The Potential Roles of Myokines in Adipose Tissue Metabolism with Exercise and Cold Exposure
Abstract
1. Introduction
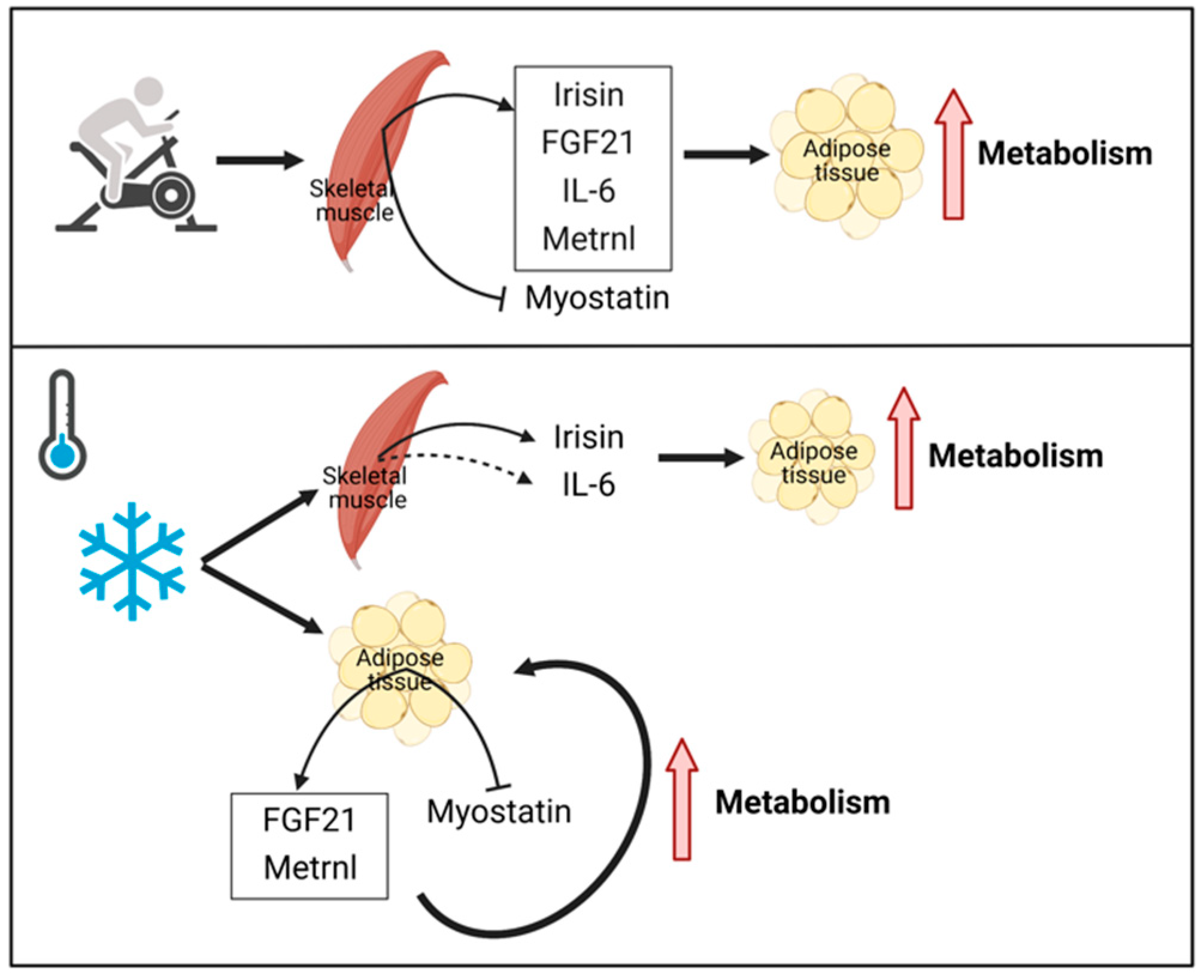
2. Similarities between Exercise- and Cold-induced Adaptations in Terms of Adipose Tissue Metabolism
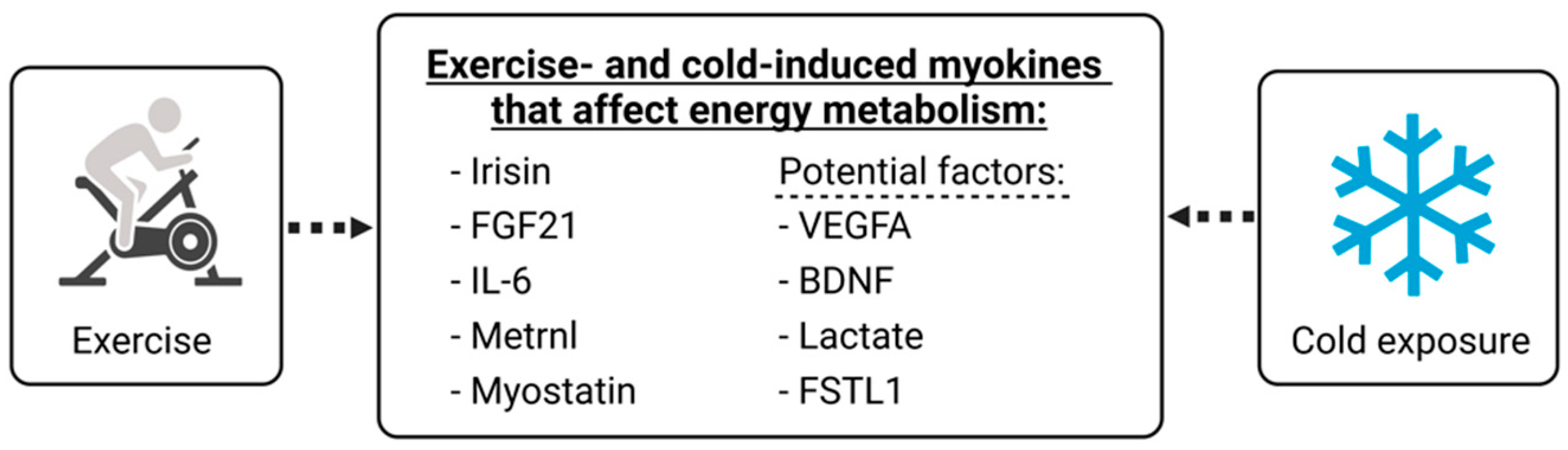
3. Key Myokines Involved in Adipose Tissue Metabolism with Exercise and Cold Exposure
3.1. Irisin
3.2. FGF21
3.3. IL-6
3.4. Metrnl
3.5. Myostatin
| Study (Year) | Population | Exercise Protocol | Temperature | Results | |||
|---|---|---|---|---|---|---|---|
| Sample Size | Mean Age | Type (Duration) | Intensity | Period (Frequency) | |||
| Ulupinar et al. (2021) [39] | 27 | 21 y | Running (40 min) | 70% HRmax | / | 0 °C, 12 °C, 24 °C | -0 °C: irisin↑ -ND in adropin |
| Ozbay et al. (2020) [42] | 32 | >18 y | Running (40 min) | 65–70% HRmax | 18 wk (4 d/wk) | Outdoor: −5–5 °C, Indoor: 21–25 °C | -Outdoor: ND in irisin, HDL-C↑ -Indoor: irisin↓, ND in adropin |
| Tsuchiya and Goto (2021) [43] | 7 | 23 y | Cycling (60 min) | 60% HRmax | / | Cold: 15–19 °C, Moderate: 24 °C, Hot: 34 °C | -Cold: ND in irisin and FGF21 -Hot: FGF21↑, myostatin↓ |
| Bubak et al. (2017) [41] | 12 | 25 y | Cycling (60 min) | 60% Wmax | / | 7 °C, 20 °C, 33 °C | -ND in FNDC5 and irisin among the 3 temperatures |
| Vosselman et al. (2015) [60] | 24 | Trained:25 y; Sedentary: 23 y | / | / | / | Cool down until shivering occurred | -Trained: FNDC5↑ -ND in irisin and IL-6 between the groups |
| Coker et al. (2017) [40] | 8 | 44 y | Running (several days) | / | / | −25–−2 °C | -Irisin↑ -ND in FGF21 and Metrnl |
| Saghebjoo et al. (2018) [69] | 13 | 25 y | Interval training (40 min) | 65% HRmax | / | Warm: 36.5–7.5 °C, Temperate: 24–25 °C, Cold: 16.5–17.5 °C | -Warm: Metrnl↑, IL-4↑ -Temperate: Metrnl↑ -Cold: Metrnl↓ |
| Jaworska et al. (2018) [63] | 20 | University students | Aerobic + Resistance (>60 min) | / | -Exercise: 2 wk (once a day) -WBC: 2 wk (5 times/wk) | −110 °C | -IGF1↑ -BDNF↑ -IL-6↓ -ND in Myostatin |
| Kozłowska-Flis et al. (2021) [44] | 65 | Training (TR): 42 y vs. Training with cryotherapy (TR-WBC): 45 y | HIIT (60 min) | 90% HRmax | -HIIT: 2 wk (3 times/wk)-WBC: 2 wk (10 times in 2 wk) | −110 °C | -TR: FGF21↑, adiponectin↑, ND in irisin |
| Jaworska et al. (2020) [45] | 25 | 20 y, Cryostimulation (CRY) vs. Control (CON) | Resistance (50 min) | 70–80% 1RM | 4 wk (3 times/wk) | −110 °C | -CRY: Myostatin↓, IL-15↑ -ND in irisin, IL-6 and BDNF |
4. Potential Circulating Factors Related to Exercise and Cold Exposure
4.1. VEGFA
4.2. BDNF
4.3. Lactate
4.4. FSTL1
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of US Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Ebi, K.L.; Capon, A.; Berry, P.; Broderick, C.; de Dear, R.; Havenith, G.; Honda, Y.; Kovats, R.S.; Ma, W.; Malik, A. Hot Weather and Heat Extremes: Health Risks. Lancet 2021, 398, 698–708. [Google Scholar] [CrossRef]
