Engineered Mesoporous Silica-Based Core-Shell Nanoarchitectures for Synergistic Chemo-Photodynamic Therapies
Abstract
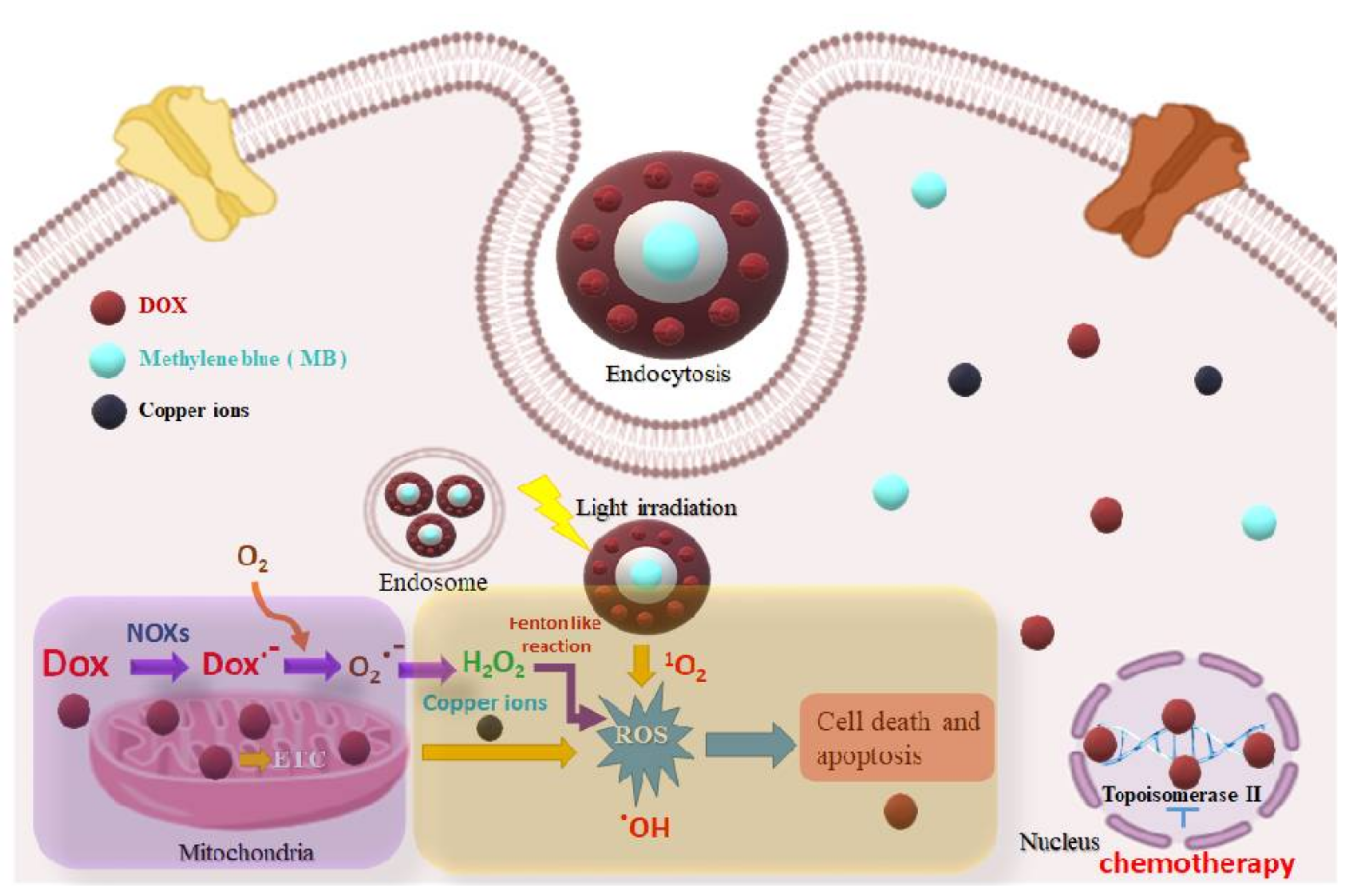
1. Introduction
2. Results and Discussion
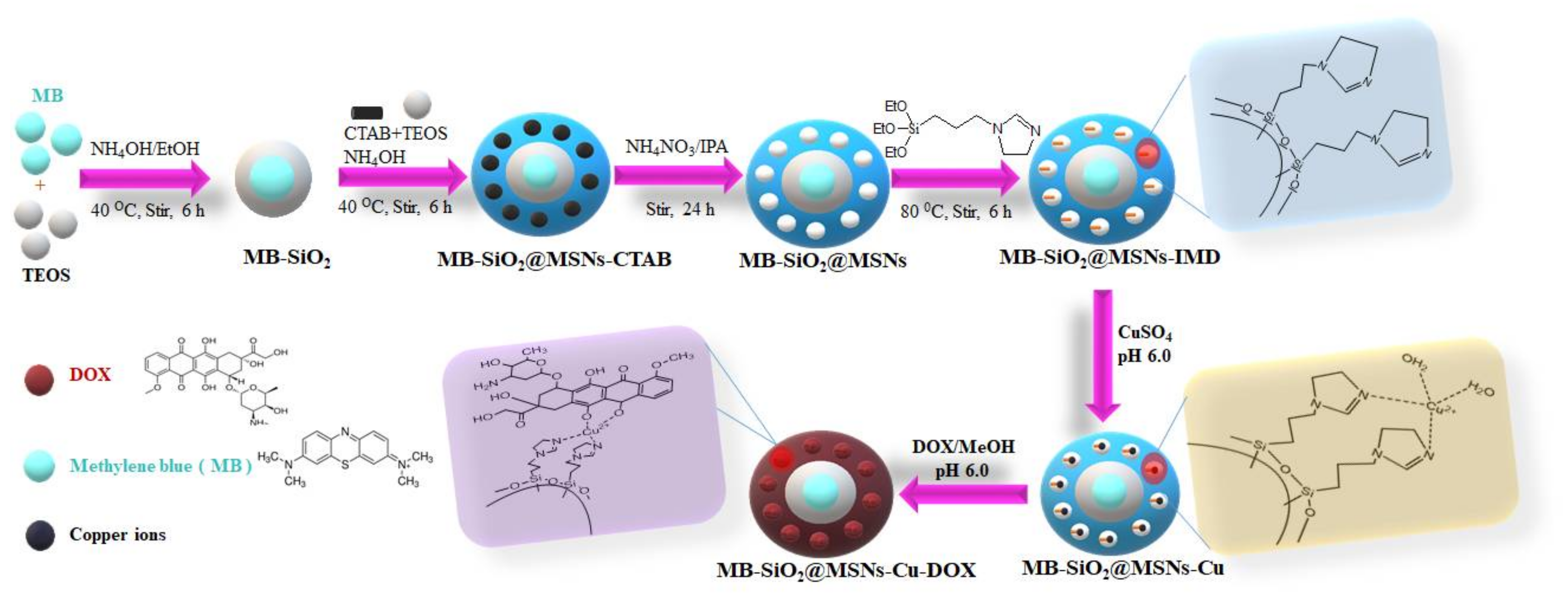
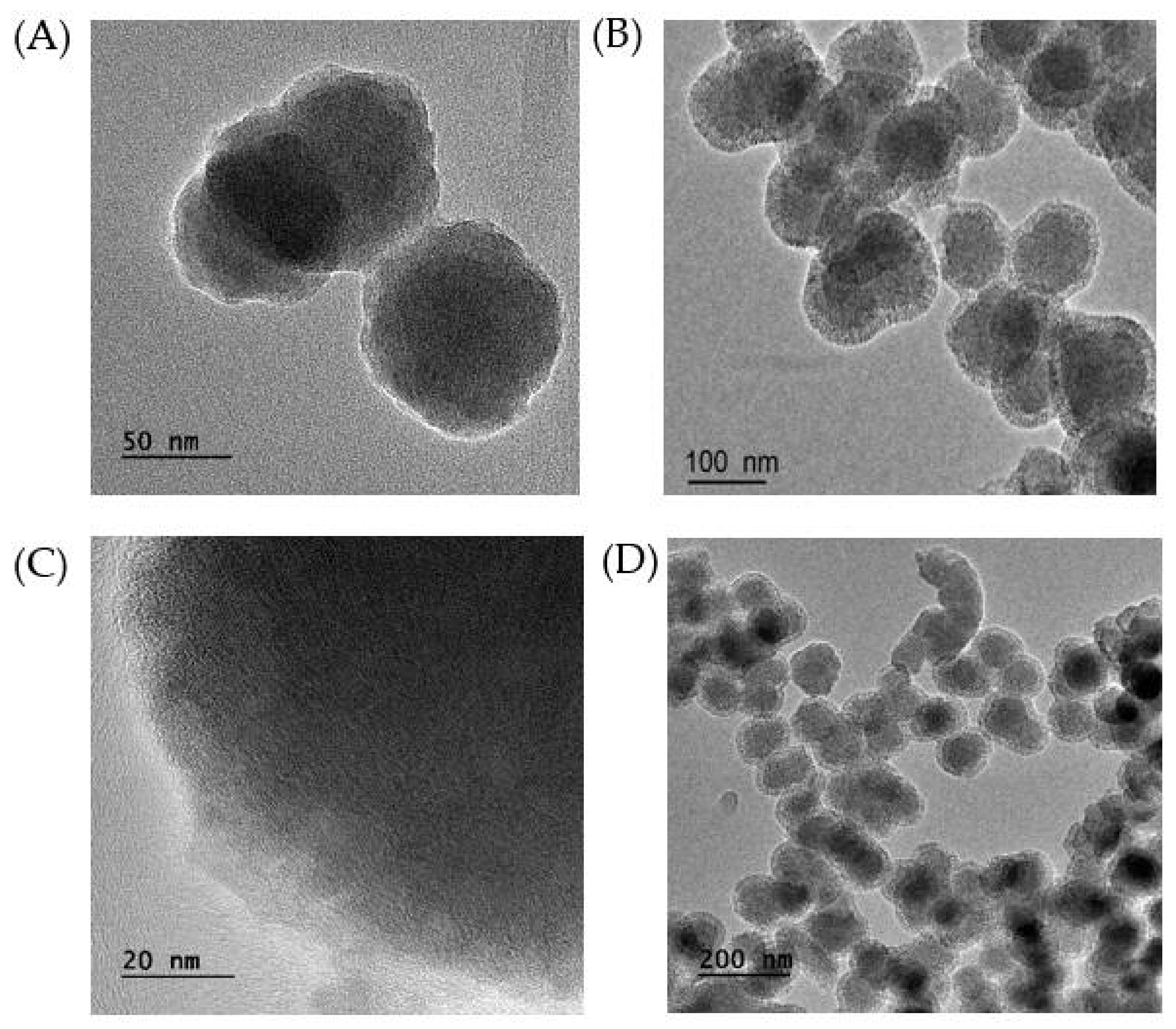
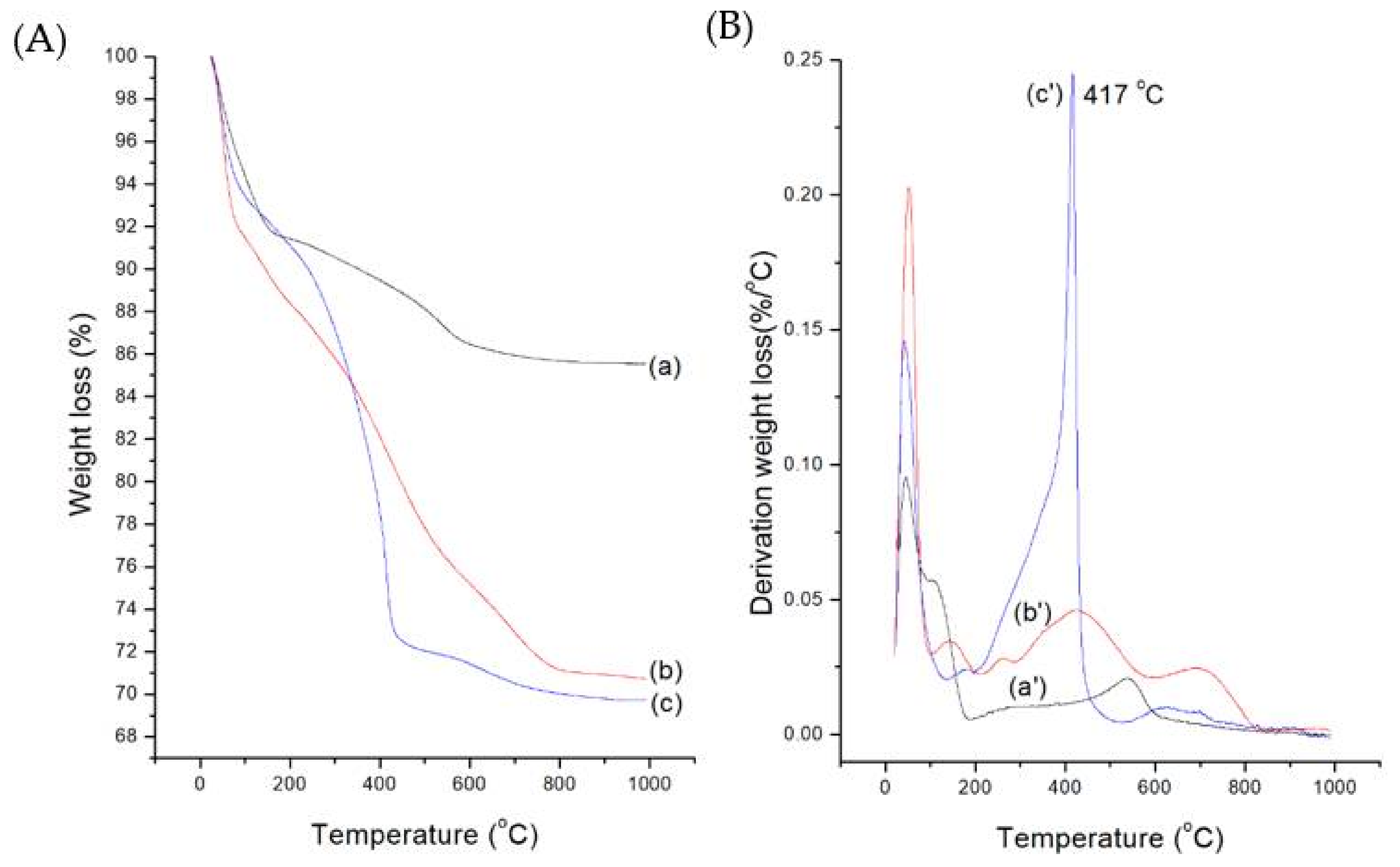
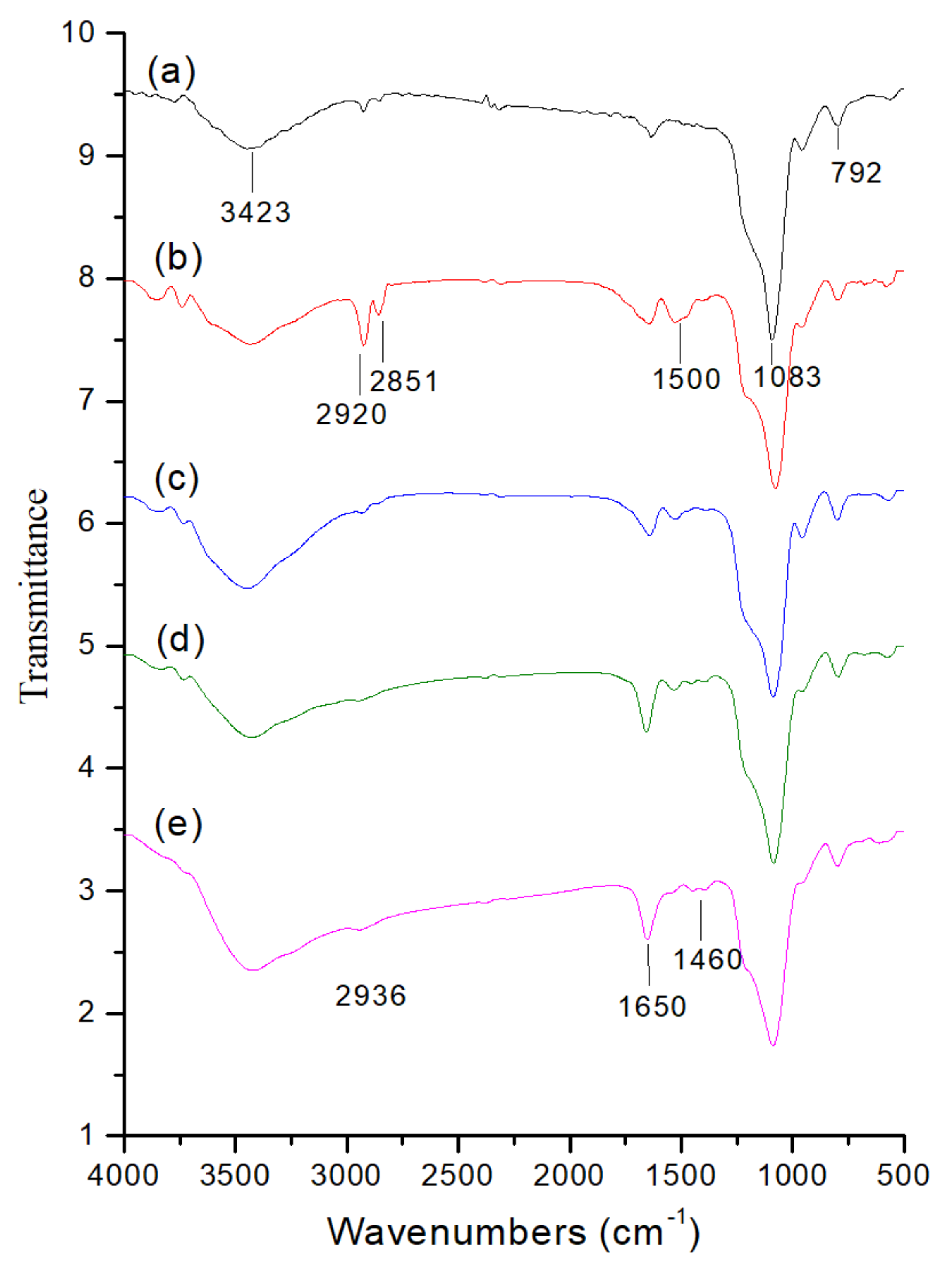
2.1. Preparation and Characterization of MB-SiO2@MSNs
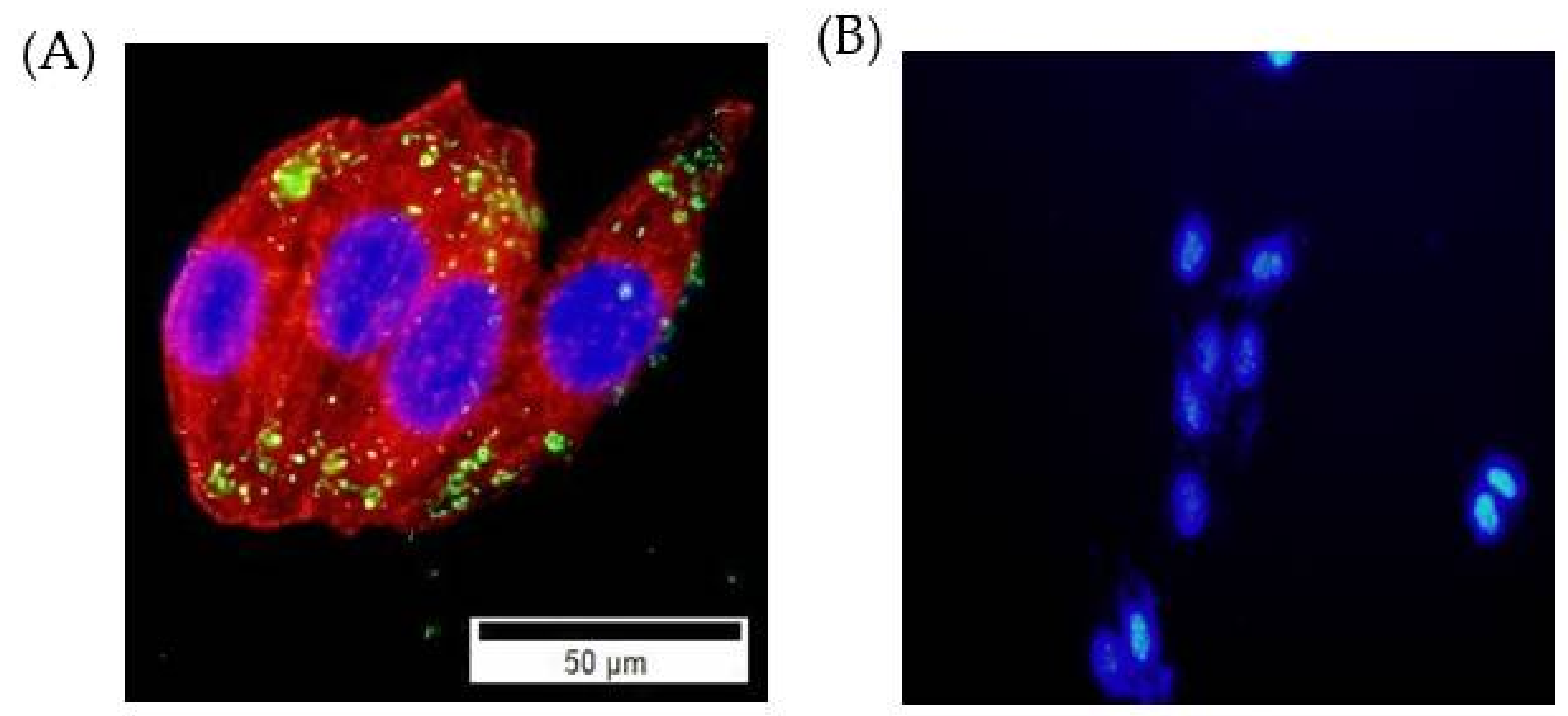
2.2. Cellular Uptake Studies
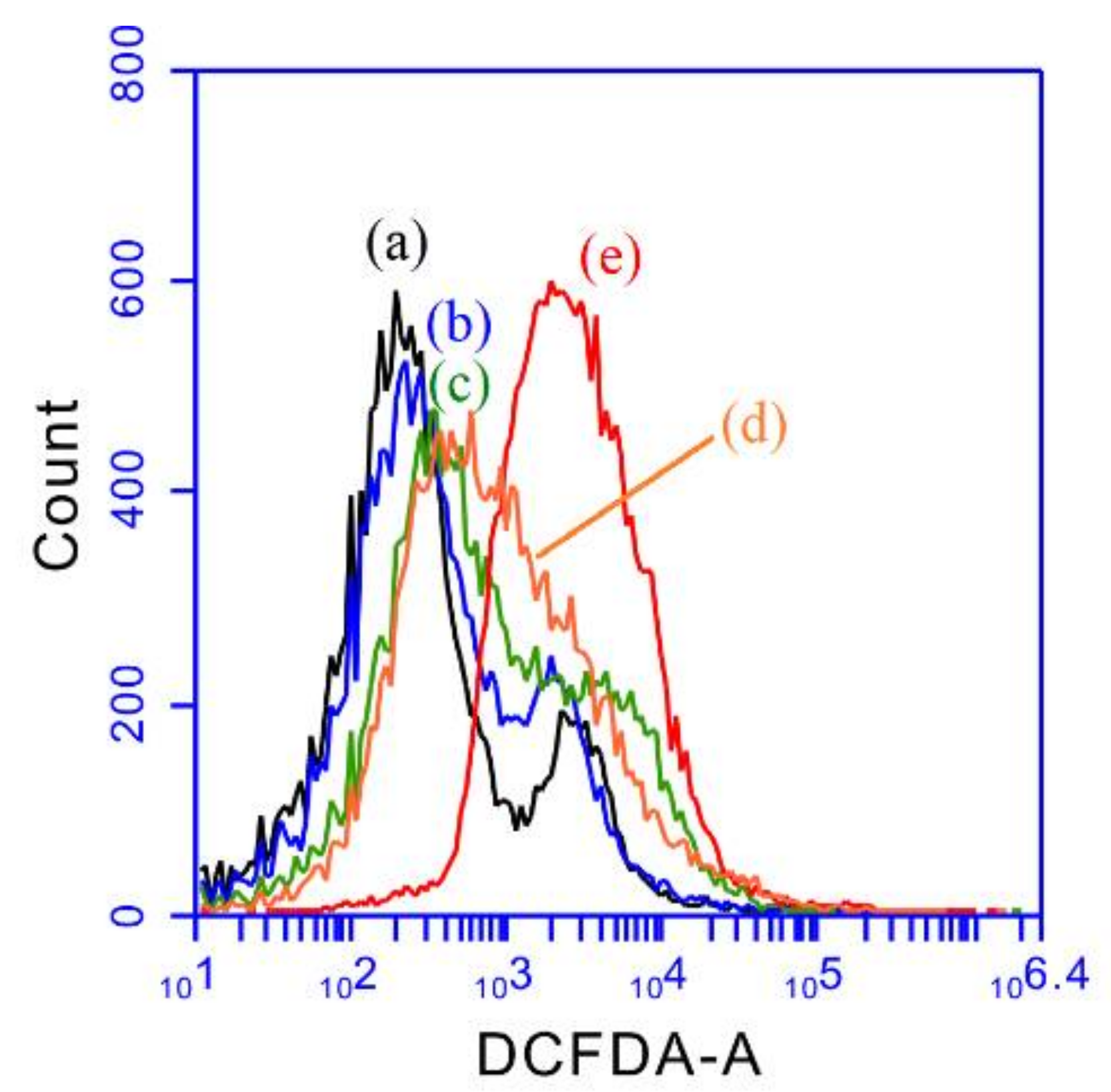
2.3. ROS Studies
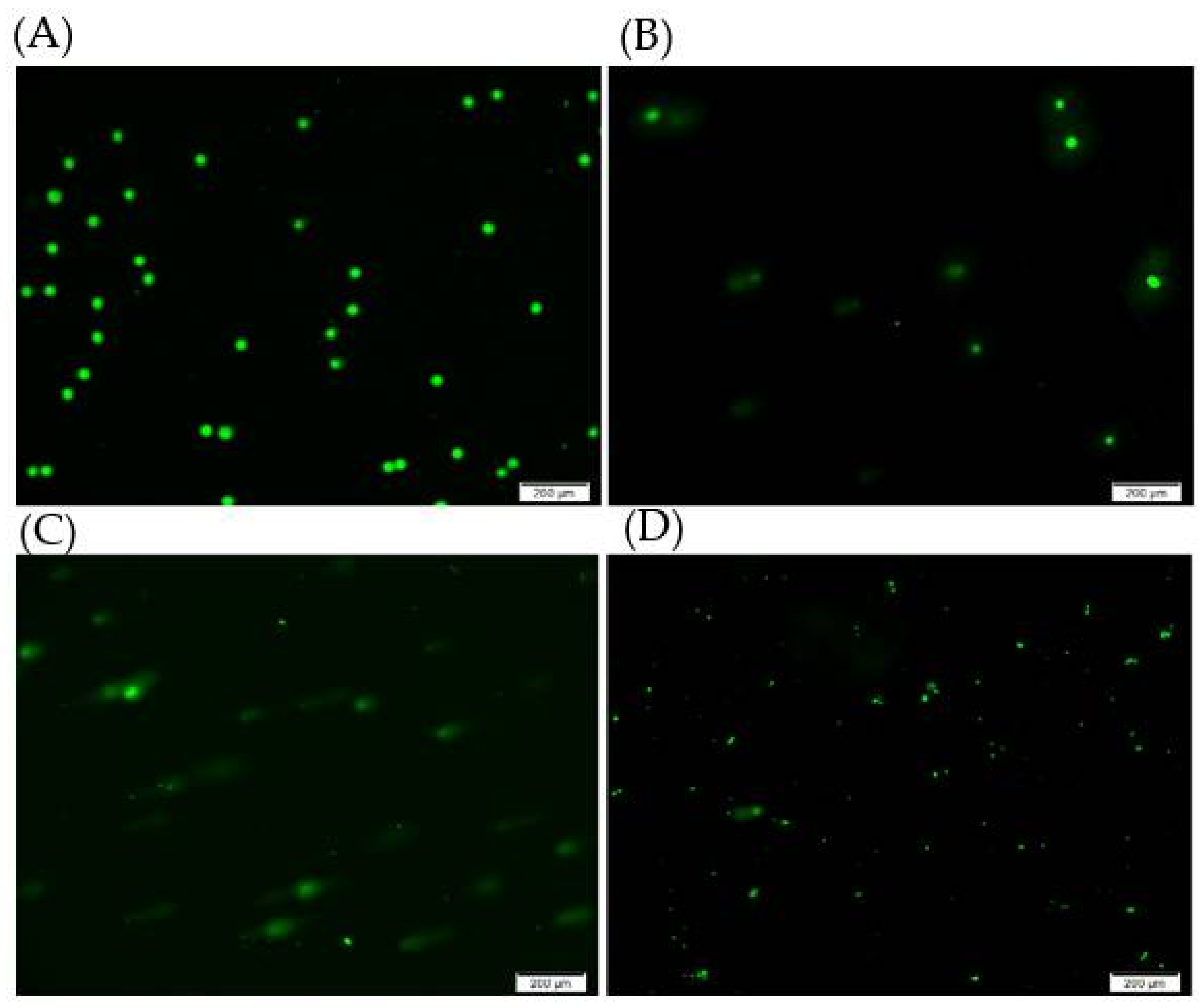
2.4. DNA Damage Studies
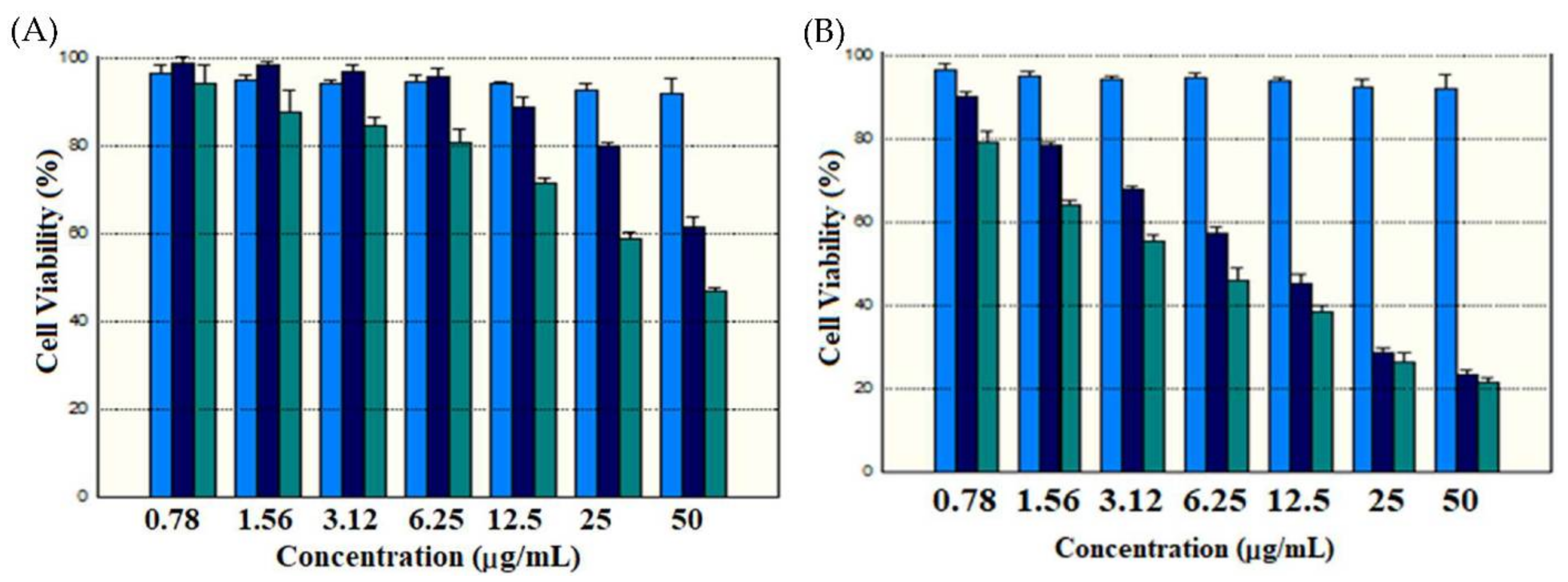
2.5. Cytotoxicity Studies
3. Materials and Methods
3.1. Materials
3.2. Characterizations
3.3. Synthesis of MB@SiO2 Nanoparticles
3.4. Synthesis of MB-SiO2@MSNs
3.5. Synthesis of Imidazole-Modified MB-SiO2@MSNs
3.6. Coordination of Cupric Ions onto MB-SiO2@MSNs-IMD Nanoparticles
3.7. Immobilization of Dox onto MB-SiO2@MSNs-Cu
3.8. Cell Culture
3.9. SRB Assay
3.10. Cellular Uptake Studies
3.11. Comet Assay
3.12. DCFH-DA Assay
3.13. ESR Spin Trapping
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef]
- Dizon, D.S.; Krilov, L.; Cohen, E.; Gangadhar, T.; Ganz, P.A.; Hensing, T.A.; Hunger, S.; Krishnamurthi, S.S.; Lassman, A.B.; Markham, M.; et al. Clinical Cancer Advances 2016: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J. Clin. Oncol. 2016, 34, 987–1011. [Google Scholar] [CrossRef]
- Bhargava, R.; Sahoo, S.; Esposito, N.N.; Chen, B. Pathology of Breast Carcinoma: Diagnostic, Prognostic, and Therapeutic Issues and Challenges. Pathol. Res. Int. 2011, 2011, 731470–731472. [Google Scholar] [CrossRef]
- Harris, T.J.R.; McCormick, F. The molecular pathology of cancer. Nat. Rev. Clin. Oncol. 2010, 7, 251–265. [Google Scholar] [CrossRef]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, Á. Assessment of the Evolution of Cancer Treatment Therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef]
- Sudhakar, A. History of Cancer, Ancient and Modern Treatment Methods. J. Cancer Sci. Ther. 2009, 1, 1–4. [Google Scholar] [CrossRef]
- Das, R.K.; Pramanik, A.; Majhi, M.; Mohapatra, S. Magnetic Mesoporous Silica Gated with Doped Carbon Dot for Site-Specific Drug Delivery, Fluorescence, and MR Imaging. Langmuir 2018, 34, 5253–5262. [Google Scholar] [CrossRef]
