Glutamic Acid and Poly-γ-glutamic Acid Enhanced the Heat Resistance of Chinese Cabbage (Brassica rapa L. ssp. pekinensis) by Improving Carotenoid Biosynthesis, Photosynthesis, and ROS Signaling
Abstract
1. Introduction
2. Results
2.1. Effect of Glu and γ-PGA on the Growth of Chinese Cabbage Seedlings under Heat Stress
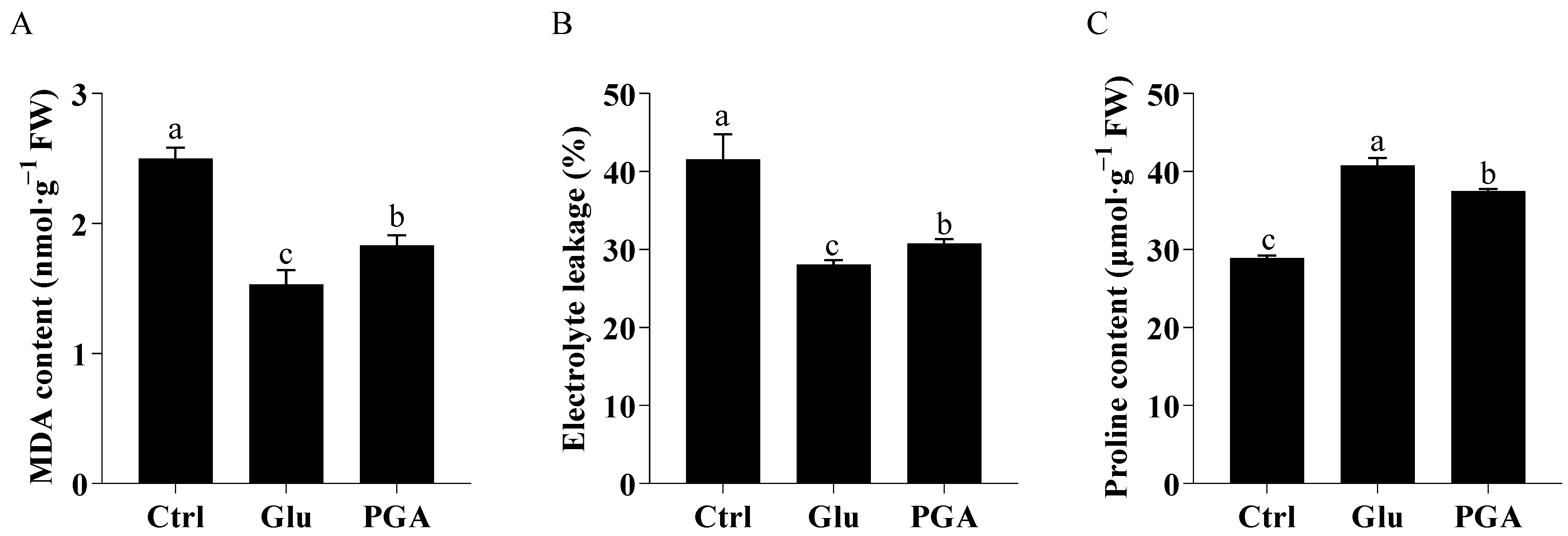
2.2. Effects of Glu and γ-PGA on MDA, Electrolyte Leakage, and Proline Content under Heat Stress
2.3. Effect of Glu and γ-PGA on Antioxidant Enzyme Activity and Superoxide Radicals under Heat Stress
2.4. Effect of Glu and γ-PGA on Photosynthetic Pigment, Gas Exchange Parameters, and Chlorophyll Fluorescence Parameters under Heat Stress
2.4.1. Photosynthetic Pigments
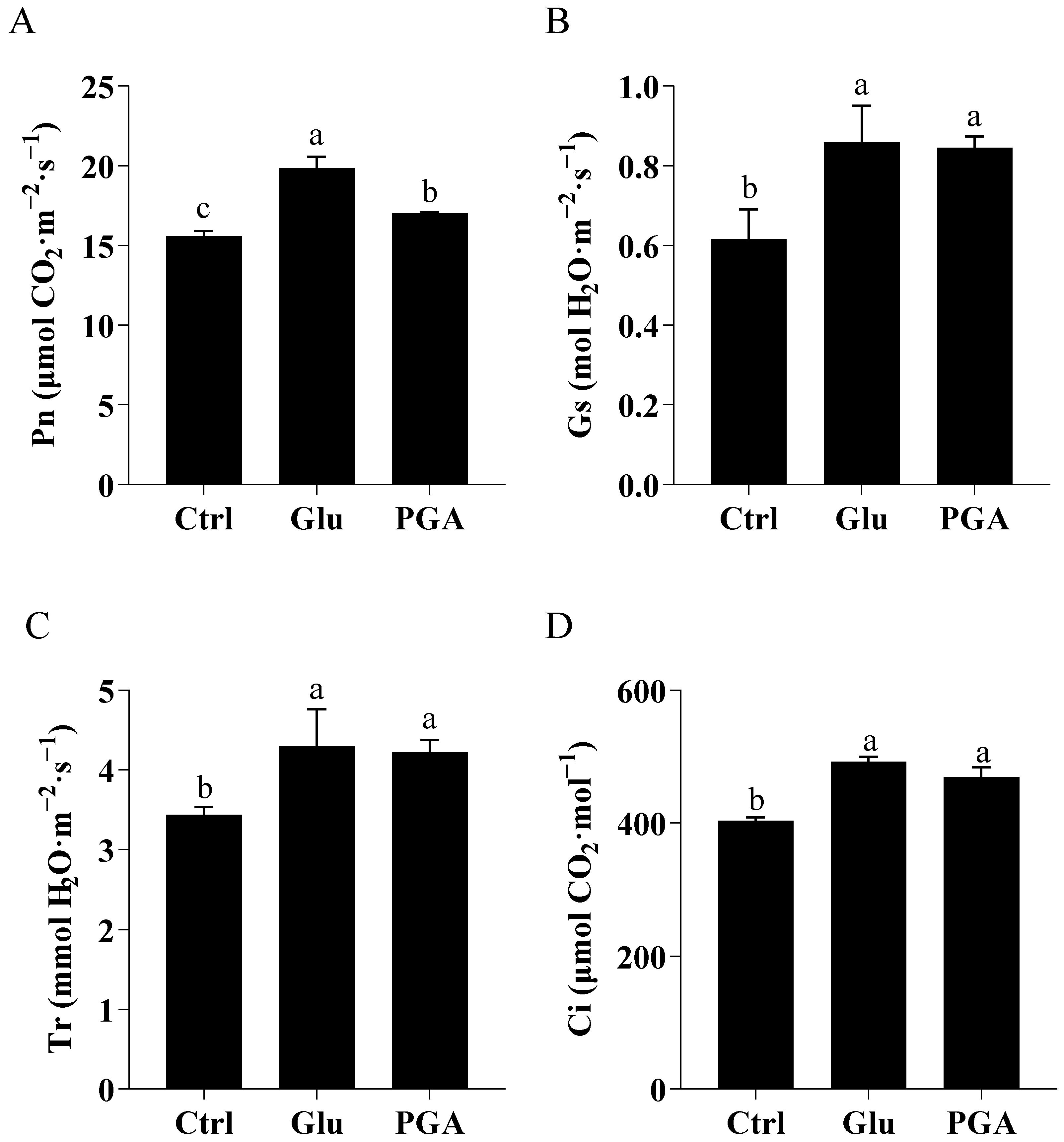
2.4.2. Gas Exchange Parameters
2.4.3. Chlorophyll Fluorescence Parameters
2.5. Transcriptome Changes in Response to Glu and γ-PGA Priming
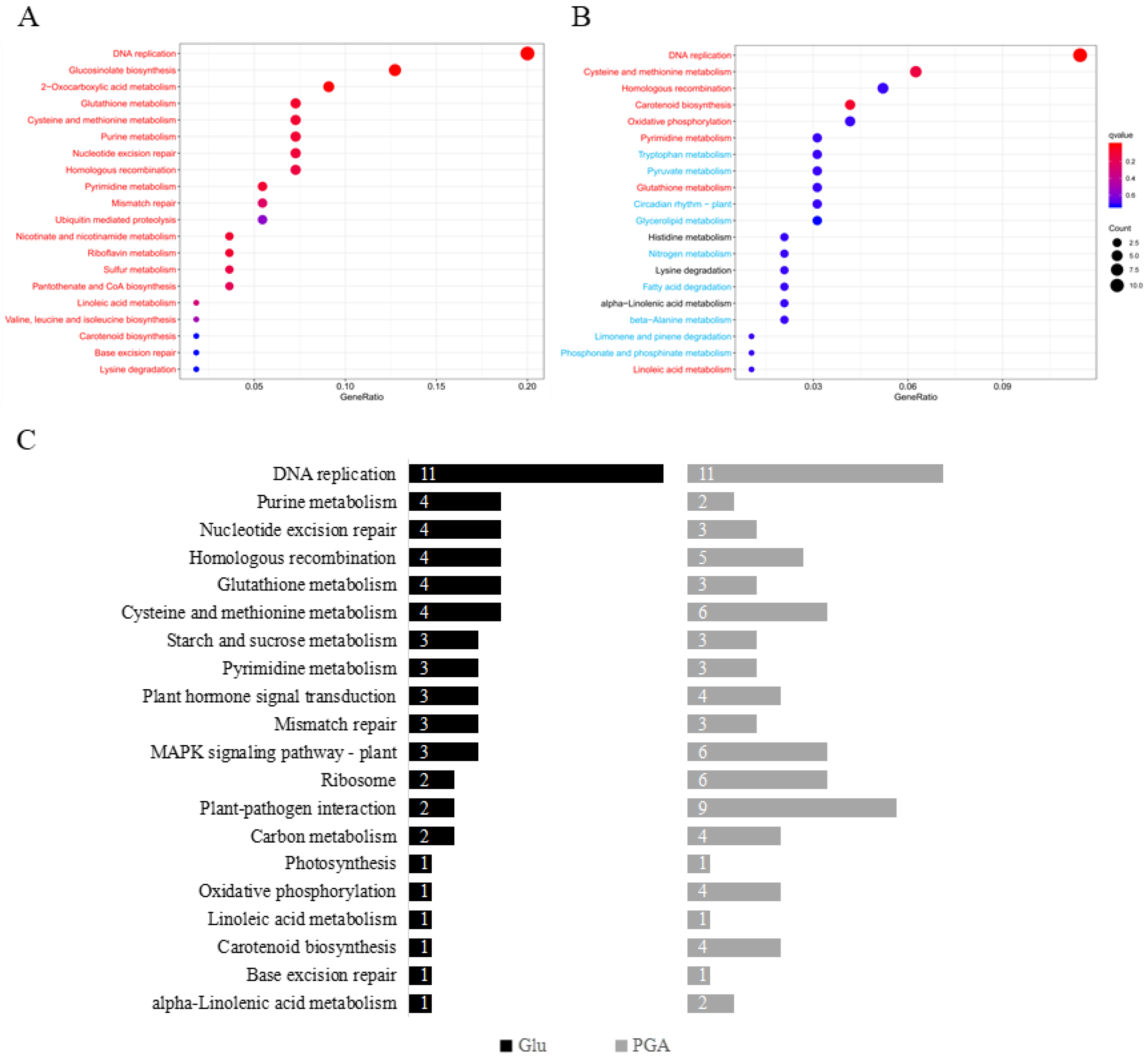
2.6. KEGG Pathway Enrichment Analysis
2.7. Validation of DEGs by qRT-PCR
3. Discussion
3.1. Molecular Aspects of Acquired Thermotolerance Conferred by Exogenous Glu and γ-PGA
3.2. Exogenous Glu and γ-PGA Improve Photosynthesis in Chinese Cabbage Seedlings under Heat Stress
3.3. Exogenous Glu and γ-PGA Mitigate the Growth Inhibition Caused by Heat Stress in Chinese Cabbage Seedlings
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Measurement of Biomass and Physiological Parameters
4.3. Measurements of Gas Exchange and Chlorophyll Fluorescence Parameters
4.4. SPAD and Spectrophotometric Measurements of Photosynthetic Pigments
4.5. Total RNA Extraction and Quality Control
4.6. RNA Sequencing and RNA-Seq Data Analysis
4.7. Transcriptomic and sRNA Data Validation by qRT-PCR
4.8. Statistical Analyses
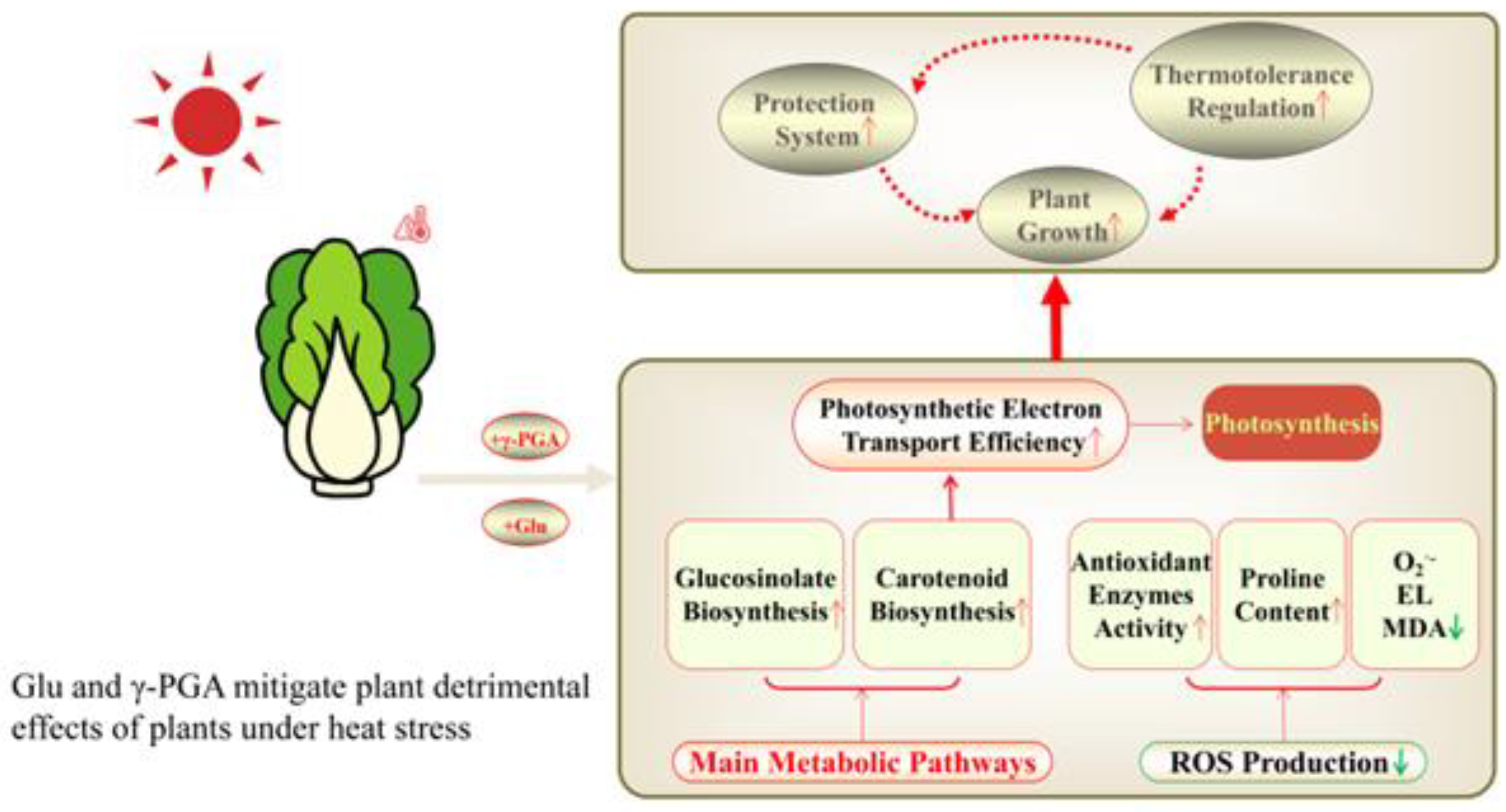
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Young, L.W.; Wilen, R.W.; Bonham-Smith, P.C. High temperature stress of Brassica napus during flowering reduces micro-and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J. Exp. Bot. 2004, 55, 485–495. [Google Scholar] [CrossRef]
- Allen, M.; Antwi-Agyei, P.; Aragon-Durand, F.; Babiker, M.; Bertoldi, P.; Bind, M.; Zickfeld, K. Technical Summary: Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Fotso-Nguemo, T.C.; Vondou, D.A.; Diallo, I.; Diedhiou, A.; Weber, T.; Tanessong, R.S.; Yepdo, Z.D. Potential impact of 1.5, 2 and 3 °C global warming levels on heat and discomfort indices changes over Central Africa. Sci. Total Environ. 2022, 804, 150099. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change; elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- Medina, E.; Kim, S.H.; Yun, M.; Choi, W.G. Recapitulation of the function and role of ROS generated in response to heat stress in plants. Plants 2021, 10, 371. [Google Scholar] [CrossRef]