- Blondin, D.P.; Labbé, S.M.; Tingelstad, H.C.; Noll, C.; Kunach, M.; Phoenix, S.; Guérin, B.; Turcotte, É.E.; Carpentier, A.C.; Richard, D. Increased Brown Adipose Tissue Oxidative Capacity in Cold-Acclimated Humans. J. Clin. Endocrinol. Metab. 2014, 99, E438–E446. [Google Scholar] [CrossRef] [PubMed]
- Gibas-Dorna, M.; Chęcińska, Z.; Korek, E.; Kupsz, J.; Sowińska, A.; Krauss, H. Cold Water Swimming Beneficially Modulates Insulin Sensitivity in Middle-Aged Individuals. J. Aging Phys. Act. 2016, 24, 547–554. [Google Scholar] [CrossRef]
- Knechtle, B.; Waśkiewicz, Z.; Sousa, C.V.; Hill, L.; Nikolaidis, P.T. Cold Water Swimming—Benefits and Risks: A Narrative Review. Int. J. Environ. Res. Public Health 2020, 17, 8984. [Google Scholar] [CrossRef]
- Munten, S.; Ménard, L.; Gagnon, J.; Dorman, S.C.; Mezouari, A.; Gagnon, D.D. High-Intensity Interval Exercise in the Cold Regulates Acute and Postprandial Metabolism. J. Appl. Physiol. 2021, 130, 408–420. [Google Scholar] [CrossRef]
- Eckardt, K.; Görgens, S.W.; Raschke, S.; Eckel, J. Myokines in Insulin Resistance and Type 2 Diabetes. Diabetologia 2014, 57, 1087–1099. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Keller, P.; Plomgaard, P.; Febbraio, M.; Saltin, B. Searching for the Exercise Factor: Is IL-6 a Candidate? J. Muscle Res. Cell Motil. 2003, 24, 113–119. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, H.-S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Krapf, S.; Schjølberg, T.; Asoawe, L.; Honkanen, S.K.; Kase, E.T.; Thoresen, G.H.; Haugen, F. Novel Methods for Cold Exposure of Skeletal Muscle in Vivo and in Vitro Show Temperature-Dependent Myokine Production. J. Therm. Biol. 2021, 98, 102930. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S.; Saito, M. A New Era in Brown Adipose Tissue Biology: Molecular Control of Brown Fat Development and Energy Homeostasis. Annu. Rev. Physiol. 2014, 76, 225. [Google Scholar] [CrossRef] [PubMed]
- Kozak, L.P.; Anunciado-Koza, R. UCP1: Its Involvement and Utility in Obesity. Int. J. Obes. 2008, 32, S32–S38. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef]
- Slivka, D.; Dumke, C.; Tucker, T.; Cuddy, J.; Ruby, B. Human MRNA Response to Exercise and Temperature. Int. J. Sports Med. 2012, 33, 94–100. [Google Scholar] [CrossRef]
- Dewal, R.S.; Stanford, K.I. Effects of Exercise on Brown and Beige Adipocytes. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 71–78. [Google Scholar] [CrossRef]
- Lehnig, A.C.; Stanford, K.I. Exercise-Induced Adaptations to White and Brown Adipose Tissue. J. Exp. Biol. 2018, 221 (Suppl. S1), jeb161570. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and Beige Adipose Tissue: A Novel Therapeutic Strategy for Obesity and Type 2 Diabetes Mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef]
