Liposome Formulation for Tumor-Targeted Drug Delivery Using Radiation Therapy
Abstract
1. Introduction
2. Results
2.1. Characterization
2.2. Payload Release from the Liposomes and Cell Viability
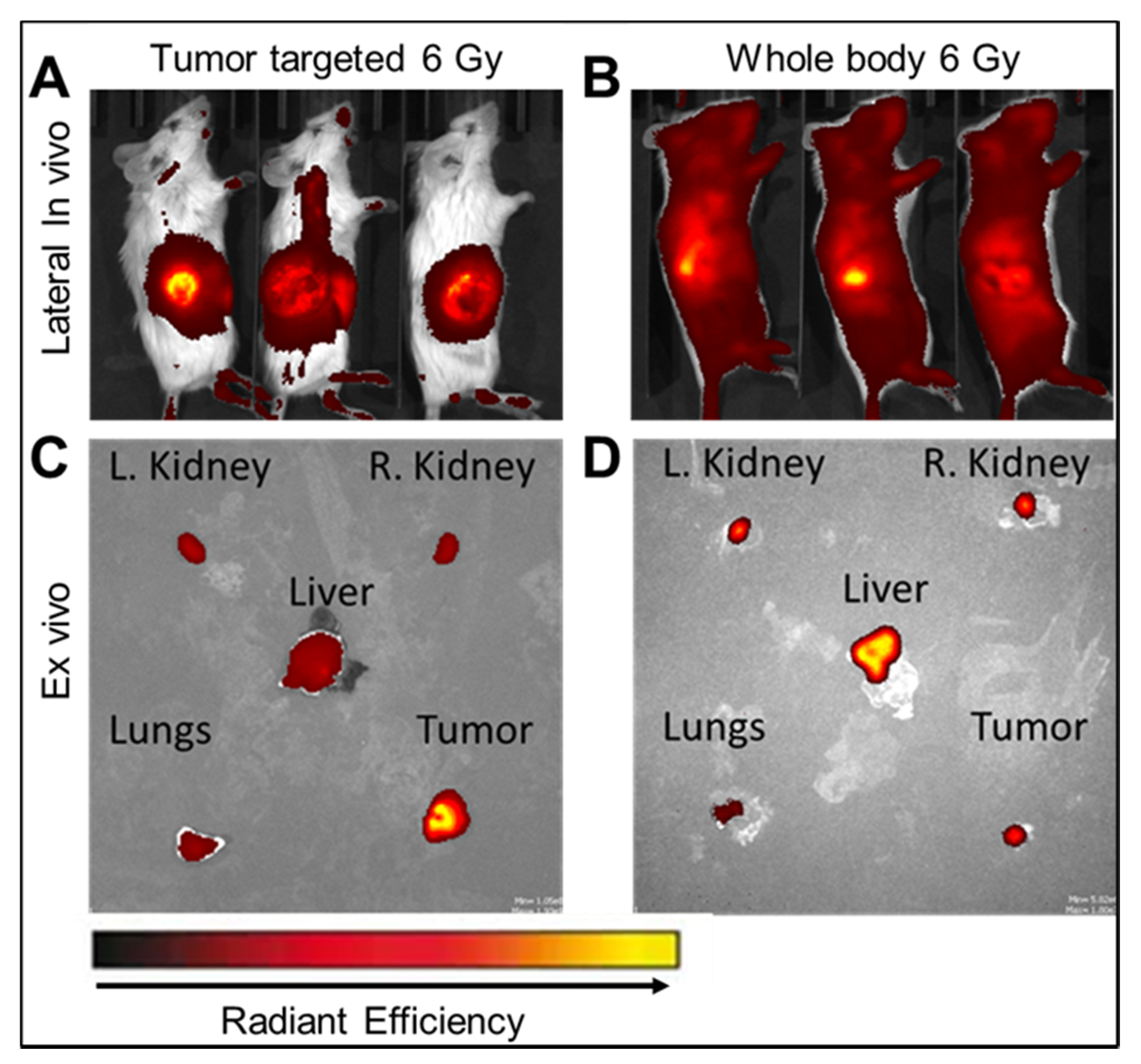
2.3. Biodistribution
2.4. In Vivo Radiation Release
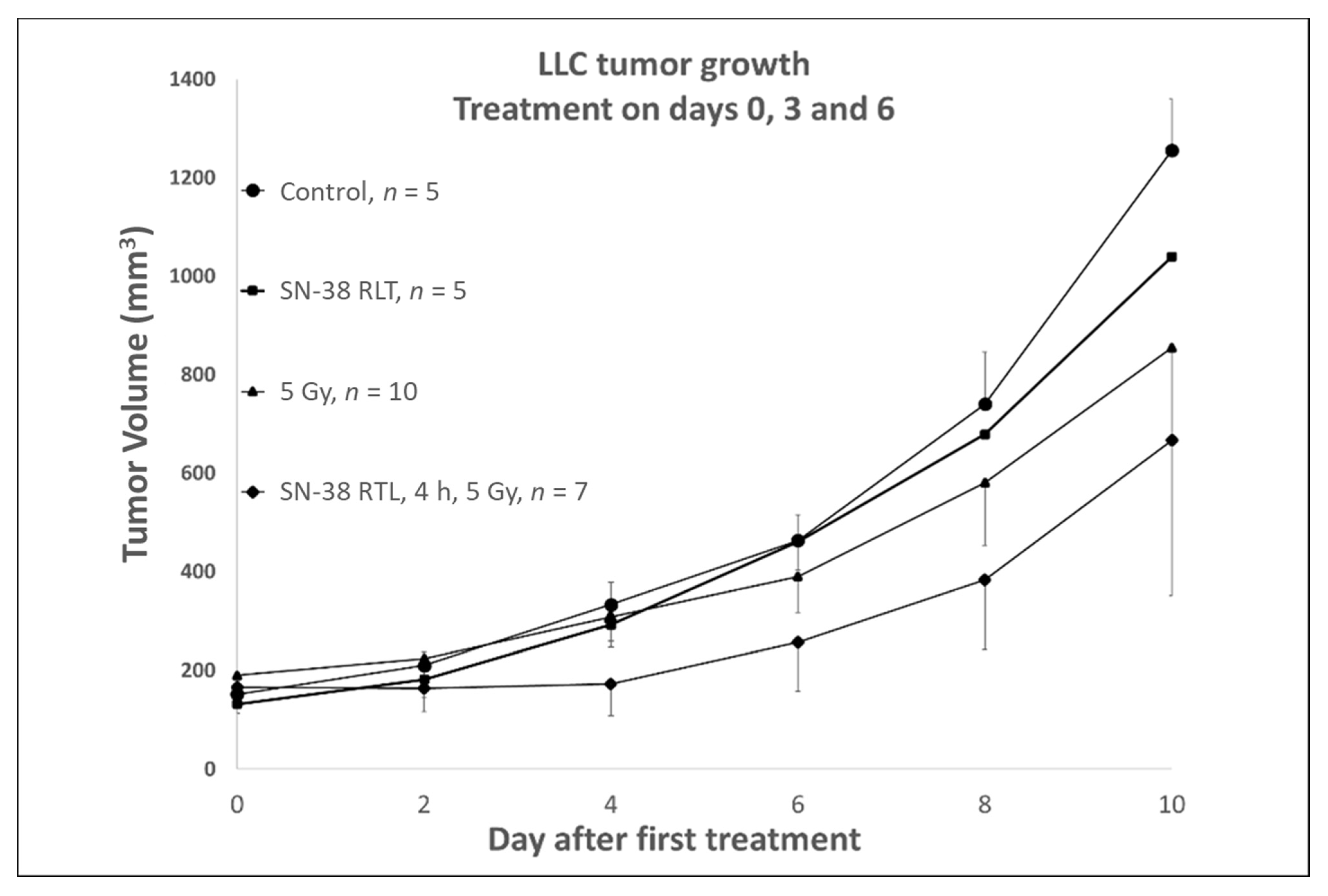
2.5. Anti-Tumor Efficacy
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Liposomes Preparation
4.3.1. Preparation of Spherical Lipid Bilayers
4.3.2. SN-38 Loaded Liposome Preparation
4.3.3. Carboxyfluorescein- and ICG- Loaded Liposome Preparation
4.3.4. Liposome Labeling with Vivo-Track 680
4.4. Characterization
4.4.1. NTA (Nanoparticle Tracking Analysis)
4.4.2. Cryo-Electron Microscopy (Cryo-EM)
4.4.3. Release of Carboxyfluorescein
4.5. In Vitro and In Vivo Efficacy
4.5.1. Cell Viability
4.5.2. Clonogenic Assay
4.5.3. Biodistribution
4.5.4. Radiation Release in the Tumor
4.5.5. Anti-Tumor efficacy
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, T.; Haemmerich, D.; Liu, H.; Seynhaeve, A.L.B.; van Rhoon, G.C.; Houtsmuller, A.B.; ten Hagen, T.L.M. Externally Triggered Smart Drug Delivery System Encapsulating Idarubicin Shows Superior Kinetics and Enhances Tumoral Drug Uptake and Response. Theranostics 2021, 11, 5700–5712. [Google Scholar] [CrossRef] [PubMed]
- Tailor, T.D.; Hanna, G.; Yarmolenko, P.S.; Dreher, M.R.; Betof, A.S.; Nixon, A.B.; Spasojevic, I.; Dewhirst, M.W. Effect of Pazopanib on Tumor Microenvironment and Liposome Delivery. Mol. Cancer Ther. 2010, 9, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.A.; Lindner, L.H.; Landon, C.D.; Park, J.Y.; Simnick, A.J.; Dreher, M.R.; Das, S.; Hanna, G.; Park, W.; Chilkoti, A.; et al. Overcoming Limitations in Nanoparticle Drug Delivery: Triggered, Intravascular Release to Improve Drug Penetration into Tumors. Cancer Res. 2012, 72, 5566–5575. [Google Scholar] [CrossRef] [PubMed]
- Dicheva, B.M.; Ten Hagen, T.L.M.; Schipper, D.; Seynhaeve, A.L.B.; Van Rhoon, G.C.; Eggermont, A.M.M.; Koning, G.A. Targeted and Heat-Triggered Doxorubicin Delivery to Tumors by Dual Targeted Cationic Thermosensitive Liposomes. J. Control. Release 2014, 195, 37–48. [Google Scholar] [CrossRef]
