Measurement of Hepatic CYP3A4 and 2D6 Activity Using Radioiodine-Labeled O-Desmethylvenlafaxine
Abstract
1. Introduction
2. Results
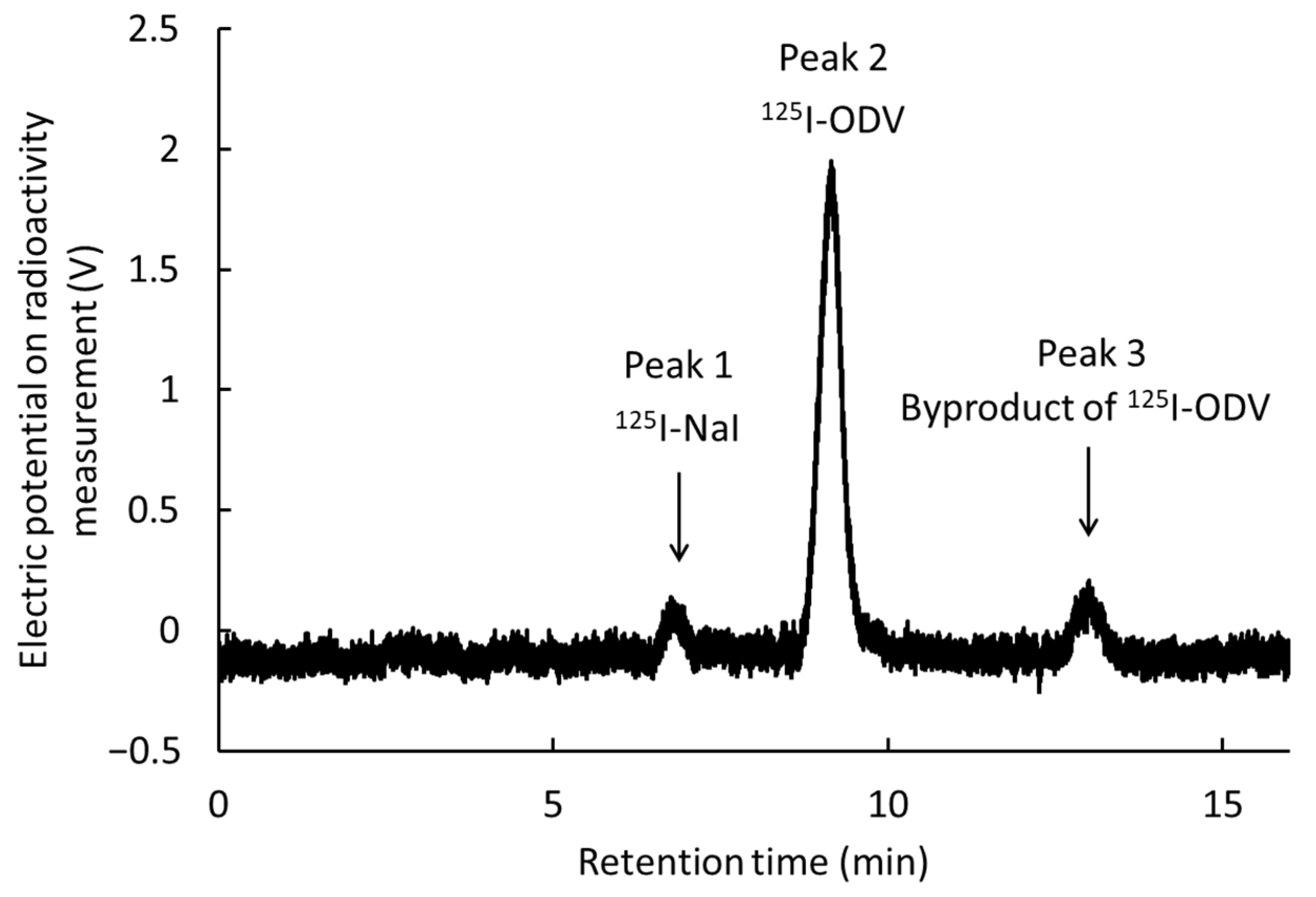