- Priyanka, D.; Nutan, K.K.; Singla-Pareek, S.L.; Pareek, A. Oxidative environment and redox homeostasis in plants: Dissecting out significant contribution of major cellular organelles. Front. Environ. Sci. 2015, 2, 70. [Google Scholar]
- Imran, M.; Aaqil Khan, M.; Shahzad, R.; Bilal, S.; Khan, M.; Yun, B.W.; Khan, A.L.; Lee, I.J. Melatonin ameliorates thermotolerance in soybean seedling through balancing redox homeostasis and modulating antioxidant defense, phytohormones and polyamines biosynthesis. Molecules 2021, 26, 5116. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Alfosea-Simón, M.; Simón-Grao, S.; Zavala-Gonzalez, E.A.; Cámara-Zapata, J.M.; Simón, I.; Martínez-Nicolás, J.J.; Lidón, V.; García-Sánchez, F. Physiological, nutritional and metabolomic responses of tomato plants after the foliar application of amino acids aspartic acid, glutamic acid and alanine. Front. Plant Sci. 2021, 11, 2138. [Google Scholar] [CrossRef] [PubMed]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef] [PubMed]
- Yaronskaya, E.; Vershilovskaya, I.; Poers, Y.; Alawady, A.E.; Averina, N.; Grimm, B. Cytokinin effects on tetrapyrrole biosynthesis and photosynthetic activity in barley seedlings. Planta 2006, 224, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liao, W.; Dawuda, M.M.; Hu, L.; Yu, J. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: A review. Plant Growth Regul. 2019, 87, 357–374. [Google Scholar] [CrossRef]
- Lv, D.G.; Yu, C.; Yang, L.; Qin, S.J.; Ma, H.Y.; Du, G.D.; Khanizadeh, S. Effects of foliar-applied L-glutamic acid on the diurnal variations of leaf gas exchange and chlorophyll fluorescence parameters in hawthorn (Crataegus pinnatifida Bge.). Eur. J. Hortic. Sci. 2009, 74, 204–209. [Google Scholar]
- Franzoni, G.; Cocetta, G.; Ferrante, A. Effect of glutamic acid foliar applications on lettuce under water stress. Physiol. Mol. Biol. Plants 2021, 27, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Forde, B.G. Glutamate signalling in roots. J. Exp. Bot. 2014, 65, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 2018, 361, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Luo, L.; Wei, J.; Chen, Q.; Yang, Y.; Hu, X.; Kong, X. The glutamate receptors AtGLR1.2 and AtGLR1.3 increase cold tolerance by regulating jasmonate signaling in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2018, 506, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Shih, L.; Van, Y.T. The production of poly-(γ-glutamic acid) from microorganisms and its various applications. Bioresour. Technol. 2001, 79, 207–225. [Google Scholar] [CrossRef]
- Ashiuchi, M. Occurrence and biosynthetic mechanism of poly-gamma-glutamic acid. In Amino-Acid Homopolymers Occurring in Nature; Springer: Berlin/Heidelberg, Germany, 2010; Volume 15, pp. 77–93. [Google Scholar]