- Mamaeva, V.; Rosenholm, J.M.; Bate-Eya, L.T.; Bergman, L.; Peuhu, E.; Duchanoy, A.; Fortelius, L.E.; Landor, S.; Toivola, D.M.; Lindén, M.; et al. Mesoporous Silica Nanoparticles as Drug Delivery Systems for Targeted Inhibition of Notch Signaling in Cancer. Mol. Ther. 2011, 19, 1538–1546. [Google Scholar] [CrossRef]
- Duo, Y.; Li, Y.; Chen, C.; Liu, B.; Wang, X.; Zeng, X.; Chen, H. DOX-loaded pH-sensitive mesoporous silica nanoparticles coated with PDA and PEG induce pro-death autophagy in breast cancer. RSC Adv. 2017, 7, 39641–39650. [Google Scholar] [CrossRef]
- Saroj, S.; Rajput, S.J. Tailor-made pH-sensitive polyacrylic acid functionalized mesoporous silica nanoparticles for efficient and controlled delivery of anti-cancer drug Etoposide. Drug Dev. Ind. Pharm. 2018, 44, 1198–1211. [Google Scholar] [CrossRef]
- Lehman, S.E.; Morris, A.S.; Mueller, P.S.; Salem, A.; Grassian, V.H.; Larsen, S.C. Silica nanoparticle-generated ROS as a predictor of cellular toxicity: Mechanistic insights and safety by design. Environ. Sci. Nano 2016, 3, 56–66. [Google Scholar] [CrossRef]
- Tao, W.; He, Z. ROS-responsive drug delivery systems for biomedical applications. Asian J. Pharm. Sci. 2018, 13, 101–112. [Google Scholar] [CrossRef]
- Joshi, G.N.; Goetjen, A.M.; Knecht, D.A. Silica particles cause NADPH oxidase–independent ROS generation and transient phagolysosomal leakage. Mol. Biol. Cell 2015, 26, 3150–3164. [Google Scholar] [CrossRef]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017, 11, 240–253. [Google Scholar] [CrossRef]
- Sui, B.; Zhong, G.; Sun, J. Drug-loadable Mesoporous Bioactive Glass Nanospheres: Biodistribution, Clearance, BRL Cellular Location and Systemic Risk Assessment via 45Ca Labelling and Histological Analysis. Sci. Rep. 2016, 6, 33443. [Google Scholar] [CrossRef]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.; Budinger, G.R.S.; Chandel, N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788. [Google Scholar] [CrossRef]
