The Medium Composition Impacts Staphylococcus aureus Biofilm Formation and Susceptibility to Antibiotics Applied in the Treatment of Bone Infections
Abstract
1. Introduction
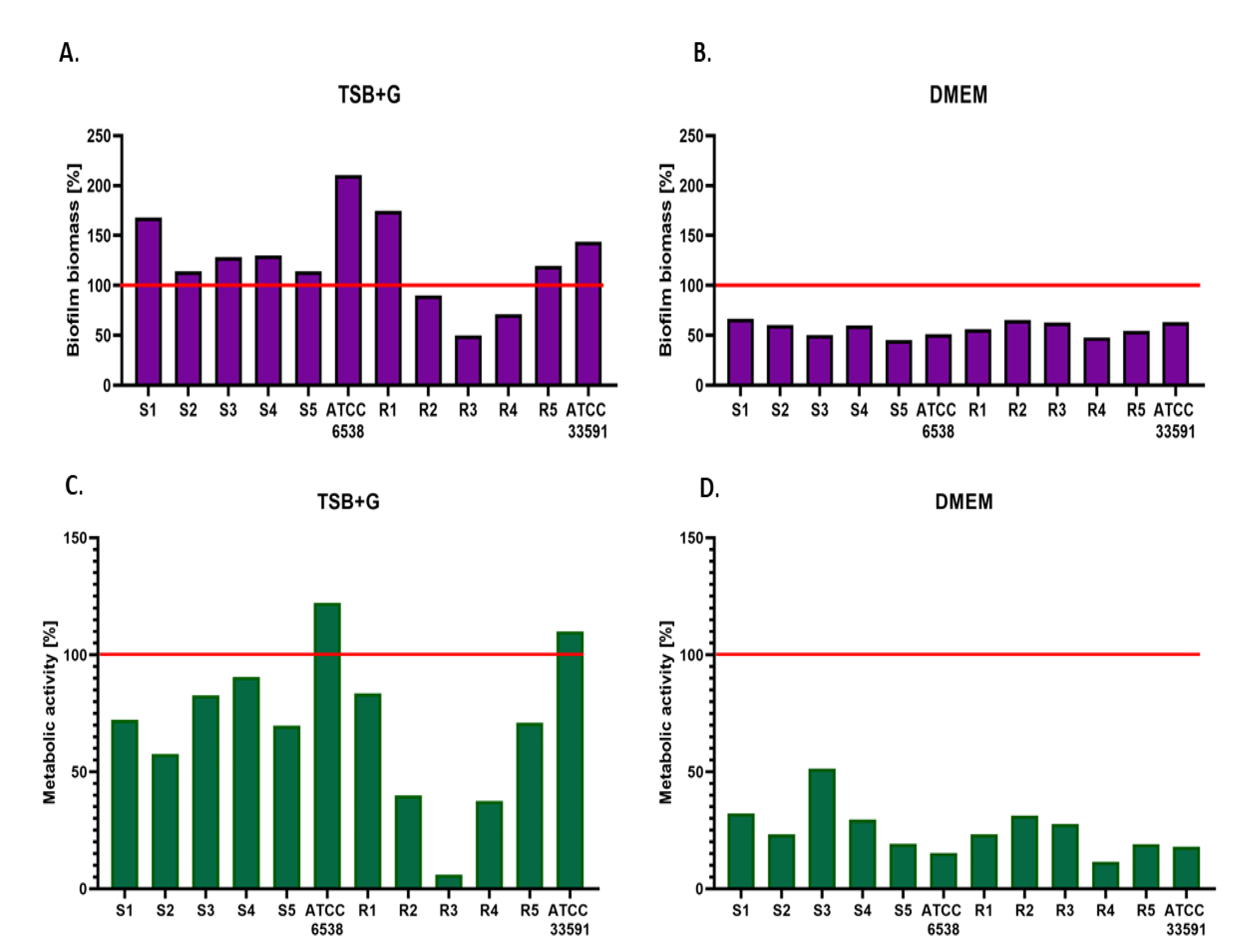
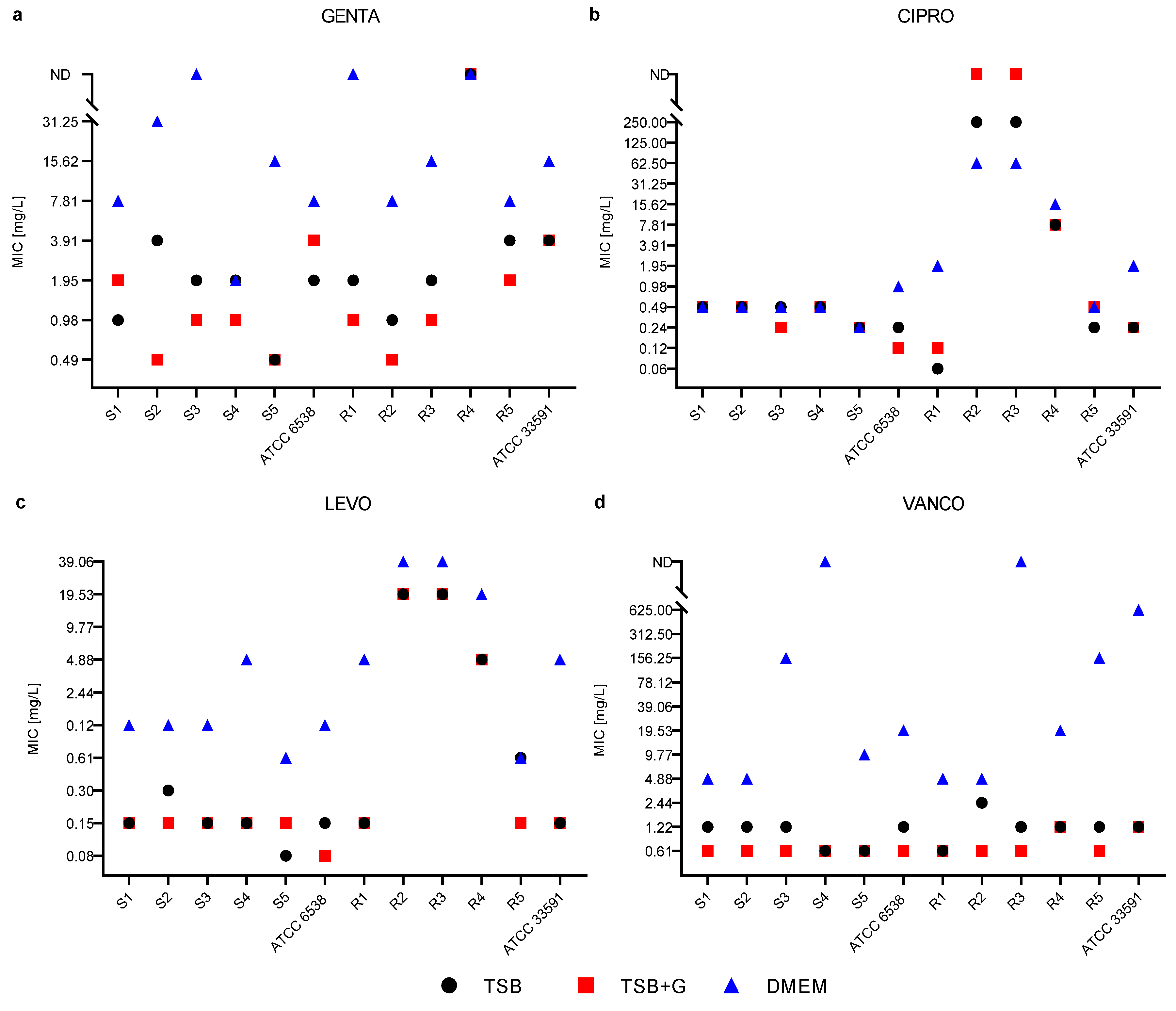
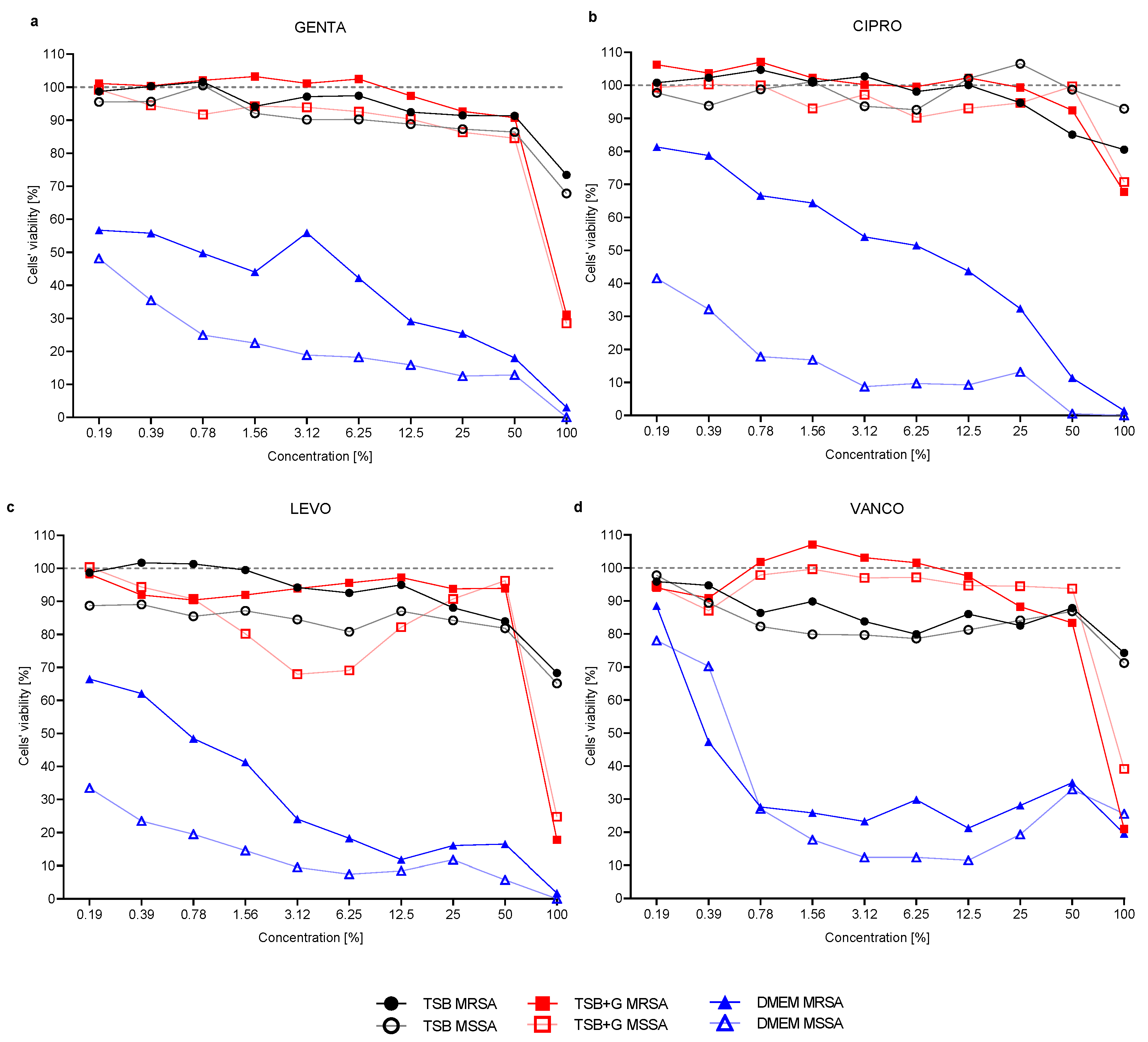
2. Results
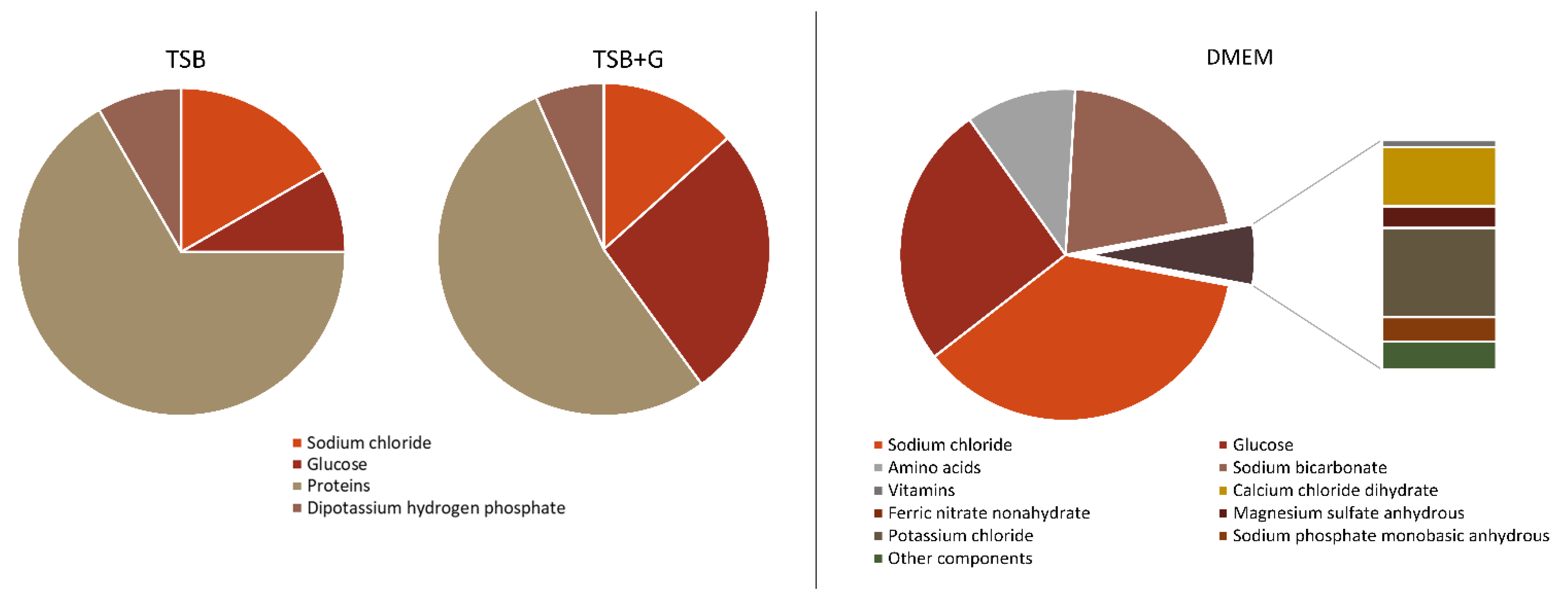
3. Discussion
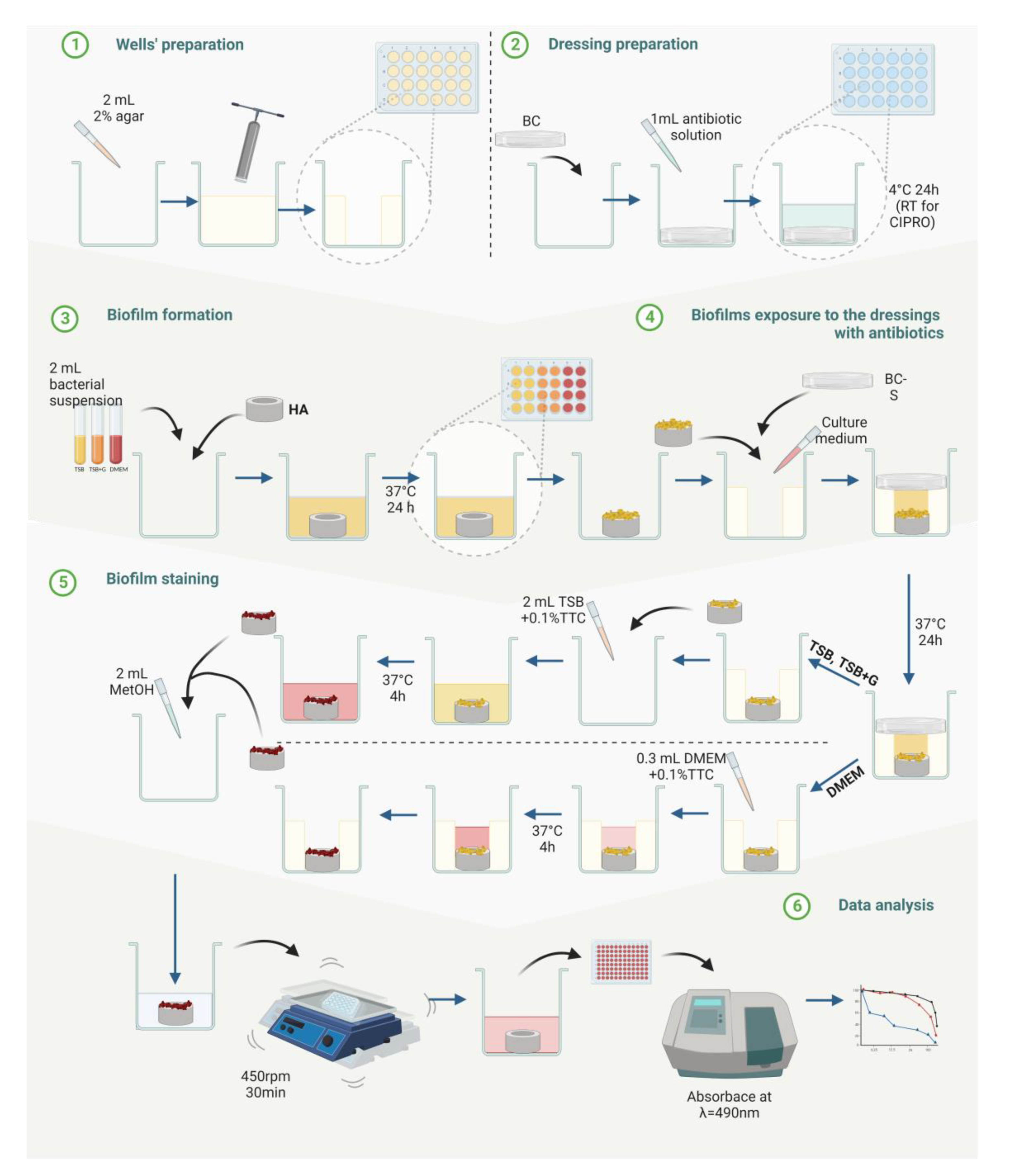
4. Materials and Methods
4.1. Tested Microorganisms
4.2. Culture Conditions
- 1.
- Tryptic Soy Broth (Biomaxima, Lublin, Poland), labeled as TSB;
