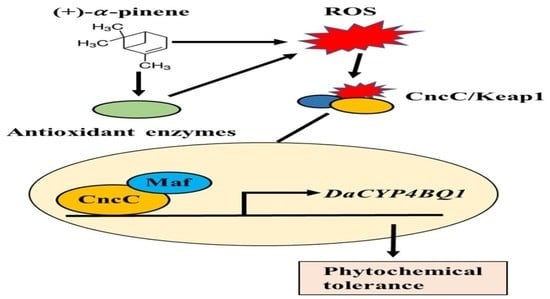
Activation of the ROS/CncC Signaling Pathway Regulates Cytochrome P450 CYP4BQ1 Responsible for (+)-α-Pinene Tolerance in Dendroctonus armandi
Abstract
1. Introduction
2. Results
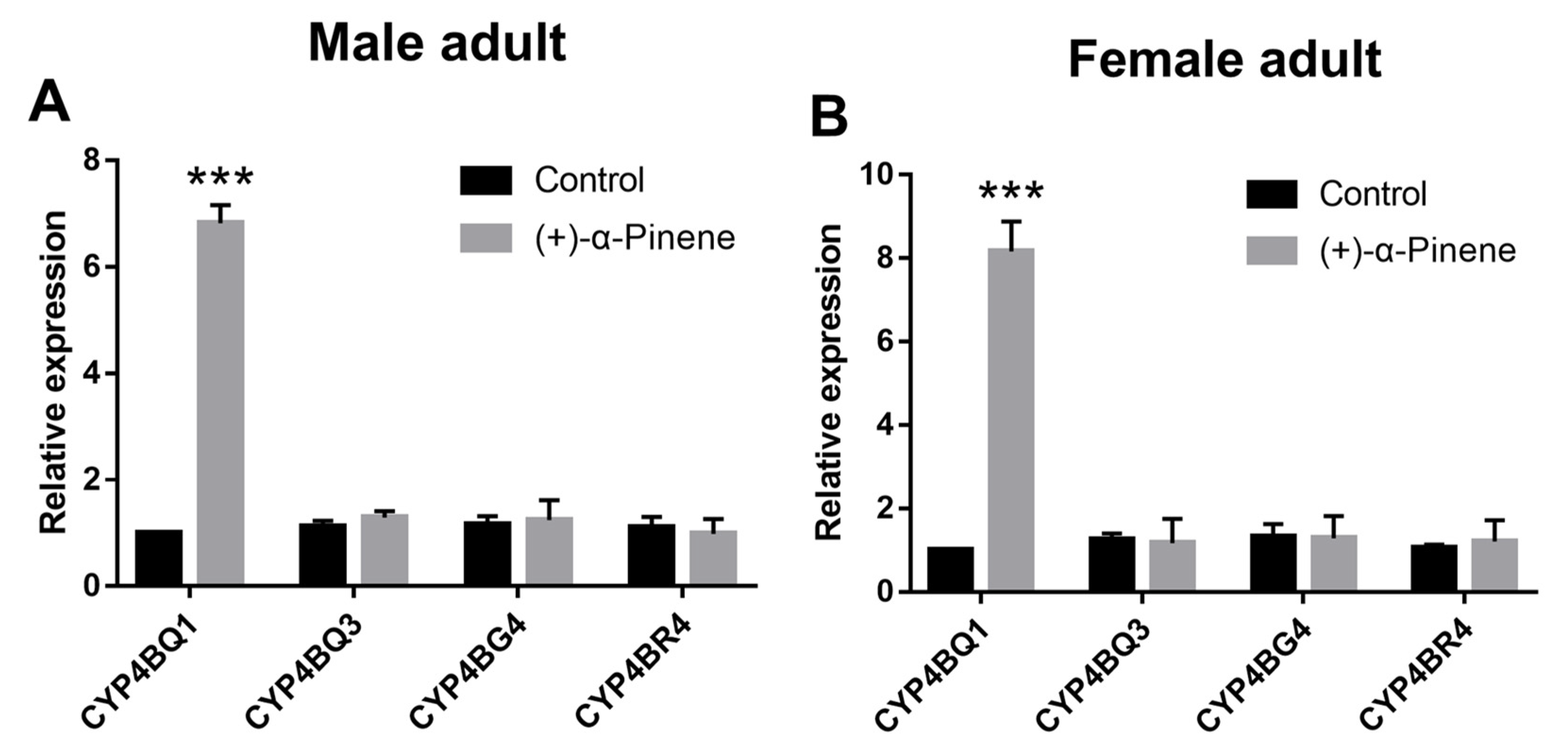
2.1. (+)-α-Pinene Exposure Induces DaCYP4BQ1 Expression
2.2. Spatiotemporal Expression Pattern of CYP4BQ1
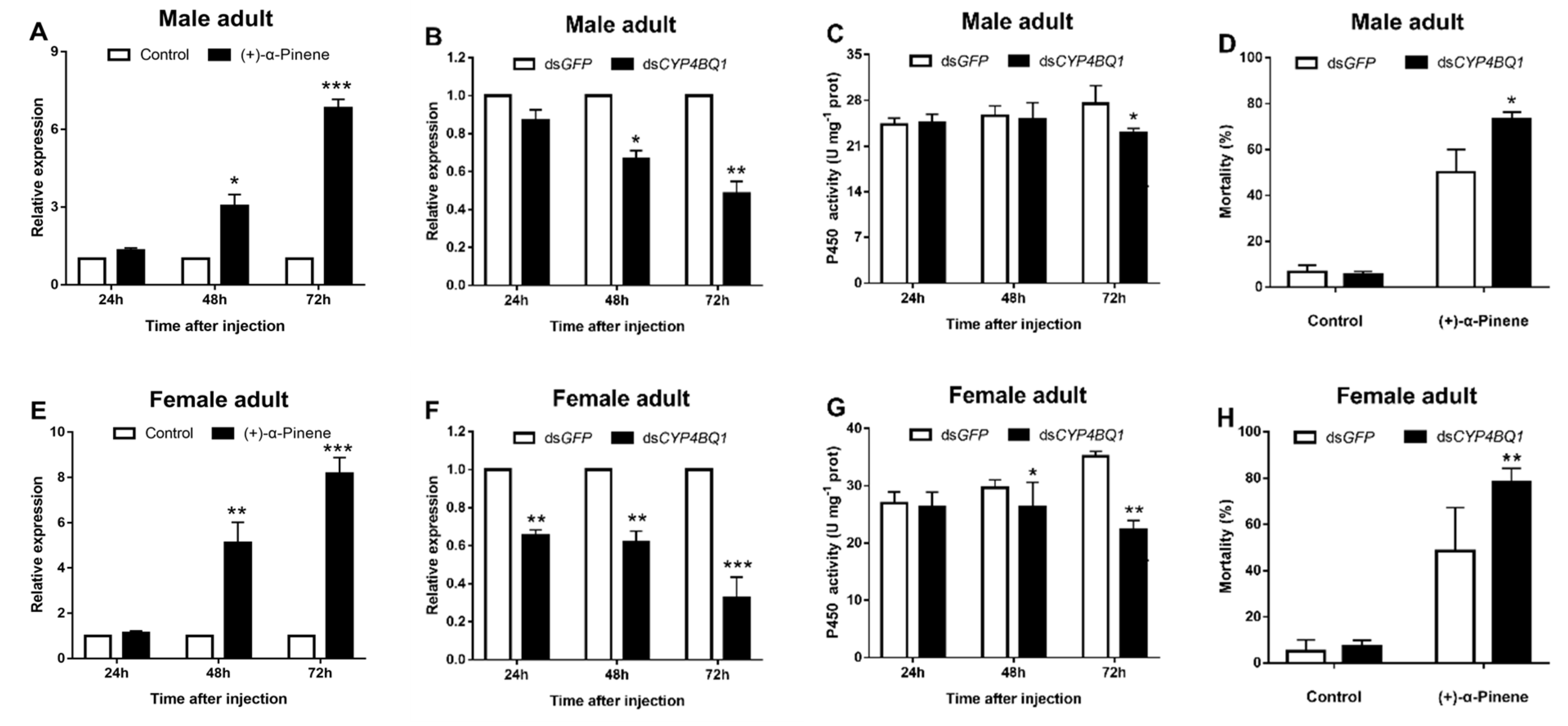
2.3. CYP4BQ1 Participates in Adults’ Tolerance to (+)-α-Pinene
2.4. (+)-α-Pinene Exposure Induces Expression of CncC and Maf
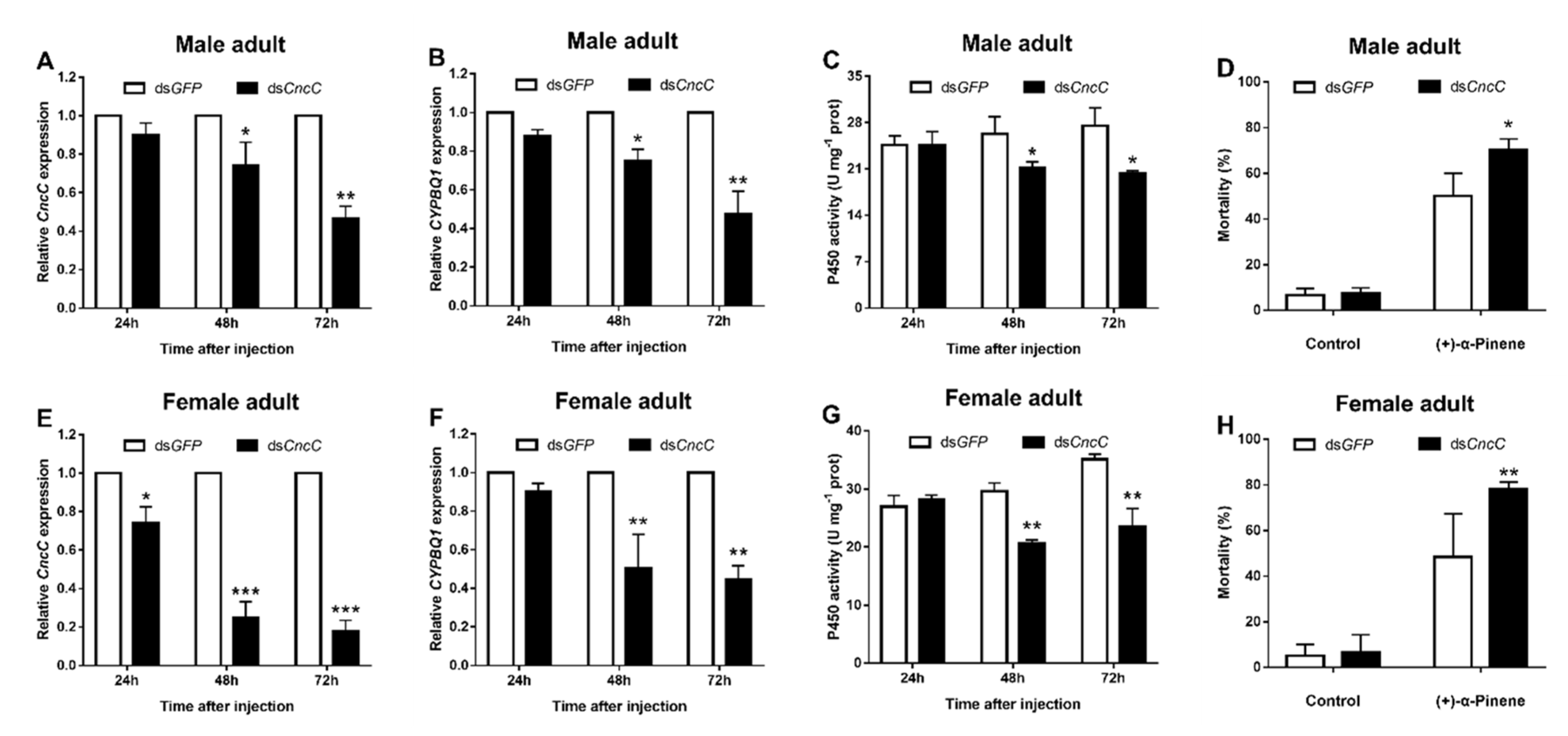
2.5. Silencing CncC Suppresses CYP4BQ1 Expression and Reduces Adults’ Tolerance to (+)-α-Pinene
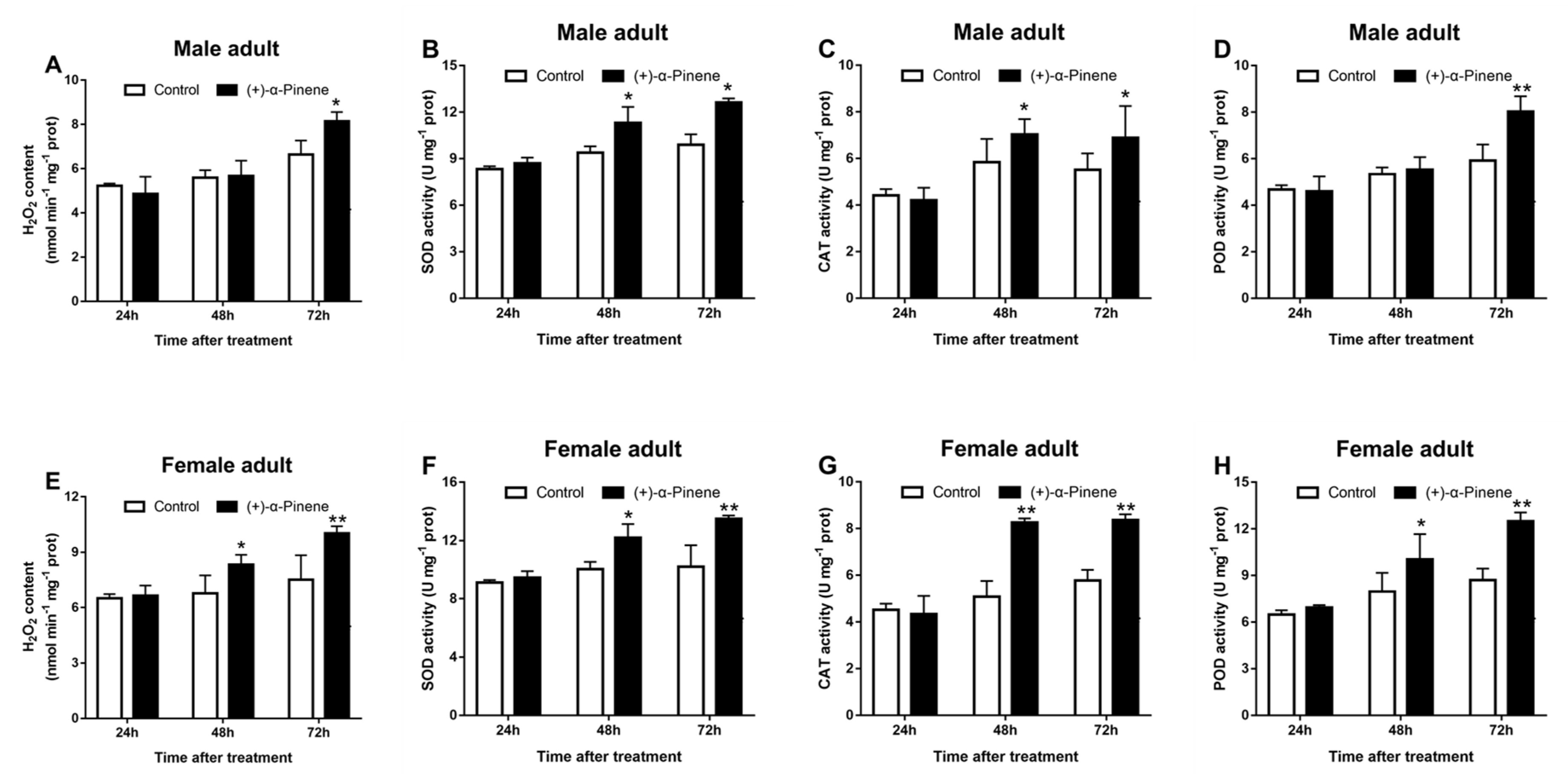
2.6. (+)-α-Pinene Exposure Enhances H2O2 Generation and the Activities of Antioxidant Enzymes
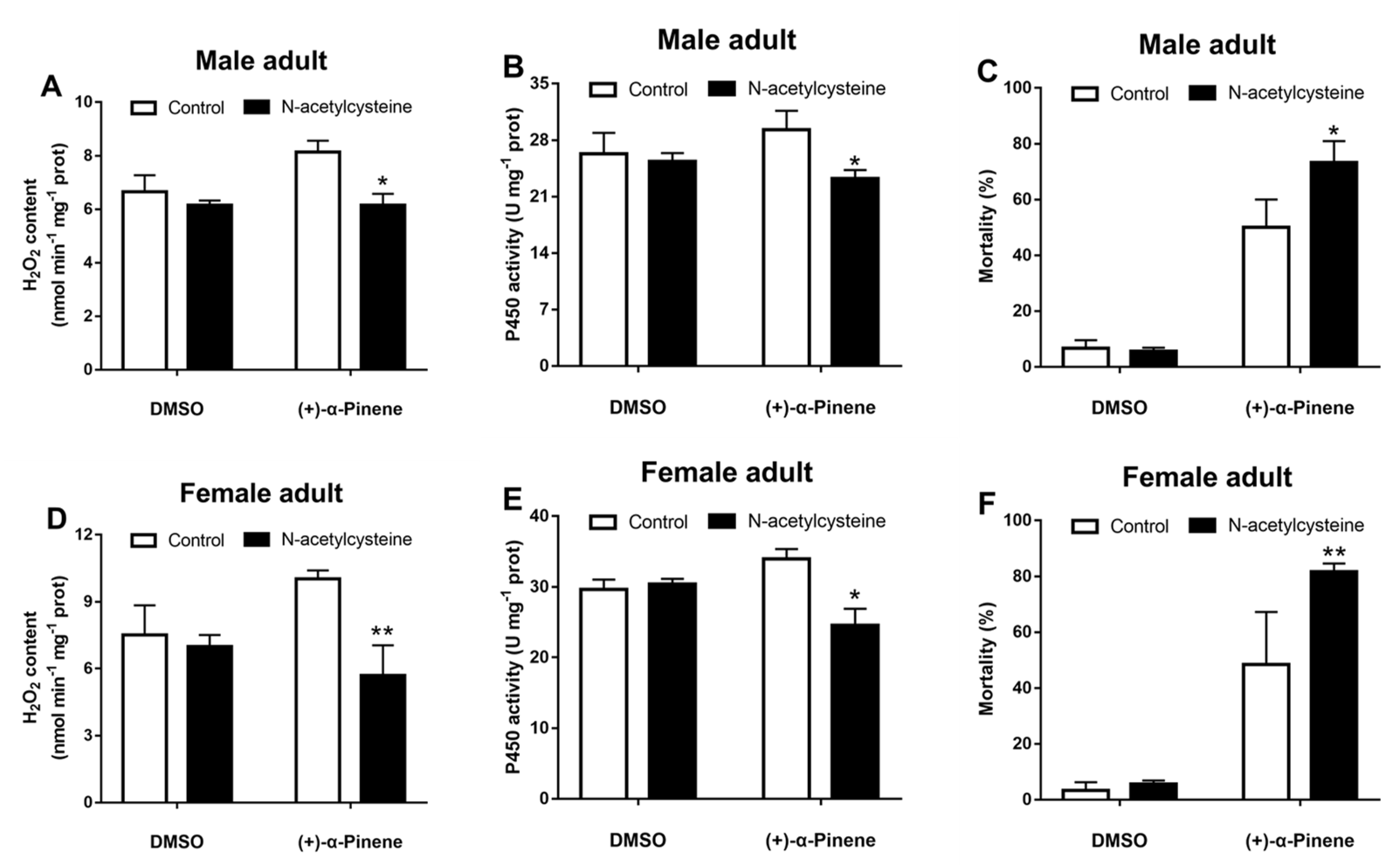
2.7. Scavenging of ROS Downregulates CncC and CYP4BQ1 Expressions and Reduces Adults’ Tolerance to (+)-α-Pinene
2.8. The CncC Agonist Curcumin Induces the Expression of CYP4BQ1 and Enhances Adults’ Tolerance to (+)-α-Pinene
3. Discussion
4. Materials and Methods
4.1. Insects and Reagents Preparation
4.2. Tolerance to (+)-α-Pinene
4.3. RNA Extraction, cDNA Synthesis, and Reverse Transcription Quantitative PCR (RT-qPCR)
4.4. Developmental and Tissue-Dependent Expression Profiles of CYP4BQ1, CncC, Maf, and Keap1
4.5. Double-Strand RNA (dsRNA) Synthesis and Injection
4.6. H2O2 Content and CAT, SOD and POD Activity Determination
4.7. Scavenging of ROS by N-Acetylcysteine (NAC) Treatment
4.8. Dietary Curcumin and (+)-α-Pinene Tolerance Analyses
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Li, Z.; Tang, M. Laboratory evaluation of flight activity of Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae). Can. Entomol. 2010, 142, 378–387. [Google Scholar] [CrossRef]