- Tang, L.; Cheng, J. Nonporous silica nanoparticles for nanomedicine application. Nano Today 2013, 8, 290–312. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, D.; Song, C.G.; Kang, P.M. Reactive oxygen species-activated nanomaterials as theranostic agents. Nanomedicine 2015, 10, 2709–2723. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Hwang, H.-M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, X.; Wang, H.; Zheng, L.; Zhong, Z.; Li, X.; Zhao, J.; Kou, J.; Jiang, Y.; Zheng, X.; et al. Upconversion nanoparticle-mediated photodynamic therapy induces THP-1 macrophage apoptosis via ROS bursts and activation of the mitochondrial caspase pathway. Int. J. Nanomed. 2015, 10, 3719–3736. [Google Scholar] [CrossRef][Green Version]
- Rao, V.N.; Han, H.S.; Lee, H.; Nguyen, V.Q.; Jeon, S.; Jung, D.-W.; Lee, J.; Yi, G.-R.; Park, J.H. ROS-responsive mesoporous silica nanoparticles for MR imaging-guided photodynamically maneuvered chemotherapy. Nanoscale 2018, 10, 9616–9627. [Google Scholar] [CrossRef]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef]
- Chou, C.-C.; Chen, W.; Hung, Y.; Mou, C.-Y. Molecular Elucidation of Biological Response to Mesoporous Silica Nanoparticles in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 22235–22251. [Google Scholar] [CrossRef]
- Tai, G.; Liu, G.; Li, Q.; Ni, W.; Zhang, N.; Zheng, X.; Wang, Y.; Shao, D. Cytotoxicity of various types of gold-mesoporous silica nanoparticles in human breast cancer cells. Int. J. Nanomed. 2015, 10, 6075–6087. [Google Scholar] [CrossRef]
- Elle, R.E.; Rahmani, S.; Lauret, C.; Morena, M.; Bidel, L.P.R.; Boulahtouf, A.; Balaguer, P.; Cristol, J.-P.; Durand, J.-O.; Charnay, C.; et al. Functionalized Mesoporous Silica Nanoparticle with Antioxidants as a New Carrier That Generates Lower Oxidative Stress Impact on Cells. Mol. Pharm. 2016, 13, 2647–2660. [Google Scholar] [CrossRef]
- Krętowski, R.; Kusaczuk, M.; Naumowicz, M.; Kotyńska, J.; Szynaka, B.; Cechowska-Pasko, M. The Effects of Silica Nanoparticles on Apoptosis and Autophagy of Glioblastoma Cell Lines. Nanomaterials 2017, 7, 230. [Google Scholar] [CrossRef]
- Tang, X.-l.; Jing, F.; Lin, B.-l.; Cui, S.; Yu, R.-t.; Shen, X.-d.; Wang, T.-w. pH-Responsive Magnetic Mesoporous Silica-Based Nanoplatform for Synergistic Photodynamic Therapy/Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 15001–15011. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, J.; Su, H.; Mu, G.; Sun, J.-H.; Tan, C.-P.; Liang, X.-J.; Ji, L.-N.; Mao, Z.-W. Co-Delivery of Cisplatin Prodrug and Chlorin e6 by Mesoporous Silica Nanoparticles for Chemo-Photodynamic Combination Therapy to Combat Drug Resistance. ACS Appl. Mater. Interfaces 2016, 8, 13332–13340. [Google Scholar] [CrossRef]
