Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways
Abstract
:1. Introduction
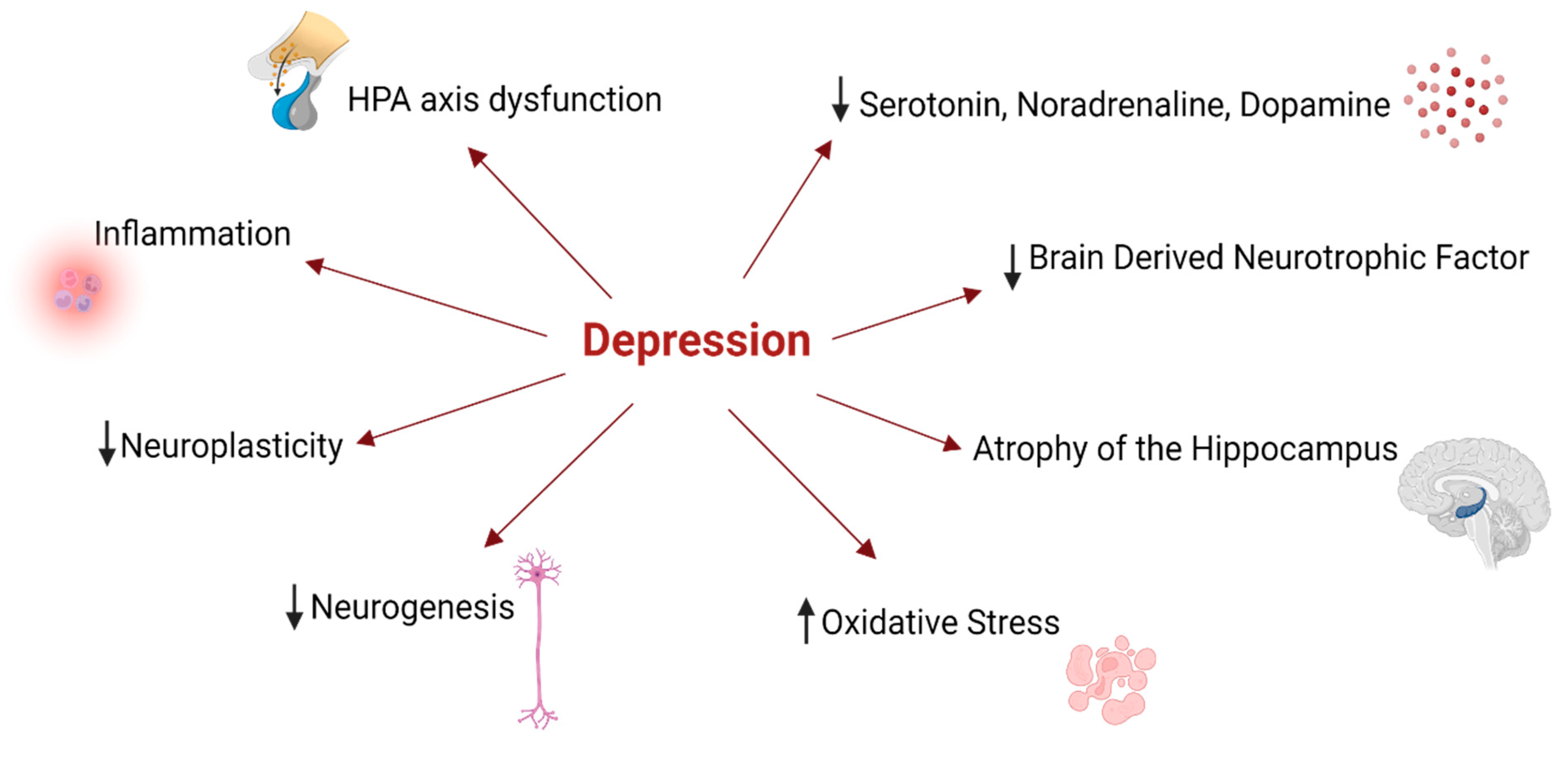
2. Depression—A Brief Contextualization
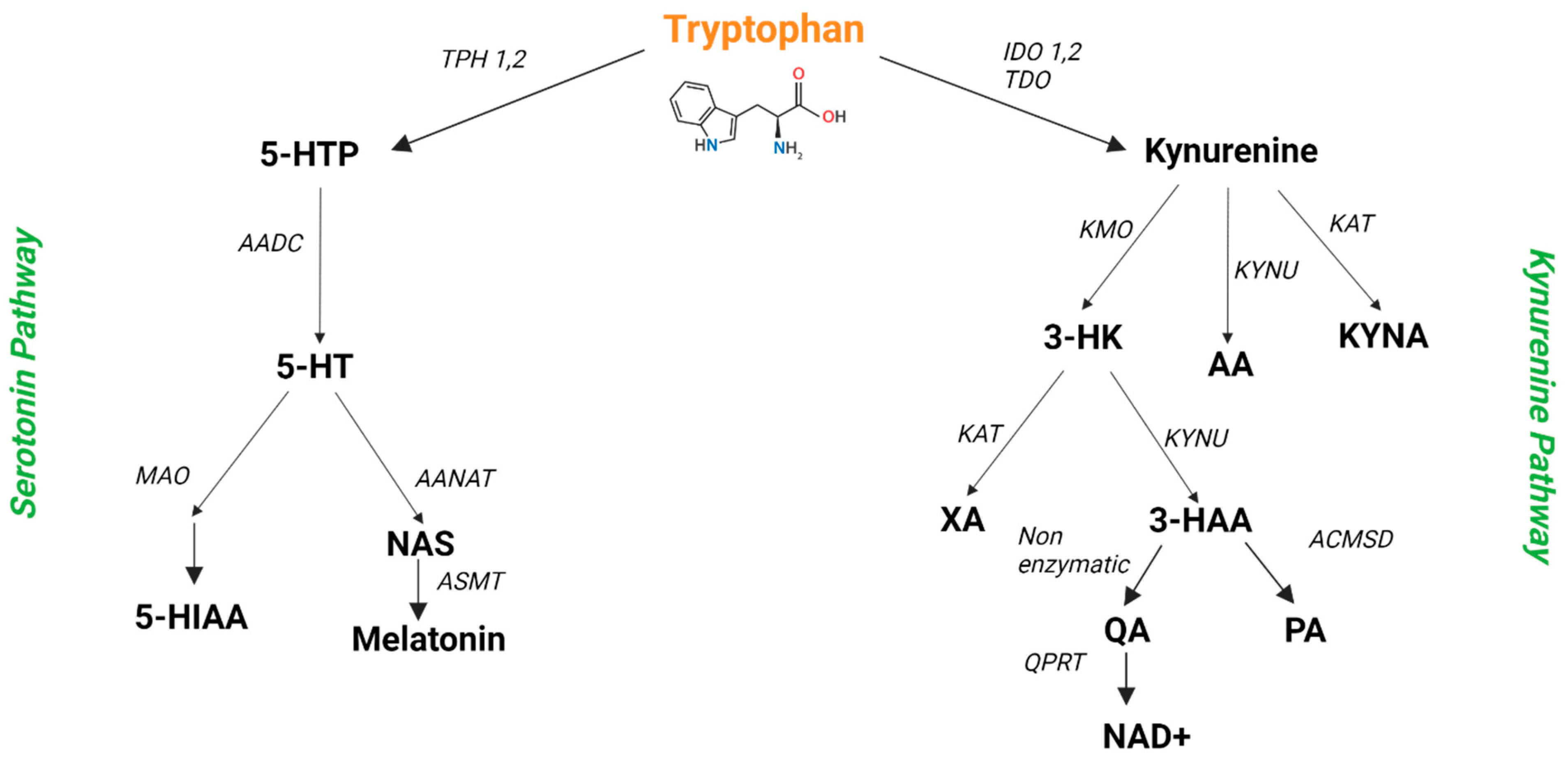
3. Trp Metabolism
4. Trp Metabolism in Depression—An Overlook
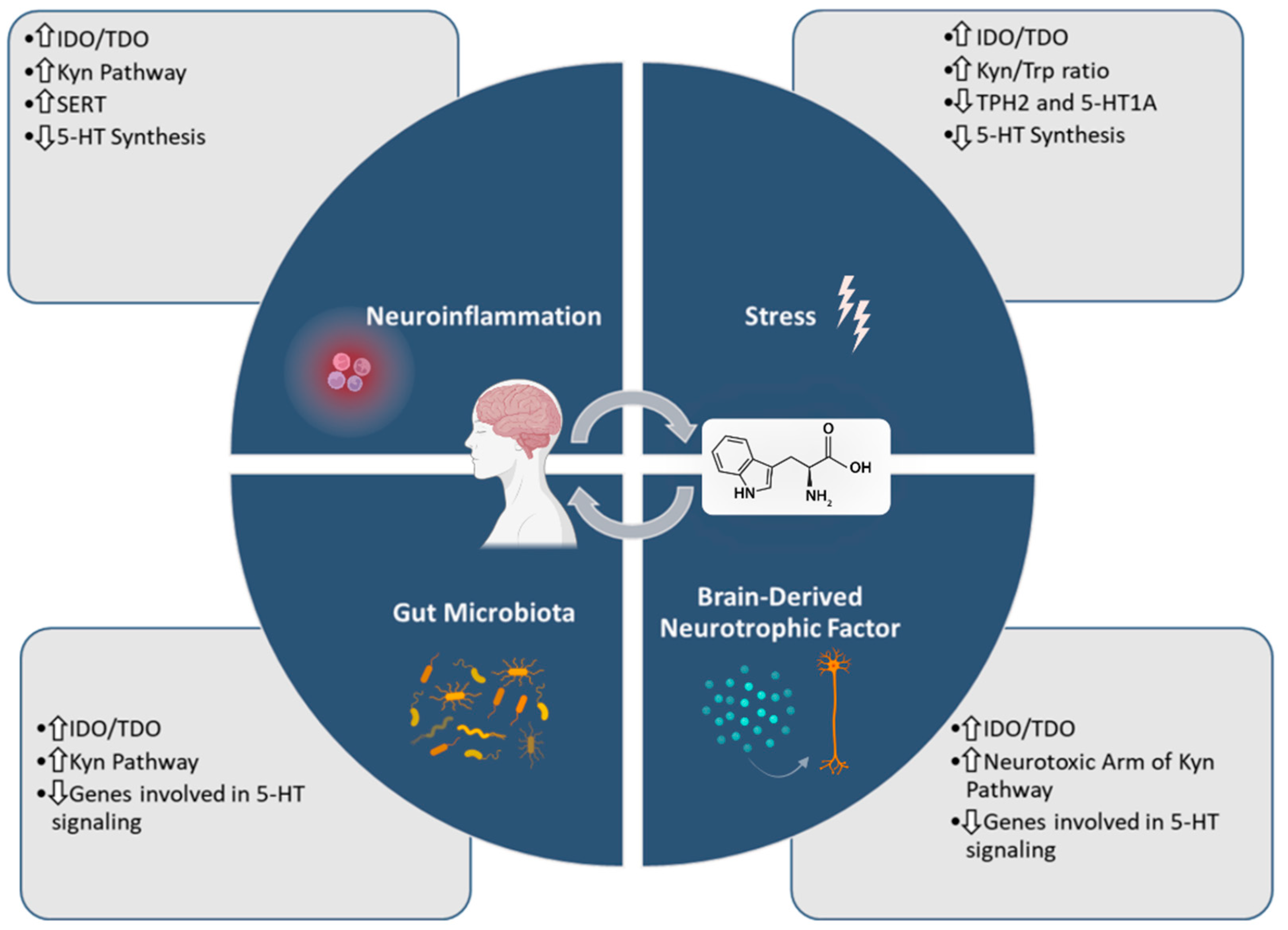
4.1. Trp Metabolism and Depression’s Associated Neuroinflammation
4.2. Trp Metabolism and Depression’s Associated Chronic Stress
4.3. Trp Metabolism and Microbiota in Depression
4.4. Trp Metabolism and Brain-Derived Neurotrophic Factor Expression in Depression
4.5. Pharmacological Modulation of Trp Metabolism in Depression—An Overlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuijpers, P. The Challenges of Improving Treatments for Depression. JAMA 2018, 320, 2529–2530. [Google Scholar] [CrossRef]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef] [Green Version]
- Comai, S.; Bertazzo, A.; Brughera, M.; Crotti, S. Tryptophan in health and disease. Adv. Clin. Chem. 2020, 95, 165–218. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2019, 25, 131–147. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [Green Version]
- Cowen, P.J.; Browning, M. What has serotonin to do with depression? World Psychiatry 2015, 14, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355. [Google Scholar] [CrossRef] [Green Version]
- BioRender. Available online: https://biorender.com/ (accessed on 15 June 2022).
- Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 14 June 2022).
- Kaltenboeck, A.; Harmer, C. The neuroscience of depressive disorders: A brief review of the past and some considerations about the future. Brain Neurosci. Adv. 2018, 2, 2398212818799269. [Google Scholar] [CrossRef] [Green Version]
- Jesulola, E.; Micalos, P.; Baguley, I.J. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model—Are we there yet? Behav. Brain Res. 2018, 341, 79–90. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Developmental Programming and Reprogramming of Hypertension and Kidney Disease: Impact of Tryptophan Metabolism. Int. J. Mol. Sci. 2020, 21, 8705. [Google Scholar] [CrossRef] [PubMed]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan metabolic pathways and brain serotonergic activity: A comparative review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindseth, G.; Helland, B.; Caspers, J. The Effects of Dietary Tryptophan on Affective Disorders. Arch. Psychiatr. Nurs. 2015, 29, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Xu, H.; Zhu, M.; Liu, K.; Lin, B.; Luo, R.; Chen, C.; Li, M. Stress inhibits tryptophan hydroxylase expression in a rat model of depression. Oncotarget 2017, 8, 63247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach-Mizrachi, H.; Underwood, M.D.; Kassir, S.A.; Bakalian, M.J.; Sibille, E.; Tamir, H.; Mann, J.J.; Arango, V. Neuronal Tryptophan Hydroxylase mRNA Expression in the Human Dorsal and Median Raphe Nuclei: Major Depression and Suicide. Neuropsychopharmacology 2006, 31, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K. Etiological classification of depression based on the enzymes of tryptophan metabolism. BMC Psychiatry 2014, 14, 372. [Google Scholar] [CrossRef] [Green Version]
- Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2020, 22, 181. [Google Scholar] [CrossRef]
- Ryan, N.D.; Birmaher, B.; Perel, J.M.; Dahl, R.E.; Meyer, V.; Al-Shabbout, M.; Iyengar, S.; Puig-Antich, J. Neuroendocrine Response to L-5-Hydroxytryptophan Challenge in Prepubertal Major Depression: Depressed vs Normal Children. Arch. Gen. Psychiatry 1992, 49, 843–851. [Google Scholar] [CrossRef]
