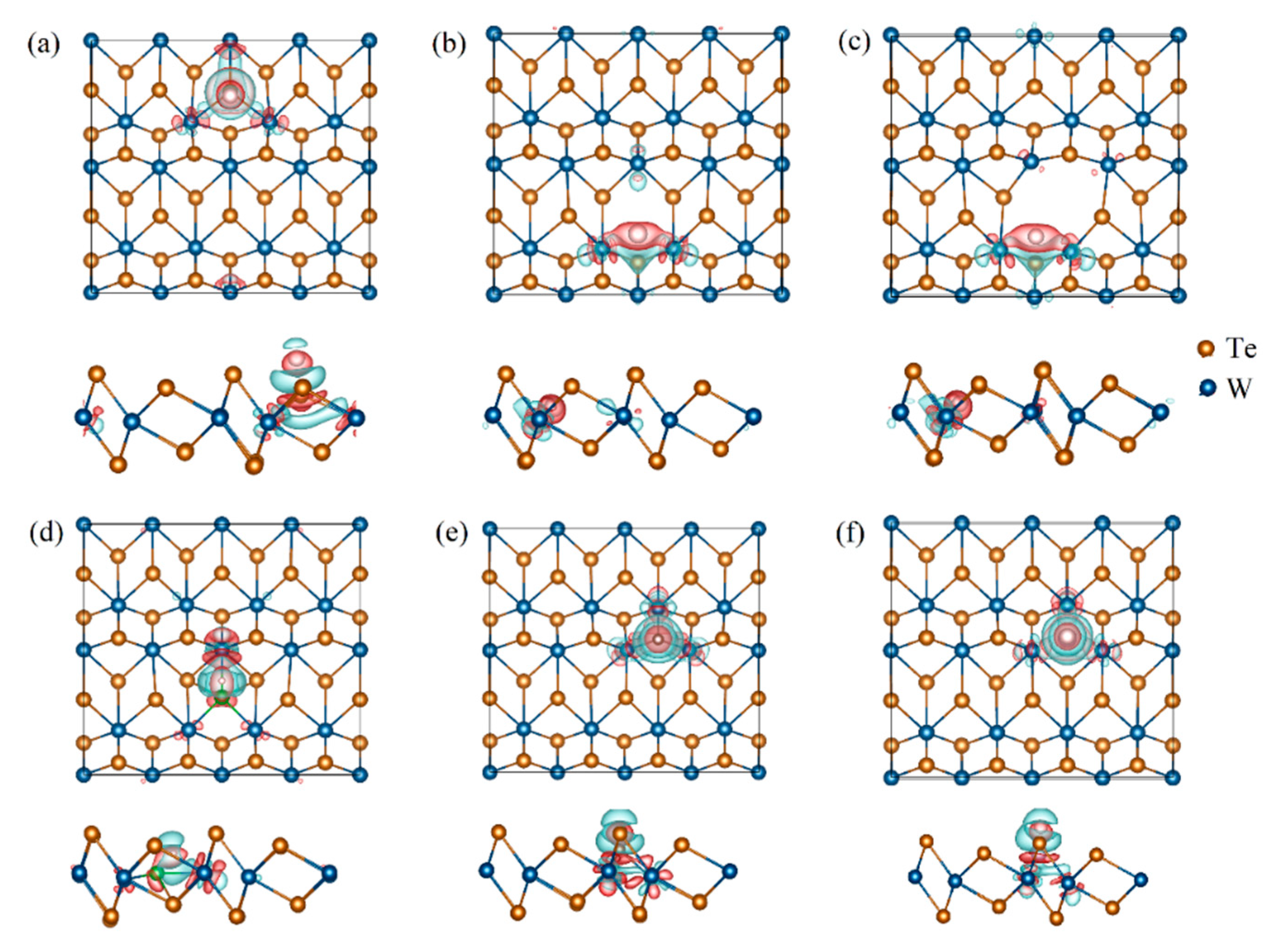
| Materials | Lattice | |
|---|---|---|
| a (Å) | b (Å) | |
| WS2 | 3.19 | 5.72 |
| WSe2 | 3.30 | 5.94 |
| WTe2 | 3.49 | 6.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Hu, G.; Hu, C.; Fan, X. Enhanced Hydrogen Evolution Reactivity of T’-Phase Tungsten Dichalcogenides (WS2, WSe2, and WTe2) Materials: A DFT Study. Int. J. Mol. Sci. 2022, 23, 11727. https://doi.org/10.3390/ijms231911727
Huang H, Hu G, Hu C, Fan X. Enhanced Hydrogen Evolution Reactivity of T’-Phase Tungsten Dichalcogenides (WS2, WSe2, and WTe2) Materials: A DFT Study. International Journal of Molecular Sciences. 2022; 23(19):11727. https://doi.org/10.3390/ijms231911727
Chicago/Turabian StyleHuang, Haihua, Guowei Hu, Chengchao Hu, and Xiaofeng Fan. 2022. "Enhanced Hydrogen Evolution Reactivity of T’-Phase Tungsten Dichalcogenides (WS2, WSe2, and WTe2) Materials: A DFT Study" International Journal of Molecular Sciences 23, no. 19: 11727. https://doi.org/10.3390/ijms231911727
APA StyleHuang, H., Hu, G., Hu, C., & Fan, X. (2022). Enhanced Hydrogen Evolution Reactivity of T’-Phase Tungsten Dichalcogenides (WS2, WSe2, and WTe2) Materials: A DFT Study. International Journal of Molecular Sciences, 23(19), 11727. https://doi.org/10.3390/ijms231911727