- Ma, H.; Li, P.; Liu, X.; Li, C.; Zhang, S.; Wang, X.; Tao, X. Poly-γ-glutamic acid enhanced the drought resistance of maize by improving photosynthesis and affecting the rhizosphere microbial community. BMC Plant Biol. 2022, 22, 11. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, P.; Pang, X.; Li, H.; Feng, X.; Xu, H. Exogenous application of poly-γ-glutamic acid enhances stress defense in Brassica napus L. seedlings by inducing cross-talks between Ca2+, H2O2, brassinolide, and jasmonic acid in leaves. Plant Physiol. Biochem. 2017, 118, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lei, P.; Feng, X.; Li, S.; Xu, H. Analysis of the metabolic pathways affected by poly (γ-glutamic acid) in Arabidopsis thaliana based on gene chip microarray. J. Agric. Food Chem. 2016, 64, 6257–6266. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ma, J.; Lei, P.; Wang, Q.; Feng, X.; Xu, H. Poly-γ-glutamic acid induces system tolerance to drought stress by promoting abscisic acid accumulation in Brassica napus L. Sci. Rep. 2020, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Fu, I.M.; Shennan, C.; Welbaum, G.E. Evaluating Chinese cabbage cultivars for high temperature tolerance. In Proceedings of the Second National Symposium New Crops: Exploration, Research, Commercialization, Indianapolis, IN, USA, 6–9 October 1991; John Wiley and Sons Ltd.: New York, NY, USA, 1993; pp. 570–573. [Google Scholar]
- Mi, B.; Liu, F.; Xie, L.; Zhou, H.; Wu, F.; Dai, X. Evaluation of the uptake capacities of heavy metals in Chinese cabbage. Ecotoxicol. Environ. Saf. 2019, 171, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Daly, P.; Tomkins, B. Production and postharvest handling of Chinese cabbage (Brassica rapa var. pekinensis). RIRDC Res. Pap. Ser. 1997, 97, 1. [Google Scholar]
- Rehmani, M.I.A.; Ding, C.; Li, G.; Ata-Ul-Karim, S.T.; Hadifa, A.; Bashir, M.A.; Ding, Y. Vulnerability of rice production to temperature extremes during rice reproductive stage in Yangtze River Valley, China. J. King Saud Univ. Sci. 2021, 33, 101599. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Palta, J.P. Leaf chlorophyll content. Remote Sens. Rev. 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Yuan, Z.; Cao, Q.; Zhang, K.; Ata-Ul-Karim, S.T.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Optimal leaf positions for SPAD meter measurement in rice. Front. Plant Sci. 2016, 7, 719. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Tapley, L. Effect of high night temperature and spikelet position on yield-related parameters of rice (Oryza sativa L.) plants. Eur. J. Agron. 2010, 33, 117–123. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- He, Y.; Mawhinney, T.P.; Preuss, M.L.; Schroeder, A.C.; Chen, B.; Abraham, L.; Jez, J.M.; Chen, S. A redox-active isopropylmalate dehydrogenase functions in the biosynthesis of glucosinolates and leucine in Arabidopsis. Plant J. 2009, 60, 679–690. [Google Scholar] [CrossRef]
