Macropore Regulation of Hydroxyapatite Osteoinduction via Microfluidic Pathway
Abstract
1. Introduction
2. Results
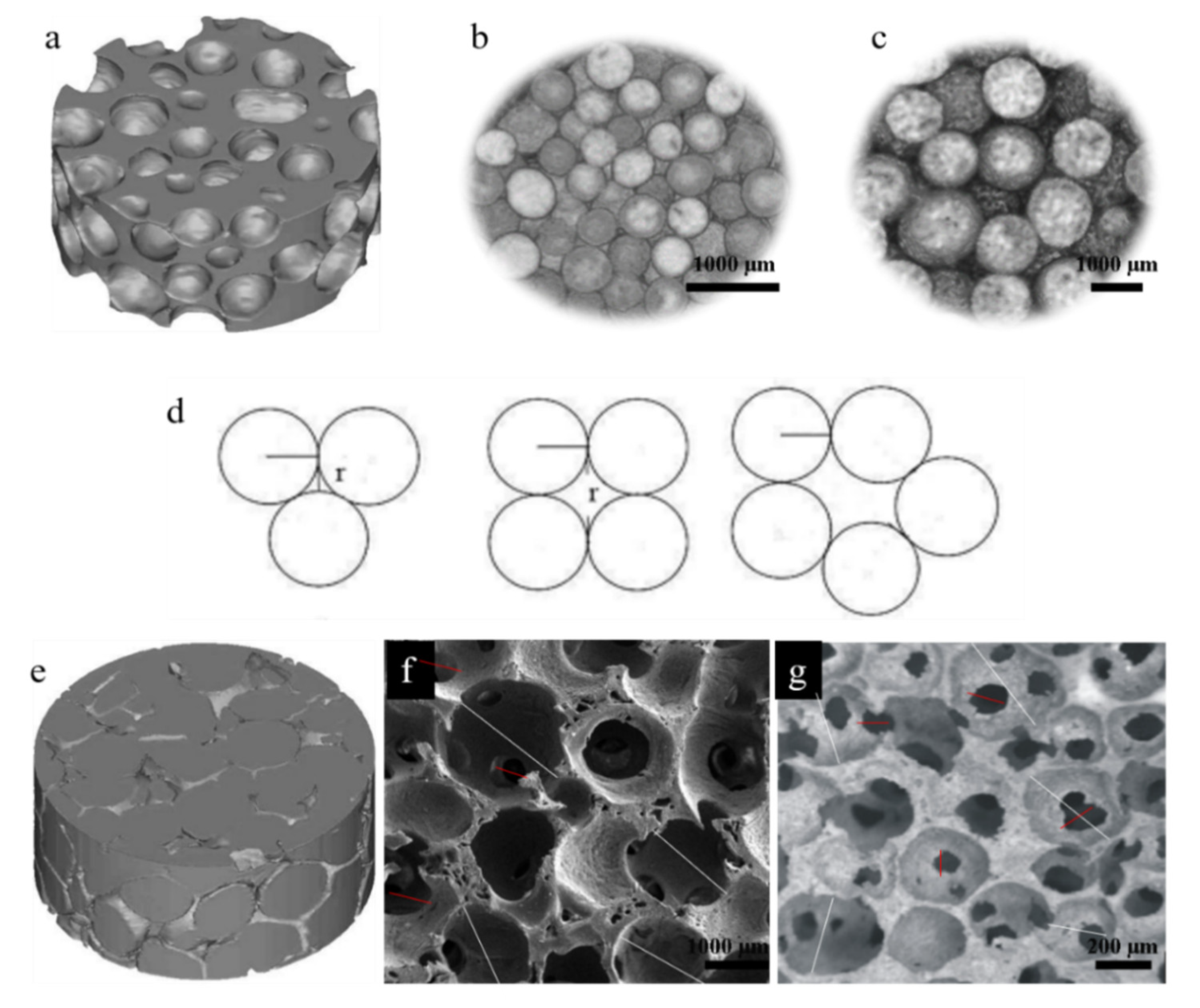
2.1. The Macro-Pore Structure Properties of HASAs and HAPPs
2.2. General Conditions of Animals
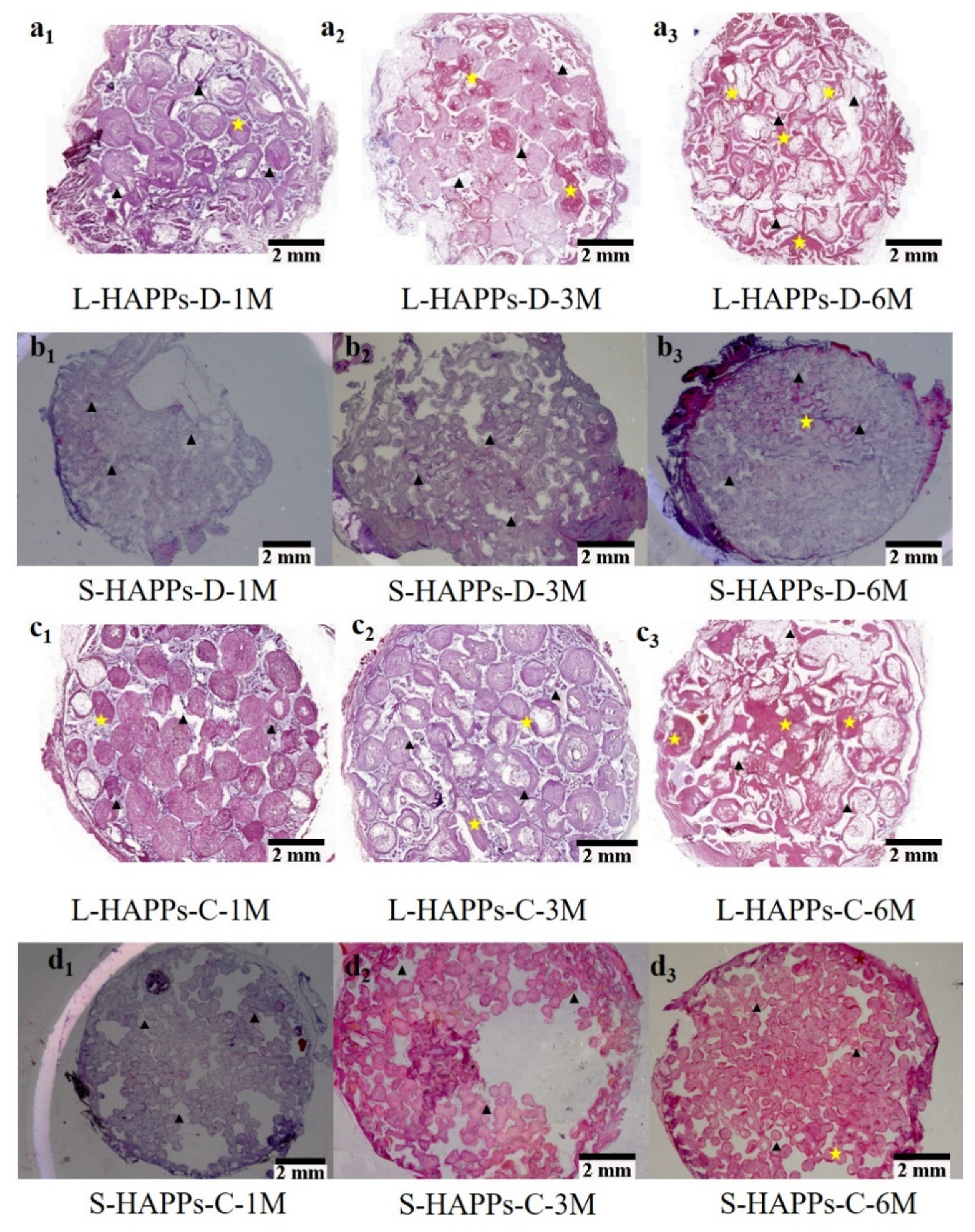
2.3. The Histological Characterization of Ectopic Bone Formation and Blood Vessel Ingrowth
2.4. Compressive Strength of Reconstructed Scaffolds
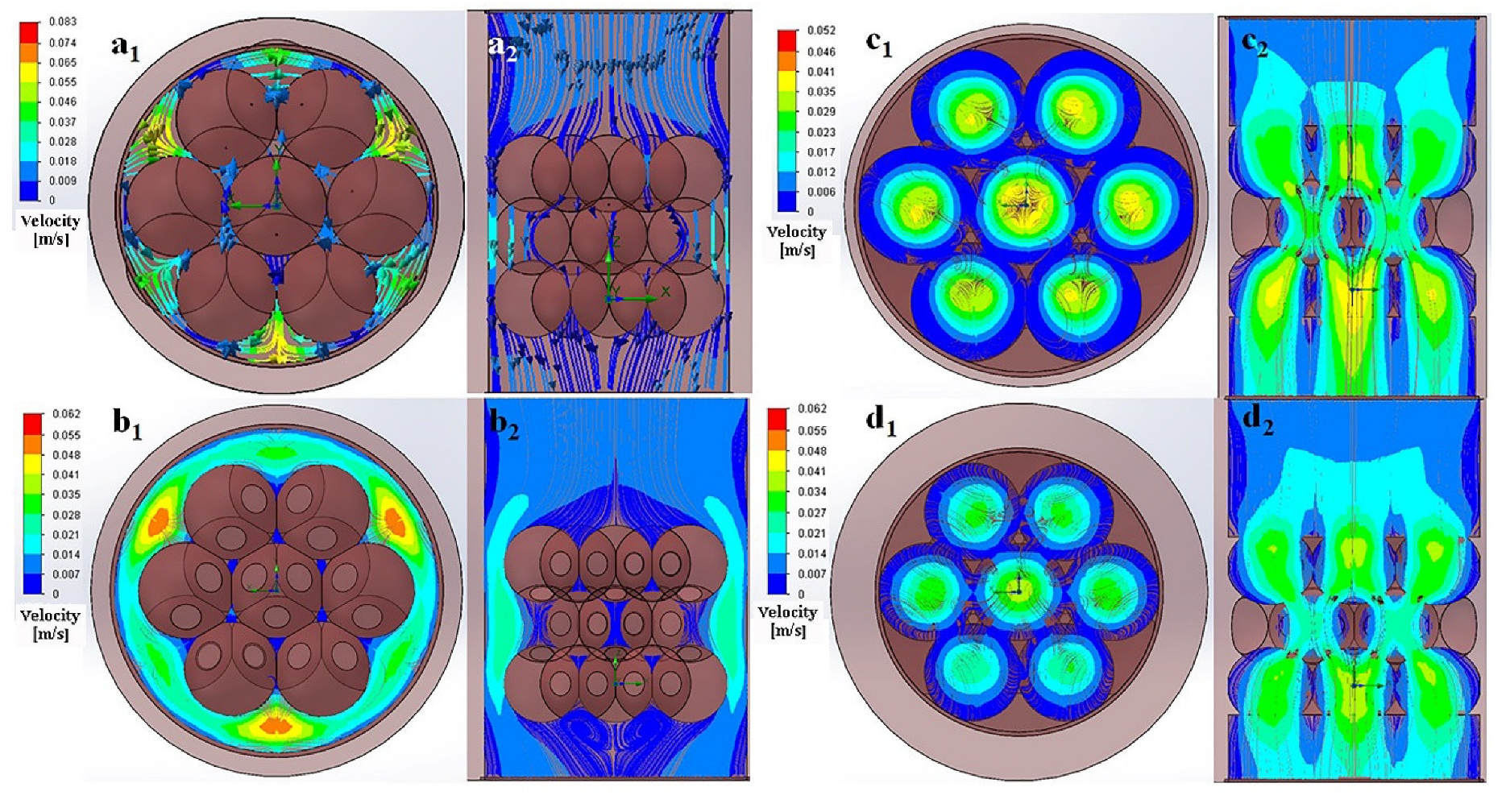
2.5. Numerical Simulation of Fluid Flow in the Scaffolds
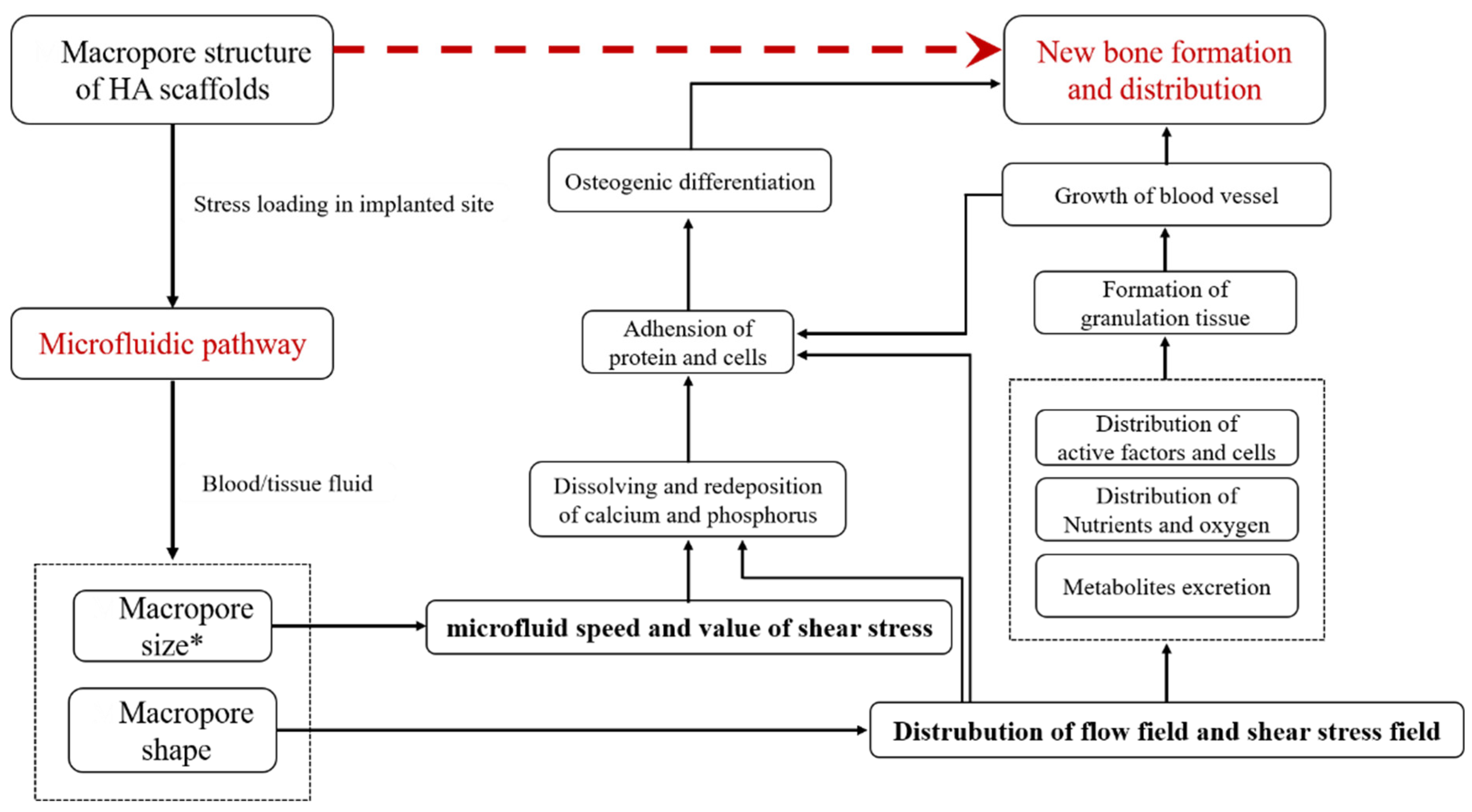
3. Discussion
4. Materials and Methods
4.1. HA Scaffolds Preparation
4.1.1. Two Macro-Pore Sizes of HASAs Preparation
4.1.2. Two Macro-Pore Sizes of HAPPs Preparation
4.2. Animal Experiment
4.3. Sample Preparation
4.4. Histology, Histomorphometry, and Compression Tests
4.5. Mathematical Models of the Macroporous Structure of 3D Scaffolds and Microfluidic Pathway
4.5.1. Geometry Reconstruction
4.5.2. CFD Model Creation
4.5.3. Mesh and Numerical Simulation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xingdong, Z.; Pin, Z.; Jianguo, Z.; Weiqun, C.; Wu, C. A Study of Hydroxyapatite Ceramics and its Osteogenesis. In Bioceramics and the Human Body; Ravaglioli, A., Krajewski, A., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 408–416. [Google Scholar] [CrossRef]
- Ripamonti, U. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials 1996, 17, 31–35. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Y.; de Bruijn, J.D.; de Groot, K.; Zhang, X. Tissue responses of calcium phosphate cement: A study in dogs. Biomaterials 2000, 21, 1283–1290. [Google Scholar] [CrossRef]
- Habibovic, P.; Gbureck, U.; Doillon, C.J.; Bassett, D.C.; van Blitterswijk, C.A.; Barralet, J.E. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials 2008, 29, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Mao, L.; Liu, J.; Yang, Z.; Zhang, W.; Shu, M.; Hu, N.; Jiang, L.; Fang, B. Fabrication, characterization and cellular biocompatibility of porous biphasic calcium phosphate bioceramic scaffolds with different pore sizes. Ceram. Int. 2016, 42, 15311–15318. [Google Scholar] [CrossRef]