- Kern, P.A.; Finlin, B.S.; Zhu, B.; Rasouli, N.; McGehee, R.E., Jr.; Westgate, P.M.; Dupont-Versteegden, E.E. The Effects of Temperature and Seasons on Subcutaneous White Adipose Tissue in Humans: Evidence for Thermogenic Gene Induction. J. Clin. Endocrinol. Metab. 2014, 99, E2772–E2779. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B. The Browning of White Adipose Tissue: Some Burning Issues. Cell Metab. 2014, 20, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. Fat Metabolism in Exercise. Skelet. Muscle Metab. Exerc. Diabetes 1998, 441, 147–156. [Google Scholar]
- Vidal, P.; Stanford, K.I. Exercise-Induced Adaptations to Adipose Tissue Thermogenesis. Front. Endocrinol. 2020, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Yan, Z.; Yan, W.; Xia, Q.; Zhang, Y. Cold Exposure Stimulates Lipid Metabolism, Induces Inflammatory Response in the Adipose Tissue of Mice and Promotes the Osteogenic Differentiation of BMMSCs via the P38 MAPK Pathway in Vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 10875. [Google Scholar]
- Badman, M.K.; Koester, A.; Flier, J.S.; Kharitonenkov, A.; Maratos-Flier, E. Fibroblast Growth Factor 21-Deficient Mice Demonstrate Impaired Adaptation to Ketosis. Endocrinology 2009, 150, 4931–4940. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef]
- Izumiya, Y.; Bina, H.A.; Ouchi, N.; Akasaki, Y.; Kharitonenkov, A.; Walsh, K. FGF21 Is an Akt-Regulated Myokine. FEBS Lett. 2008, 582, 3805–3810. [Google Scholar] [CrossRef]
- Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; Spiegelman, B.M. FGF21 Regulates PGC-1α and Browning of White Adipose Tissues in Adaptive Thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar]
- Jonsdottir, I.H.; Schjerling, P.; Ostrowski, K.; Asp, S.; Richter, E.A.; Pedersen, B.K. Muscle Contractions Induce Interleukin-6 MRNA Production in Rat Skeletal Muscles. J. Physiol. 2000, 528, 157–163. [Google Scholar] [CrossRef]
- Steensberg, A.; Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of Interleukin-6 in Contracting Human Skeletal Muscles Can Account for the Exercise-induced Increase in Plasma Interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like Is a Hormone That Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lawler, A.M.; Lee, S.-J. Regulation of Skeletal Muscle Mass in Mice by a New TGF-p Superfamily Member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Olesen, J.L.; Schjerling, P.; Haddad, F.; Langberg, H.; Baldwin, K.M.; Kjaer, M. Short-Term Strength Training and the Expression of Myostatin and IGF-I Isoforms in Rat Muscle and Tendon: Differential Effects of Specific Contraction Types. J. Appl. Physiol. 2007, 102, 573–581. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kong, X.; Yao, T.; Zhou, P.; Kazak, L.; Tenen, D.; Lyubetskaya, A.; Dawes, B.A.; Tsai, L.; Kahn, B.B.; Spiegelman, B.M.; et al. Brown Adipose Tissue Controls Skeletal Muscle Function via the Secretion of Myostatin. Cell Metab. 2018, 28, 631–643.e3. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G. The Myokine Irisin Increases Cortical Bone Mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef]
