Growth Hormone (GH) Crosses the Blood–Brain Barrier (BBB) and Induces Neuroprotective Effects in the Embryonic Chicken Cerebellum after a Hypoxic Injury
Abstract
1. Introduction
2. Results
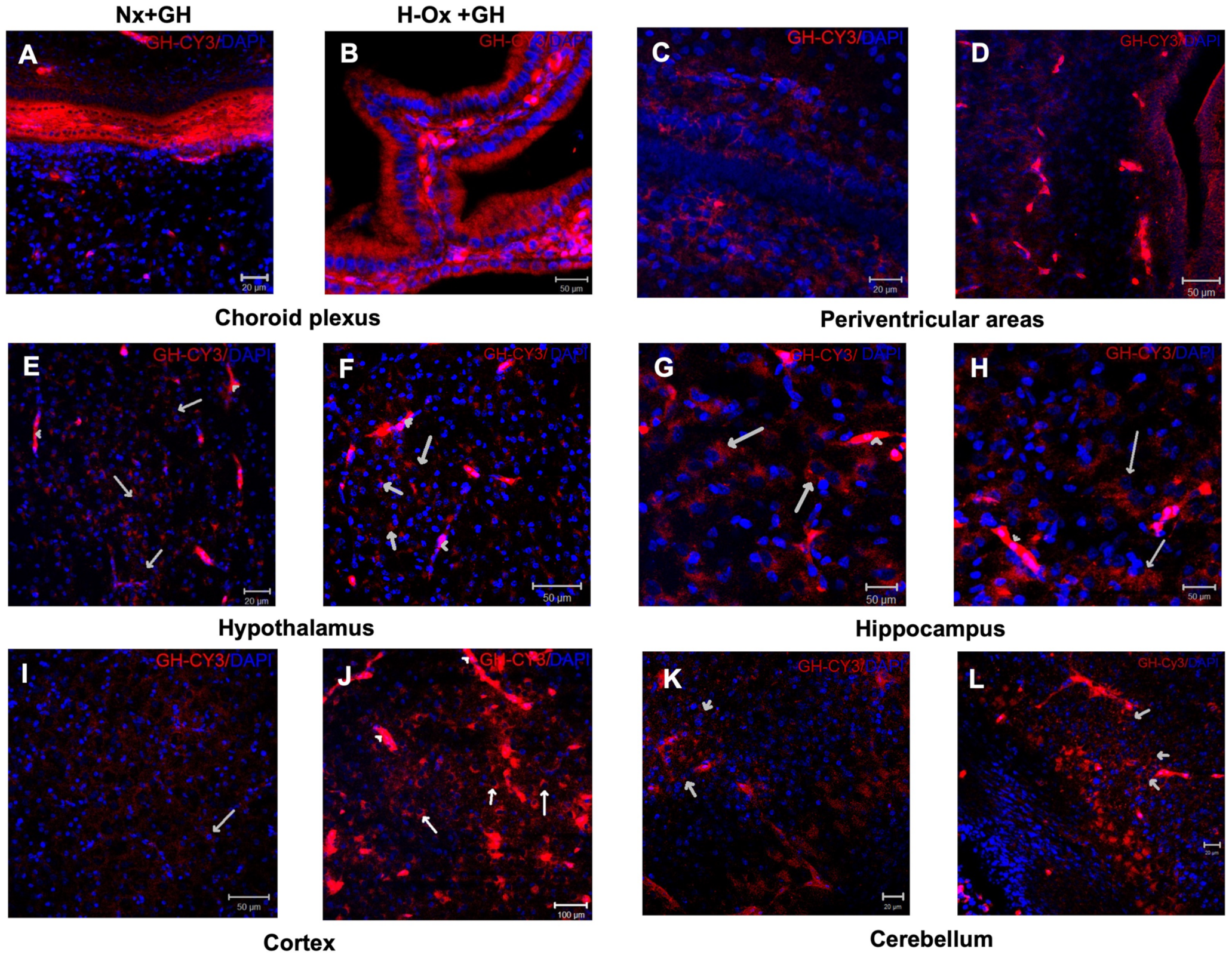
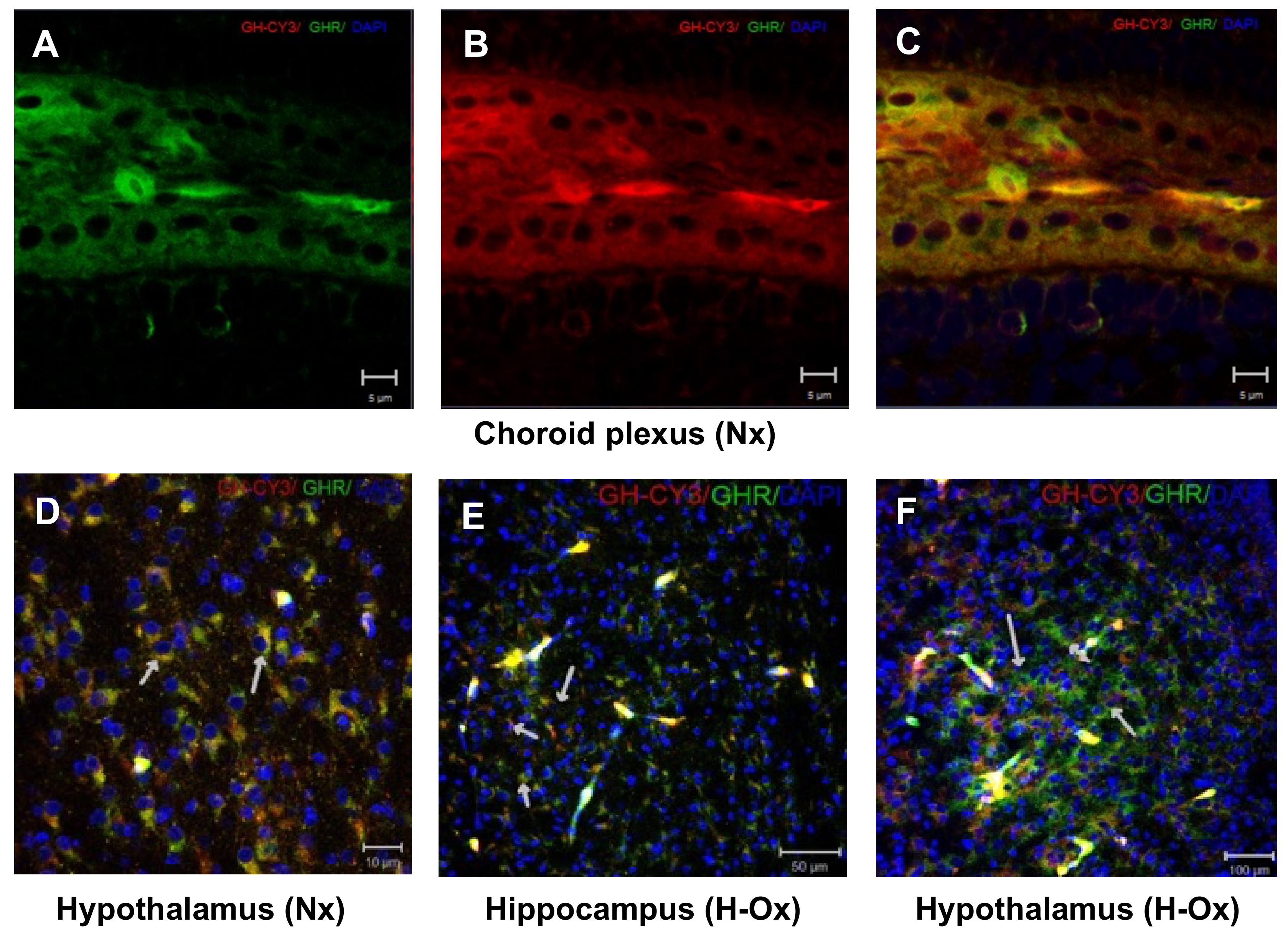
2.1. Distribution of Cy3-GH in the Chicken Brain Exposed to Hypoxia
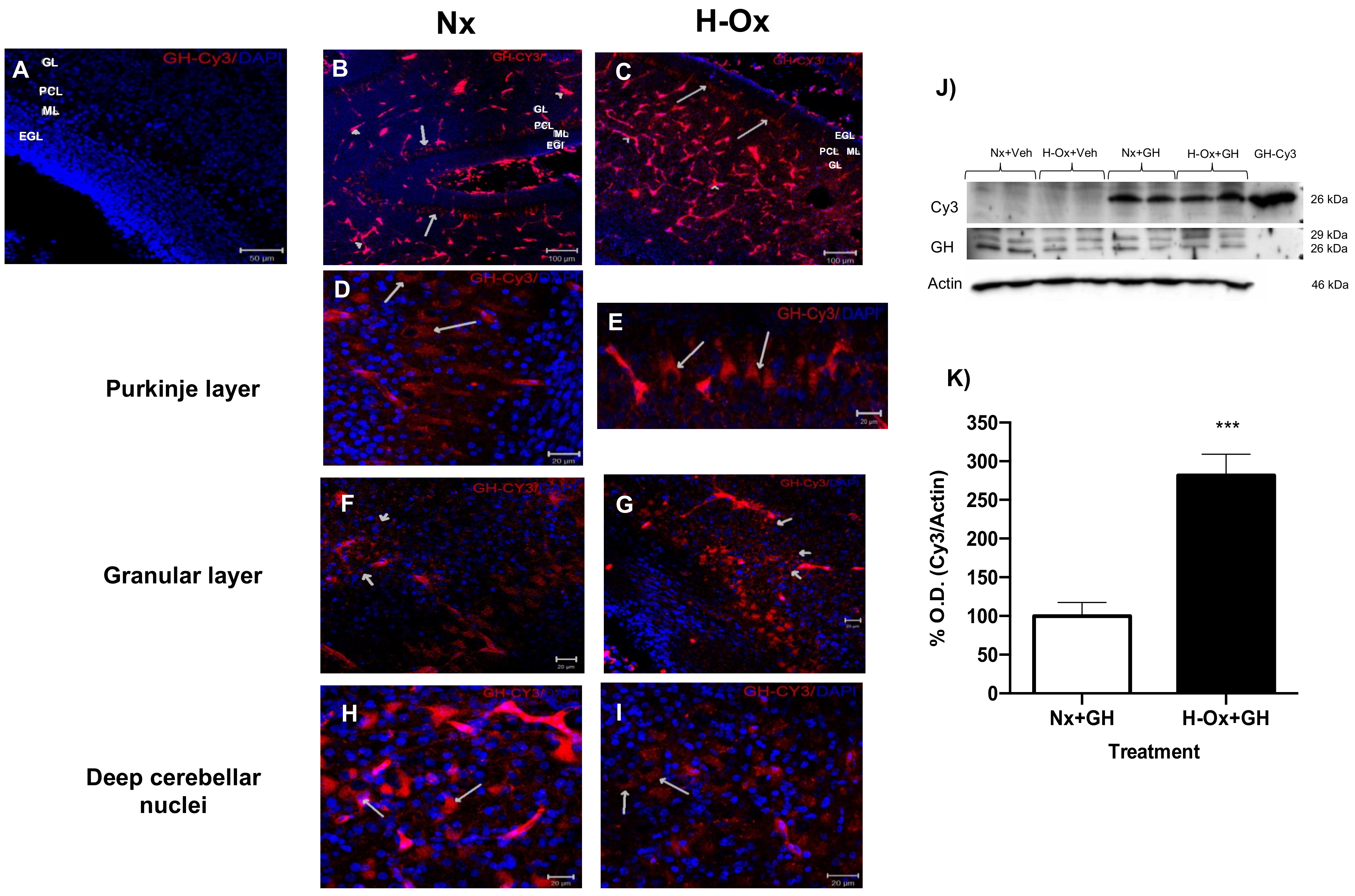
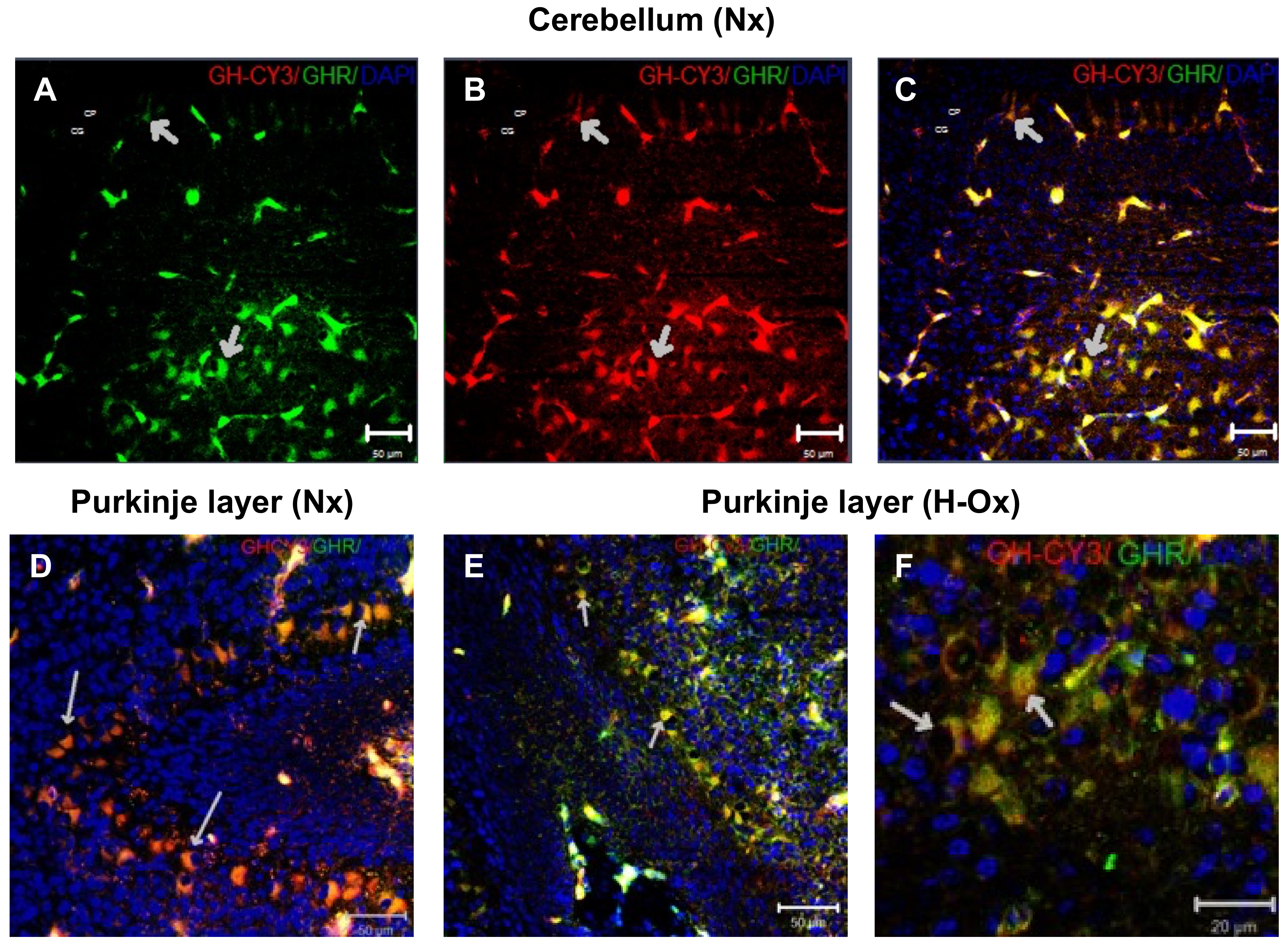
2.2. Distribution of Cy3-GH in the Hypoxic Cerebellum Strata
2.3. Effects of Hypoxia and GH Treatment on Cerebellar Cytostructure
2.4. Neuroprotective Actions of GH in the Hypoxic Cerebellum
2.5. Effect of GH upon Lipid Peroxidation and iNOS Gene Expression
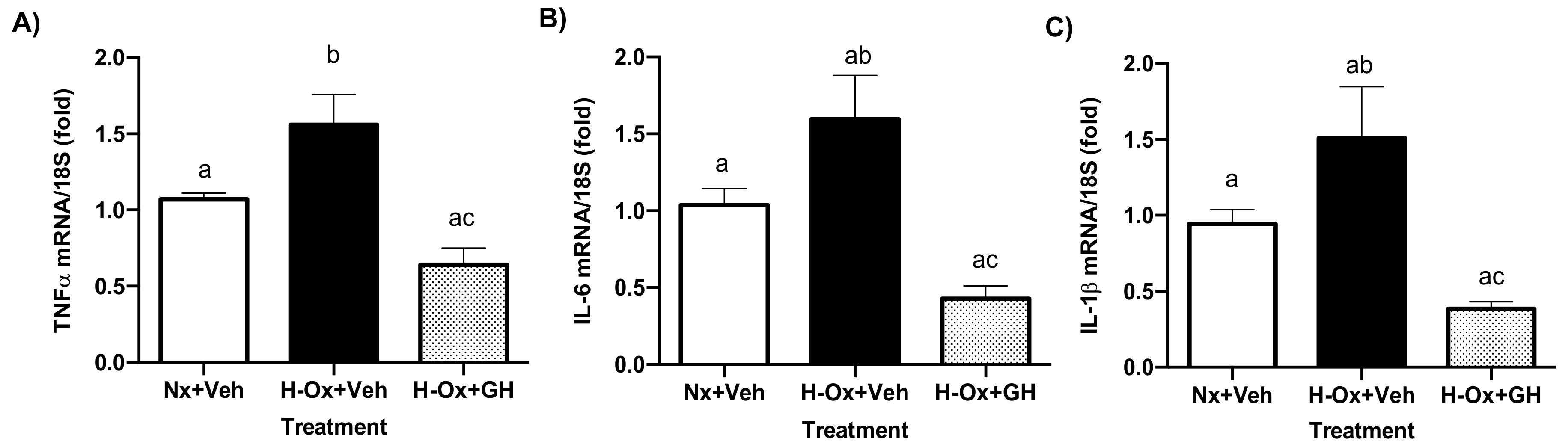
2.6. GH Suppressed Hypoxia-Induced mRNA Expression Levels of Pro-Inflammatory Mediators in the Cerebellum
2.7. Differential Modulation of Neurotrophic Factors in Response to GH Treatment in Hypoxic Cerebellum Injury
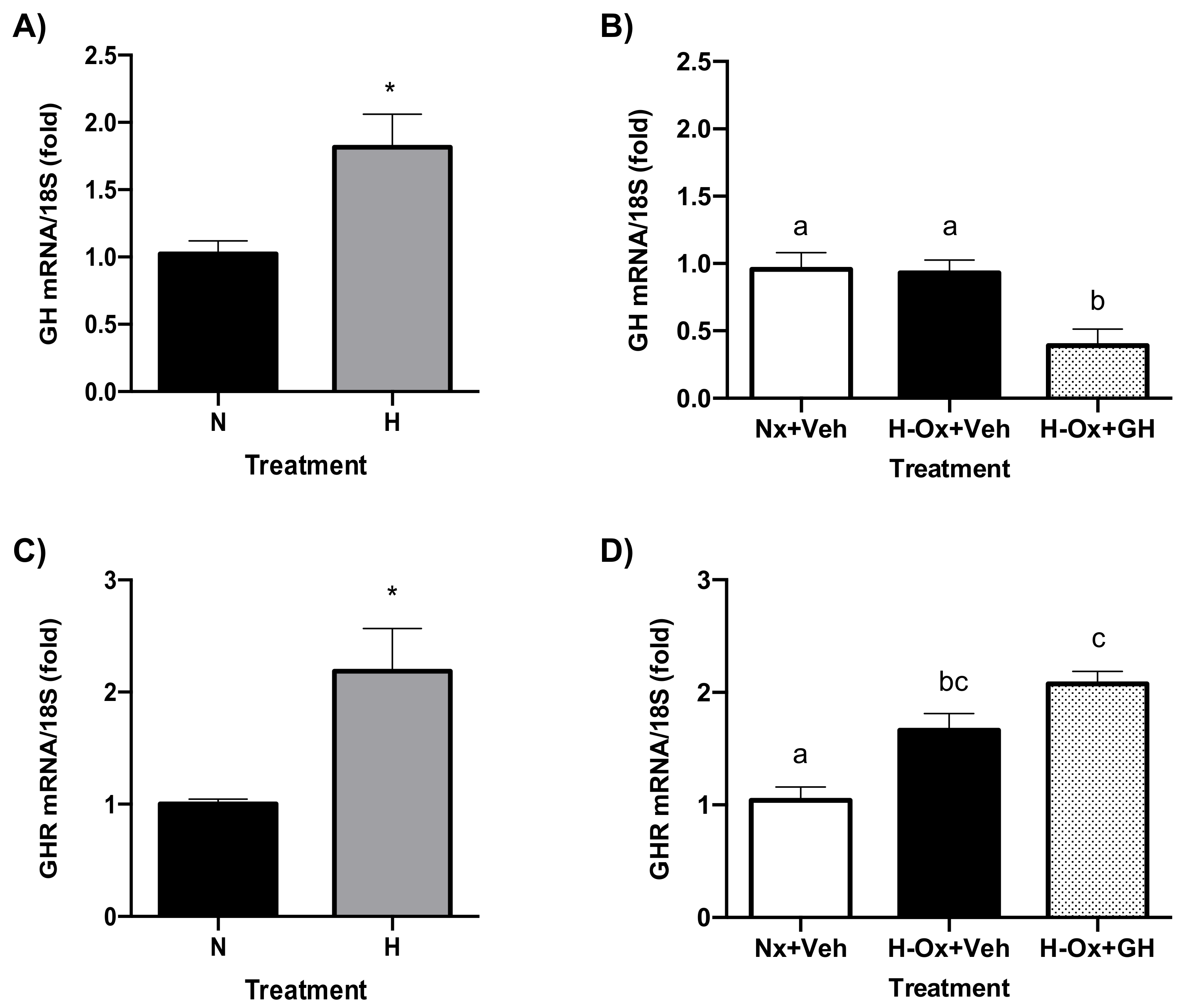
2.8. Endogenous GH and GHR Expression in Hypoxia Cerebellum Injury
2.9. Endogenous GH and GHR Expression in Hypoxia Cerebellum Injury
3. Discussion
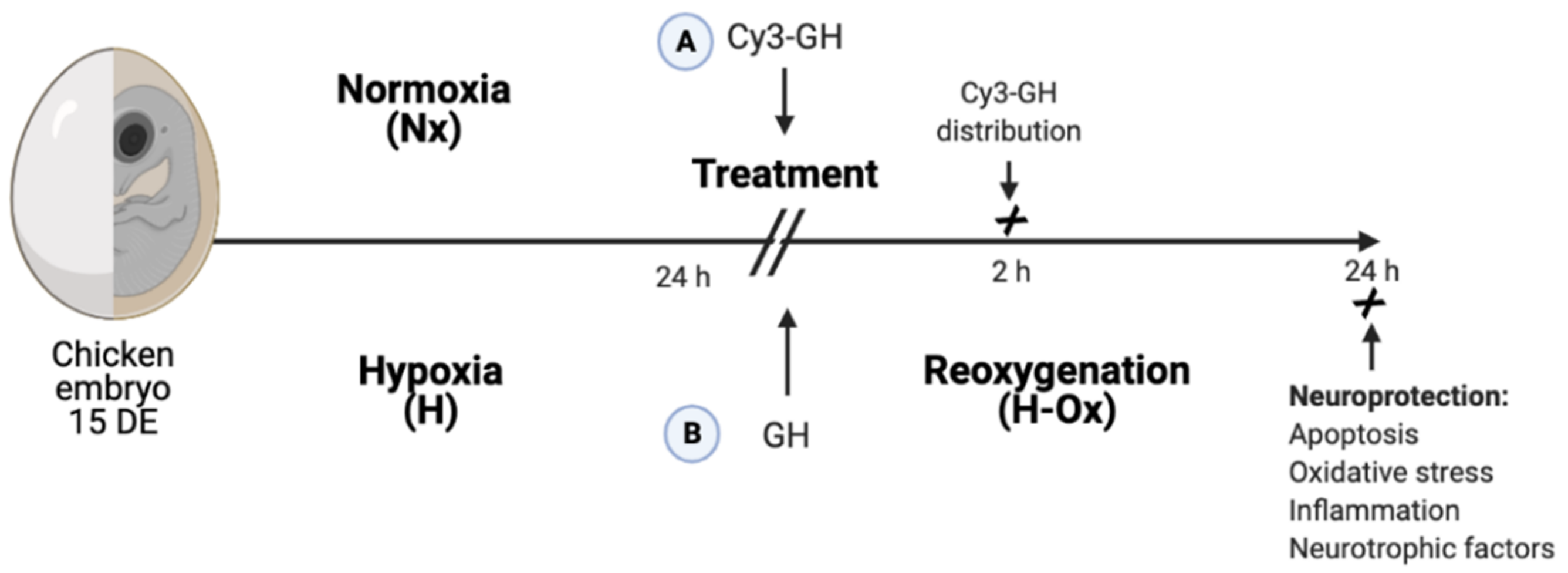
4. Materials and Methods
4.1. Animals
4.2. Treatments
4.3. Histological Analysis
4.4. Immunohistochemistry
4.5. Western Blot Analysis
4.6. Determination of Lipid Peroxidation (LPO)
4.7. Evaluation of Apoptosis
4.8. RT-PCR
4.9. Quantitative PCR (qPCR)
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rainaldi, M.A.; Perlman, J.M. Pathophysiology of Birth Asphyxia. Clin. Perinatol. 2016, 43, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Marschitz, M.; Neira-Pena, T.; Rojas-Mancilla, E.; Espina-Marchant, P.; Esmar, D.; Perez, R.; Muñoz, V.; Gutierrez-Hernandez, M.; Rivera, B.; Simola, N.; et al. Perinatal asphyxia: CNS development and deficits with delayed onset. Front. Neurosci. 2014, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Hassell, K.J.; Ezzati, M.; Alonso-Alconada, D.; Hausenloy, D.J.; Robertson, N.J. New horizons for newborn brain protection: Enhancing endogenous neuroprotection. Arch. Dis. Child Fetal. Neonatal. 2015, 100, F541–F552. [Google Scholar] [CrossRef] [PubMed]
- Biran, V.; Heine, V.M.; Verney, C.; Sheldon, R.A.; Spadafora, R.; Vexler, Z.S.; Rowitch, D.H.; Ferriero, D.M. Cerebellar abnormalities following hypoxia alone compared to hypoxic-ischemic forebrain injury in the developing rat brain. Neurobiol. Dis. 2011, 41, 138–146. [Google Scholar] [CrossRef]
- Annink, K.V.; Meerts, L.; van der Aa, N.E.; Alderliesten, T.; Nikkels, P.G.J.; Nijboer, C.H.A.; Groenendaal, F.; de Vries, L.S.; Benders, M.J.N.L.; Hoebeek, F.E.; et al. Cerebellar injury in term neonates with hypoxic-ischemic encephalopathy is underestimated. Pediatr. Res. 2021, 89, 1171–1178. [Google Scholar] [CrossRef]