- Guo, X.; Li, M.; Qi, W.; Bai, H.; Nie, Z.; Hu, Z.; Xiao, Y.; de Bruijn, J.D.; Bao, C.; Yuan, H. Serial cellular events in bone formation initiated by calcium phosphate ceramics. Acta Biomater. 2021, 134, 730–743. [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Sumo, S.; Bayrak, E.S.; Brey, E.M.; Cinar, A. Three-dimensional modeling of angiogenesis in porous biomaterial scaffolds. Biomaterials 2013, 34, 2875–2887. [Google Scholar] [CrossRef]
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C 2016, 61, 922–939. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, W.; Liu, D.; Zhang, H.; Gao, P.; Geng, L.; Yuan, Y.; Lu, J.; Wang, Z. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3K/Akt pathways. Sci. Rep. 2015, 5, 9409. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wiraja, C.; Muhammad, H.B.; Xu, C.; Wang, D.-A. Improvement of endothelial progenitor outgrowth cell (EPOC)-mediated vascularization in gelatin-based hydrogels through pore size manipulation. Acta Biomater. 2017, 58, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.-i.; Rodan, G.A. Control of osteoblast function and regulation of bone mass. Nature 2003, 423, 349–355. [Google Scholar] [CrossRef]
- Bodde, E.W.H.; Cammaert, C.T.R.; Wolke, J.G.C.; Spauwen, P.H.M.; Jansen, J.A. Investigation as to the osteoinductivity of macroporous calcium phosphate cement in goats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83B, 161–168. [Google Scholar] [CrossRef]
- Wang, H.; Zhi, W.; Lu, X.; Li, X.; Duan, K.; Duan, R.; Mu, Y.; Weng, J. Comparative studies on ectopic bone formation in porous hydroxyapatite scaffolds with complementary pore structures. Acta Biomater 2013, 9, 8413–8421. [Google Scholar] [CrossRef]