- Mahgoub, M.O.; D’Souza, C.; Al Darmaki, R.S.; Baniyas, M.M.; Adeghate, E. An Update on the Role of Irisin in the Regulation of Endocrine and Metabolic Functions. Peptides 2018, 104, 15–23. [Google Scholar] [CrossRef]
- Dulian, K.; Laskowski, R.; Grzywacz, T.; Kujach, S.; Flis, D.J.; Smaruj, M.; Ziemann, E. The Whole Body Cryostimulation Modifies Irisin Concentration and Reduces Inflammation in Middle Aged, Obese Men. Cryobiology 2015, 71, 398–404. [Google Scholar] [CrossRef]
- Ulupınar, S.; Özbay, S.; Gençoğlu, C.; Altınkaynak, K.; Şebin, E.; Oymak, B. Exercise in the Cold Causes Greater Irisin Release but May Not Be Enough for Adropin. Chin. J. Physiol. 2021, 64, 6. [Google Scholar]
- Coker, R.H.; Weaver, A.N.; Coker, M.S.; Murphy, C.J.; Gunga, H.-C.; Steinach, M. Metabolic Responses to the Yukon Arctic Ultra: Longest and Coldest in the World. Med. Sci. Sports Exerc. 2017, 49, 357–362. [Google Scholar] [CrossRef]
- Bubak, M.P.; Heesch, M.W.; Shute, R.J.; Dinan, N.E.; Laursen, T.L.; La Salle, D.T.; Slivka, D.R. Irisin and Fibronectin Type III Domain-Containing 5 Responses to Exercise in Different Environmental Conditions. Int. J. Exerc. Sci. 2017, 10, 666. [Google Scholar] [PubMed]
- Ozbay, S.; Ulupınar, S.; Şebin, E.; Altınkaynak, K. Acute and Chronic Effects of Aerobic Exercise on Serum Irisin, Adropin, and Cholesterol Levels in the Winter Season: Indoor Training versus Outdoor Training. Chin. J. Physiol. 2020, 63, 21. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Goto, K. Myokine Secretion Following Moderate-Intensity Endurance Exercise under Different Environmental Temperatures. Cytokine 2021, 144, 155553. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska-Flis, M.; Rodziewicz-Flis, E.; Micielska, K.; Kortas, J.; Jaworska, J.; Borkowska, A.; Sansoni, V.; Perego, S.; Lombardi, G.; Ziemann, E. Short and Long-Term Effects of High-Intensity Interval Training Applied Alone or with Whole-Body Cryostimulation on Glucose Homeostasis and Myokine Levels in Overweight to Obese Subjects. Front. Biosci.-Landmark 2021, 26, 1132–1146. [Google Scholar]
- Jaworska, J.; Rodziewicz-Flis, E.; Kortas, J.; Kozłowska, M.; Micielska, K.; Babińska, A.; Laskowski, R.; Lombardi, G.; Ziemann, E. Short-Term Resistance Training Supported by Whole-Body Cryostimulation Induced a Decrease in Myostatin Concentration and an Increase in Isokinetic Muscle Strength. IJERPH 2020, 17, 5496. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a Novel FGF, FGF-21, Preferentially Expressed in the Liver. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 2000, 1492, 203–206. [Google Scholar] [CrossRef]