- Huber, D.P.W.; Robert, J.A. The proteomics and transcriptomics of early host colonization and overwintering physiology in the Mountain Pine Beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Curculionidae). In Pine Bark Beetles; Tittiger, C., Blomquist, G.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 50; pp. 101–128. [Google Scholar]
- Chen, H.; Tang, M.; Gao, J.; Chen, X.; Li, Z. Changes in the composition of volatile monoterpenes and sesquiterpenes of Pinus armandi, P-tabulaeformis, and P-bungeana in Northwest China. Chem. Nat. Compd. 2006, 42, 534–538. [Google Scholar] [CrossRef]
- Bohlmann, J. Pine terpenoid defences in the mountain pine beetle epidemic and in other conifer pest interactions: Specialized enemies are eating holes into a diverse, dynamic and durable defence system. Tree Physiol. 2012, 32, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Kang, X.; An, H.; Liu, B.; Chen, H. Two Key Volatiles of Chinese White Pine (Pinus armandii) (Pinales: Pinaceae: Pinoideae) Phloem Resist Invasion by Chinese White Pine Beetle (Dendroctonus armandii) (Coleoptera: Curculionidae: Scolytinae). Appl. Ecol. Environ. Res. 2022, 20, 587–600. [Google Scholar] [CrossRef]
- Chiu, C.C.; Keeling, C.I.; Bohlmann, J. The cytochrome P450 CYP6DE1 catalyzes the conversion of alpha-pinene into the mountain pine beetle aggregation pheromone trans-verbenol. Sci. Rep. 2019, 9, 1477. [Google Scholar] [CrossRef]
- Lucia, A.; Audino, P.G.; Seccacini, E.; Licastro, S.; Zerba, E.; Masuh, H. Larvicidal effect of Eucalyptus Grandis essential oil and turpentine and their major components on Aedes Aegypti larvae. J. Am. Mosq. Control Assoc. 2007, 23, 299–303. [Google Scholar] [CrossRef]
- Mercier, B.; Prost, J.; Prost, M. The essential oil of turpentine and its major volatile fraction (alpha- and beta-pinenes): A review. Int. J. Occup. Med. Environ. Health 2009, 22, 331–342. [Google Scholar] [CrossRef]
- Shahriari, M.; Zibaee, A.; Sahebzadeh, N.; Shamakhi, L. Effects of alpha-pinene, trans-anethole, and thymol as the essential oil constituents on antioxidant system and acetylcholine esterase of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Pestic. Biochem. Physiol. 2018, 150, 40–47. [Google Scholar] [CrossRef]