- 2.
- Tryptic Soy Broth (Biomaxima, Lublin, Poland) with 1% of glucose supplementation (w/v; Chempur, Piekary Slaskie, Poland), labeled as TSB + G;
- 3.
- Dulbecco’s Modified Eagle’s Medium High Glucose (Biowest, Riverside, MO, USA; cat no. L0103) supplemented with 10% of fetal bovine serum (Biowest, Riverside, MO, USA), labeled as DMEM.
4.3. Tested Antibiotics
- 1.
- Gentamycin as gentamycin sulfate, powder for the solution of final concentration 2 g/L (Pol-Aura, Dywity, Poland), labeled as GENTA;
- 2.
- Ciprofloxacin as monohydrate ciprofloxacin hydrochloride, solution for infusion in concentration 2 g/L of the active substance (Cipronex®) (Polpharma, Starogard Gdanski, Poland), labeled as CIPRO;
- 3.
- Levofloxacin as hemihydrate levofloxacin; solution for infusion of 5 g/L active substance (Levofloxacin Kabi®) (Fresenius Kabi, Warsaw, Poland), labeled as LEVO;
- 4.
- Vancomycin as vancomycin hydrochloride; powder for infusion solution of final concentration 5 g/L (Vancomycin-MIP®) (MIP Pharma, Gdansk, Poland), labeled as VANCO.