- Parmar, B.; Lewis, J.E.; Samms, R.J.; Ebling, F.J.; Cheng, C.C.; Adams, A.C.; Mallinson, J.; Cooper, S.; Taylor, T.; Ghasemi, R. Eccentric Exercise Increases Circulating Fibroblast Activation Protein α but Not Bioactive Fibroblast Growth Factor 21 in Healthy Humans. Exp. Physiol. 2018, 103, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, Y.; Aoi, W.; Takanami, Y.; Kawai, Y.; Mizushima, K.; Naito, Y.; Yoshikawa, T. Acute Exercise Increases Fibroblast Growth Factor 21 in Metabolic Organs and Circulation. Physiol. Rep. 2016, 4, e12828. [Google Scholar] [CrossRef] [PubMed]
- Kruse, R.; Vienberg, S.G.; Vind, B.F.; Andersen, B.; Højlund, K. Effects of Insulin and Exercise Training on FGF21, Its Receptors and Target Genes in Obesity and Type 2 Diabetes. Diabetologia 2017, 60, 2042–2051. [Google Scholar] [CrossRef]
- Martin, A.R.; Chung, S.; Koehler, K. Is Exercise a Match for Cold Exposure? Common Molecular Framework for Adipose Tissue Browning. Int. J. Sports Med. 2020, 41, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, R.; Kobayashi, L.; Shirai, T.; Takemasa, T. Effects of Exercise Intensity on White Adipose Tissue Browning and Its Regulatory Signals in Mice. Physiol. Rep. 2022, 10, e15205. [Google Scholar] [CrossRef]
- Hansen, J.S.; Clemmesen, J.O.; Secher, N.H.; Hoene, M.; Drescher, A.; Weigert, C.; Pedersen, B.K.; Plomgaard, P. Glucagon-to-Insulin Ratio Is Pivotal for Splanchnic Regulation of FGF-21 in Humans. Mol. Metab. 2015, 4, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.M.; Verdich, C.; Toubro, S.; Astrup, A.; Richelsen, B. Association between Measures of Insulin Sensitivity and Circulating Levels of Interleukin-8, Interleukin-6 and Tumor Necrosis Factor-Alpha. Effect of Weight Loss in Obese Men. Eur. J. Endocrinol. 2003, 148, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Van Hall, G.; Steensberg, A.; Sacchetti, M.; Fischer, C.; Keller, C.; Schjerling, P.; Hiscock, N.; Møller, K.; Saltin, B.; Febbraio, M.A. Interleukin-6 Stimulates Lipolysis and Fat Oxidation in Humans. J. Clin. Endocrinol. Metab. 2003, 88, 3005–3010. [Google Scholar] [CrossRef]
- Raschke, S.; Eckel, J. Adipo-Myokines: Two Sides of the Same Coin—Mediators of Inflammation and Mediators of Exercise. Mediat. Inflamm. 2013, 2013, 320724. [Google Scholar] [CrossRef]
- Bal, N.C.; Maurya, S.K.; Pani, S.; Sethy, C.; Banerjee, A.; Das, S.; Patnaik, S.; Kundu, C.N. Mild Cold Induced Thermogenesis: Are BAT and Skeletal Muscle Synergistic Partners? Biosci. Rep. 2017, 37, BSR20171087. [Google Scholar] [CrossRef]
- Knudsen, J.G.; Murholm, M.; Carey, A.L.; Biensø, R.S.; Basse, A.L.; Allen, T.L.; Hidalgo, J.; Kingwell, B.A.; Febbraio, M.A.; Hansen, J.B.; et al. Role of IL-6 in Exercise Training- and Cold-Induced UCP1 Expression in Subcutaneous White Adipose Tissue. PLoS ONE 2014, 9, e84910. [Google Scholar] [CrossRef]
- Yildirim, N.C.; Yurekli, M. The Effect of Adrenomedullin and Cold Stress on Interleukin-6 Levels in Some Rat Tissues. Clin. Exp. Immunol. 2010, 161, 171–175. [Google Scholar] [CrossRef]