- Barradas, A.M.; Yuan, H.; van Blitterswijk, C.A.; Habibovic, P. Osteoinductive biomaterials: Current knowledge of properties, experimental models and biological mechanisms. Eur. Cell Mater. 2011, 21, 407–429; discussion 429. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Chang, B.; Song, W.; Han, T.; Yan, J.; Li, F.; Zhao, L.; Kou, H.; Zhang, Y. Influence of pore size of porous titanium fabricated by vacuum diffusion bonding of titanium meshes on cell penetration and bone ingrowth. Acta Biomater. 2016, 33, 311–321. [Google Scholar] [CrossRef]
- Choi, D.J.; Park, S.J.; Gu, B.K.; Kim, Y.-J.; Chung, S.; Kim, C.-H. Effect of the pore size in a 3D bioprinted gelatin scaffold on fibroblast proliferation. J. Ind. Eng. Chem. 2018, 67, 388–395. [Google Scholar] [CrossRef]
- Yeatts, A.B.; Fisher, J.P. Bone tissue engineering bioreactors: Dynamic culture and the influence of shear stress. Bone 2011, 48, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Lacroix, D.; Ito, K.; van Rietbergen, B.; Hofmann, S. Changes in scaffold porosity during bone tissue engineering in perfusion bioreactors considerably affect cellular mechanical stimulation for mineralization. Bone Rep. 2020, 12, 100265. [Google Scholar] [CrossRef] [PubMed]
- Montorsi, M.; Genchi, G.G.; De Pasquale, D.; De Simoni, G.; Sinibaldi, E.; Ciofani, G. Design, fabrication, and characterization of a multimodal reconfigurable bioreactor for bone tissue engineering. Biotechnol. Bioeng. 2022, 119, 1965–1979. [Google Scholar] [CrossRef]
- Shanglong, X.; Pingan, D.; Youzhuan, X.; Yang, Y. Cell distribution in a scaffold with random architectures under the influence of fluid dynamics. J. Biomater. Appl. 2008, 23, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.; Zauel, R.; Stockman, H.; Guldberg, R.; Fyhrie, D. 3-D computational modeling of media flow through scaffolds in a perfusion bioreactor. J. Biomech. 2005, 38, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, P.; Weng, J.; Zhi, W.; Hu, Y.; Feng, H.; Yao, Y.; Li, S.; Xia, T. Histomorphological researches on large porous hydroxyapatite cylinder tubes with polylactic acid surface coating in different nonskeletal sites in vivo. J. Biomed. Mater. Res. A 2012, 100, 1203–1208. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, N.; Zhu, M.; Qiu, Q.; Zhao, P.; Zheng, C.; Bai, Q.; Zeng, Q.; Lu, T. The contribution of pore size and porosity of 3D printed porous titanium scaffolds to osteogenesis. Biomater. Adv. 2022, 133, 112651. [Google Scholar] [CrossRef]
- Peng, Q.; Jiang, F.; Huang, P.; Zhou, S.; Weng, J.; Bao, C.; Zhang, C.; Yu, H. A novel porous bioceramics scaffold by accumulating hydroxyapatite spherules for large bone tissue engineering in vivo. I. Preparation and characterization of scaffold. J. Biomed. Mater. Res. Part A 2010, 93A, 920–929. [Google Scholar] [CrossRef]
- Zhao, J.; Li, J.-Y.; Zhi, W.; Lu, X.; Jia, Z.-B.; Weng, J. Preparation and Optimization of Porous HA Ceramic Scaffolds by Wax Spheres Leaching Method. J. Inorg. Mater. 2013, 28, 74–78. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, L.Y.; Yang, X.B.; Weng, J. Preparation of bioactive porous HA/PCL composite scaffolds. Appl. Surf. Sci. 2008, 255, 2942–2946. [Google Scholar] [CrossRef]
- Mastrogiacomo, M.; Scaglione, S.; Martinetti, R.; Dolcini, L.; Beltrame, F.; Cancedda, R.; Quarto, R. Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials 2006, 27, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Wang, Z.; Lu, J.; Liu, J.; Chen, G.; Lv, R.; Wang, J.; Lin, K.; Zhang, J.; Huang, X. The Correlation Between the Internal Structure and Vascularization of Controllable Porous Bioceramic Materials In Vivo: A Quantitative Study. Tissue Eng. Part A 2010, 16, 3791–3803. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, T.; Hou, W.; Liu, F.; Qing, W.; Huang, L.; Ma, G.; Mu, Y.; Weng, J. The response of host blood vessels to graded distribution of macro-pores size in the process of ectopic osteogenesis. Mater. Sci. Eng. C 2020, 109, 110641. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Xu, T.; Li, J.; Guo, T.; Lu, X.; Ren, J.; Ren, X.; Mu, Y.; Weng, J. Effect of the interconnecting window diameter of hydroxyapatite scaffolds on vascularization and osteoinduction. Ceram. Int. 2022, 48, 25070–25078. [Google Scholar] [CrossRef]