- Xu, B.-B.; Liu, Z.-D.; Sun, J.-H. The effects of α-pinene on the feeding performance and pheromone production of Dendroctonus valens. Entomol. Exp. Appl. 2014, 150, 269–278. [Google Scholar] [CrossRef]
- Nishida, R. Chemical ecology of insect-plant interactions: Ecological significance of plant secondary metabolites. Biosci. Biotechnol. Biochem. 2014, 78, 1–13. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect CYP genes and P450 enzymes. In Insect Molecular Biology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2012; pp. 236–316. [Google Scholar]
- Cheng, T.; Wu, J.; Wu, Y.; Chilukuri, R.V.; Huang, L.; Yamamoto, K.; Feng, L.; Li, W.; Chen, Z.; Guo, H.; et al. Genomic adaptation to polyphagy and insecticides in a major East Asian noctuid pest. Nat. Ecol. Evol. 2017, 1, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Kaplanoglu, E.; Chapman, P.; Scott, I.M.; Donly, C. Overexpression of a cytochrome P450 and a UDP-glycosyltransferase is associated with imidacloprid resistance in the Colorado potato beetle, Leptinotarsa decemlineata. Sci. Rep. 2017, 7, 1762. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Pan, Y.; Gao, X.; Xi, J.; Zhang, L.; Yang, C.; Bi, R.; Yang, S.; Xin, X.; Shang, Q. Cytochrome P450 CYP6DA2 regulated by cap ‘n’ collar isoform C (CncC) is associated with gossypol tolerance in Aphis gossypii Glover. Insect Mol. Biol. 2016, 25, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Cano-Ramirez, C.; Lopez, M.F.; Cesar-Ayala, A.K.; Pineda-Martinez, V.; Sullivan, B.T.; Zuniga, G. Isolation and expression of cytochrome P450 genes in the antennae and gut of pine beetle Dendroctonus rhizophagus (Curculionidae: Scolytinae) following exposure to host monoterpenes. Gene 2013, 520, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, R.; Tang, J.; Du, L.; Wang, Y.; Wang, J.; Liu, L.; Gao, S.; Li, B. Three cytochrome P450 CYP4 family genes regulated by the CncC signaling pathway mediate phytochemical susceptibility in the red flour beetle. Tribolium castaneum. Pest. Manag. Sci. 2022, 78, 3508–3518. [Google Scholar] [CrossRef]