- Vosselman, M.J.; Hoeks, J.; Brans, B.; Pallubinsky, H.; Nascimento, E.B.M.; van der Lans, A.A.J.J.; Broeders, E.P.M.; Mottaghy, F.M.; Schrauwen, P.; van Marken Lichtenbelt, W.D. Low Brown Adipose Tissue Activity in Endurance-Trained Compared with Lean Sedentary Men. Int. J. Obes. 2015, 39, 1696–1702. [Google Scholar] [CrossRef]
- Rhind, S.G.; Castellani, J.W.; Brenner, I.K.; Shephard, R.J.; Zamecnik, J.; Montain, S.J.; Young, A.J.; Shek, P.N. Intracellular Monocyte and Serum Cytokine Expression Is Modulated by Exhausting Exercise and Cold Exposure. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 281, R66–R75. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, E.; Olek, R.A.; Kujach, S.; Grzywacz, T.; Antosiewicz, J.; Garsztka, T.; Laskowski, R. Five-Day Whole-Body Cryostimulation, Blood Inflammatory Markers, and Performance in High-Ranking Professional Tennis Players. J. Athl. Train. 2012, 47, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, J.; Micielska, K.; Kozłowska, M.; Wnorowski, K.; Skrobecki, J.; Radzimiński, L.; Babińska, A.; Rodziewicz, E.; Lombardi, G.; Ziemann, E. A 2-Week Specific Volleyball Training Supported by the Whole Body Cryostimulation Protocol Induced an Increase of Growth Factors and Counteracted Deterioration of Physical Performance. Front. Physiol. 2018, 9, 1711. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y. Aerobic Exercise Increases Meteorin-Like Protein in Muscle and Adipose Tissue of Chronic High-Fat Diet-Induced Obese Mice. BioMed Res. Int. 2018, 2018, 6283932. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Song, J.; Zheng, S.-L.; Fan, M.-B.; Guan, Y.-F.; Qu, Y.; Xu, J.; Wang, P.; Miao, C.-Y. Adipocyte Metrnl Antagonizes Insulin Resistance through PPARγ Signaling. Diabetes 2015, 64, 4011–4022. [Google Scholar] [CrossRef]
- Amor, M.; Itariu, B.K.; Moreno-Viedma, V.; Keindl, M.; Jürets, A.; Prager, G.; Langer, F.; Grablowitz, V.; Zeyda, M.; Stulnig, T.M. Serum Myostatin Is Upregulated in Obesity and Correlates with Insulin Resistance in Humans. Exp. Clin. Endocrinol. Diabetes 2019, 127, 550–556. [Google Scholar] [CrossRef]
- Dong, J.; Dong, Y.; Chen, F.; Mitch, W.E.; Zhang, L. Inhibition of Myostatin in Mice Improves Insulin Sensitivity via Irisin-Mediated Cross Talk between Muscle and Adipose Tissues. Int. J. Obes. 2016, 40, 434–442. [Google Scholar] [CrossRef]
- Kozłowska, M.; Kortas, J.; Żychowska, M.; Antosiewicz, J.; Żuczek, K.; Perego, S.; Lombardi, G.; Ziemann, E. Beneficial Effects of Whole-Body Cryotherapy on Glucose Homeostasis and Amino Acid Profile Are Associated with a Reduced Myostatin Serum Concentration. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Saghebjoo, M.; Einaloo, A.; Mogharnasi, M.; Ahmadabadi, F. The Response of Meteorin-like Hormone and Interleukin-4 in Overweight Women during Exercise in Temperate, Warm and Cold Water. Horm. Mol. Biol. Clin. Investig. 2018, 36, 20180027. [Google Scholar] [CrossRef]