- Habibovic, P.; Sees, T.M.; Doel, M.A.V.D.; Blitterswijk, C.A.V.; Groot, K.D. Osteoinduction by biomaterials—Physicochemical and structural influences. J. Biomed. Mater. Res. Part A 2006, 77, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Crooks, J.; Kirkbride, A.N. Sintered porous hydroxyapatites with intrinsic osteoinductive activity: Geometric induction of bone formation. S. Afr. J. Sci. 1999, 95, 335–343. [Google Scholar] [CrossRef]
- Yuan, H.; Fernandes, H.; Habibovic, P.; de Boer, J.; Barradas, A.M.C.; de Ruiter, A.; Walsh, W.R.; van Blitterswijk, C.A.; de Bruijn, J.D. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc. Natl. Acad. Sci. USA 2010, 107, 13614–13619. [Google Scholar] [CrossRef]
- Yuan, H.P.; Yang, Z.J.; Li, Y.B.; Zhang, X.D.; De Bruijn, J.D.; De Groot, K. Osteoinduction by calcium phosphate biomaterials. J. Mater. Sci.-Mater. Med. 1998, 9, 723–726. [Google Scholar] [CrossRef]
- Yuan, H.; van Blitterswijk, C.A.; de Groot, K.; de Bruijn, J.D. A comparison of bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) implanted in muscle and bone of dogs at different time periods. J. Biomed. Mater. Res. Part A 2006, 78A, 139–147. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, L.; Ji, X.; He, Y.; Cui, Y.; Liu, X.; Xuan, C.; Wang, Z.; Yang, W.; Chai, M.; et al. High-Strength Hydroxyapatite Scaffolds with Minimal Surface Macrostructures for Load-Bearing Bone Regeneration. Adv. Funct. Mater. 2022, 32, 2204182. [Google Scholar] [CrossRef]
- Ripamonti, U.; Van Den Heever, B.; Crooks, J.; Tucker, M.M.; Sampath, T.K.; Rueger, D.C.; Reddi, A.H. Long-term evaluation of bone formation by osteogenic protein 1 in the baboon and relative efficacy of bone-derived bone morphogenetic proteins delivered by irradiated xenogeneic collagenous matrices. J. Bone Miner. Res. 2000, 15, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, C.; Gao, H.; Jia, L.; Zeng, H.; Zheng, L.; Wang, C.; Zhang, H.; Wang, L.; Song, J.; et al. Biomechanical Effects of 3D-Printed Bioceramic Scaffolds With Porous Gradient Structures on the Regeneration of Alveolar Bone Defect: A Comprehensive Study. Front. Bioeng Biotechnol. 2022, 10, 882631. [Google Scholar] [CrossRef] [PubMed]