- Li, X.; Deng, Z.; Chen, X. Regulation of insect P450s in response to phytochemicals. Curr. Opin. Insect Sci. 2021, 43, 108–116. [Google Scholar] [CrossRef]
- Wilding, C.S. Regulating resistance: CncC:Maf, antioxidant response elements and the overexpression of detoxification genes in insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 89–96. [Google Scholar] [CrossRef]
- Misra, J.R.; Horner, M.A.; Lam, G.; Thummel, C.S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 2011, 25, 1796–1806. [Google Scholar] [CrossRef]
- Yang, X.; Deng, S.; Wei, X.; Yang, J.; Zhao, Q.; Yin, C.; Du, T.; Guo, Z.; Xia, J.; Yang, Z.; et al. MAPK-directed activation of the whitefly transcription factor CREB leads to P450-mediated imidacloprid resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 10246–10253. [Google Scholar] [CrossRef]
- Nakata, K.; Tanaka, Y.; Nakano, T.; Adachi, T. Nuclear receptor-mediated transcriptional regulation in phase I, II, and III xenobiotic metabolizing systems. Drug Metab. Pharmacokinet. 2006, 21, 437–457. [Google Scholar] [CrossRef]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, M.; Palli, S.R. Transcription factors, CncC and Maf, regulate expression of CYP6BQ genes responsible for deltamethrin resistance in Tribolium castaneum. Insect Biochem. Mol. Biol. 2015, 65, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Cheng, Y.; Li, W.; Li, Y.; Zeng, R.; Song, Y. Activation of CncC pathway by ROS burst regulates cytochrome P450 CYP6AB12 responsible for lambda-cyhalothrin tolerance in Spodoptera litura. J. Hazard. Mater. 2020, 387, 121698. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, M.; Palli, S.R. Transcription factor cap n collar C regulates multiple cytochrome P450 genes conferring adaptation to potato plant allelochemicals and resistance to imidacloprid in Leptinotarsa decemlineata (Say). Insect Biochem. Mol. Biol. 2017, 83, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, G.J.; Tittiger, C.; MacLean, M.; Keeling, C.I. Cytochromes P450: Terpene detoxification and pheromone production in bark beetles. Curr. Opin. Insect Sci. 2021, 43, 97–102. [Google Scholar] [CrossRef]
- Li, F.; Ma, K.; Chen, X.; Zhou, J.J.; Gao, X. The regulation of three new members of the cytochrome P450 CYP6 family and their promoters in the cotton aphid Aphis gossypii by plant allelochemicals. Pest. Manag. Sci. 2019, 75, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Z.; Li, M.; Fang, Y.; Qu, J.; Mao, T.; Chen, J.; Li, F.; Sun, H.; Li, B. Responses of detoxification enzymes in the midgut of Bombyx mori after exposure to low-dose of acetamiprid. Chemosphere 2020, 251, 126438. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, J.; Wu, H.; Zhang, H.; Zhang, J.; Ma, E. Knockdown of cytochrome P450 CYP6 family genes increases susceptibility to carbamates and pyrethroids in the migratory locust, Locusta migratoria. Chemosphere 2019, 223, 48–57. [Google Scholar] [CrossRef]
- Lao, S.-H.; Huang, X.-H.; Huang, H.-J.; Liu, C.-W.; Zhang, C.-X.; Bao, Y.-Y. Genomic and transcriptomic insights into the cytochrome P450 monooxygenase gene repertoire in the rice pest brown planthopper, Nilaparvata lugens. Genomics 2015, 106, 301–309. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, X.; Wu, H.; Silver, K.; Zhang, J.; Ma, E.; Zhu, K.Y. Transcriptome-wide survey, gene expression profiling and exogenous chemical-induced transcriptional responses of cytochrome P450 superfamily genes in migratory locust (Locusta migratoria). Insect Biochem. Mol. Biol. 2018, 100, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Maibeche-Coisne, M.; Monti-Dedieu, L.; Aragon, S.; Dauphin-Villemant, C. A new cytochrome P450 from Drosophila melanogaster, CYP4G15, expressed in the nervous system. Biochem. Biophys. Res. Commun. 