- Yan, T.; Cheng, J.; Liu, Z.; Cheng, F.; Wei, X.; He, J. pH-Sensitive mesoporous silica nanoparticles for chemo-photodynamic combination therapy. Colloids Surf. B Biointerfaces 2018, 161, 442–448. [Google Scholar] [CrossRef]
- Li, H.; Wei, M.; Lv, X.; Hu, Y.; Shao, J.; Song, X.; Yang, D.; Wang, W.; Li, B.; Dong, X. Cerium-based Nanoparticles for Cancer Photodynamic Therapy. J. Innov. Opt. HealthSci. 2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, H.; He, P.; Shen, C.; Li, Q.; Duan, Y.; Shao, Z.; Mu, F.; Huang, F.; Li, P.; et al. Exfoliated FePS3 nanosheets for T1-weighted magnetic resonance imaging-guided near-infrared photothermal therapy in vivo. Sci. China Mater. 2021, 64, 2613–2623. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Wang, J.; Yuan, A.; Sun, M.; Wu, J.; Hu, Y. Self-assembled IR780-loaded transferrin nanoparticles as an imaging, targeting and PDT/PTT agent for cancer therapy. Sci. Rep. 2016, 6, 27421. [Google Scholar] [CrossRef]
- Hovan, A.; Datta, S.; Kruglik, S.G.; Jancura, D.; Miskovsky, P.; Bánó, G. Phosphorescence Kinetics of Singlet Oxygen Produced by Photosensitization in Spherical Nanoparticles. Part I. Theory. J. Phys. Chem. B 2018, 122, 5147–5153. [Google Scholar] [CrossRef]
- Kankala, R.K.; Han, Y.-H.; Na, J.; Lee, C.-H.; Sun, Z.; Wang, S.-B.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A.-Z.; et al. Nanoarchitectured Structure and Surface Biofunctionality of Mesoporous Silica Nanoparticles. Adv. Mater. 2020, 32, 1907035. [Google Scholar] [CrossRef]
- Kankala, R.K.; Han, Y.-H.; Xia, H.-Y.; Wang, S.-B.; Chen, A.-Z. Nanoarchitectured prototypes of mesoporous silica nanoparticles for innovative biomedical applications. J. Nanobiotechnol. 2022, 20, 126. [Google Scholar] [CrossRef]
- Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Nanoarchitecting Hierarchical Mesoporous Siliceous Frameworks: A New Way Forward. iScience 2020, 23, 101687. [Google Scholar] [CrossRef]
- Gartmann, N.; Brühwiler, D. Controlling and Imaging the Functional-Group Distribution on Mesoporous Silica. Angewandte Chem. Int. Ed. 2009, 48, 6354–6356. [Google Scholar] [CrossRef]