- Aliño, J.L.-I.; Gutierrez, J.A.; Iglesias, M.M. 5-Hydroxytryptophan (5-HTP) and a MAOI (nialamide) in the treatment of depressions. A double-blind controlled study. Int. Pharmacopsychiatry 1976, 11, 8–15. [Google Scholar] [CrossRef]
- Mendlewicz, J.; Youdim, M.B.H. Antidepressant potentiation of 5-hydroxytryptophan by L-deprenil in affective illness. J. Affect. Disord. 1980, 2, 137–146. [Google Scholar] [CrossRef]
- Vahid-Ansari, F.; Albert, P.R. Rewiring of the Serotonin System in Major Depression. Front. Psychiatry 2021, 12, 2275. [Google Scholar] [CrossRef] [PubMed]
- Thase, M.E. The role of monoamine oxidase inhibitors in depression treatment guidelines. J. Clin. Psychiatry 2012, 73, 10–16. [Google Scholar] [CrossRef]
- Jones, D.N.; Raghanti, M.A. The role of monoamine oxidase enzymes in the pathophysiology of neurological disorders. J. Chem. Neuroanat. 2021, 114, 101957. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, S.; Wilson, A.A.; Miler, L.; Rusjan, P.M.; Vasdev, N.; Kish, S.J.; Rajkowska, G.; Wang, J.; Bagby, M.; Mizrahi, R.; et al. Monoamine Oxidase B Total Distribution Volume in the Prefrontal Cortex of Major Depressive Disorder: An [11C]SL25.1188 Positron Emission Tomography Study. JAMA Psychiatry 2019, 76, 634–641. [Google Scholar] [CrossRef] [Green Version]
- Placidi, G.P.A.; Oquendo, M.A.; Malone, K.M.; Huang, Y.Y.; Ellis, S.P.; Mann, J.J. Aggressivity, suicide attempts, and depression: Relationship to cerebrospinal fluid monoamine metabolite levels. Biol. Psychiatry 2001, 50, 783–791. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin in Aging and Disease—Multiple Consequences of Reduced Secretion, Options and Limits of Treatment. Aging Dis. 2012, 3, 194. [Google Scholar] [PubMed]
- Tonon, A.C.; Pilz, L.K.; Markus, R.P.; Hidalgo, M.P.; Elisabetsky, E. Melatonin and Depression: A Translational Perspective From Animal Models to Clinical Studies. Front. Psychiatry 2021, 12, 452. [Google Scholar] [CrossRef] [PubMed]
- Ogłodek, E.A.; Just, M.J.; Szromek, A.R.; Araszkiewicz, A. Melatonin and neurotrophins NT-3, BDNF, NGF in patients with varying levels of depression severity. Pharmacol. Rep. 2016, 68, 945–951. [Google Scholar] [CrossRef]
- Ali, T.; Rahman, S.U.; Hao, Q.; Li, W.; Liu, Z.; Ali Shah, F.; Murtaza, I.; Zhang, Z.; Yang, X.; Liu, G.; et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J. Pineal Res. 2020, 69, e12667. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, N.; Zhang, X.; Han, X.; Zhai, X.; Lu, Y. IDO and TDO as a potential therapeutic target in different types of depression. Metab. Brain Dis. 2018, 33, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Smythe, G.; Takikawa, O.; Brew, B.J. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 2005, 49, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Smith, D.G.; Kerr, S.J.; Smythe, G.A.; Kapoor, V.; Armati, P.J.; Brew, B.J. Characterisation of kynurenine pathway metabolism in human astrocytes and implications in neuropathogenesis. Redox Rep. 2000, 5, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Wigner, P.; Czarny, P.; Synowiec, E.; Bijak, M.; Białek, K.; Talarowska, M.; Galecki, P.; Szemraj, J.; Sliwinski, T. Association between single nucleotide polymorphisms of TPH1 and TPH2 genes, and depressive disorders. J. Cell. Mol. Med. 2018, 22, 1778–1791. [Google Scholar] [CrossRef] [Green Version]