- Textor, S.; Bartram, S.; Kroymann, J.; Falk, K.L.; Hick, A.; Pickett, J.A.; Gershenzon, J. Biosynthesis of methionine-derived glucosinolates in Arabidopsis thaliana: Recombinant expression and characterization of methylthioalkylmalate synthase, the condensing enzyme of the chain-elongation cycle. Planta 2004, 218, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Schuster, J.; Knill, T.; Reichelt, M.; Gershenzon, J.; Binder, S. Branched-chain aminotransferase 4 is part of the chain elongation pathway in the biosynthesis of methionine-derived glucosinolates in Arabidopsis. Plant Cell 2006, 18, 2664–2679. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, M.; Schemenewit, A.; Lopukhin, A.; Müller, A.; Janowitz, T.; Weiler, E.; Oecking, C. Desulfoglucosinolate sulfotransferases from Arabidopsis thaliana catalyze the final step in the biosynthesis of the glucosinolate core structure. J. Biol. Chem. 2004, 279, 50717–50725. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.U.; Park, J.I.; Seo, M.S.; Kumar, T.S.; Lee, I.H.; Park, B.S.; Nou, I.S. Identification and expression analysis of chitinase genes related to biotic stress resistance in Brassica. Mol. Biol. Rep. 2015, 39, 3649–3657. [Google Scholar] [CrossRef]
- Bell, E.; Creelman, R.A.; Mullet, J.E. A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc. Natl. Acad. Sci. USA 1995, 92, 8675–8679. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, M.; Léon-Kloosterzie, K.M.; Schwart, S.H.; Zeevaart, J.A. The genetic and molecular dissection of abscisic acid biosynthesis and signal transduction in Arabidopsis. Plant Physiol. Biochem. 1998, 36, 83–89. [Google Scholar] [CrossRef]
- Aklilu, B.B.; Culligan, K.M. Molecular evolution and functional diversification of replication protein A1 in plants. Front. Plant Sci. 2016, 7, 33. [Google Scholar] [CrossRef]
- Essers, J.; Theil, A.F.; Baldeyron, C.; van Cappellen, W.A.; Houtsmuller, A.B.; Kanaar, R.; Vermeulen, W. Nuclear dynamics of PCNA in DNA replication and repair. Mol. Cell. Biol. 2005, 25, 9350–9359. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Tran, N.Q.; Dang, H.Q.; Tuteja, R. Plant MCM proteins: Role in DNA replication and beyond. Plant Mol. Biol. 2011, 77, 537–545. [Google Scholar] [CrossRef]
- Kantidze, O.L.; Velichko, A.K.; Razin, S.V. Heat stress-induced DNA damage. Acta Nat. 2016, 8, 75–78. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerat, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed]