- Hutton, L.C.; Yan, E.; Yawno, T.; Castillo-Melendez, M.; Hirst, J.J.; Walker, D.W. Injury of the developing cerebellum: A brief review of the effects of endotoxin and asphyxial challenges in the late gestation sheep fetus. Cerebellum 2014, 13, 777–786. [Google Scholar] [CrossRef]
- Zhang, Z.; Narayan, S.; Su, L.; Al-Alawyat, H.; Liu, J.; Kannan, S. Cerebellar injury and impaired function in a rabbit model of maternal inflammation-induced neonatal brain injury. Neurobiol. Learn Mem. 2019, 165, 106901. [Google Scholar] [CrossRef]
- Campanille, V.; Saraceno, G.E.; Rivière, S.; Logica, T.; Kölliker, R.; Capani, F.; Castilla, R. Long lasting cerebellar alterations after perinatal asphyxia in rats. Brain Res. Bull. 2015, 116, 57–66. [Google Scholar] [CrossRef]
- Lee, C.; Kim, D.W.; Jeon, G.S.; Roh, E.J.; Seo, J.H.; Wang, K.C.; Cho, S.S. Cerebellar alterations induced by chronic hypoxia: An immunohistochemical study using a chick embryonic model. Brain Res. 2001, 901, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Millar, L.J.; Shi, L.; Hoerder-Suabedissen, A.; Molnár, Z. Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges. Front. Cell. Neurosci. 2017, 11, 78. [Google Scholar] [CrossRef]
- Devesa, J.; Almengló, C.; Devesa, P. Multiple Effects of Growth Hormone in the Body: Is It Really the Hormone for Growth? Clin. Med. Insights Endocrinol. Diabetes 2016, 9, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moreno, C.G.; Arámburo, C. Growth Hormone (GH) and Synaptogenesis. Vitam. Horm. 2020, 114, 91–123. [Google Scholar] [CrossRef] [PubMed]
- Arce, V.M.; Devesa, P.; Devesa, J. Role of growth hormone (GH) in the treatment of neural diseases: From neuroprotection to neural repair. Neurosci. Res. 2013, 76, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Alba-Betancourt, C.; Arámburo, C.; Avila-Mendoza, J.; Ahumada-Solórzano, S.M.; Carranza, M.; Rodríguez-Méndez, A.J.; Harvey, S.; Luna, M. Expression, cellular distribution, and heterogeneity of growth hormone in the chicken cerebellum during development. Gen. Comp. Endocrinol. 2011, 170, 528–540. [Google Scholar] [CrossRef]
- Baltazar-Lara, R.; Ávila-Mendoza, J.; Martínez-Moreno, C.G.; Carranza, M.; Pech-Pool, S.; Vázquez-Martínez, O.; Díaz-Muñoz, M.; Luna, M.; Arámburo, C. Neuroprotective Effects of Growth Hormone (GH) and Insulin-Like Growth Factor Type 1 (IGF-1) after Hypoxic-Ischemic Injury in Chicken Cerebellar Cell Cultures. Int. J. Mol. Sci. 2020, 22, 256. [Google Scholar] [CrossRef]
- Alba-Betancourt, C.; Luna-Acosta, J.L.; Ramírez-Martínez, C.E.; Avila-González, D.; Granados-Ávalos, E.; Carranza, M.; Martínez-Coria, H.; Arámburo, C.; Luna, M. Neuro-protective effects of growth hormone (GH) after hypoxia-ischemia injury in embryonic chicken cerebellum. Gen. Comp. Endocrinol. 2013, 183, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Stern, W.C.; Miller, M.; Resnick, O.; Morgane, P.J. Distribution of 125I-labeled rat growth hormone in regional brain areas and peripheral tissue of the rat. Am. J. Anat. 1975, 144, 503–507. [Google Scholar] [CrossRef]