- Freitas, L.B.d.O.; Corgosinho, L.d.M.; Faria, J.A.Q.A.; dos Santos, V.M.; Resende, J.M.; Leal, A.S.; Gomes, D.A.; Sousa, E.M.B.d. Multifunctional mesoporous silica nanoparticles for cancer-targeted, controlled drug delivery and imaging. Microporous Mesoporous Mater. 2017, 242, 271–283. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef]
- Shi, S.; Chen, F.; Cai, W. Biomedical Applications of Functionalized Hollow Mesoporous Silica Nanoparticles: Focusing on Molecular Imaging. Nanomedicine 2013, 8, 10–2217. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, Q.; Li, J.; Shen, X.; Mu, C.; Cai, K. Dendrimerlike Mesoporous Silica Nanoparticles as pH-Responsive Nanocontainers for Targeted Drug Delivery and Bioimaging. ACS Appl. Mater. Interfaces 2015, 7, 7357–7372. [Google Scholar] [CrossRef] [PubMed]
- Maggini, L.; Cabrera, I.; Ruiz-Carretero, A.; Prasetyanto, E.A.; Robinet, E.; De Cola, L. Breakable mesoporous silica nanoparticles for targeted drug delivery. Nanoscale 2016, 8, 7240–7247. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Na, H.-K.; Kim, Y.-K.; Ryoo, S.-R.; Cho, H.S.; Lee, K.E.; Jeon, H.; Ryoo, R.; Min, D.-H. Facile Synthesis of Monodispersed Mesoporous Silica Nanoparticles with Ultralarge Pores and Their Application in Gene Delivery. ACS Nano 2011, 5, 3568–3576. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.-P.; Kuang, L.-Y.; Huang, C.-A.; Jane, W.-N.; Hung, Y.; Hsing, Y.-i.C.; Mou, C.-Y. A simple plant gene delivery system using mesoporous silica nanoparticles as carriers. J. Mater. Chem. B 2013, 1, 5279–5287. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Zhang, H.; Liu, C.-G.; Kanubaddi, K.R.; Lee, C.-H.; Wang, S.-B.; Cui, W.; Santos, H.A.; Lin, K.; Chen, A.-Z. Metal Species–Encapsulated Mesoporous Silica Nanoparticles: Current Advancements and Latest Breakthroughs. Adv. Funct. Mater. 2019, 29, 1902652. [Google Scholar] [CrossRef]
- Planas, O.; Bresolí-Obach, R.; Nos, J.; Gallavardin, T.; Ruiz-González, R.; Agut, M.; Nonell, S. Synthesis, Photophysical Characterization, and Photoinduced Antibacterial Activity of Methylene Blue-loaded Amino- and Mannose-Targeted Mesoporous Silica Nanoparticles. Molecules 2015, 20, 6284–6298. [Google Scholar] [CrossRef]