4.4. Evaluation of Biofilm Biomass Level Using the Crystal Violet Dying Method [CV] in 96-Well Microtiter Plate
4.5. Evaluation of Biofilm Metabolic Activity Using Richard’s Method [RM] in 96-Well Microtiter Plate
4.6. Evaluation of Antimicrobial Susceptibility of Antibiotics Using Disc and Strip Diffusion Methods
4.7. Evaluation of Minimal Inhibitory Concentration [MIC] of Antibiotics Using Spectrophotometric Assessment and Richard’s Method in 96-Well Microtiter Plate
4.8. Evaluation of Minimal Biofilm Eradication Concentration [MBEC] of Antibiotics Using Spectrophotometric Assessment and Richard’s Method in 96-Well Microtiter Plate
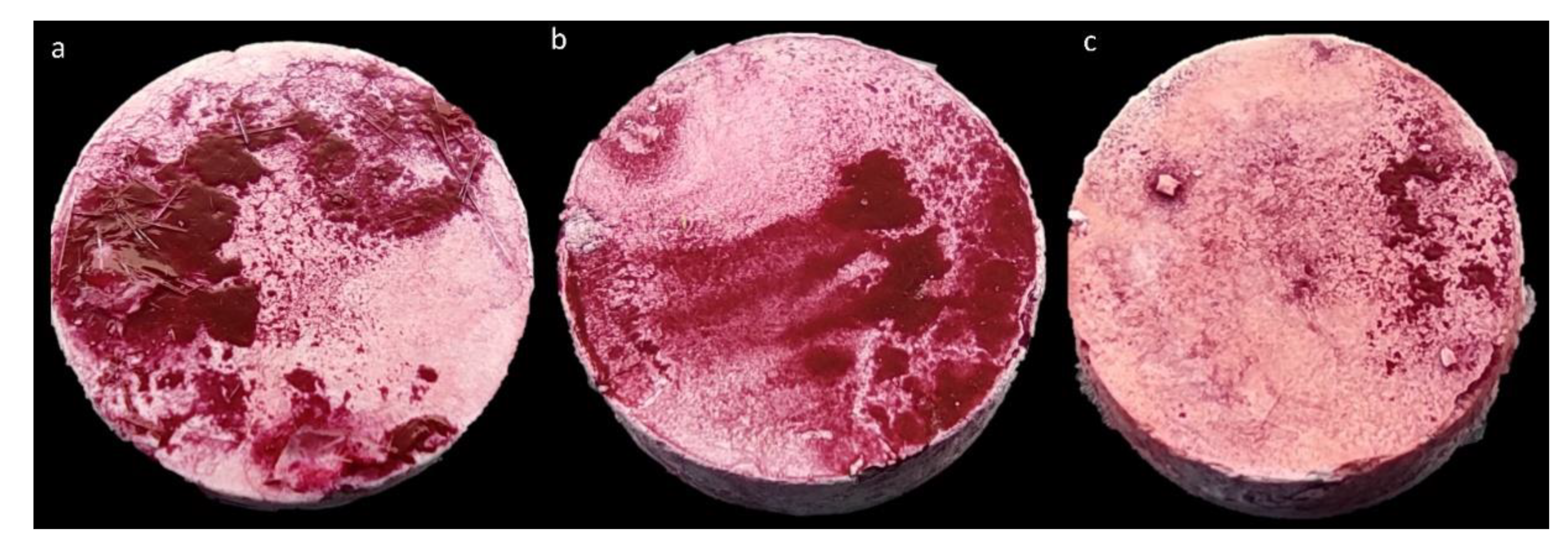
4.9. Evaluation of Biofilm Eradication from Bone Hydroxyapatite Using Modified Antibiofilm Dressing’s Activity Measurement [A.D.A.M.]
4.9.1. Preparation of Bacterial Cellulose Dressings Chemisorbed with Antibiotics
4.9.2. Preparation of Hydroxyapatite Discs Coated with Biofilm
4.9.3. Biofilm Eradication Activity of Chemisorbed Dressings
4.10. Statistical Analysis
5. Conclusions
- Staphylococcus aureus cultured in DMEM displayed lower biofilm-forming ability and decreased metabolic activity than biofilms cultured in TSB and TSB + G.
- The effectiveness of antibiotics in inhibiting growth and eradicating staphylococcal biofilm depends on the culture medium in which the bacteria are cultivated.
- It is necessary to conduct analyses on the efficacy of antibiotics in models that mimic the site of infection as closely as possible because of the large number of factors that influence their activity.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwarz, E.M.; Parvizi, J.; Gehrke, T.; Aiyer, A.; Battenberg, A.; Brown, S.A.; Callaghan, J.J.; Citak, M.; Egol, K.; Garrigues, G.E.; et al. 2018 International Consensus Meeting on Musculoskeletal Infection: Research Priorities from the General Assembly Questions. J. Orthop. Res. 2019, 37, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Kremers, H.M.; Nwojo, M.E.; Ransom, J.E.; Wood-Wentz, C.M.; Melton, L.J.; Huddleston, P.M. Trends in the Epidemiology of Osteomyelitis: A Population-Based Study, 1969 to 2009. J. Bone Jt. Surg. Am. 2015, 97, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, J.H.; Manring, M.M.; Shirtliff, M. Osteomyelitis of the Long Bones. Semin. Plast. Surg. 2009, 23, 059–072. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.A.; Trombetta, R.P.; de Mesy Bentley, K.L.; Boyce, B.F.; Gill, A.L.; Gill, S.R.; Nishitani, K.; Ishikawa, M.; Morita, Y.; Ito, H.; et al. Evolving Concepts in Bone Infection: Redefining “Biofilm”, “Acute vs. Chronic Osteomyelitis”, “the Immune Proteome” and “Local Antibiotic Therapy”. Bone Res. 2019, 7, 1–18. [Google Scholar] [CrossRef]
- Flammier, S.; Rasigade, J.-P.; Badiou, C.; Henry, T.; Vandenesch, F.; Laurent, F.; Trouillet-Assant, S. Human Monocyte-Derived Osteoclasts Are Targeted by Staphylococcal Pore-Forming Toxins and Superantigens. PLoS ONE 2016, 11, e0150693. [Google Scholar] [CrossRef]
- Jin, T.; Zhu, Y.L.; Li, J.; Shi, J.; He, X.Q.; Ding, J.; Xu, Y.Q. Staphylococcal Protein A, Panton-Valentine Leukocidin and Coagulase Aggravate the Bone Loss and Bone Destruction in Osteomyelitis. Cell Physiol. Biochem. 2013, 32, 322–333. [Google Scholar] [CrossRef]
- Trouillet-Assant, S.; Lelièvre, L.; Martins-Simões, P.; Gonzaga, L.; Tasse, J.; Valour, F.; Rasigade, J.-P.; Vandenesch, F.; Muniz Guedes, R.L.; Ribeiro de Vasconcelos, A.T.; et al. Adaptive Processes of Staphylococcus aureus Isolates during the Progression from Acute to Chronic Bone and Joint Infections in Patients. Cell. Microbiol. 2016, 18, 1405–1414. [Google Scholar] [CrossRef]
- Ahmed, S.; Meghji, S.; Williams, R.J.; Henderson, B.; Brock, J.H.; Nair, S.P. Staphylococcus aureus Fibronectin Binding Proteins Are Essential for Internalization by Osteoblasts but Do Not Account for Differences in Intracellular Levels of Bacteria. Infect. Immun. 2001, 69, 2872–2877. [Google Scholar] [CrossRef]
- Elasri, M.O.; Thomas, J.R.; Skinner, R.A.; Blevins, J.S.; Beenken, K.E.; Nelson, C.L.; Smelter, M.S. Staphylococcus aureus Collagen Adhesin Contributes to the Pathogenesis of Osteomyelitis. Bone 2002, 30, 275–280. [Google Scholar] [CrossRef]
- Vazquez, V.; Liang, X.; Horndahl, J.K.; Ganesh, V.K.; Smeds, E.; Foster, T.J.; Hook, M. Fibrinogen Is a Ligand for the Staphylococcus aureus Microbial Surface Components Recognizing Adhesive Matrix Molecules (MSCRAMM) Bone Sialoprotein-Binding Protein (Bbp). J. Biol. Chem. 2011, 286, 29797–29805. [Google Scholar] [CrossRef]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus Biofilm: A Complex Developmental Organism: Molecular Mechanisms of Staphylococcus Aureus Biofilm Development. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef]
- Wijesinghe, G.; Dilhari, A.; Gayani, B.; Kottegoda, N.; Samaranayake, L.; Weerasekera, M. Influence of Laboratory Culture Media on in Vitro Growth, Adhesion, and Biofilm Formation of Pseudomonas Aeruginosa and Staphylococcus aureus. Med. Princ. Pract. 2019, 28, 28–35. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2021, 10, 3. [Google Scholar] [CrossRef]