- Steiner, J.; Walter, M.; Gos, T.; Guillemin, G.J.; Bernstein, H.G.; Sarnyai, Z.; Mawrin, C.; Brisch, R.; Bielau, H.; zu Schwabedissen, L.M.; et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: Evidence for an immune-modulated glutamatergic neurotransmission? J. Neuroinflammation 2011, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, H.; Ozaki, N.; Sawada, M.; Isobe, K.; Ohta, T.; Nagatsu, T. A link between stress and depression: Shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress 2008, 11, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, J.; Zhu, P.; Xie, H.; Lu, L.; Bai, W.; Pan, W.; Shi, R.; Ye, J.; Xia, B.; et al. Tryptophan-rich diet ameliorates chronic unpredictable mild stress induced depression- and anxiety-like behavior in mice: The potential involvement of gut-brain axis. Food Res. Int. 2022, 157, 111289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, R.; She, Y.; Liu, X.; Zhao, H.; Li, C.; Jia, Y. A new perspective on depression and neuroinflammation: Non-coding RNA. J. Psychiatr. Res. 2022, 148, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Cardoso, A.; Vale, N. Highlighting Immune System and Stress in Major Depressive Disorder, Parkinson’s, and Alzheimer’s Diseases, with a Connection with Serotonin. Int. J. Mol. Sci. 2021, 22, 8525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, Inflammation and Pain. Int. Anesthesiol. Clin. 2007, 45, 27. [Google Scholar] [CrossRef] [Green Version]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Carvalho, L.A.; Pariante, C.M. Glucocorticoids, cytokines and brain abnormalities in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 722–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, C.; Macedo e Cordeiro, T.; Suchting, R.; de Dios, C.; Cuellar Leal, V.A.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of Immune Activation on the Kynurenine Pathway and Depression Symptoms—A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2020, 118, 514. [Google Scholar] [CrossRef]
- Kopra, E.; Mondelli, V.; Pariante, C.; Nikkheslat, N. Ketamine’s effect on inflammation and kynurenine pathway in depression: A systematic review. J. Psychopharmacol. 2021, 35, 934. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Li, C.T.; Lin, W.C.; Hong, C.J.; Tu, P.C.; Bai, Y.M.; Cheng, C.M.; Su, T.P. Rapid inflammation modulation and antidepressant efficacy of a low-dose ketamine infusion in treatment-resistant depression: A randomized, double-blind control study. Psychiatry Res. 2018, 269, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Kadriu, B.; Farmer, C.A.; Yuan, P.; Park, L.T.; Deng, Z.-D.; Moaddel, R.; Henter, I.D.; Shovestul, B.; Ballard, E.D.; Kraus, C.; et al. The Kynurenine Pathway and Bipolar Disorder: Intersection of the Monoaminergic and Glutamatergic Systems and Immune Response. Mol. Psychiatry 2021, 26, 4085–4095. [Google Scholar] [CrossRef]
- Kiraly, D.D.; Horn, S.R.; Van Dam, N.T.; Costi, S.; Schwartz, J.; Kim-Schulze, S.; Patel, M.; Hodes, G.E.; Russo, S.J.; Merad, M.; et al. Altered peripheral immune profiles in treatment-resistant depression: Response to ketamine and prediction of treatment outcome. Transl. Psychiatry 2017, 7, e1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zheng, W.; Liu, W.; Wang, C.; Zhan, Y.; Li, H.; Chen, L.; Li, M.; Ning, Y. Antidepressant effect of repeated ketamine administration on kynurenine pathway metabolites in patients with unipolar and bipolar depression. Brain Behav. Immun. 2018, 74, 205–212. [Google Scholar] [CrossRef]
- Daly, M.; Robinson, E. Depression and anxiety during COVID-19. Lancet 2022, 399, 518. [Google Scholar] [CrossRef]
- Dewulf, J.P.; Martin, M.; Marie, S.; Oguz, F.; Belkhir, L.; De Greef, J.; Yombi, J.C.; Wittebole, X.; Laterre, P.-F.; Jadoul, M.; et al. Urine metabolomics links dysregulation of the tryptophan-kynurenine pathway to inflammation and severity of COVID-19. Sci. Rep. 2022, 12, 9959. [Google Scholar] [CrossRef] [PubMed]