- Lv, J.; Wu, G.; He, Y.; Zhang, L.; Yi, Y. Methylene blue-loaded gold nanobipyramids @SiO2 enhanced singlet oxygen generation for phototherapy of cancer cells. Opt. Mater. Express 2017, 7, 409–414. [Google Scholar] [CrossRef]
- Hanafi-Bojd, M.Y.; Jaafari, M.R.; Ramezanian, N.; Xue, M.; Amin, M.; Shahtahmassebi, N.; Malaekeh-Nikouei, B. Surface functionalized mesoporous silica nanoparticles as an effective carrier for epirubicin delivery to cancer cells. Eur. J. Pharm. Biopharm. 2015, 89, 248–258. [Google Scholar] [CrossRef]
- Wani, A.; Muthuswamy, E.; Savithra, G.H.L.; Mao, G.; Brock, S.; Oupický, D. Surface Functionalization of Mesoporous Silica Nanoparticles Controls Loading and Release Behavior of Mitoxantrone. Pharm. Res. 2012, 29, 2407–2418. [Google Scholar] [CrossRef]
- Smith, M.A.; Ilasi, M.G.; Zoelle, A. A Novel Approach to Calibrate Mesopore Size from Nitrogen Adsorption Using X-ray Diffraction: An SBA-15 Case Study. J. Phys. Chem. C 2013, 117, 17493–17502. [Google Scholar] [CrossRef]
- Huang, I.P.; Sun, S.-P.; Cheng, S.-H.; Lee, C.-H.; Wu, C.-Y.; Yang, C.-S.; Lo, L.-W.; Lai, Y.-K. Enhanced Chemotherapy of Cancer Using pH-Sensitive Mesoporous Silica Nanoparticles to Antagonize P-Glycoprotein-Mediated Drug Resistance. Mol. Cancer Ther. 2011, 10, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, D.; Ceña, V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9 (Suppl. S1), 51–63. [Google Scholar] [CrossRef]
- Belhekar, A.A.; Awate, S.V.; Anand, R. Photocatalytic activity of titania modified mesoporous silica for pollution control. Catal. Commun. 2002, 3, 453–458. [Google Scholar] [CrossRef]
- Haque, E.; Jun, J.W.; Jhung, S.H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater. 2011, 185, 507–511. [Google Scholar] [CrossRef]
- Shibu, E.S.; Hamada, M.; Murase, N.; Biju, V. Nanomaterials formulations for photothermal and photodynamic therapy of cancer. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 53–72. [Google Scholar] [CrossRef]
- Ahmed, N.; Fessi, H.; Elaissari, A. Theranostic applications of nanoparticles in cancer. Drug Discov. Today 2012, 17, 928–934. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Su, Z.; Wang, C.; Liao, Y.; Fu, Q. Multifunctional Hollow Mesoporous Silica Nanocages for Cancer Cell Detection and the Combined Chemotherapy and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2011, 3, 2479–2486. [Google Scholar] [CrossRef]