2000, 273, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.D.; Unnithan, G.C.; Andersen, J.F.; Evans, P.H.; Murataliev, M.B.; Szabo, L.Z.; Mash, E.A.; Bowers, W.S.; Feyereisen, R. A cytochrome P450 terpenoid hydroxylase linked to the suppression of insect juvenile hormone synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 12884–12889. [Google Scholar] [CrossRef]
- Maibeche-Coisne, M.; Merlin, C.; Francois, M.C.; Porcheron, P.; Jacquin-Joly, E. P450 and P450 reductase cDNAs from the moth Mamestra brassicae: Cloning and expression patterns in male antennaeb. Gene 2005, 346, 195–203. [Google Scholar] [CrossRef]
- Maibeche-Coisne, M.; Jacquin-Joly, E.; Francois, M.C.; Nagnan-Le Meillour, P. cDNA cloning of biotransformation enzymes belonging to the cytochrome P450 family in the antennae of the noctuid moth Mamestra brassicae. Insect Mol. Biol. 2002, 11, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.J.; Stevens, J.L.; Andersen, J.F.; Feyereisen, R. Expression of cytochrome P450 genes of the CYP4 family in midgut and fat body of the tobacco hornworm, Manduca sexta. Arch. Biochem. Biophys. 1995, 321, 13–20. [Google Scholar] [CrossRef]
- Londono, D.K.; Siegfried, B.D.; Lydy, M.J. Atrazine induction of a family 4 cytochrome P450 gene in Chironomus tentans (Diptera: Chironomidae). Chemosphere 2004, 56, 701–706. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Cui, R.; Zeng, X.; Gao, X. Cloning and Expression of Multiple Cytochrome P450 Genes: Induction by Fipronil in Workers of the Red Imported Fire Ant (Solenopsis invicta Buren). PLoS ONE 2016, 11, e0150915. [Google Scholar] [CrossRef]
- Ma, K.; Li, F.; Tang, Q.; Liang, P.; Liu, Y.; Zhang, B.; Gao, X. CYP4CJ1-mediated gossypol and tannic acid tolerance in Aphis gossypii Glover. Chemosphere 2019, 219, 961–970. [Google Scholar] [CrossRef]