- Mingoti, M.E.D.; Bertollo, A.G.; Simões, J.L.B.; Francisco, G.R.; Bagatini, M.D.; Ignácio, Z.M. COVID-19, Oxidative Stress, and Neuroinflammation in the Depression Route. J. Mol. Neurosci. 2022, 72, 1166–1181. [Google Scholar] [CrossRef]
- Dawood, S.; Bano, S.; Badawy, A.A.-B. Inflammation and serotonin deficiency in major depressive disorder: Molecular docking of antidepressant and anti-inflammatory drugs to tryptophan and indoleamine 2,3-dioxygenases. Biosci. Rep. 2022, 42. [Google Scholar] [CrossRef] [PubMed]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Bay-Richter, C.; Linderholm, K.R.; Lim, C.K.; Samuelsson, M.; Träskman-Bendz, L.; Guillemin, G.J.; Erhardt, S.; Brundin, L. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav. Immun. 2015, 43, 110–117. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.M.; De Smedt-Peyrusse, V.; Morael, J.; Vancassel, S.; Capuron, L.; Gaudout, D.; Pourtau, L.; Castanon, N. Prevention of Stress-Induced Depressive-like Behavior by Saffron Extract Is Associated with Modulation of Kynurenine Pathway and Monoamine Neurotransmission. Pharmaceutics 2021, 13, 2155. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Ye, F.; Xu, C.X.; Chang, Q.; Liu, X.M.; Pan, R. Le Effect of Radix Polygalae extract on the colonic dysfunction in rats induced by chronic restraint stress. J. Ethnopharmacol. 2022, 294, 115349. [Google Scholar] [CrossRef] [PubMed]
- Delgado, I.; Cussotto, S.; Anesi, A.; Dexpert, S.; Aubert, A.; Aouizerate, B.; Beau, C.; Forestier, D.; Ledaguenel, P.; Magne, E.; et al. Association between the indole pathway of tryptophan metabolism and subclinical depressive symptoms in obesity: A preliminary study. Int. J. Obes. 2022, 46, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Achtyes, E.; Keaton, S.A.; Smart, L.; Burmeister, A.R.; Heilman, P.L.; Krzyzanowski, S.; Nagalla, M.; Guillemin, G.; Galvis, M.L.E.; Lim, E.; et al. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav. Immun. 2020, 83, 239. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2015, 16, 22–34. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Jiang, T.; Chen, P.; Ouyang, J.; Xu, G.; Zeng, Z.; Sun, Y. Emerging tendency towards autoimmune process in major depressive patients: A novel insight from Th17 cells. Psychiatry Res. 2011, 188, 224–230. [Google Scholar] [CrossRef]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef]
- Miller, A.H. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: Psychoneuroimmunology at the translational interface. Brain Behav. Immun. 2009, 23, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.-B.; Blakely, R.D.; A Hewlett, W. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 2006, 31, 2121–2131. [Google Scholar] [CrossRef] [Green Version]
- Tsao, C.W.; Lin, Y.S.; Cheng, J.T.; Chang, W.W.; Chen, C.L.; Wu, S.R.; Fan, C.W.; Lo, H.Y. Serotonin transporter mRNA expression is decreased by lamivudine and ribavirin and increased by interferon in immune cells. Scand. J. Immunol. 2006, 63, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-B.; Lindler, K.M.; Owens, A.W.; Daws, L.C.; Blakely, R.D.; Hewlett, W.A. Interleukin-1 Receptor Activation by Systemic Lipopolysaccharide Induces Behavioral Despair Linked to MAPK Regulation of CNS Serotonin Transporters. Neuropsychopharmacology 2010, 35, 2510–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himmerich, H.; Patsalos, O.; Lichtblau, N.; Ibrahim, M.A.A.; Dalton, B. Cytokine research in depression: Principles, challenges, and open questions. Front. Psychiatry 2019, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Fanet, H.; Capuron, L.; Castanon, N.; Calon, F.; Vancassel, S. Tetrahydrobioterin (BH4) Pathway: From Metabolism to Neuropsychiatry. Curr. Neuropharmacol. 2021, 19, 591. [Google Scholar] [CrossRef]