- Shao, X.R.; Wei, X.Q.; Zhang, S.; Fu, N.; Lin, Y.F.; Cai, X.X.; Peng, Q. Effects of Micro-Environmental PH of Liposome on Chemical Stability of Loaded Drug. Nanoscale Res. Lett. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Nekkanti, V.; Kalepu, S. Recent Advances in Liposomal Drug Delivery: A Review. Pharm. Nanotechnol. 2015, 3, 35–55. [Google Scholar] [CrossRef]
- Vieira, D.B.; Gamarra, L.F. Getting into the Brain: Liposome-Based Strategies for Effective Drug Delivery across the Blood–Brain Barrier. Int. J. Nanomed. 2016, 11, 5381. [Google Scholar] [CrossRef]
- Bevers, S.; Kooijmans, S.A.A.; Van de Velde, E.; Evers, M.J.W.; Seghers, S.; Gitz-Francois, J.J.J.M.; van Kronenburg, N.C.H.; Fens, M.H.A.M.; Mastrobattista, E.; Hassler, L.; et al. MRNA-LNP Vaccines Tuned for Systemic Immunization Induce Strong Antitumor Immunity by Engaging Splenic Immune Cells. Mol. Ther. 2022, 30, 3078–3094. [Google Scholar] [CrossRef]
- Shimon, M.B.; Shapira, S.; Seni, J.; Arber, N. The Big Potential of Small Particles: Lipid-Based Nanoparticles and Exosomes in Vaccination. Vaccines 2022, 10, 1119. [Google Scholar] [CrossRef]
- Fukuda, A.; Tahara, K.; Hane, Y.; Matsui, T.; Sasaoka, S.; Hatahira, H.; Motooka, Y.; Hasegawa, S.; Naganuma, M.; Abe, J.; et al. Comparison of the Adverse Event Profiles of Conventional and Liposomal Formulations of Doxorubicin Using the FDA Adverse Event Reporting System. PLoS ONE 2017, 12, e0185654. [Google Scholar] [CrossRef]
- Rafiyath, S.M.; Rasul, M.; Lee, B.; Wei, G.; Lamba, G.; Liu, D. Comparison of Safety and Toxicity of Liposomal Doxorubicin vs. Conventional Anthracyclines: A Meta-Analysis. Exp. Hematol. Oncol. 2012, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Ohkohchi, N.; Yasunaga, M.; Kuroda, J.I.; Koga, Y.; Kenmotsu, H.; Kinoshita, T.; Matsumura, Y. Detailed Distribution of NK012, an SN-38-Incorporating Micelle, in the Liver and Its Potent Antitumor Effects in Mice Bearing Liver Metastases. Clin. Cancer Res. 2010, 16, 4822–4831. [Google Scholar] [CrossRef] [PubMed]