- Suvarnapathaki, S.; Wu, X.; Lantigua, D.; Nguyen, M.A.; Camci-Unal, G. Hydroxyapatite-Incorporated Composite Gels Improve Mechanical Properties and Bioactivity of Bone Scaffolds. Macromol. Biosci. 2020, 20, 2000176. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Hoff, B.; Suvarnapathaki, S.; Lantigua, D.; McCarthy, C.; Wu, B.; Camci-Unal, G. Mineralized Hydrogels Induce Bone Regeneration in Critical Size Cranial Defects. Adv. Healthc. Mater. 2021, 10, 2001101. [Google Scholar] [CrossRef]
- Jaasma, M.J.; Plunkett, N.A.; O’Brien, F.J. Design and validation of a dynamic flow perfusion bioreactor for use with compliant tissue engineering scaffolds. J. Biotechnol. 2008, 133, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Bonvin, C.; Overney, J.; Shieh, A.C.; Dixon, J.B.; Swartz, M.A. A multichamber fluidic device for 3D cultures under interstitial flow with live imaging: Development, characterization, and applications. Biotechnol. Bioeng. 2010, 105, 982–991. [Google Scholar] [CrossRef]
- Nihouannen, D.L.; Daculsi, G.; Saffarzadeh, A.; Gauthier, O.; Delplace, S.; Pilet, P.; Layrolle, P. Ectopic bone formation by microporous calcium phosphate ceramic particles in sheep muscles. Bone 2005, 36, 1086. [Google Scholar] [CrossRef]
- Le Huec, J.C.; Clément, D.; Brouillaud, B.; Barthe, N.; Dupuy, B.; Foliguet, B.; Basse-Cathalinat, B. Evolution of the local calcium content around irradiated β-tricalcium phosphate ceramic implants: In vivo study in the rabbit. Biomaterials 1998, 19, 733–738. [Google Scholar] [CrossRef]
- Roux, S.; Orcel, P. Bone loss: Factors that regulate osteoclast differentiation—An update. Arthritis Res. Ther. 2000, 2, 451–456. [Google Scholar] [CrossRef]