- Nyberg, F. Growth hormone in the brain: Characteristics of specific brain targets for the hormone and their functional significance. Front. Neuroendocrinol. 2000, 21, 330–348. [Google Scholar] [CrossRef]
- Hallberg, M.; Nyberg, F. Growth Hormone Receptors in the Brain and Their Potential as Therapeutic Targets in Central Nervous System Disorders. Open Endocrinol. J. 2012, 6, 27–33. [Google Scholar] [CrossRef][Green Version]
- Åberg, D. Role of the growth hormone/insulin-like growth factor 1 axis in neurogenesis. Endocr. Dev. 2010, 17, 63–76. [Google Scholar] [CrossRef]
- Pan, W.; Yu, Y.; Cain, C.M.; Nyberg, F.; Couraud, P.O.; Kastin, A.J. Permeation of growth hormone across the blood-brain barrier. Endocrinology 2005, 146, 4898–4904. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.J.; Slaby, F.J.; Posner, B.L. A receptor-mediated mechanism for the transport of prolactin from blood to cerebrospinal fluid. Endocrinology 1987, 120, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S.; Wyatt, A.K.; Herbison, R.E.; Knowles, P.J.; Ladyman, S.R.; Binart, N.; Banks, W.A.; Grattan, D.R. Prolactin transport into mouse brain is independent of prolactin receptor. FASEB J. 2016, 30, 1002–1010. [Google Scholar] [CrossRef]
- Horvat, S.; Baker, G.; Kirstein’s, L.; Lawrence, A.M. Growth hormone (GH), immunoreactivity in the rodent and primate CNS: Distribution, characterization and presence posthipophysectomy. Brain Res. 1982, 239, 543–557. [Google Scholar]
- Wakai, S.; Hirokawa, N. Development of the blood-brain barrier to horseradish peroxidase in the chick embryo. Cell Tissue Res. 1978, 195, 195–203. [Google Scholar] [CrossRef]
- Grange-Messent, V.; Raison, D.; Bouchaud, C. Astrocyte-endothelial cell relationships during the establishment of the blood-brain barrier in the chick embryo. Biol. Cell 1996, 86, 45–51. [Google Scholar] [CrossRef]
- Fleming, T.; Martínez-Moreno, C.G.; Mora, J.; Aizouki, M.; Luna, M.; Arámburo, C.; Harvey, S. Internalization and synaptogenic effect of GH in retinal ganglion cells (RGCs). Gen. Comp. Endocrinol. 2016, 1, 151–160. [Google Scholar] [CrossRef]
- Kaur, C.; Ling, E.A. Blood-brain barrier in hypoxic-ischemic conditions. Curr. Neurovasc. Res. 2008, 5, 71–81. [Google Scholar] [CrossRef]
- Ek, C.J.; D’Angelo, B.; Baburamani, A.A.; Lehner, C.; Levin, A.L.; Smith, P.L.; Mallard, C. Brain barrier properties and cerebral blood flow in neonatal mice exposed to cerebral hypoxia-ischemia. J. Cereb. Blood Flow Metab. 2015, 35, 818–827. [Google Scholar] [CrossRef]
- Mustafa, A.; Sharma, H.S.; Olsson, Y.; Gordh, T.; Thoren, P.; Sjöquist, P.O.; Nyberg, F. Vascular permeability to growth hormone in the rat central nervous system after focal spinal cord injury. Influence of a new anti-oxidant H 290/51 and age. Neurosci. Res. 1995, 23, 185–194. [Google Scholar] [CrossRef]