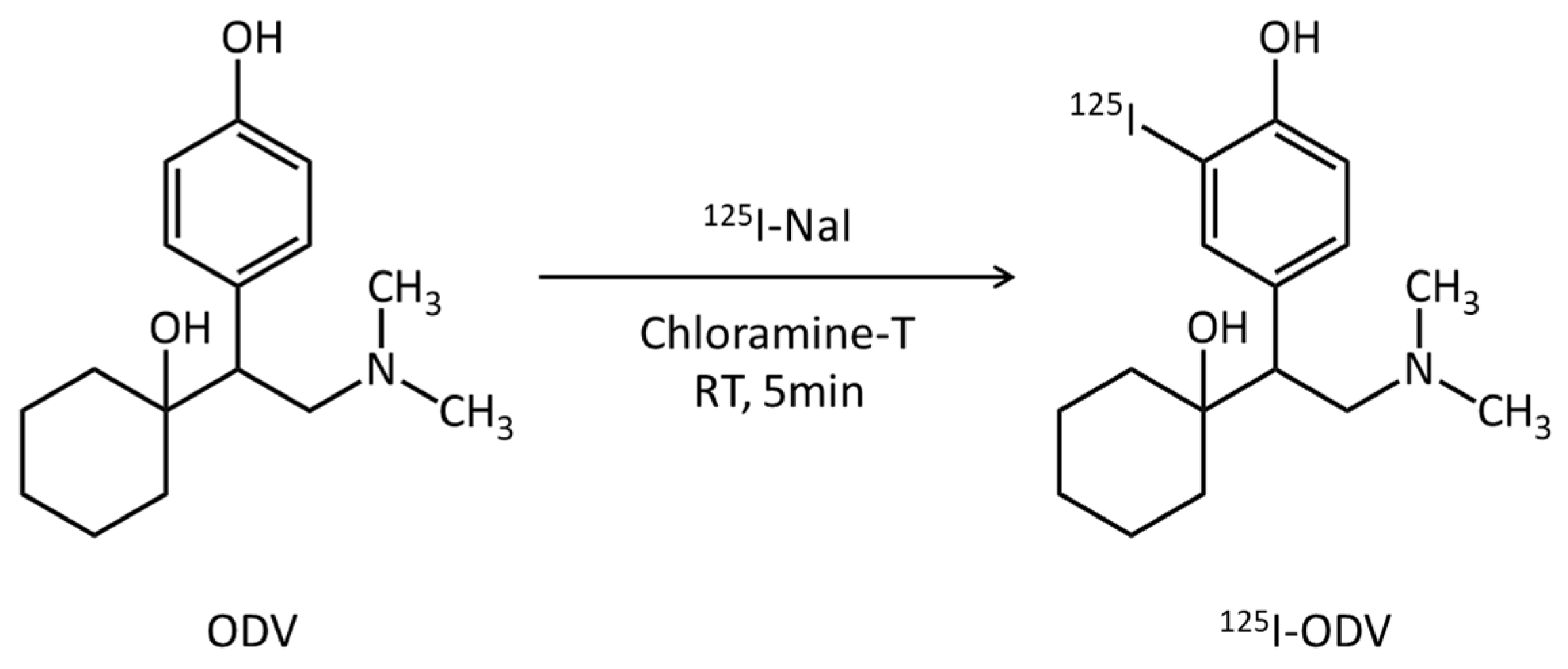
2.1. Labeling of 125I-O-Desmethylvenlafaxine