- Palli, S.R. CncC/Maf-mediated xenobiotic response pathway in insects. Arch. Insect Biochem. Physiol. 2020, 104, e21674. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Cheng, Y.; Li, Y.; Li, W.; Ma, Y.; Zhou, Q.; Lu, K. Adipokinetic hormone regulates cytochrome P450-mediated imidacloprid resistance in the brown planthopper, Nilaparvata lugens. Chemosphere 2020, 259, 127490. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, M.; Zhang, Y.; Shen, G.; Di, H.; Wang, Y.; He, L. The expression of P450 genes mediating fenpropathrin resistance is regulated by CncC and Maf in Tetranychus cinnabarinus (Boisduval). Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2017, 198, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Li, Y.; Cheng, Y.; Li, W.; Zeng, B.; Gu, C.; Zeng, R.; Song, Y. Activation of the ROS/CncC and 20-Hydroxyecdysone Signaling Pathways Is Associated with Xanthotoxin-Induced Tolerance to lambda-Cyhalothrin in Spodoptera litura. J. Agric. Food Chem. 2021, 69, 13425–13435. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mao, T.; Wang, H.; Lu, Z.; Qu, J.; Fang, Y.; Chen, J.; Li, M.; Cheng, X.; Hu, J.; et al. The CncC/keap1 pathway is activated in high temperature-induced metamorphosis and mediates the expression of Cyp450 genes in silkworm, Bombyx mori. Biochem. Biophys. Res. Commun. 2019, 514, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fu, D.; Gao, H.; Ning, H.; Sun, Y.; Chen, H.; Tang, M. Cloning and Expression of the Neuropeptide F and Neuropeptide F Receptor Genes and Their Regulation of Food Intake in the Chinese White Pine Beetle Dendroctonus armandi. Front. Physiol. 2021, 12, 662651. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Ma, M.; Wang, C.; Shi, Q.; Zhang, R.; Chen, H. Cytochrome P450s from the Chinese white pine beetle, Dendroctonus armandi (Curculionidae: Scolytinae): Expression profiles of different stages and responses to host allelochemicals. Insect Biochem. Mol. Biol. 2015, 65, 35–46. [Google Scholar] [CrossRef]