- Kraus, R.M.; Stallings III, H.W.; Yeager, R.C.; Gavin, T.P. Circulating Plasma VEGF Response to Exercise in Sedentary and Endurance-Trained Men. J. Appl. Physiol. 2004, 96, 1445–1450. [Google Scholar] [CrossRef]
- Ohno, H.; Shirato, K.; Sakurai, T.; Ogasawara, J.; Sumitani, Y.; Sato, S.; Imaizumi, K.; Ishida, H.; Kizaki, T. Effect of Exercise on HIF-1 and VEGF Signaling. J. Phys. Fit. Sports Med. 2012, 1, 5–16. [Google Scholar] [CrossRef]
- Olfert, I.M.; Howlett, R.A.; Tang, K.; Dalton, N.D.; Gu, Y.; Peterson, K.L.; Wagner, P.D.; Breen, E.C. Muscle-specific VEGF Deficiency Greatly Reduces Exercise Endurance in Mice. J. Physiol. 2009, 587, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Kusminski, C.M.; Luby-Phelps, K.; Spurgin, S.B.; An, Y.A.; Wang, Q.A.; Holland, W.L.; Scherer, P.E. Brown Adipose Tissue Derived VEGF-A Modulates Cold Tolerance and Energy Expenditure. Mol. Metab. 2014, 3, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Matthews, V.B.; Åström, M.-B.; Chan, M.H.S.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Åkerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-Derived Neurotrophic Factor Is Produced by Skeletal Muscle Cells in Response to Contraction and Enhances Fat Oxidation via Activation of AMP-Activated Protein Kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Griffin, É.W.; Mullally, S.; Foley, C.; Warmington, S.A.; O’Mara, S.M.; Kelly, Á.M. Aerobic Exercise Improves Hippocampal Function and Increases BDNF in the Serum of Young Adult Males. Physiol. Behav. 2011, 104, 934–941. [Google Scholar] [CrossRef]
- Xu, B.; Goulding, E.H.; Zang, K.; Cepoi, D.; Cone, R.D.; Jones, K.R.; Tecott, L.H.; Reichardt, L.F. Brain-Derived Neurotrophic Factor Regulates Energy Balance Downstream of Melanocortin-4 Receptor. Nat. Neurosci. 2003, 6, 736–742. [Google Scholar] [CrossRef]
- Dun, S.L.; Lyu, R.-M.; Chen, Y.-H.; Chang, J.-K.; Luo, J.J.; Dun, N.J. Irisin-Immunoreactivity in Neural and Non-Neural Cells of the Rodent. Neuroscience 2013, 240, 155–162. [Google Scholar] [CrossRef]
- Zsuga, J.; Tajti, G.; Papp, C.; Juhasz, B.; Gesztelyi, R. FNDC5/Irisin, a Molecular Target for Boosting Reward-Related Learning and Motivation. Med. Hypotheses 2016, 90, 23–28. [Google Scholar] [CrossRef]
- Papp, C.; Pak, K.; Erdei, T.; Juhasz, B.; Seres, I.; Szentpeteri, A.; Kardos, L.; Szilasi, M.; Gesztelyi, R.; Zsuga, J. Alteration of the Irisin–Brain-Derived Neurotrophic Factor Axis Contributes to Disturbance of Mood in COPD Patients. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2023. [Google Scholar] [CrossRef]
- Brooks, G.A. Cell–Cell and Intracellular Lactate Shuttles. J. Physiol. 2009, 587, 5591–5600. [Google Scholar] [CrossRef]