- Scheepens, A.; Sirimanne, E.; Beilharz, E.; Breier, B.H.; Waters, M.J.; Gluckman, P.D.; Williams, C.E. Alterations in the neural growth hormone axis following hypoxic-ischemic brain injury. Mol. Brain Res. 1999, 68, 88–100. [Google Scholar] [CrossRef]
- Lobie, P.E.; García-Aragón, J.; Lincoln, D.T.; Barnard, R.; Wilcox, J.N.; Waters, M.J. Localization and ontogeny of growth hormone receptor gene expression in the central nervous system. Dev. Brain Res. 1993, 74, 225–233. [Google Scholar] [CrossRef]
- Benterud, T.; Manueldas, S.; Rivera, S.; Henckel, E.; Løberg, E.M.; Norgren, S.; Baumbusch, L.O.; Solberg, R.; Saugstad, O.D. Cerebellum Susceptibility to Neonatal Asphyxia: Possible Protective Effects of N-Acetylcysteine Amide. Dis. Markers 2018, 2018, 5046372. [Google Scholar] [CrossRef] [PubMed]
- Sathyanesan, A.; Kratimenos, P.; Gallo, V. Disruption of neonatal Purkinje cell function underlies injury-related learning deficits. Proc. Natl. Acad. Sci. USA 2021, 16, e2017876118. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, M.; Berciano, M.T.; Blanco, M. Ectopic Purkinje cells in the cerebellar white matter of normal adult rodents: A Golgi study. Acta Anat. 1986, 127, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Kan, E.M.; Lu, J.; Hao, A.; Dheen, S.T.; Kaur, C.; Ling, E.A. Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: Role of TLR4 in hypoxic microglia. J. Neuroinflammation 2013, 10, 785. [Google Scholar] [CrossRef]
- Glickstein, M.; Strata, P.; Voogd, J. Cerebellum: History. Neuroscience 2009, 162, 549–559. [Google Scholar] [CrossRef]
- Noguchi, T.; Sugiasaki, T.; Tsukada, Y. Microcephalic cerebrum with hypomyelination in the growth hormone-deficient mouse (lit). Neurochem. Res. 1985, 10, 1097–1106. [Google Scholar] [CrossRef]
- Noguchi, T.; Sugiasaki, T.; Nishikawa, N.; Tsukada, Y. Restoration of microcephalic cerebrum with hypomyelination in the growth hormone-deficient mouse (lit): Stimulatory effects of GH restricted to the first 20 days of postnatal life. Neurochem. Res. 1988, 13, 249–252. [Google Scholar] [CrossRef]
- Puka-Sundvall, M.; Wallin, C.; Gilland, E.; Hallin, U.; Wang, X.; Sandberg, M.; Karlsson, J.; Blomgren, K.; Hagberg, H. Impairment of mitochondrial respiration after cerebral hypoxia-ischemia in immature rats: Relationship to activation of caspase-3 and neuronal injury. Dev. Brain Res. 2000, 125, 43–50. [Google Scholar] [CrossRef]