- Liu, B.; Fu, D.; Ning, H.; Tang, M.; Chen, H. Identification of the Short Neuropeptide F and Short Neuropeptide F Receptor Genes and Their Roles of Food Intake in Dendroctonus armandi. Insects 2021, 12, 844. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, B.; Fu, D.; Ning, H.; Tang, M.; Chen, H. Knockdown of CYP6CR2 and CYP6DE5 reduces tolerance to host plant allelochemicals in the Chinese white pine beetle Dendroctonus armandi. Pestic. Biochem. Physiol. 2022, 187, 105180. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fu, D.; Ning, H.; Tang, M.; Chen, H. Identification and functional characterization of the sulfakinin and sulfakinin receptor in the Chinese white pine beetle Dendroctonus armandi. Front. Physiol. 2022, 13, 927890. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Tang, M.; Chen, H. Activation of the ROS/CncC Signaling Pathway Regulates Cytochrome P450 CYP4BQ1 Responsible for (+)-α-Pinene Tolerance in Dendroctonus armandi. Int. J. Mol. Sci. 2022, 23, 11578. https://doi.org/10.3390/ijms231911578
Liu B, Tang M, Chen H. Activation of the ROS/CncC Signaling Pathway Regulates Cytochrome P450 CYP4BQ1 Responsible for (+)-α-Pinene Tolerance in Dendroctonus armandi. International Journal of Molecular Sciences. 2022; 23(19):11578. https://doi.org/10.3390/ijms231911578
Chicago/Turabian StyleLiu, Bin, Ming Tang, and Hui Chen. 2022. "Activation of the ROS/CncC Signaling Pathway Regulates Cytochrome P450 CYP4BQ1 Responsible for (+)-α-Pinene Tolerance in Dendroctonus armandi" International Journal of Molecular Sciences 23, no. 19: 11578. https://doi.org/10.3390/ijms231911578
APA StyleLiu, B., Tang, M., & Chen, H. (2022). Activation of the ROS/CncC Signaling Pathway Regulates Cytochrome P450 CYP4BQ1 Responsible for (+)-α-Pinene Tolerance in Dendroctonus armandi. International Journal of Molecular Sciences, 23(19), 11578. https://doi.org/10.3390/ijms231911578