- Yang, Z.J.; Yuan, H.P.; Zou, P.; Tong, W.D.; Qu, S.X.; Zhang, X.D. Osteogenic responses to extraskeletally implanted synthetic porous calcium phosphate ceramics: An early stage histomorphological study in dogs. J. Mater. Sci.-Mater. Med. 1997, 8, 697–701. [Google Scholar] [CrossRef]
- Habibovic, P.; Yuan, H.P.; van der Valk, C.M.; Meijer, G.; van Blitterswijk, C.A.; de Groot, K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials 2005, 26, 3565–3575. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Qu, S.X.; Lu, X.Y.; Weng, J. Fabricating Hydroxyapatite Spherulites with Characteristic Microporous Structure by Chitin Sol Emulsified in Oil and Gelatinized In Situ. Key Eng. Mater. 2005, 284–286, 419–422. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, X.; Duan, K.; Guo, L.Y.; Zhou, S.B.; Weng, J. Improving mechanical and biological properties of macroporous HA scaffolds through composite coatings. Colloids Surf. B Biointerfaces 2009, 74, 159–166. [Google Scholar] [CrossRef]
- Zhi, W.; Zhang, C.; Duan, K.; Li, X.; Qu, S.; Wang, J.; Zhu, Z.; Huang, P.; Xia, T.; Liao, G.; et al. A novel porous bioceramics scaffold by accumulating hydroxyapatite spherulites for large bone tissue engineering in vivo. II. Construct large volume of bone grafts. J. Biomed. Mater. Res. Part A 2014, 102, 2491–2501. [Google Scholar] [CrossRef] [PubMed]
- Karimipour-Fard, P.; Jeffrey, M.P.; JonesTaggart, H.; Pop-Iliev, R.; Rizvi, G. Development, processing and characterization of Polycaprolactone/Nano-Hydroxyapatite/Chitin-Nano-Whisker nanocomposite filaments for additive manufacturing of bone tissue scaffolds. J. Mech. Behav. Biomed. Mater. 2021, 120, 104583. [Google Scholar] [CrossRef]

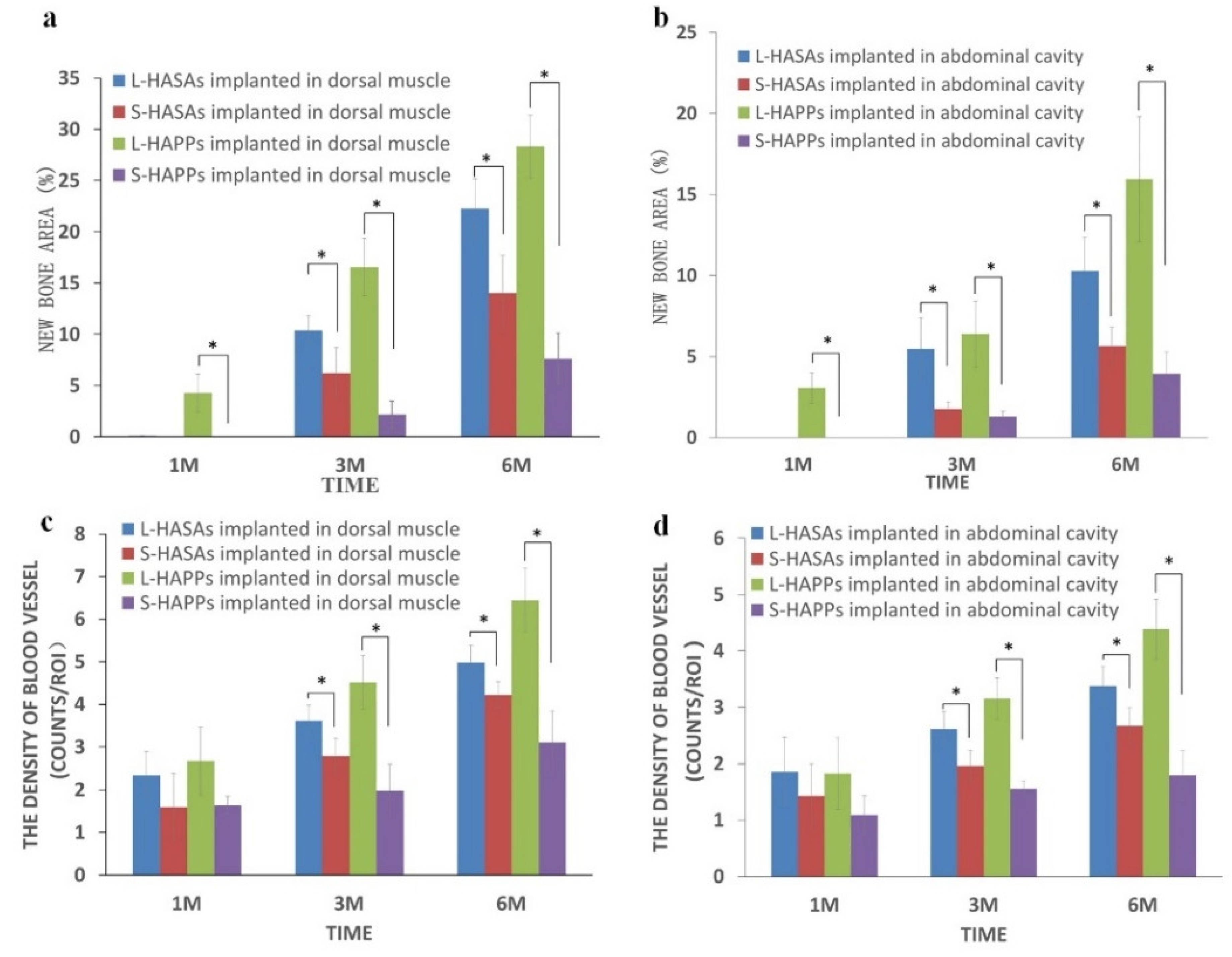

| Implant Time | Scaffold | Dorsal Muscle | Abdominal Cavity | ||||
|---|---|---|---|---|---|---|---|
| New Bone Location | New Bone Formation (%) | Vessel Density (mm−2) | New Bone Location | New Bone Formation (%) | Vessel Density (mm−2) | ||
| 1 M | L-HASAs | \ | \ | 2.34 ± 0.55 | \ | \ | 1.86 ± 0.31 |