- Raison, C.L.; Woolwine, B.J.; Demetrashvili, M.F.; Borisov, A.S.; Weinreib, R.; Staab, J.P.; Zajecka, J.M.; Bruno, C.J.; Henderson, M.A.; Reinus, J.F.; et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment. Pharmacol. Ther. 2007, 25, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Ominique, D.; Usselman, L.M.; Awson, A.H.L.; Ane, J.; Umnick, F.G.; Mita, A.; Anatunga, K.M.; Uzanne, S.; Enna, P.; Ebecca, R.; et al. Paroxetine for the Prevention of Depression Induced by High-Dose Interferon Alfa. N. Engl. J. Med. 2001, 344, 961–966. [Google Scholar] [CrossRef]
- Russell, G.; Lightman, S. The human stress response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, L.A.; Pérez-Padilla, E.A.; García-Oscos, F.; Salgado, H.; Atzori, M.; Pineda, J.C. A new theory of depression based on the serotonin/kynurenine relationship and the hypothalamicpituitary- adrenal axis. Biomedica 2018, 38, 437–450. [Google Scholar] [CrossRef] [Green Version]
- Messaoud, A.; Mensi, R.; Douki, W.; Neffati, F.; Najjar, M.F.; Gobbi, G.; Valtorta, F.; Gaha, L.; Comai, S. Reduced peripheral availability of tryptophan and increased activation of the kynurenine pathway and cortisol correlate with major depression and suicide. World J. Biol. Psychiatry 2019, 20, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.F. Tryptophan–Kynurenine Metabolism as a Common Mediator of Genetic and Environmental Impacts in Major Depressive Disorder: The Serotonin Hypothesis Revisited 40 Years Later. Isr. J. Psychiatry Relat. Sci. 2010, 47, 56. [Google Scholar]
- La Torre, D.; Dalile, B.; de Loor, H.; Van Oudenhove, L.; Verbeke, K. Changes in kynurenine pathway metabolites after acute psychosocial stress in healthy males: A single-arm pilot study. Stress 2021, 24, 920–930. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, K.; Harkin, A. Stress-related regulation of the kynurenine pathway: Relevance to neuropsychiatric and degenerative disorders. Neuropharmacology 2017, 112, 307–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibney, S.M.; Fagan, E.M.; Waldron, A.M.; O’Byrne, J.; Connor, T.J.; Harkin, A. Inhibition of stress-induced hepatic tryptophan 2,3-dioxygenase exhibits antidepressant activity in an animal model of depressive behaviour. Int. J. Neuropsychopharmacol. 2014, 17, 917–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, M.A.; Parrott, J.M.; McCusker, R.H.; Dantzer, R.; Kelley, K.W.; O’Connor, J.C. Intracerebroventricular administration of lipopolysaccharide induces indoleamine-2,3-dioxygenase-dependent depression-like behaviors. J. Neuroinflammation 2013, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, J.C.; Lawson, M.A.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal Muscle PGC-1α1 Modulates Kynurenine Metabolism and Mediates Resilience to Stress-Induced Depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Bethea, C.L.; Centeno, M.L.; Cameron, J.L. Neurobiology of stress-induced reproductive dysfunction in female macaques. Mol. Neurobiol. 2008, 38, 199–230. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhu, X.; Yu, P.; Sheng, T.; Wang, Y.; Ye, Y. Crocin ameliorates depressive-like behaviors induced by chronic restraint stress via the NAMPT-NAD+-SIRT1 pathway in mice. Neurochem. Int. 2022, 157, 105343. [Google Scholar] [CrossRef]