- Wang, X.; Karlsson, J.O.; Zhu, C.; Bahr, B.A.; Hagberg, H.; Blomgren, K. Caspase-3 activation after neonatal rat cerebral hypoxia-ischemia. Biol. Neonate 2001, 79, 172–179. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X.; Hagberg, H.; Blomgren, K. Correlation between caspase-3 activation and three different markers of DNA damage in neonatal cerebral hypoxia-ischemia. J. Neurochem. 2000, 75, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Mehmet, H.; Penrice, J.; Cooper, C.; Cady, E.; Wyatt, J.S.; Squier, M.V. Apoptosis and necrosis in the newborn piglet brain following transient cerebral hypoxia-ischemia. Neuropathol. Appl. Neurobiol. 1997, 23, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Han, T.R.; Chun, M.H.; Jang, H.D.; Kim, K.-S.; Lim, H.K.; Cho, H.J. Neuroprotective effects of growth hormone against hypoxic-ischemic brain injury in neonatal rats: H magnetic resonance spectroscopy study. J. Korean Med. Sci. 2007, 22, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Lee, E.; Kim, J.W.; Kwon, B.S.; Jung, M.K.; Jee, Y.H.; Kim, J.; Bae, S.R.; Chang, Y.P. Protective effect of growth hormone on neuronal apoptosis after hypoxia-ischemia in the neonatal rat brain. Neurosci. Lett. 2004, 354, 64–68. [Google Scholar] [CrossRef]
- Harvey, S.; Baudet, M.L.; Sanders, E.J. Growth hormone and cell survival in the neural retina: Caspase dependence and independence. Neuroreport 2006, 17, 1715–1718. [Google Scholar] [CrossRef]
- Frago, L.M.; Pañeda, C.; Dickson, S.L.; Hewson, A.K.; Argente, J.; Chowen, J.A. Growth hormone (GH) and GH-releasing peptide-6 increase brain insulin-like growth factor-I expression and activate intracellular signaling pathways involved in neuroprotection. Endocrinology 2002, 143, 4113–4122. [Google Scholar] [CrossRef]
- Heredia, M.; Fuente, A.; Criado, J.; Yajeya, J.; Devesa, J.; Riolobos, A.S. Early growth hormone (GH) treatment promotes relevant motor functional improvement after severe frontal cortex lesion in adult rats. Behav. Brain Res. 2013, 247, 48–58. [Google Scholar] [CrossRef]
- Li, R.C.; Guo, S.Z.; Raccurt, M.; Moudilou, E.; Morel, G.; Brittian, K.R.; Gozal, D. Exogenous growth hormone attenuates cognitive deficits induced by intermittent hypoxia in rats. Neuroscience 2011, 196, 237–250. [Google Scholar] [CrossRef]
- Olivares-Hernández, J.D.; Carranza, M.; Balderas-Márquez, J.E.; Epardo, D.; Baltazar-Lara, R.; Ávila-Mendoza, J.; Martínez-Moreno, C.G.; Luna, M.; Arámburo, C. Neuroprotective and regenerative effects of growth hormone (GH) in the embryonic chicken cerebral pallium exposed to hypoxic-ischemic (HI) injury. Int. J. Mol. Sci. 2022, 23, 9054. [Google Scholar] [CrossRef]
- Back, S.A. Encephalopathy of Prematurity: Pathophysiology. In Volpe’s Neurology of the Newborn, 6th ed.; Volpe, J., Terrie, I., Darras, B., de Vries, L., du Plessis, A., Neil, J., Perlman, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 405–424. [Google Scholar]