- Jeanson, Y.; Ribas, F.; Galinier, A.; Arnaud, E.; Ducos, M.; André, M.; Chenouard, V.; Villarroya, F.; Casteilla, L.; Carrière, A. Lactate Induces FGF21 Expression in Adipocytes through a P38-MAPK Pathway. Biochem. J. 2016, 473, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Carrière, A.; Jeanson, Y.; Berger-Müller, S.; André, M.; Chenouard, V.; Arnaud, E.; Barreau, C.; Walther, R.; Galinier, A.; Wdziekonski, B. Browning of White Adipose Cells by Intermediate Metabolites: An Adaptive Mechanism to Alleviate Redox Pressure. Diabetes 2014, 63, 3253–3265. [Google Scholar] [CrossRef]
- Görgens, S.W.; Raschke, S.; Holven, K.B.; Jensen, J.; Eckardt, K.; Eckel, J. Regulation of Follistatin-like Protein 1 Expression and Secretion in Primary Human Skeletal Muscle Cells. Arch. Physiol. Biochem. 2013, 119, 75–80. [Google Scholar] [CrossRef]
- Horak, M.; Kuruczova, D.; Zlamal, F.; Tomandl, J.; Bienertova-Vasku, J. Follistatin-like 1 Is Downregulated in Morbidly and Super Obese Central-European Population. Dis. Markers 2018, 2018, 4140815. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Shi, X.; Lu, T.; Ruan, H.; Gao, Y. The Glycoprotein Follistatin-like 1 Promotes Brown Adipose Thermogenesis. Metabolism 2019, 98, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Kon, M.; Ebi, Y.; Nakagaki, K. Effects of Acute Sprint Interval Exercise on Follistatin-like 1 and Apelin Secretions. Arch. Physiol. Biochem. 2021, 127, 223–227. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, T.; Mokou, M.; Li, L.; Li, P.; Song, J.; Liu, H.; Zhu, Z.; Liu, D.; Yang, M.; et al. Follistatin-like 1 as a Novel Adipomyokine Related to Insulin Resistance and Physical Activity. J. Clin. Endocrinol. Metab. 2020, 105, e4499–e4509. [Google Scholar] [CrossRef]
| Myokine | Related to Exercise | Related to Cold Exposure | ||
|---|---|---|---|---|
| Rodent | Human | Rodent | Human | |
| Irisin | Yes [26] | Yes [26] | Not sure | Yes [27] |
| FGF21 | Yes [28] | Not sure | Yes [29] | Yes [27] |
| IL-6 | Yes [30] | Yes [31] | Not sure | Not sure |
| Metrnl | Yes [32] | Yes [32] | Yes [32] | Not sure |
| Myostatin | Yes [33] | Yes [34] | Yes [35] | Not sure |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Bae, J.-H.; Wang, Y.; Song, W. The Potential Roles of Myokines in Adipose Tissue Metabolism with Exercise and Cold Exposure. Int. J. Mol. Sci. 2022, 23, 11523. https://doi.org/10.3390/ijms231911523
Jiang S, Bae J-H, Wang Y, Song W. The Potential Roles of Myokines in Adipose Tissue Metabolism with Exercise and Cold Exposure. International Journal of Molecular Sciences. 2022; 23(19):11523. https://doi.org/10.3390/ijms231911523
Chicago/Turabian StyleJiang, Shu, Jun-Hyun Bae, Yangwenjie Wang, and Wook Song. 2022. "The Potential Roles of Myokines in Adipose Tissue Metabolism with Exercise and Cold Exposure" International Journal of Molecular Sciences 23, no. 19: 11523. https://doi.org/10.3390/ijms231911523
APA StyleJiang, S., Bae, J.-H., Wang, Y., & Song, W. (2022). The Potential Roles of Myokines in Adipose Tissue Metabolism with Exercise and Cold Exposure. International Journal of Molecular Sciences, 23(19), 11523. https://doi.org/10.3390/ijms231911523