- Vangara, K.K.; Ali, H.I.; Lu, D.; Liu, J.L.; Kolluru, S.; Palakurthi, S. SN-38-Cyclodextrin Complexation and Its Influence on the Solubility, Stability, and In Vitro Anticancer Activity Against Ovarian Cancer. AAPS PharmSciTech 2014, 15, 472. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; Papadopoulos, K.P.; Tolcher, A.W.; Beeram, M.; Urien, S.; Schaaf, L.J.; Tahiri, S.; Bekaii-Saab, T.; Lokiec, F.M.; Rezaï, K.; et al. Phase I Dose-Escalation Study of EZN-2208 (PEG-SN38), a Novel Conjugate of Poly(Ethylene) Glycol and SN38, Administered Weekly in Patients With Advanced Cancer. Cancer Chemother. Pharmacol. 2013, 71, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimnejad, P.; Dinarvand, R.; Sajadi, A.; Jaafari, M.R.; Nomani, A.R.; Azizi, E.; Rad-Malekshahi, M.; Atyabi, F. Preparation and in Vitro Evaluation of Actively Targetable Nanoparticles for SN-38 Delivery against HT-29 Cell Lines. Nanomedicine 2010, 6, 478–485. [Google Scholar] [CrossRef]
- Vangara, K.K.; Liu, J.L.; Palakurthi, S. Hyaluronic Acid-Decorated PLGA-PEG Nanoparticles for Targeted Delivery of SN-38 to Ovarian Cancer. Anticancer Res. 2013, 33, 2425–2434. [Google Scholar]
- Zhang, J.A.; Xuan, T.; Parmar, M.; Ma, L.; Ugwu, S.; Ali, S.; Ahmad, I. Development and Characterization of a Novel Liposome-Based Formulation of SN-38. Int. J. Pharm. 2004, 270, 93–107. [Google Scholar] [CrossRef]
- Carie, A.; Rios-Doria, J.; Costich, T.; Burke, B.; Slama, R.; Skaff, H.; Sill, K. IT-141, a Polymer Micelle Encapsulating SN-38, Induces Tumor Regression in Multiple Colorectal Cancer Models. J. Drug Deliv. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Gu, Q.; Xing, J.Z.; Huang, M.; He, C.; Chen, J. SN-38 Loaded Polymeric Micelles to Enhance Cancer Therapy. Nanotechnology 2012, 23, 205101. [Google Scholar] [CrossRef]
- Zhang, H. Onivyde for the Therapy of Multiple Solid Tumors. Onco. Targets. Ther. 2016, 9, 3001. [Google Scholar] [CrossRef]