- Kaur, C.; Rathnasamy, G.; Ling, E.-A. Roles of Activated Microglia in Hypoxia-Induced Neuroinflammation in the Developing Brain and the Retina. J. Neuroimmune Pharmacol. 2013, 8, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Viswanathan, S.; Zhirong, Z.; Eng, A.L. Microglia-Derived Proinflammatory Cytokines Tumor Necrosis Factor-Alpha and Interleukin-1beta Induce Purkinje Neuronal Apoptosis via Their Receptors in Hypoxic Neonatal Rat Brain. Brain Struct. Funct. 2014, 219, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Kumar, P.A.; Sun, J.; Aggarwal, A.; Fan, Y.; Sperling, M.A.; Lumeng, C.N.; Menon, R.K. Targeted deletion of growth hormone (GH) receptor in macrophage reveals novel osteopontin-mediated effects of GH on glucose homeostasis and insulin sensitivity in diet-induced obesity. J. Biol. Chem. 2013, 288, 15725–15735. [Google Scholar] [CrossRef] [PubMed]
- Haeffner, A.; Thieblemont, N.; Déas, O.; Marelli, O.; Charpentier, B.; Senik, A.; Wright, S.D.; Haeffner-Cavaillon, N.; Hirsch, F. Inhibitory effect of growth hormone on TNF-alpha secretion and nuclear factor-kappaB translocation in lipopolysaccharide-stimulated human monocytes. J. Immunol. 1997, 158, 1310–1314. [Google Scholar] [PubMed]
- Schmidt, H.; Grune, T.; Muller, R.; Siems, W.G.; Wauer, R.R. Increased levels of lipid peroxidation products malondialdehyde and 4-hydroxynonenal after perinatal hypoxia. Pediatr. Res. 1996, 40, 15–20. [Google Scholar] [CrossRef]
- Buonocore, G.; Zani, S.; Perrone, S.; Caciotti, B.; Bracci, R. Intraerythrocyte nonprotein-bound iron and plasma malondialdehyde in the hypoxic newborn. Free Radic. Biol. Med. 1998, 25, 766–770. [Google Scholar] [CrossRef]
- Stock, M.K.; Silvernail, K.K.; Metcalfe, J. Prenatal oxidative stress: I. Malondialdehyde in hypoxic and hyperoxic chick embryos. Free Radic. Biol. Med. 1990, 8, 313–318. [Google Scholar] [CrossRef]
- Buonocore, G.; Groenendaal, F. Anti-oxidant strategies. Semin. Fetal Neonatal. 2007, 2, 287–295. [Google Scholar] [CrossRef]
- Martínez-Moreno, C.G.; Fleming, T.; Carranza, M.; Ávila-Mendoza, J.; Luna, M.; Harvey, S.; Arámburo, C. Growth hormone protects against kainate excitotoxicity and induces BDNF and NT3 expression in chicken neuroretinal cells. Exp. Eye Res. 2018, 166, 1–12. [Google Scholar] [CrossRef]
- Martinez-Moreno, C.G.; Epardo, D.; Balderas-Márquez, J.E.; Fleming, T.; Carranza, M.; Luna, M.; Harvey, S.; Arámburo, C. Regenerative Effect of Growth Hormone (GH) in the Retina after Kainic Acid Excitotoxic Damage. Int. J. Mol. Sci. 2019, 20, 4433. [Google Scholar] [CrossRef]
- Olivares-Hernández, J.D.; Balderas-Márquez, J.E.; Carranza, M.; Luna, M.; Martínez-Moreno, C.G.; Arámburo, C. Growth Hormone (GH) Enhances Endogenous Mechanisms of Neuroprotection and Neuroplasticity after Oxygen and Glucose Deprivation Injury (OGD) and Reoxygenation (OGD/R) in Chicken Hippocampal Cell Cultures. Neural Plast. 2021, 2021, 9990166. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Reimunde, P.; Devesa, P.; Barberá, M.; Arce, V. Growth hormone (GH) and brain trauma. Horm. Behav. 