| S-HASAs | \ | \ | 1.60 ± 0.79 | \ | \ | 1.43 ± 1.02 | |
| L-HAPPs | Central | 4.27 ± 1.85 | 2.67 ± 0.79 | Central | 3.06 ± 0.94 | 1.83 ± 0.23 | |
| S-HAPPs | \ | \ | 1.64 ± 0.21 | \ | \ | 1.39 ± 0.83 | |
| 3 M | L-HASAs | Outer | 10.36 ± 1.48 | 3.18 ± 0.37 | Outer | 5.47 ± 1.93 | 2.59 ± 0.41 |
| S-HASAs | Outer | 6.18 ± 2.5 | 3.31 ± 0.72 | Outer | 1.74 ± 0.47 | 2.13 ± 0.47 | |
| L-HAPPs | All | 16.57 ± 2.79 | 4.12 ± 0.83 | All | 6.38 ± 2.05 | 3.15 ± 0.37 | |
| S-HAPPs | Central | 2.14 ± 1.33 | 2.58 ± 0.62 | Central | 1.28 ± 0.34 | 1.75 ± 0.15 | |
| 6 M | L-HASAs | All | 22.27 ± 2.89 | 4.02 ± 0.46 | Outer | 10.28 ± 2.06 | 3.17 ± 0.51 |
| S-HASAs | Outer | 14.0 ± 3.67 | 4.78 ± 0.45 | Outer | 5.65 ± 1.17 | 2.87 ± 0.39 | |
| L-HAPPs | All | 28.31 ± 3.06 | 6.44 ± 0.75 | All | 15.93 ± 3.87 | 4.38 ± 0.53 | |
| S-HAPPs | All | 7.61 ± 2.48 | 3.11 ± 1.03 | Central | 3.94 ± 1.33 | 1.90 ± 0.63 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, F.; Fang, X.; Zhou, T.; Huang, X.; Duan, K.; Wang, J.; Qu, S.; Zhi, W.; Weng, J. Macropore Regulation of Hydroxyapatite Osteoinduction via Microfluidic Pathway. Int. J. Mol. Sci. 2022, 23, 11459. https://doi.org/10.3390/ijms231911459
Shi F, Fang X, Zhou T, Huang X, Duan K, Wang J, Qu S, Zhi W, Weng J. Macropore Regulation of Hydroxyapatite Osteoinduction via Microfluidic Pathway. International Journal of Molecular Sciences. 2022; 23(19):11459. https://doi.org/10.3390/ijms231911459
Chicago/Turabian StyleShi, Feng, Xin Fang, Teng Zhou, Xu Huang, Ke Duan, Jianxin Wang, Shuxin Qu, Wei Zhi, and Jie Weng. 2022. "Macropore Regulation of Hydroxyapatite Osteoinduction via Microfluidic Pathway" International Journal of Molecular Sciences 23, no. 19: 11459. https://doi.org/10.3390/ijms231911459
APA StyleShi, F., Fang, X., Zhou, T., Huang, X., Duan, K., Wang, J., Qu, S., Zhi, W., & Weng, J. (2022). Macropore Regulation of Hydroxyapatite Osteoinduction via Microfluidic Pathway. International Journal of Molecular Sciences, 23(19), 11459. https://doi.org/10.3390/ijms231911459