- Milano, G.; Innocenti, F.; Minami, H. Liposomal Irinotecan (Onivyde): Exemplifying the Benefits of Nanotherapeutic Drugs. Cancer Sci. 2022, 113, 2224. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xu, M.Q.; Zhang, W.; Ma, S.; Guo, W.; Wang, Y.; Zhang, Y.; Gou, T.; Chen, Y.; Liang, X.J.; et al. ICAM-1-Targeted Liposomes Loaded with Liver X Receptor Agonists Suppress PDGF-Induced Proliferation of Vascular Smooth Muscle Cells. Nanoscale Res. Lett. 2017, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, F.; Wen, Z.; Zhan, C.; Feng, L.; Liu, Y.; Wei, X.; Xie, C.; Lu, W. LyP-1-Conjugated PEGylated Liposomes: A Carrier System for Targeted Therapy of Lymphatic Metastatic Tumor. J. Control. Release 2012, 157, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Zhan, C.; Wang, S.; Yao, B.; Hu, X.; Song, X.; Zhang, M.; Wei, X.; Xiong, Y.; Lu, W. Liposome-Based Systemic Glioma-Targeted Drug Delivery Enabled by All-d Peptides. ACS Appl. Mater. Interfaces 2016, 8, 29977–29985. [Google Scholar] [CrossRef] [PubMed]
- Noble, G.T.; Stefanick, J.F.; Ashley, J.D.; Kiziltepe, T.; Bilgicer, B. Ligand-Targeted Liposome Design: Challenges and Fundamental Considerations. Trends Biotechnol. 2014, 32, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Shen, Q.; Liu, Y.; Yan, Z.; Wei, X.; Zhan, C.; Gao, J.; Xie, C.; Yao, B.; Lu, W. Stabilized Heptapeptide A7R for Enhanced Multifunctional Liposome-Based Tumor-Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 13232–13241. [Google Scholar] [CrossRef]
- Saveyn, H.; De Baets, B.; Thas, O.; Hole, P.; Smith, J.; Van der Meeren, P. Accurate Particle Size Distribution Determination by Nanoparticle Tracking Analysis Based on 2-D Brownian Dynamics Simulation. J. Colloid Interface Sci. 2010, 352, 593–600. [Google Scholar] [CrossRef]