- Mah, T.F.; O’Toole, G.A. Mechanisms of Biofilm Resistance to Antimicrobial Agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-Resistant Staphylococcus aureus (MRSA): Antibiotic-Resistance and the Biofilm Phenotype. Med. Chem. Commun. 2019, 10, 1231–1241. [Google Scholar] [CrossRef]
- Kim, C.; Milheiriço, C.; Gardete, S.; Holmes, M.A.; Holden, M.T.G.; de Lencastre, H.; Tomasz, A. Properties of a Novel PBP2A Protein Homolog from Staphylococcus aureus Strain LGA251 and Its Contribution to the β-Lactam-Resistant Phenotype. J. Biol. Chem. 2012, 287, 36854–36863. [Google Scholar] [CrossRef]
- Morgenstern, M.; Erichsen, C.; Militz, M.; Xie, Z.; Peng, J.; Stannard, J.; Metsemakers, W.-J.; Schaefer, D.; Alt, V.; Søballe, K.; et al. The AO Trauma CPP Bone Infection Registry: Epidemiology and Outcomes of Staphylococcus aureus Bone Infection. J. Orthop. Res. 2021, 39, 136–146. [Google Scholar] [CrossRef]
- Putnam, N.E.; Fulbright, L.E.; Curry, J.M.; Ford, C.A.; Petronglo, J.R.; Hendrix, A.S.; Cassat, J.E. MyD88 and IL-1R Signaling Drive Antibacterial Immunity and Osteoclast-Driven Bone Loss during Staphylococcus aureus Osteomyelitis. PLoS Pathog. 2019, 15, e1007744. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal Infections: Mechanisms of Biofilm Maturation and Detachment as Critical Determinants of Pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef]
- Hasanzadeh, A.; Gholipour, B.; Rostamnia, S.; Eftekhari, A.; Tanomand, A.; Valizadeh, K.A.; Khaksar, S.; Khalilov, R. Biosynthesis of AgNPs onto the Urea-Based Periodic Mesoporous Organosilica (AgxNPs/Ur-PMO) for Antibacterial and Cell Viability Assay. J. Colloid Interface Sci. 2021, 585, 676–683. [Google Scholar] [CrossRef]
- Negahdari, R.; Sharifi, S.; Ghavimi, M.A.; Memar, M.Y.; Khaneshi, B.; Maleki Dizaj, S.; Eftekhari, A.; Cucchiarini, M. Curcumin Nanocrystals: Production, Physicochemical Assessment, and In Vitro Evaluation of the Antimicrobial Effects against Bacterial Loading of the Implant Fixture. Appl. Sci. 2020, 10, 8356. [Google Scholar] [CrossRef]
- Alves, C.; Mendes, D.; Marques, F.B. Fluoroquinolones and the Risk of Tendon Injury: A Systematic Review and Meta-Analysis. Eur. J. Clin. Pharmacol. 2019, 75, 1431–1443. [Google Scholar] [CrossRef]
- Bruniera, F.R.; Ferreira, F.M.; Saviolli, L.R.M.; Bacci, M.R.; Feder, D.; da Luz Gonçalves Pedreira, M.; Sorgini Peterlini, M.A.; Azzalis, L.A.; Campos Junqueira, V.B.; Fonseca, F.L.A. The Use of Vancomycin with Its Therapeutic and Adverse Effects: A Review. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 694–700. [Google Scholar]
- Gorelik, E.; Masarwa, R.; Perlman, A.; Rotshild, V.; Abbasi, M.; Muszkat, M.; Matok, I. Fluoroquinolones and Cardiovascular Risk: A Systematic Review, Meta-Analysis and Network Meta-Analysis. Drug Saf. 2019, 42, 529–538. [Google Scholar] [CrossRef]
- Hayward, R.S.; Harding, J.; Molloy, R.; Land, L.; Longcroft-Neal, K.; Moore, D.; Ross, J.D.C. Adverse Effects of a Single Dose of Gentamicin in Adults: A Systematic Review. Br. J. Clin. Pharmacol. 2018, 84, 223–238. [Google Scholar] [CrossRef]
- Caplin, J.D.; García, A.J. Implantable Antimicrobial Biomaterials for Local Drug Delivery in Bone Infection Models. Acta Biomater. 2019, 93, 2–11. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Lozano, D.; González, B.; Izquierdo-Barba, I. Biomaterials against Bone Infection. Adv. Healthc. Mater. 2020, 9, 2000310. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.; Wu, P.; Chen, B. Update on New Medicinal Applications of Gentamicin: Evidence-Based Review. J. Formos. Med. Assoc. 2014, 113, 72–82. [Google Scholar] [CrossRef]
- Maczynska, B.; Secewicz, A.; Smutnicka, D.; Szymczyk, P.; Dudek-Wicher, R.; Junka, A.; Bartoszewicz, M. In Vitro Efficacy of Gentamicin Released from Collagen Sponge in Eradication of Bacterial Biofilm Preformed on Hydroxyapatite Surface. PLoS ONE 2019, 14, e0217769. [Google Scholar] [CrossRef]
- Ojkic, N.; Lilja, E.; Direito, S.; Dawson, A.; Allen, R.J.; Waclaw, B. A Roadblock-and-Kill Mechanism of Action Model for the DNA-Targeting Antibiotic Ciprofloxacin. Antimicrob. Agents Chemother. 2020, 64, e02487-19. [Google Scholar] [CrossRef]
- Hurst, M.; Lamb, H.M.; Scott, L.J.; Figgitt, D.P. Levofloxacin. Drugs 2002, 62, 2127–2167. [Google Scholar] [CrossRef] [PubMed]
- Milatovic, D.; Schmitz, F.-J.; Brisse, S.; Verhoef, J.; Fluit, A.C. In Vitro Activities of Sitafloxacin (DU-6859a) and Six Other Fluoroquinolones against 8796 Clinical Bacterial Isolates. Antimicrob. Agents Chemother. 2000, 44, 1102–1107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Peng, H.; Rao, X. Molecular Events for Promotion of Vancomycin Resistance in Vancomycin Intermediate Staphylococcus aureus. Front. Microbiol. 2016, 7, 1601. [Google Scholar] [CrossRef] [PubMed]
- Valour, F.; Trouillet-Assant, S.; Riffard, N.; Tasse, J.; Flammier, S.; Rasigade, J.-P.; Chidiac, C.; Vandenesch, F.; Ferry, T.; Laurent, F. Antimicrobial Activity against Intraosteoblastic Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Lipsky, B.A. Systemic Antibiotic Therapy for Chronic Osteomyelitis in Adults. Clin. Infect. Dis. 2012, 54, 393–407. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Disk Diffusion Test Methodology. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2022_manuals/Manual_v_10.0_EUCAST_Disk_Test_2022.pdf (accessed on 1 January 2022).