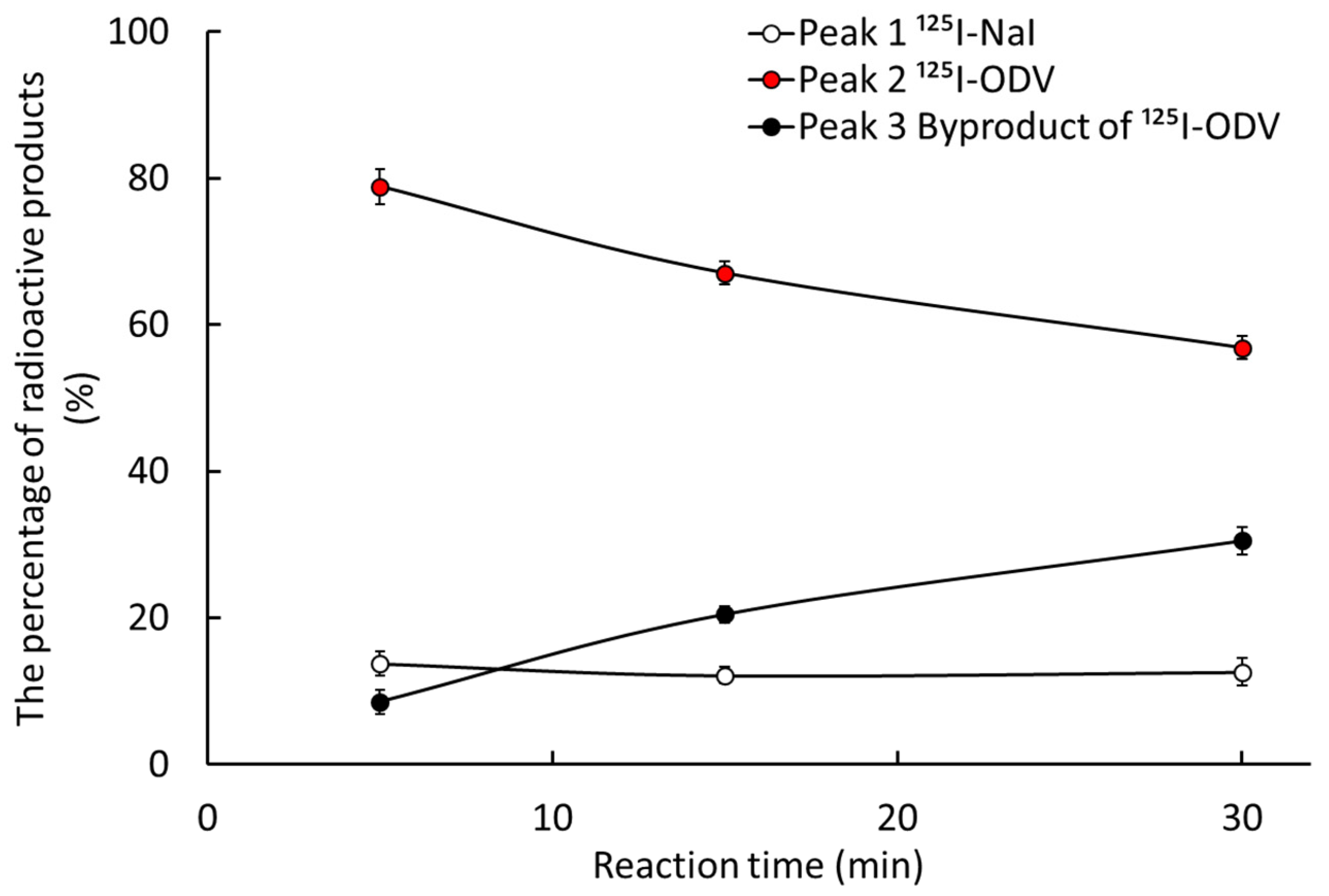
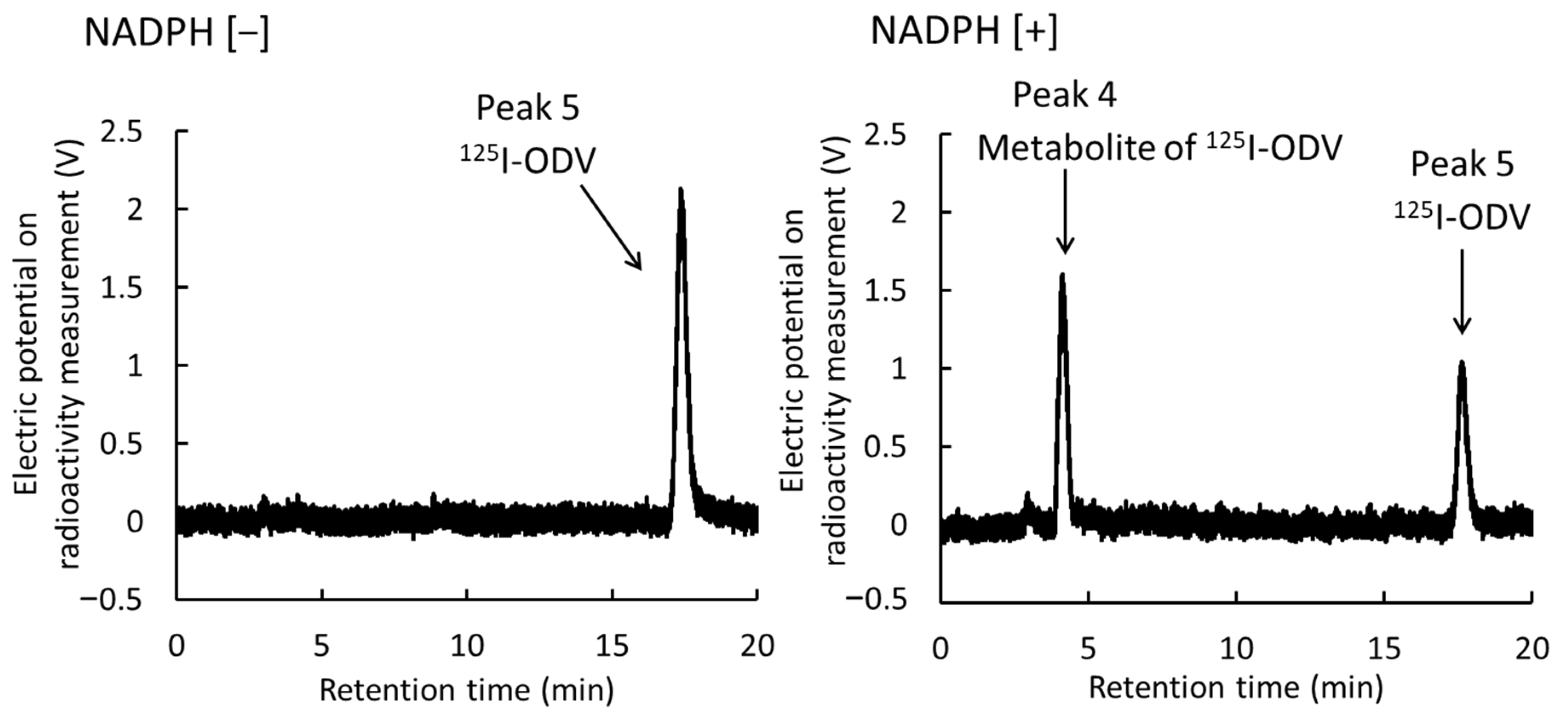
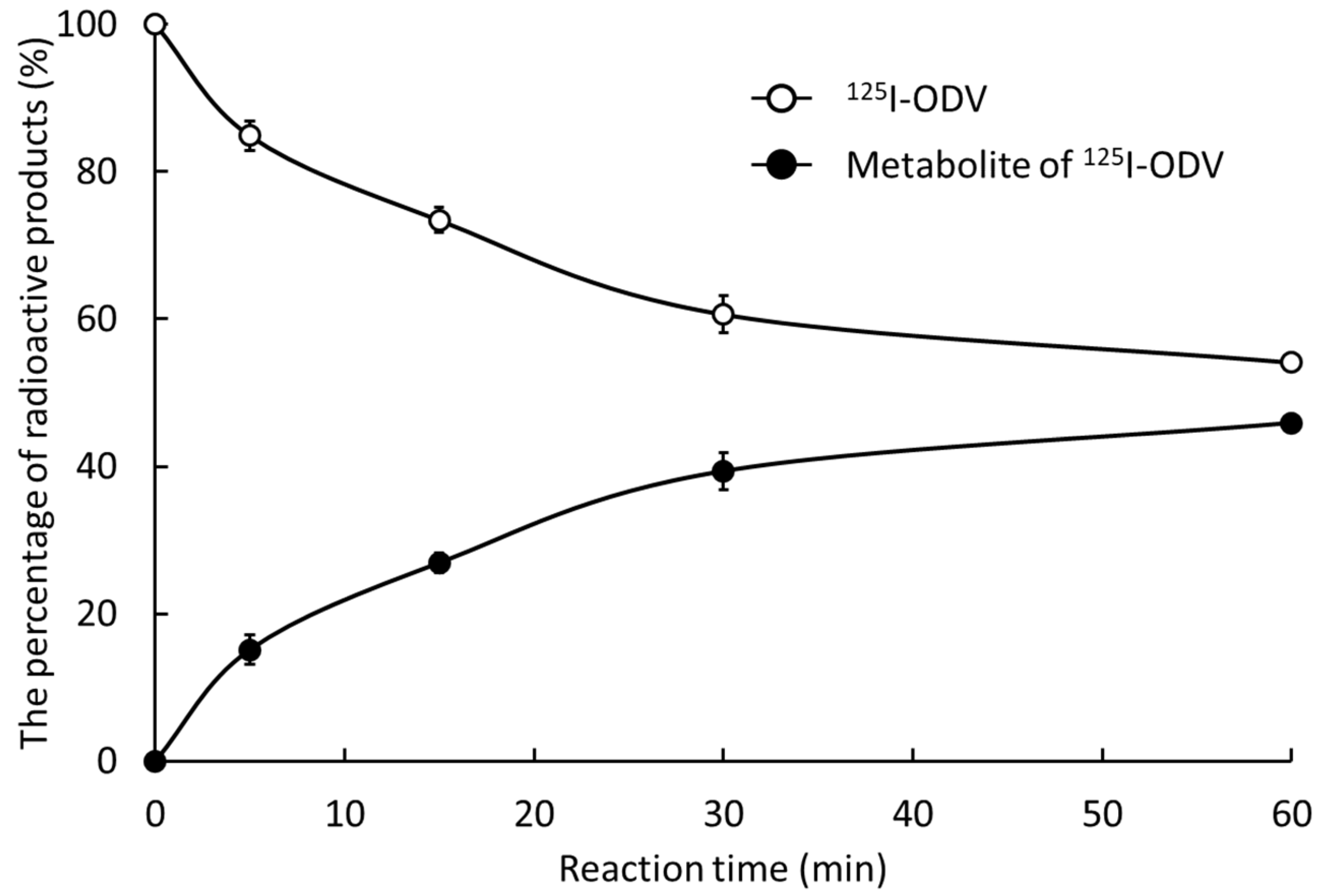