- Reshetov, V.; Zorin, V.; Siupa, A.; D’Hallewin, M.A.; Guillemin, F.; Bezdetnaya, L. Interaction of Liposomal Formulations of Meta-Tetra(Hydroxyphenyl)Chlorin (Temoporfin) with Serum Proteins: Protein Binding and Liposome Destruction. Photochem. Photobiol. 2012, 88, 1256–1264. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. The Carrier Potential of Liposomes in Biology and Medicine. N. Engl. J. Med. 2009, 295, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. Liposomes in Drug Delivery: How It All Happened. Pharmaceutics 2016, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Yatvin, M.B.; Weinstein, J.N.; Dennis, W.H.; Blumenthal, R. Design of Liposomes for Enhanced Local Release of Drugs by Hyperthermia. Science 1978, 202, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth Liposomes: Review of the Basic Science, Rationale, and Clinical Applications, Existing and Potential. Int. J. Nanomed. 2006, 1, 297. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolarz, A.J.; Chhetri, B.P.; Borrelli, M.J.; Jenkins, S.V.; Jamshidi-Parsian, A.; Phillips, J.H.; Fologea, D.; Gandy, J.; Griffin, R.J. Liposome Formulation for Tumor-Targeted Drug Delivery Using Radiation Therapy. Int. J. Mol. Sci. 2022, 23, 11662. https://doi.org/10.3390/ijms231911662
Stolarz AJ, Chhetri BP, Borrelli MJ, Jenkins SV, Jamshidi-Parsian A, Phillips JH, Fologea D, Gandy J, Griffin RJ. Liposome Formulation for Tumor-Targeted Drug Delivery Using Radiation Therapy. International Journal of Molecular Sciences. 2022; 23(19):11662. https://doi.org/10.3390/ijms231911662
Chicago/Turabian StyleStolarz, Amanda J., Bijay P. Chhetri, Michael J. Borrelli, Samir V. Jenkins, Azemat Jamshidi-Parsian, Joshua H. Phillips, Daniel Fologea, Jay Gandy, and Robert J. Griffin. 2022. "Liposome Formulation for Tumor-Targeted Drug Delivery Using Radiation Therapy" International Journal of Molecular Sciences 23, no. 19: 11662. https://doi.org/10.3390/ijms231911662
APA StyleStolarz, A. J., Chhetri, B. P., Borrelli, M. J., Jenkins, S. V., Jamshidi-Parsian, A., Phillips, J. H., Fologea, D., Gandy, J., & Griffin, R. J. (2022). Liposome Formulation for Tumor-Targeted Drug Delivery Using Radiation Therapy. International Journal of Molecular Sciences, 23(19), 11662. https://doi.org/10.3390/ijms231911662