- Veis, D.J.; Cassat, J.E. Infectious Osteomyelitis: Marrying Bone Biology and Microbiology to Shed New Light on a Persistent Clinical Challenge. J. Bone Miner. Res. 2021, 36, 636–643. [Google Scholar] [CrossRef]
- Meeker, D.G.; Beenken, K.E.; Mills, W.B.; Loughran, A.J.; Spencer, H.J.; Lynn, W.B.; Smeltzer, M.S. Evaluation of Antibiotics Active against Methicillin-Resistant Staphylococcus aureus Based on Activity in an Established Biofilm. Antimicrob. Agents Chemother. 2016, 60, 5688–5694. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Pourhajibagher, M.; Chiniforush, N.; Soltanian, A.R.; Alikhani, M.Y.; Bahador, A. Biofilm Formation and Antibiotic Resistance in Meticillin-Resistant and Meticillin-Sensitive Staphylococcus aureus Isolated from Burns. J. Wound Care 2019, 28, 66–73. [Google Scholar] [CrossRef]
- Junka, A.; Wojtowicz, W.; Ząbek, A.; Krasowski, G.; Smutnicka, D.; Bakalorz, B.; Boruta, A.; Dziadas, M.; Młynarz, P.; Sedghizadeh, P.P.; et al. Metabolic Profiles of Exudates from Chronic Leg Ulcerations. J. Pharm. Biomed. Anal. 2017, 137, 13–22. [Google Scholar] [CrossRef]
- Power, G.; Moore, Z.; O’Connor, T. Measurement of PH, Exudate Composition and Temperature in Wound Healing: A Systematic Review. J. Wound Care 2017, 26, 381–397. [Google Scholar] [CrossRef]
- Paleczny, J.; Junka, A.; Brożyna, M.; Dydak, K.; Oleksy-Wawrzyniak, M.; Ciecholewska-Juśko, D.; Dziedzic, E.; Bartoszewicz, M. The High Impact of Staphylococcus aureus Biofilm Culture Medium on In Vitro Outcomes of Antimicrobial Activity of Wound Antiseptics and Antibiotic. Pathogens 2021, 10, 1385. [Google Scholar] [CrossRef]
- Nishitani, K.; Sutipornpalangkul, W.; de Mesy Bentley, K.L.; Varrone, J.J.; Bello-Irizarry, S.N.; Ito, H.; Matsuda, S.; Kates, S.L.; Daiss, J.L.; Schwarz, E.M. Quantifying the Natural History of Biofilm Formation in Vivo during the Establishment of Chronic Implant-Associated Staphylococcus aureus Osteomyelitis in Mice to Identify Critical Pathogen and Host Factors. J. Orthop. Res. 2015, 33, 1311–1319. [Google Scholar] [CrossRef]
- Reizner, W.; Hunter, J.G.; O’Malley, N.T.; Southgate, R.D.; Schwarz, E.M.; Kates, S.L. A Systematic Review of Animal Models for Staphylococcus aureus Osteomyelitis. Eur. Cell Mater. 2014, 27, 196–212. [Google Scholar] [CrossRef]
- Miao, J.; Lin, S.; Soteyome, T.; Peters, B.M.; Li, Y.; Chen, H.; Su, J.; Li, L.; Li, B.; Xu, Z.; et al. Biofilm Formation of Staphylococcus aureus under Food Heat Processing Conditions: First Report on CML Production within Biofilm. Sci. Rep. 2019, 9, 1312. [Google Scholar] [CrossRef]
- Foulston, L.; Elsholz, A.K.W.; DeFrancesco, A.S.; Losick, R. The Extracellular Matrix of Staphylococcus aureus Biofilms Comprises Cytoplasmic Proteins That Associate with the Cell Surface in Response to Decreasing PH. mBio 2014, 5, e01667-14. [Google Scholar] [CrossRef]
- Reffuveille, F.; Josse, J.; Velard, F.; Lamret, F.; Varin-Simon, J.; Dubus, M.; Haney, E.F.; Hancock, R.E.W.; Mongaret, C.; Gangloff, S.C. Bone Environment Influences Irreversible Adhesion of a Methicillin-Susceptible Staphylococcus aureus Strain. Front. Microbiol. 2018, 9, 2865. [Google Scholar] [CrossRef]
- Waldrop, R.; McLaren, A.; Bse, F.C.; McLemore, R. Biofilm Growth Has a Threshold Response to Glucose in Vitro. Clin. Orthop. Relat. Res. 2014, 472, 3305–3310. [Google Scholar] [CrossRef]
- Suzuki, A.; Iwata, J. Amino Acid Metabolism and Autophagy in Skeletal Development and Homeostasis. Bone 2021, 146, 115881. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Kolodkin-Gal, I.; Foulston, L.; Kolter, R.; Aizenberg, J.; Losick, R. Inhibitory Effects of D-Amino Acids on Staphylococcus aureus Biofilm Development. J. Bacteriol. 2011, 193, 5616–5622. [Google Scholar] [CrossRef]
- Huang, L.; Lou, Y.; Zhang, D.; Ma, L.; Qian, H.; Hu, Y.; Ju, P.; Xu, D.; Li, X. D-Cysteine Functionalised Silver Nanoparticles Surface with a “Disperse-Then-Kill” Antibacterial Synergy. Chem. Eng. J. 2020, 381, 122662. [Google Scholar] [CrossRef]
- Shukla, S.K.; Rao, T.S. Effect of Calcium on Staphylococcus aureus Biofilm Architecture: A Confocal Laser Scanning Microscopic Study. Colloids Surf. B Biointerfaces 2013, 103, 448–454. [Google Scholar] [CrossRef]
- Abraham, N.M.; Lamlertthon, S.; Fowler, V.G.; Jefferson, K.K. Chelating Agents Exert Distinct Effects on Biofilm Formation in Staphylococcus aureus Depending on Strain Background: Role for Clumping Factor B. J. Med. Microbiol. 2012, 61, 1062–1070. [Google Scholar] [CrossRef]