- Makhadmeh, G.N.; Abdul Aziz, A.; Abdul Razak, K. The efficacy of methylene blue encapsulated in silica nanoparticles compared to naked methylene blue for photodynamic applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1018–1022. [Google Scholar] [CrossRef]
- Kohle, F.F.E.; Li, S.; Turker, M.Z.; Wiesner, U.B. Ultrasmall PEGylated and Targeted Core–Shell Silica Nanoparticles Carrying Methylene Blue Photosensitizer. ACS Biomater. Sci. Eng. 2020, 6, 256–264. [Google Scholar] [CrossRef]
- Malinge, J.; Allain, C.; Galmiche, L.; Miomandre, F.; Audebert, P. Preparation, Photophysical, Electrochemical, and Sensing Properties of Luminescent Tetrazine-Doped Silica Nanoparticles. Chem. Mater. 2011, 23, 4599–4605. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Lee, C.-H.; Yang, C.-S.; Tseng, F.-G.; Mou, C.-Y.; Lo, L.-W. Mesoporous silica nanoparticles functionalized with an oxygen-sensing probe for cell photodynamic therapy: Potential cancer theranostics. J. Mater. Chem. 2009, 19, 1252–1257. [Google Scholar] [CrossRef]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef]
- Yang, P.; Gai, S.; Lin, J. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679–3698. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Park, Y.; Choi, K.; Lee, J.S.; Yi, J. Ordered mesoporous silica (SBA-15) derivatized with imidazole-containing functionalities as a selective adsorbent of precious metal ions. J. Mater. Chem. 2004, 14, 1043–1049. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lin, T.-S.; Mou, C.-Y. (VO)2+ Ions Immobilized on Functionalized Surface of Mesoporous Silica and Their Activity toward the Hydroxylation of Benzene. J. Phys. Chem. B 2003, 107, 2543–2551. [Google Scholar] [CrossRef]
- Kankala, R.K.; Tsai, P.-Y.; Kuthati, Y.; Wei, P.-R.; Liu, C.-L.; Lee, C.-H. Overcoming multidrug resistance through co-delivery of ROS-generating nano-machinery in cancer therapeutics. J. Mater. Chem. B 2017, 5, 1507–1517. [Google Scholar] [CrossRef]
- Shen, J.; He, Q.; Gao, Y.; Shi, J.; Li, Y. Mesoporous silica nanoparticles loading doxorubicin reverse multidrug resistance: Performance and mechanism. Nanoscale 2011, 3, 4314–4322. [Google Scholar] [CrossRef]