- Czerniawski, P.; Bednarek, P. Glutathione s-transferases in the biosynthesis of sulfur-containing secondary metabolites in Brassicaceae plants. Front. Plant Sci. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Akram, W.; Khan, W.U.; Shah, A.A.; Yasin, N.A.; Li, G. Liquiritoside alleviated Pb induced stress in Brassica rapa subsp. Parachinensis: Modulations in glucosinolate content and some physiochemical attributes. Front. Plant Sci. 2021, 12, 722498. [Google Scholar] [CrossRef]
- Ludwig-Muller, J.; Krishna, P.; Forreiter, C. A glucosinolate mutant of Arabidopsis is thermosensitive and defective in cytosolic Hsp90 expression after heat stress. Plant Physiol. 2000, 123, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen Martínez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [PubMed]
- Taviano, M.F.; Melchini, A.; Filocamo, A.; Costa, C.; Catania, S.; Raciti, R.; Saha, S.; Needs, P.; Bisignano, G.G.; Miceli, N. Contribution of the glucosinolate fraction to the overall antioxidant potential, cytoprotection against oxidative insult and antimicrobial activity of Eruca sativa Mill. leaves extract. Pharmacogn. Mag. 2017, 13, 738–743. [Google Scholar] [CrossRef]
- Troufflard, S.; Mullen, W.; Larson, T.R.; Graham, I.A.; Crozier, A.; Amtmann, A.; Armengaud, P. Potassium deficiency induces the biosynthesis of oxylipins and glucosinolates in Arabidopsis thaliana. BMC Plant Biol. 2010, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Koschmieder, J.; Wüst, F.; Schaub, P.; Álvarez, D.; Trautmann, D.; Krischke, M.; Rustenholz, C.; Mano, J.; Mueller, M.J.; Bartels, D.; et al. Plant apocarotenoid metabolism utilizes defense mechanisms against reactive carbonyl species and xenobiotics. Plant Physiol. 2021, 185, 331–351. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zhou, Y.; Deng, H.; Lin, L.; Deng, Q.; Wang, J.; Lv, X.; Zhang, X.; Liang, D. Melatonin improves heat tolerance in Actinidia deliciosa via carotenoid biosynthesis and heat shock proteins expression. Physiol. Plant. 2021, 172, 1582–1593. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Endo, A.; Sawada, Y.; Takahashi, H.; Okamoto, M.; Ikegami, K.; Koiwai, H.; Seo, M.; Toyomas, T.; Mitsuhash, W.; Shinozaki, K.; et al. Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol. 2008, 147, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef]
- Di, F.; Jian, H.; Wang, T.; Chen, X.; Ding, Y.; Du, H.; Lu, K.; Li, J.; Liu, L. Genome-wide analysis of the PYL gene family and identification of PYL genes that respond to abiotic stress in Brassica napus. Genes 2018, 9, 156. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; El-Yazied, A.A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.M.; Ibrahim, M.F.M.; et al. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef]