- Samant, N.P.; Gupta, G.L. Gossypetin- based therapeutics for cognitive dysfunction in chronic unpredictable stress- exposed mice. Metab. Brain Dis. 2022, 37, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Youssef, B.; Ramadan, K.S.; ElShebiney, S.; Ibrahim, E.A. Antidepressant-like effects of aqueous extracts of miswak (Salvadora persica) and date palm (Phoenix dactylifera) on depression-like behaviors using CUMS model in male rats. J. Food Biochem. 2022, e14164. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sun-Waterhouse, D.; Tao, Q.; Li, W.; Shu, D.; Cui, C. The enhanced serotonin (5-HT) synthesis and anti-oxidative roles of Trp oligopeptide in combating anxious depression C57BL/6 mice. J. Funct. Foods 2020, 67, 103859. [Google Scholar] [CrossRef]
- Bastiaanssen, T.F.S.; Cussotto, S.; Claesson, M.J.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Gutted! Unraveling the Role of the Microbiome in Major Depressive Disorder. Harv. Rev. Psychiatry 2020, 28, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004, 558, 263. [Google Scholar] [CrossRef]
- Gareau, M.G.; Jury, J.; MacQueen, G.; Sherman, P.M.; Perdue, M.H. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 2007, 56, 1522. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the microbiota-gut-brain axis in chronic restraint stress: Disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 2021, 13, 1–16. [Google Scholar] [CrossRef]
- Marin, I.A.; Goertz, J.E.; Ren, T.; Rich, S.S.; Onengut-Gumuscu, S.; Farber, E.; Wu, M.; Overall, C.C.; Kipnis, J.; Gaultier, A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 2017, 7, srep43859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Babaei, F.; Mirzababaei, M.; Mohammadi, G.; Dargahi, L.; Nassiri-Asl, M. Saccharomyces boulardii attenuates lipopolysaccharide-induced anxiety-like behaviors in rats. Neurosci. Lett. 2022, 778, 136600. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Zhou, Y.; Zhao, F.; Gao, X.; Wu, C.; Wu, T.; Jiang, L.; Zhang, D. Correlation between intestinal microbiotal imbalance and 5-HT metabolism, immune inflammation in chronic unpredictable mild stress male rats. Genes. Brain Behav. 2022, 21. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Farzi, A.; Ke, X.; Yu, Y.; Chen, C.; Chen, S.; Yu, T.; Wang, H.; Li, Y. Oral administration of Lactococcus lactis WHH2078 alleviates depressive and anxiety symptoms in mice with induced chronic stress. Food Funct. 2022, 13, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, Y.; Shi, Z.; Zhou, N.; Ren, G.; Hao, X.; Zou, L.; Yao, Y. Mung Bean Protein Suppresses Undernutrition-Induced Growth Deficits and Cognitive Dysfunction in Rats via Gut Microbiota-TLR4/NF-kB Pathway. J. Agric. Food Chem. 2021, 69, 12566–12577. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, L.; Aceto, S.; Agnisola, C.; De Paolo, S.; Dipineto, L.; Stilling, R.M.; Dinan, T.G.; Cryan, J.F.; Menna, L.F.; Fioretti, A. Probiotic modulation of the microbiota-gut-brain axis and behaviour in zebrafish. Sci. Rep. 2016, 6, 30046. [Google Scholar] [CrossRef] [Green Version]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateos-Aparicio, P.; Rodríguez-Moreno, A. The impact of studying brain plasticity. Front. Cell. Neurosci. 2019, 13, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumeister, A.; Yuan, P.; Young, T.A.; Bonne, O.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H. Effects of tryptophan depletion on serum levels of brain-derived neurotrophic factor in unmedicated patients with remitted depression and healthy subjects. Am. J. Psychiatry 2005, 162, 805–807. [Google Scholar] [CrossRef]
- Dugan, A.M.; Parrott, J.M.; Redus, L.; Hensler, J.G.; O’Connor, J.C. Low-Level Stress Induces Production of Neuroprotective Factors in Wild-Type but Not BDNF+/− Mice: Interleukin-10 and Kynurenic Acid. Int. J. Neuropsychopharmacol. 2016, 19, pyv089. [Google Scholar] [CrossRef] [Green Version]