- Kankala, R.K.; Liu, C.-G.; Chen, A.-Z.; Wang, S.-B.; Xu, P.-Y.; Mende, L.K.; Liu, C.-L.; Lee, C.-H.; Hu, Y.-F. Overcoming multidrug resistance through the synergistic effects of hierarchical pH-sensitive, ROS-generating nanoreactors. ACS Biomater. Sci. Eng. 2017, 3, 2431–2442. [Google Scholar] [CrossRef]
- Shahabi, S.; Döscher, S.; Bollhorst, T.; Treccani, L.; Maas, M.; Dringen, R.; Rezwan, K. Enhancing Cellular Uptake and Doxorubicin Delivery of Mesoporous Silica Nanoparticles via Surface Functionalization: Effects of Serum. ACS Appl. Mater. Interfaces 2015, 7, 26880–26891. [Google Scholar] [CrossRef]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.Y. Effect of Surface Functionalization of MCM-41-Type Mesoporous Silica Nanoparticles on the Endocytosis by Human Cancer Cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed]


| Sample | Surface Area a (m2 g−1) | Pore Volume b (cm3 g−1) | Pore Diameter c (nm) |
|---|---|---|---|
| MB-SiO2@MSNs-CTA+ | 256 | 0.650 | N.D. d |
| MB-SiO2@MSNs | 818 | 1.044 | 2.5 |
| MB-SiO2@MSNs-IMD | 386 | 0.592 | 2.1 |
| MB-SiO2@MSNs-Cu | 380 | 0.486 | N.D. d |
| MB-SiO2@MSNs-Cu-Dox | 249 | 0.439 | N.D. d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.-M.; Chiu, S.-H.; Busa, P.; Liu, C.-L.; Kankala, R.K.; Lee, C.-H. Engineered Mesoporous Silica-Based Core-Shell Nanoarchitectures for Synergistic Chemo-Photodynamic Therapies. Int. J. Mol. Sci. 2022, 23, 11604. https://doi.org/10.3390/ijms231911604
Gao Y-M, Chiu S-H, Busa P, Liu C-L, Kankala RK, Lee C-H. Engineered Mesoporous Silica-Based Core-Shell Nanoarchitectures for Synergistic Chemo-Photodynamic Therapies. International Journal of Molecular Sciences. 2022; 23(19):11604. https://doi.org/10.3390/ijms231911604
Chicago/Turabian StyleGao, Yue-Mei, Shih-Han Chiu, Prabhakar Busa, Chen-Lun Liu, Ranjith Kumar Kankala, and Chia-Hung Lee. 2022. "Engineered Mesoporous Silica-Based Core-Shell Nanoarchitectures for Synergistic Chemo-Photodynamic Therapies" International Journal of Molecular Sciences 23, no. 19: 11604. https://doi.org/10.3390/ijms231911604
APA StyleGao, Y.-M., Chiu, S.-H., Busa, P., Liu, C.-L., Kankala, R. K., & Lee, C.-H. (2022). Engineered Mesoporous Silica-Based Core-Shell Nanoarchitectures for Synergistic Chemo-Photodynamic Therapies. International Journal of Molecular Sciences, 23(19), 11604. https://doi.org/10.3390/ijms231911604