- Feng, B.; Liu, P.; Li, G.; Dong, S.T.; Wang, F.H.; Kong, L.A.; Zhang, J.W. Effect of heat stress on the photosynthetic characteristics in flag leaves at the grain-filling stage of different heat-resistant winter wheat varieties. J. Agron. Crop Sci. 2013, 200, 143–155. [Google Scholar] [CrossRef]
- Logan, B.A. Chlorophyll a fluorescence: A signature of photosynthesis. J. Torrey Bot. Soc. 2005, 132, 650. [Google Scholar] [CrossRef]
- Li, H.; Ahammed, G.J.; Zhou, G.; Xia, X.; Zhou, J.; Shi, K.; Yu, J.; Zhou, Y. Unraveling main limiting sites of photosynthesis under below-and above-ground heat stress in cucumber and the alleviatory role of luffa rootstock. Front. Plant Sci. 2016, 7, 746. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylla and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal. Res. 2009, 46, 58–63. [Google Scholar] [CrossRef]
- Bokari, U.G. Chlorophyll, dry matter, and photosynthetic conversion-efficiency relationships in warm-season grasses. J. Range Manag. 1983, 36, 431–434. [Google Scholar] [CrossRef]
- Kura-Hotta, M.; Satoh, K.; Katoh, S. Relationship between photosynthesis and chlorophyll content during leaf senescence of rice seedlings. Plant Cell Physiol. 1987, 28, 1321–1329. [Google Scholar]
- Horton, P. Optimization of light harvesting and photoprotection: Molecular mechanisms and physiological consequences. Philos. Trans. R. Soc. B 2012, 367, 3455–3465. [Google Scholar] [CrossRef]
- Demmig-Adams, B. Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant Cell Physiol. 1998, 39, 474–482. [Google Scholar] [CrossRef]
- Cogdell, R.J.; Gardiner, A.T. Functions of carotenoids in photosynthesis. Method Enzymol. 1993, 214, 185–193. [Google Scholar]
- Szafrańska, K.; Reiter, R.J.; Posmyk, M.M. Melatonin application to Pisum sativum L. seeds positively influences the function of the photosynthetic apparatus in growing seedlings during paraquat-induced oxidative stress. Front. Plant Sci. 2016, 7, 1663. [Google Scholar] [CrossRef]
- Xu, P.; Chukhutsina, V.U.; Nawrocki, W.J.; Schansker, G.; Bielczynski, L.W.; Lu, Y.; Karcher, D.; Roberta, R.B. Photosynthesis without β-carotene. eLife Sci. 2020, 9, e58984. [Google Scholar] [CrossRef]
- Cignoni, E.; Lapillo, M.; Cupellini, L.; Acosta-Gutiérrez, S.; Ger, F.L. A different perspective for nonphotochemical quenching in plant antenna complexes. Nat. Commun. 2021, 12, 7152. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Wang, P.; Richter, A.S.; Kleeberg, J.R.W.; Geimer, S.; Grimm, B. Post-translational coordination of chlorophyll biosynthesis and breakdown by BCMs maintains chlorophyll homeostasis during leaf development. Nat. Commun. 2020, 11, 1254. [Google Scholar] [CrossRef] [PubMed]
- Driedonks, N.; Xu, J.; Peters, J.L.; Park, S.; Rieu, I. Multi-level interactions between heat shock factors, heat shock proteins, and the redox system regulate acclimation to heat. Front. Plant Sci. 2015, 6, 999. [Google Scholar] [CrossRef] [PubMed]