- Taglialegna, A.; Navarro, S.; Ventura, S.; Garnett, J.A.; Matthews, S.; Penades, J.R.; Lasa, I.; Valle, J. Staphylococcal Bap Proteins Build Amyloid Scaffold Biofilm Matrices in Response to Environmental Signals. PLoS Pathog. 2016, 12, e1005711. [Google Scholar] [CrossRef]
- Dorschner, R.A.; Lopez-Garcia, B.; Peschel, A.; Kraus, D.; Morikawa, K.; Nizet, V.; Gallo, R.L. The Mammalian Ionic Environment Dictates Microbial Susceptibility to Antimicrobial Defense Peptides. FASEB J. 2006, 20, 35–42. [Google Scholar] [CrossRef]
- Jaikumpun, P.; Ruksakiet, K.; Stercz, B.; Pállinger, É.; Steward, M.; Lohinai, Z.; Dobay, O.; Zsembery, Á. Antibacterial Effects of Bicarbonate in Media Modified to Mimic Cystic Fibrosis Sputum. Int. J. Mol. Sci. 2020, 21, 8614. [Google Scholar] [CrossRef]
- Dobay, O.; Laub, K.; Stercz, B.; Kéri, A.; Balázs, B.; Tóthpál, A.; Kardos, S.; Jaikumpun, P.; Ruksakiet, K.; Quinton, P.M.; et al. Bicarbonate Inhibits Bacterial Growth and Biofilm Formation of Prevalent Cystic Fibrosis Pathogens. Front. Microbiol. 2018, 9, 2245. [Google Scholar] [CrossRef]
- Piątkowska, E.; Paleczny, J.; Dydak, K.; Letachowicz, K. Antimicrobial Activity of Hemodialysis Catheter Lock Solutions in Relation to Other Compounds with Antiseptic Properties. PLoS ONE 2021, 16, e0258148. [Google Scholar] [CrossRef]
- Senobar Tahaei, S.A.; Stájer, A.; Barrak, I.; Ostorházi, E.; Szabó, D.; Gajdács, M. Correlation Between Biofilm-Formation and the Antibiotic Resistant Phenotype in Staphylococcus aureus Isolates: A Laboratory-Based Study in Hungary and a Review of the Literature. Infect. Drug Resist. 2021, 14, 1155–1168. [Google Scholar] [CrossRef]
- Martí, M.; Trotonda, M.P.; Tormo-Más, M.Á.; Vergara-Irigaray, M.; Cheung, A.L.; Lasa, I.; Penadés, J.R. Extracellular Proteases Inhibit Protein-Dependent Biofilm Formation in Staphylococcus aureus. Microbes Infect. 2010, 12, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liang, Y.; Lin, S.; Chen, D.; Li, B.; Li, L.; Deng, Y. Crystal Violet and XTT Assays on Staphylococcus aureus Biofilm Quantification. Curr. Microbiol. 2016, 73, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Digranes, A.; Dibb, W.L.; Benonisen, E.; Ostervold, B. The in Vitro Activity of Gentamicin, Tobramycin and Netilmicin against 500 Clinical Isolates of Bacteria. A Comparative Study Using Three Different Test Media. Acta Pathol. Microbiol. Immunol. Scand. B 1983, 91, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Bock, L.J.; Hind, C.K.; Sutton, J.M.; Wand, M.E. Growth Media and Assay Plate Material Can Impact on the Effectiveness of Cationic Biocides and Antibiotics against Different Bacterial Species. Lett. Appl. Microbiol. 2018, 66, 368–377. [Google Scholar] [CrossRef]
- Nanavaty, J.; Mortensen, J.E.; Shryock, T.R. The Effects of Environmental Conditions on the in Vitro Activity of Selected Antimicrobial Agents against Escherichia coli. Curr. Microbiol. 1998, 36, 212–215. [Google Scholar] [CrossRef]
- Walsh, T.R.; Bolmström, A.; Qwärnström, A.; Ho, P.; Wootton, M.; Howe, R.A.; MacGowan, A.P.; Diekema, D. Evaluation of Current Methods for Detection of Staphylococci with Reduced Susceptibility to Glycopeptides. J. Clin. Microbiol. 2001, 39, 2439–2444. [Google Scholar] [CrossRef]
- Girard, L.P.; Ceri, H.; Gibb, A.P.; Olson, M.; Sepandj, F. MIC versus MBEC to Determine the Antibiotic Sensitivity of Staphylococcus aureus in Peritoneal Dialysis Peritonitis. Perit. Dial. Int. 2010, 30, 652–656. [Google Scholar] [CrossRef]
- Chen, X.; Thomsen, T.R.; Winkler, H.; Xu, Y. Influence of Biofilm Growth Age, Media, Antibiotic Concentration and Exposure Time on Staphylococcus aureus and Pseudomonas Aeruginosa Biofilm Removal in Vitro. BMC Microbiol. 2020, 20, 264. [Google Scholar] [CrossRef]
- Junka, A.F.; Żywicka, A.; Szymczyk, P.; Dziadas, M.; Bartoszewicz, M.; Fijałkowski, K.A.D.A.M. Test (Antibiofilm Dressing’s Activity Measurement)—Simple Method for Evaluating Anti-Biofilm Activity of Drug-Saturated Dressings against Wound Pathogens. J. Microbiol. Methods 2017, 143, 6–12. [Google Scholar] [CrossRef]
- Krasowski, G.; Wicher-Dudek, R.; Paleczny, J.; Bil-Lula, I.; Fijałkowski, K.; Sedghizadeh, P.P.; Szymczyk, P.; Dudek, B.; Bartoszewicz, M.; Junka, A. Potential of Novel Bacterial Cellulose Dressings Chemisorbed with Antiseptics for the Treatment of Oral Biofilm Infections. Appl. Sci. 2019, 9, 5321. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 12.0. 2022. Available online: https://eucast.org/clinical_breakpoints/ (accessed on 1 January 2022).