2013, 63, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Boie, G.; Doerr, H.G.; Trollmann, R. Oxygen-sensitive regulation and neuroprotective effects of growth hormone-dependent growth factors during early postnatal development. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R539–R548. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Terörde, K.; Dörr, H.G.; Trollmann, R. Recombinant Human Growth Hormone Activates Neuroprotective Growth Factors in Hypoxic Brain Injury in Neonatal Mice. Endocrinology 2021, 162, bqab008. [Google Scholar] [CrossRef]
- Nonomura, T.; Kubo, T.; Oka, T.; Shimoke, K.; Yamada, M.; Enokido, Y.; Hatanaka, H. Signaling pathways and survival effects of BDNF and NT-3 on cultured cerebellar granule cells. Dev. Brain Res. 1996, 97, 42–50. [Google Scholar] [CrossRef]
- Roumes, H.; Dumont, U.; Sanchez, S.; Mazuel, L.; Blanc, J.; Raffard, G.; Chateil, J.F.; Pellerin, L.; Bouzier-Sore, A.K. Neuroprotective role of lactate in rat neonatal hypoxia-ischemia. J. Cereb. Blood Flow Metab. 2021, 41, 342–358. [Google Scholar] [CrossRef]
- Ong, L.K.; Chow, W.Z.; TeBay, C.; Kluge, M.; Pietrogrande, G.; Zalewska, K.; Crock, P.; Aberg, D.; Bivard, A.; Johnson, S.J.; et al. Growth hormone improves cognitive function after experimental stroke. Stroke 2018, 49, 1257–1266. [Google Scholar] [CrossRef]
- Song, J.; Park, K.; Lee, H.; Kim, M. The effect of recombinant human growth hormone therapy in patients with completed stroke: A pilot trial. Ann. Rehabil. Med. 2012, 36, 447–457. [Google Scholar] [CrossRef]
- Szarka, N.; Szellar, D.; Kiss, S.; Farkas, N.; Szakacs, Z.; Czigler, A.; Ungvari, Z.; Hegyi, P.; Buki, A.; Toth, P. Effect of Growth Hormone on Neuropsychological Outcomes and Quality of Life of Patients with Traumatic Brain Injury: A Systematic Review. J. Neurotrauma 2021, 38, 1467–1483. [Google Scholar] [CrossRef]
- Stochholm, K.; Kiess, W. Long-term safety of growth hormone—A combined registry analysis. Clin. Endocrinol. 2018, 88, 515–528. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by the thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed]


| Target | Host/Type | Dilution | Source | Cat. No. |
|---|---|---|---|---|
| GHR | mouse/monoclonal | 1:200 | Thermo Fisher Scientific | MA1-82444 |
| HIF-1α | rabbit/polyclonal | 1:1000 | Cell Signaling | 3716S |
| Cy3 | mouse/monoclonal | 1:1000 | Abcam | ab52060 |
| GH | rabbit/polyclonal | 1:10,000 | LabD01 | CAP2 |
| Actin | mouse/monoclonal | 1:3000 | Santa Cruz Biotechnology | Sc-58673 |
| Goat anti-Mouse IgG (H + L) Cross-Adsorbed Secondary Antibody, HRP | goat/polyclonal | 1:5000 | Thermo Fisher Scientific | G-21040 |
| Goat anti-Rabbit Ig (H + L) Secondary Antibody, HRP | goat/polyclonal | 1:5000 | Invitrogen | 656120 |
| Rabbit anti-Mouse IgG (H + L) Secondary Antibody, FITC | rabbit/polyclonal | 1:2000 | Invitrogen | A16161 |