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat stress phenotypes of Arabidopsis mutants implicate mul-tiple signalling pathways in the acquisition of thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jiang, J.; Li, S.; Li, M.; Tan, Y.; Song, S.; Shu, Q.; Huang, J. Glutamate alleviates cadmium toxicity in rice via suppressing cadmium uptake and translocation. J. Hazard. Mater. 2020, 384, 121319. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Xu, Z.; Ding, Y.; Tang, B.; Zhang, Y.; Li, H.; Feng, X.; Xu, H. Effect of poly (γ-glutamic acid) on the physiological responses and calcium signaling of rape seedlings (Brassica napus L.) under cold stress. J. Agric. Food Chem. 2015, 63, 10399–10406. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Koca, H.; Bor, M.; Özdemir, F.; Türkan, İ. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 2007, 60, 344–351. [Google Scholar] [CrossRef]
- Thalhammer, A.; Pagter, M.; Hincha, D.K.; Zuther, E. Measuring freezing tolerance of leaves and rosettes: Electrolyte leakage and chlorophyll fluorescence assays. Methods Mol. Biol. 2020, 2156, 9–21. [Google Scholar]
- Han, Z.; Wei, X.; Wan, D.; He, W.; Wang, X.; Xiong, Y. Effect of molybdenum on plant physiology and cadmium uptake and translocation in rape (Brassica napus L.) under different levels of cadmium stress. Int. J. Environ. Res. 2020, 17, 2355. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR. Methods 2002, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Tsujimoto, T.; Uyama, H.; Sung, M.H.; Kim, k.; Kuramitsu, S. Enhancement of enzyme activity and stability by poly(γ-glutamic acid). Polym. J. 2010, 42, 818–822. [Google Scholar] [CrossRef]
- Nishida, T.; Kamezawa, H.; Hara, T.; Matsumoto, Y. Heat resistance and local structure of FeCl2-absorbed crosslinked poly(γ-glutamic acid). J. Radioanal. Nucl. Chem. 2001, 250, 547–550. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant 2013, 35, 1381–1396. [Google Scholar] [CrossRef]




| Treatment | Fresh Weight (g∙Plant−1) | Dry Weight (g∙Plant−1) | Plant Height (cm) | Hypocotyl Diameter (mm) | Leaf Length (cm) | Leaf Width (cm) |
|---|---|---|---|---|---|---|
| Ctrl | 3.51 ± 0.17 c | 0.33 ± 0.02 b | 12.60 ± 0.36 b | 2.25 ± 0.06 b | 11.63 ± 0.35 b | 4.67 ± 0.31 b |
| Glu | 5.18 ± 0.19 a | 0.50 ± 0.02 a | 16.10 ± 0.32 a | 2.56 ± 0.06 a | 15.76 ± 0.28 a | 6.68 ± 0.29 a |
| PGA | 4.82 ± 0.16 b | 0.46 ± 0.02 a | 15.93 ± 0.33 a | 2.46 ± 0.05 a | 15.44 ± 0.25 a | 6.13 ± 0.19 a |
| Treatments | Antioxidant Enzyme (U·g−1 FW) | Superoxide Radical Content (μmol·g−1 FW) | |||
|---|---|---|---|---|---|
| SOD | APX | CAT | POD | O2·− | |
| Ctrl | 255.31 ± 0.32 b | 0.56 ± 0.13 b | 333.44 ± 0.12 b | 32.29 ± 1.45 b | 23.39 ± 0.82 a |