- Ieraci, A.; Beggiato, S.; Ferraro, L.; Barbieri, S.S.; Popoli, M. Kynurenine pathway is altered in BDNF Val66Met knock-in mice: Effect of physical exercise. Brain Behav. Immun. 2020, 89, 440–450. [Google Scholar] [CrossRef]
- Myint, K.; Jacobs, K.; Myint, A.M.; Lam, S.K.; Henden, L.; Hoe, S.Z.; Guillemin, G.J. Effects of stress associated with academic examination on the kynurenine pathway profile in healthy students. PLoS ONE 2021, 16, e0252668. [Google Scholar] [CrossRef]
- Gao, L.; Gao, T.; Zeng, T.; Huang, P.; Wong, N.K.; Dong, Z.; Li, Y.; Deng, G.; Wu, Z.; Lv, Z. Blockade of Indoleamine 2, 3-dioxygenase 1 ameliorates hippocampal neurogenesis and BOLD-fMRI signals in chronic stress precipitated depression. Aging 2021, 13, 5875–5891. [Google Scholar] [CrossRef] [PubMed]
- Eadie, B.D.; Redila, V.A.; Christie, B.R. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J. Comp. Neurol. 2005, 486, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homberg, J.R.; Molteni, R.; Calabrese, F.; Riva, M.A. The serotonin–BDNF duo: Developmental implications for the vulnerability to psychopathology. Neurosci. Biobehav. Rev. 2014, 43, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.G.; Jin, S.L.; Li, G.Y.; Li, Q.Q.; Li, Z.R.; Ma, H.X.; Zhuo, C.J.; Jiang, R.H.; Ye, M.J. Serotonin regulates brain-derived neurotrophic factor expression in select brain regions during acute psychological stress. Neural Regen. Res. 2016, 11, 1471. [Google Scholar] [CrossRef]
- Martinowich, K.; Lu, B. Interaction between BDNF and Serotonin: Role in Mood Disorders. Neuropsychopharmacology 2008, 33, 73–83. [Google Scholar] [CrossRef]
- Rios, M.; Lambe, E.K.; Liu, R.; Teillon, S.; Liu, J.H.; Akbarian, S.; Roffler-Tarlov, S.; Jaenisch, R.; Aghajanian, G.K. Severe deficits in 5-HT2A -mediated neurotransmission in BDNF conditional mutant mice. J. Neurobiol. 2006, 66, 408–420. [Google Scholar] [CrossRef]
- Hensler, J.G.; Advani, T.; Monteggia, L.M. Regulation of serotonin-1A receptor function in inducible brain-derived neurotrophic factor knockout mice after administration of corticosterone. Biol. Psychiatry 2007, 62, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Son, S.Y.; Lee, A.Y.; Park, H.G.; Lee, W.L.; Lee, C.H. Metabolite Profiling Revealed That a Gardening Activity Program Improves Cognitive Ability Correlated with BDNF Levels and Serotonin Metabolism in the Elderly. Int. J. Environ. Res. Public Health 2020, 17, 541. [Google Scholar] [CrossRef] [Green Version]
- Evsiukova, V.S.; Bazovkina, D.; Bazhenova, E.; Kulikova, E.A.; Kulikov, A.V. Tryptophan Hydroxylase 2 Deficiency Modifies the Effects of Fluoxetine and Pargyline on the Behavior, 5-HT- and BDNF-Systems in the Brain of Zebrafish ( Danio rerio). Int. J. Mol. Sci. 2021, 22, 12851. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Castorina, S.; Imbesi, R.; Szychlinska, M.A.; Scuderi, S.; Loreto, C.; Giunta, S. Changes in serotonin (5-HT) and brain-derived neurotrophic factor (BDFN) expression in frontal cortex and hippocampus of aged rat treated with high tryptophan diet. Brain Res. Bull. 2015, 119, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Pietrelli, A.; Matković, L.; Vacotto, M.; Lopez-Costa, J.J.; Basso, N.; Brusco, A. Aerobic exercise upregulates the BDNF-Serotonin systems and improves the cognitive function in rats. Neurobiol. Learn. Mem. 2018, 155, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Ogata, N.; de Souza Dantas, L.M.; Crowell-Davis, S.L. Selective Serotonin Reuptake Inhibitors. In Veterinary Psychopharmacology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 103–128. [Google Scholar] [CrossRef]