| Goat anti-Rabbit IgG Antibody, Cy3 conjugate | goat/polyclonal | 1:5000 | Millipore | AP132C |
| Target | Primer | Sequence (5′-3′) | Size | Accession # |
|---|---|---|---|---|
| GH | Fwd Rev | CGCACCTATATTCCGGAGGAC GGCAGCTCCATGTCTGACT | 128 bp | NM_204359 |
| GHR | Fwd Rev | ACTTCACCATGGACAATGCCTA GGGGTTTCTGCCATTGAAGCTC | 180 bp | NM_001001293.1 |
| IGF-1 | Fwd Rev | TACCTTGGCCTGTGTTTGCT CCCTTGTGGTGTAAGCGTCT | 170 bp | NM_001004384 |
| IGF-1R | Fwd Rev | TCCAACACAACACTGAAGAATC ACCATATTCCAGCTATTGGAGC | 166 bp | NM_205032.1 |
| 18S | Fwd Rev | CTCTTTCTCGATTCCGTGGGT TTAGCATGCCAGAGTCTCGT | 100 bp | M59389 |
| BDNF | Fwd Rev | AGCAGTCAAGTGCCTTTGGA TCCGCTGCTGTTACCCACTCG | 167 bp | NM_001031616 |
| VEGF | Fwd Rev | CAATTGAGACCCTGGTGGAC TCTCATCAGAGGCACACAGG | 85 pb | NM_205042.2 |
| NT-3 | Fwd Rev | AGGCAGCAGAGACGCTACAAC AGCACAGTTACCTGGTGTCCT | 248 bp | NM_001109762 |
| HIF-1𝑎 | Fwd Rev | GACTCCTGTTTCCACTGTAAC CAGACTCAGACACCATCAAC | 153 bp | XM_040700545 |
| TNF-𝑎 | Fwd Rev | GAGCAGGGCTGACACGGAT GCACAAAAGAGCTGATGGCAG | 149 bp | NM_204267.1 |
| IL-6 | Fwd Rev | AAATCCCTCCTCGCCAATCT CCCTCACGGTCTTCTCCATAAA | 205 bp | NM_204628.1 |
| IL-1β | Fwd Rev | GGATTCTGAGCACACCACAGT CAGGCGGTAGAAGATGAAGC | 552 pb | XM_015297469.2 |
| iNOS | Fwd Rev | CCAGCTGATTGGGTGTGGAT TACAGCCTTGGCCAAAATGC | 194 pb | NM_204961.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baltazar-Lara, R.; Zenil, J.M.; Carranza, M.; Ávila-Mendoza, J.; Martínez-Moreno, C.G.; Arámburo, C.; Luna, M. Growth Hormone (GH) Crosses the Blood–Brain Barrier (BBB) and Induces Neuroprotective Effects in the Embryonic Chicken Cerebellum after a Hypoxic Injury. Int. J. Mol. Sci. 2022, 23, 11546. https://doi.org/10.3390/ijms231911546
Baltazar-Lara R, Zenil JM, Carranza M, Ávila-Mendoza J, Martínez-Moreno CG, Arámburo C, Luna M. Growth Hormone (GH) Crosses the Blood–Brain Barrier (BBB) and Induces Neuroprotective Effects in the Embryonic Chicken Cerebellum after a Hypoxic Injury. International Journal of Molecular Sciences. 2022; 23(19):11546. https://doi.org/10.3390/ijms231911546
Chicago/Turabian StyleBaltazar-Lara, Rosario, Janeth Mora Zenil, Martha Carranza, José Ávila-Mendoza, Carlos G. Martínez-Moreno, Carlos Arámburo, and Maricela Luna. 2022. "Growth Hormone (GH) Crosses the Blood–Brain Barrier (BBB) and Induces Neuroprotective Effects in the Embryonic Chicken Cerebellum after a Hypoxic Injury" International Journal of Molecular Sciences 23, no. 19: 11546. https://doi.org/10.3390/ijms231911546
APA StyleBaltazar-Lara, R., Zenil, J. M., Carranza, M., Ávila-Mendoza, J., Martínez-Moreno, C. G., Arámburo, C., & Luna, M. (2022). Growth Hormone (GH) Crosses the Blood–Brain Barrier (BBB) and Induces Neuroprotective Effects in the Embryonic Chicken Cerebellum after a Hypoxic Injury. International Journal of Molecular Sciences, 23(19), 11546. https://doi.org/10.3390/ijms231911546