- Ciechanska, D.; Wietecha, J.; Kaźmierczak, D.; Kazimierczak, J. Biosynthesis of Modified Bacterial Cellulose in a Tubular Form. Fibres Text. East. Eur. 2010, 82, 98–104. [Google Scholar]
- Krzyżek, P.; Gościniak, G.; Fijałkowski, K.; Migdał, P.; Dziadas, M.; Owczarek, A.; Czajkowska, J.; Aniołek, O.; Junka, A. Potential of Bacterial Cellulose Chemisorbed with Anti-Metabolites, 3-Bromopyruvate or Sertraline, to Fight against Helicobacter Pylori Lawn Biofilm. Int. J. Mol. Sci. 2020, 21, 9507. [Google Scholar] [CrossRef]
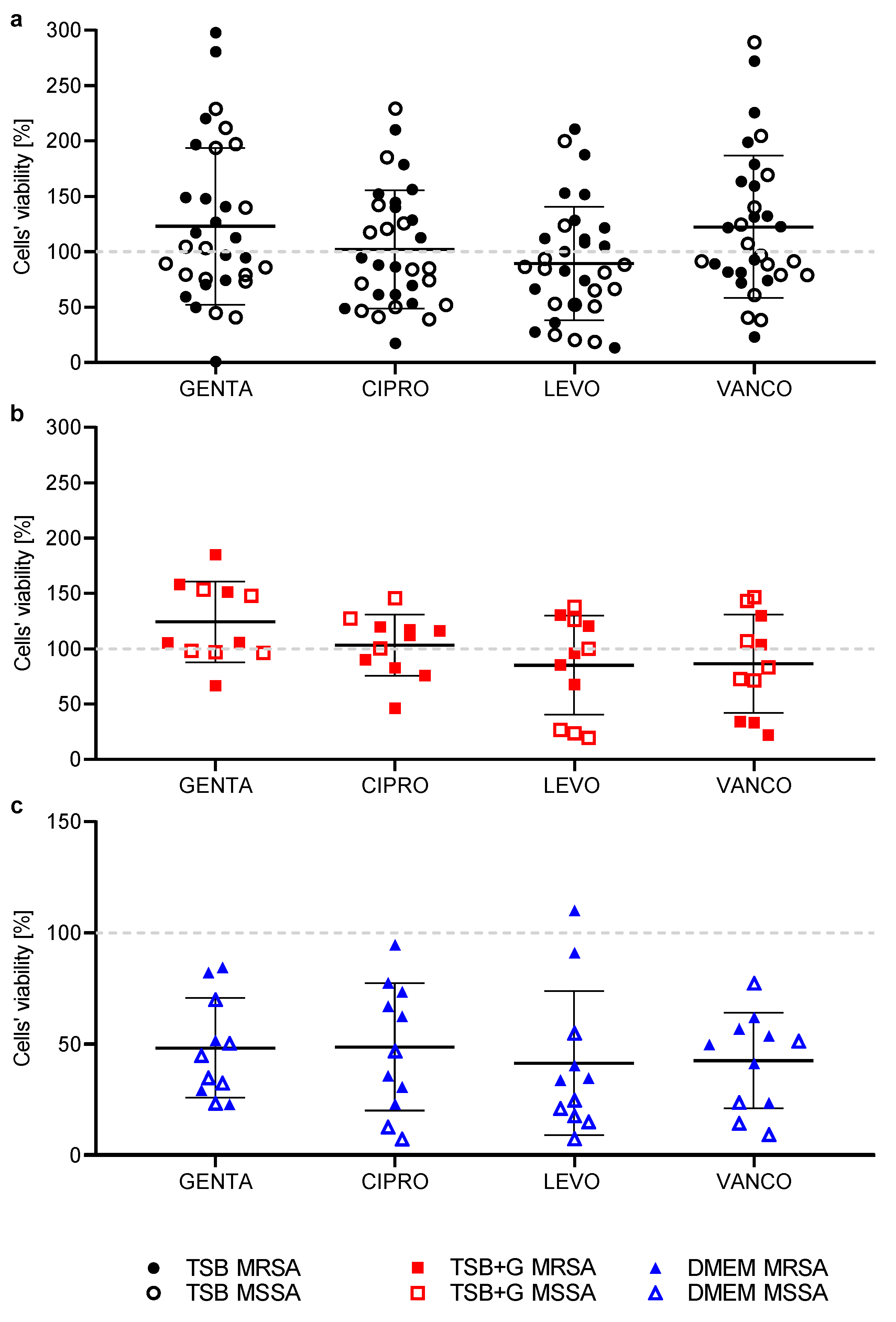
| Crystal Violet vs. Richard’s Methods Correlations | |||
|---|---|---|---|
| Tested Group | TSB | TSB + G | DMEM |
| All strains | p = 0.454 | p < 0.0001 | p < 0.0001 |
| r = 0.82 | r = 0.61 | ||
| MRSA | p = 0.307 | p < 0.0001 | p = 0.003 |
| r = 0.87 | r = 0.66 | ||
| MSSA | p = 0.951 | p = 0.003 | p = 0.027 |
| r = 0.66 | r = 0.52 | ||
| Substance | Comparison of MIC Values in a Specific Medium | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TSB vs. TSB + G | TSB | vs. | DMEM | p | TSB + G | vs. | DMEM | p | |
| GENTA | ns | ↓ | ↑ | * | ↓ | ↑ | *** | ||
| CIPRO | ns | ns | ns | ns | ns | ns | ns | ||
| LEVO | ns | ↓ | ↑ | * | ↓ | ↑ | * | ||
| VANCO | ns | ↓ | ↑ | * | ↓ | ↑ | *** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paleczny, J.; Brożyna, M.; Dudek-Wicher, R.; Dydak, K.; Oleksy-Wawrzyniak, M.; Madziała, M.; Bartoszewicz, M.; Junka, A. The Medium Composition Impacts Staphylococcus aureus Biofilm Formation and Susceptibility to Antibiotics Applied in the Treatment of Bone Infections. Int. J. Mol. Sci. 2022, 23, 11564. https://doi.org/10.3390/ijms231911564
Paleczny J, Brożyna M, Dudek-Wicher R, Dydak K, Oleksy-Wawrzyniak M, Madziała M, Bartoszewicz M, Junka A. The Medium Composition Impacts Staphylococcus aureus Biofilm Formation and Susceptibility to Antibiotics Applied in the Treatment of Bone Infections. International Journal of Molecular Sciences. 2022; 23(19):11564. https://doi.org/10.3390/ijms231911564
Chicago/Turabian StylePaleczny, Justyna, Malwina Brożyna, Ruth Dudek-Wicher, Karolina Dydak, Monika Oleksy-Wawrzyniak, Marcin Madziała, Marzenna Bartoszewicz, and Adam Junka. 2022. "The Medium Composition Impacts Staphylococcus aureus Biofilm Formation and Susceptibility to Antibiotics Applied in the Treatment of Bone Infections" International Journal of Molecular Sciences 23, no. 19: 11564. https://doi.org/10.3390/ijms231911564
APA StylePaleczny, J., Brożyna, M., Dudek-Wicher, R., Dydak, K., Oleksy-Wawrzyniak, M., Madziała, M., Bartoszewicz, M., & Junka, A. (2022). The Medium Composition Impacts Staphylococcus aureus Biofilm Formation and Susceptibility to Antibiotics Applied in the Treatment of Bone Infections. International Journal of Molecular Sciences, 23(19), 11564. https://doi.org/10.3390/ijms231911564