- Laban, T.S.; Saadabadi, A. Monoamine Oxidase Inhibitors (MAOI). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sansone, R.A.; Sansone, L.A. Serotonin Norepinephrine Reuptake Inhibitors: A Pharmacological Comparison. Innov. Clin. Neurosci. 2014, 11, 37. [Google Scholar]
- Sheffler, Z.M.; Abdijadid, S. Antidepressants. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Moraczewski, J.; Aedma, K.K. Tricyclic Antidepressants. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022; pp. 231–256. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Antidepressants in Alzheimer’s Disease: A Focus on the Role of Mirtazapine. Pharmaceuticals 2021, 14, 930. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Huang, X.F.; Newell, K.A. The kynurenine pathway in major depression: What we know and where to next. Neurosci. Biobehav. Rev. 2021, 127, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Halaris, A.; Myint, A.M.; Savant, V.; Meresh, E.; Lim, E.; Guillemin, G.; Hoppensteadt, D.; Fareed, J.; Sinacore, J. Does escitalopram reduce neurotoxicity in major depression? J. Psychiatr. Res. 2015, 66–67, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kocki, T.; Urbańska, E.M.; Kocki, J.; Kloc, R.; Kocka, K.; Olajossy, M.; Owe-Larsson, B. Prolonged therapy with antidepressants increases hippocampal level of kynurenic acid and expression of Kat1 and Kat2 genes. Pharmacol. Rep. 2018, 70, 737–745. [Google Scholar] [CrossRef]
- Modoux, M.; Rolhion, N.; Mani, S.; Sokol, H. Tryptophan Metabolism as a Pharmacological Target. Trends Pharmacol. Sci. 2021, 42, 60–73. [Google Scholar] [CrossRef] [PubMed]
| Drug Class | Brief Description | Examples |
|---|---|---|
| SSRIs—Selective 5-HT Reuptake Inhibitors | Inhibit SERT at the presynaptic axon terminal, increasing the amount of 5-HT in the synaptic cleft [115] | Fluoxetine, sertraline, escitalopram, paroxetine [115] |
| MAOIs—Monoamine Oxidase Inhibitors | Block MAO enzyme, inhibiting the breakdown of 5-HT and other neurotransmitters, increasing their levels [116] | Moclobemide, tranylcypromine, phenelzine, isocarboxazid [116] |
| SNRIs—Serotonin–Noradrenaline Reuptake Inhibitors | Inhibit the reuptake of both 5-HT and norepinephrine, by blocking reuptake transporters, increasing their amount in the synaptic cleft [117] | Venlafaxine, duloxetine, desvenlafaxine [118] |
| TCAs—Tricyclic Antidepressants | Block the reuptake of 5-HT and norepinephrine, act as antagonists on post-synaptic cholinergic (alpha1 and alpha2), muscarinic, and histaminergic receptors (H1), enhancing neurotransmission [119] | Amitriptyline, imipramine, desipramine, clomipramine [118] |
| NaSSAs—Noradrenergic and Specific Serotonergic Antidepressants | Antagonism of 5-HT2 (5-HT2A and 5-HT2C) and 5-HT3 receptors, block α2 receptors, enhancing neurotransmission [120] | Mirtazapine [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23, 8493. https://doi.org/10.3390/ijms23158493
Correia AS, Vale N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. International Journal of Molecular Sciences. 2022; 23(15):8493. https://doi.org/10.3390/ijms23158493
Chicago/Turabian StyleCorreia, Ana Salomé, and Nuno Vale. 2022. "Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways" International Journal of Molecular Sciences 23, no. 15: 8493. https://doi.org/10.3390/ijms23158493
APA StyleCorreia, A. S., & Vale, N. (2022). Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. International Journal of Molecular Sciences, 23(15), 8493. https://doi.org/10.3390/ijms23158493