| Glu | 303.98 ± 0.61 a | 0.72 ± 0.29 a | 447.44 ± 0.21 a | 46.97 ± 1.93 a | 15.65 ± 0.19 c |
| PGA | 289.29 ± 0.75 a | 0.71 ± 0.28 a | 444.19 ± 0.18 a | 44.90 ± 1.93 a | 18.12 ± 0.16 b |
| Pigment | Treatments | ||
|---|---|---|---|
| Ctrl | Glu | PGA | |
| SPAD | 29.13 ± 0.54 b | 32.73 ± 0.46 a | 32.23 ± 0.19 a |
| chl a (mg·g−1 FW) | 1.54 ± 0.03 b | 2.13 ± 0.05 a | 1.88 ± 0.03 a |
| chl b (mg·g−1 FW) | 0.45 ± 0.01 c | 0.69 ± 0.03 a | 0.57 ± 0.07 b |
| total chl (mg·g−1 FW) | 1.99 ± 0.04 c | 2.83 ± 0.05 a | 2.45 ± 0.02 b |
| carotenoid (mg·g−1 FW) | 0.25 ± 0.05 b | 0.33 ± 0.06 a | 0.31 ± 0.04 a |
| chl a/b | 3.39 ± 0.04 b | 3.06 ± 0.08 a | 3.29 ± 0.10 b |
| chl/car | 8.07 ± 0.19 ab | 8.54 ± 0.30 a | 7.82 ± 0.16 b |
| Treatment | Fv/Fo | Fv/Fm | ETR | ΦPSII | NPQ | qP |
|---|---|---|---|---|---|---|
| Ctrl | 3.15 ± 0.06 c | 0.77 ± 0.04 b | 31.70 ± 0.12 c | 0.37 ± 0.01 b | 0.27 ± 0.01 a | 0.56 ± 0.02 b |
| Glu | 4.08 ± 0.04 a | 0.80 ± 0.04 a | 38.23 ± 1.14 a | 0.45 ± 0.03 a | 0.23 ± 0.02 b | 0.63 ± 0.01 a |
| PGA | 3.74 ± 0.04 b | 0.79 ± 0.01 a | 36.13 ± 0.54 b | 0.43 ± 0.01 a | 0.24 ± 0.02 ab | 0.62 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, J.; Zheng, W.; Tan, J.; Li, Z.; Wu, M.; Hong, S.-B.; Zhao, Y.; Zhu, Z.; Zang, Y. Glutamic Acid and Poly-γ-glutamic Acid Enhanced the Heat Resistance of Chinese Cabbage (Brassica rapa L. ssp. pekinensis) by Improving Carotenoid Biosynthesis, Photosynthesis, and ROS Signaling. Int. J. Mol. Sci. 2022, 23, 11671. https://doi.org/10.3390/ijms231911671
Quan J, Zheng W, Tan J, Li Z, Wu M, Hong S-B, Zhao Y, Zhu Z, Zang Y. Glutamic Acid and Poly-γ-glutamic Acid Enhanced the Heat Resistance of Chinese Cabbage (Brassica rapa L. ssp. pekinensis) by Improving Carotenoid Biosynthesis, Photosynthesis, and ROS Signaling. International Journal of Molecular Sciences. 2022; 23(19):11671. https://doi.org/10.3390/ijms231911671
Chicago/Turabian StyleQuan, Jin, Weiwei Zheng, Jingru Tan, Zewei Li, Meifang Wu, Seung-Beom Hong, Yanting Zhao, Zhujun Zhu, and Yunxiang Zang. 2022. "Glutamic Acid and Poly-γ-glutamic Acid Enhanced the Heat Resistance of Chinese Cabbage (Brassica rapa L. ssp. pekinensis) by Improving Carotenoid Biosynthesis, Photosynthesis, and ROS Signaling" International Journal of Molecular Sciences 23, no. 19: 11671. https://doi.org/10.3390/ijms231911671
APA StyleQuan, J., Zheng, W., Tan, J., Li, Z., Wu, M., Hong, S.-B., Zhao, Y., Zhu, Z., & Zang, Y. (2022). Glutamic Acid and Poly-γ-glutamic Acid Enhanced the Heat Resistance of Chinese Cabbage (Brassica rapa L. ssp. pekinensis) by Improving Carotenoid Biosynthesis, Photosynthesis, and ROS Signaling. International Journal of Molecular Sciences, 23(19), 11671. https://doi.org/10.3390/ijms231911671