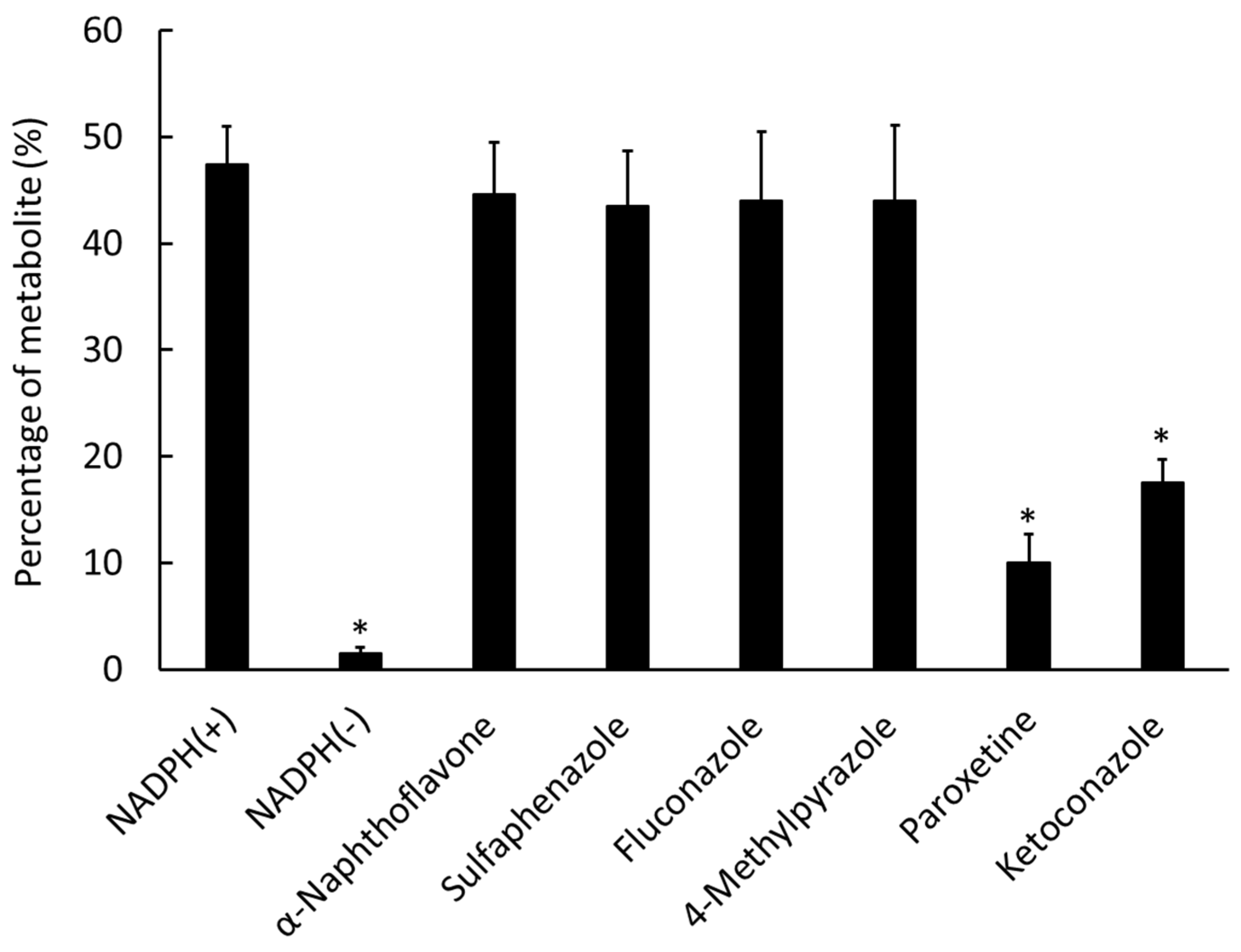
2.2. Metabolism of 125I-ODV In Vitro
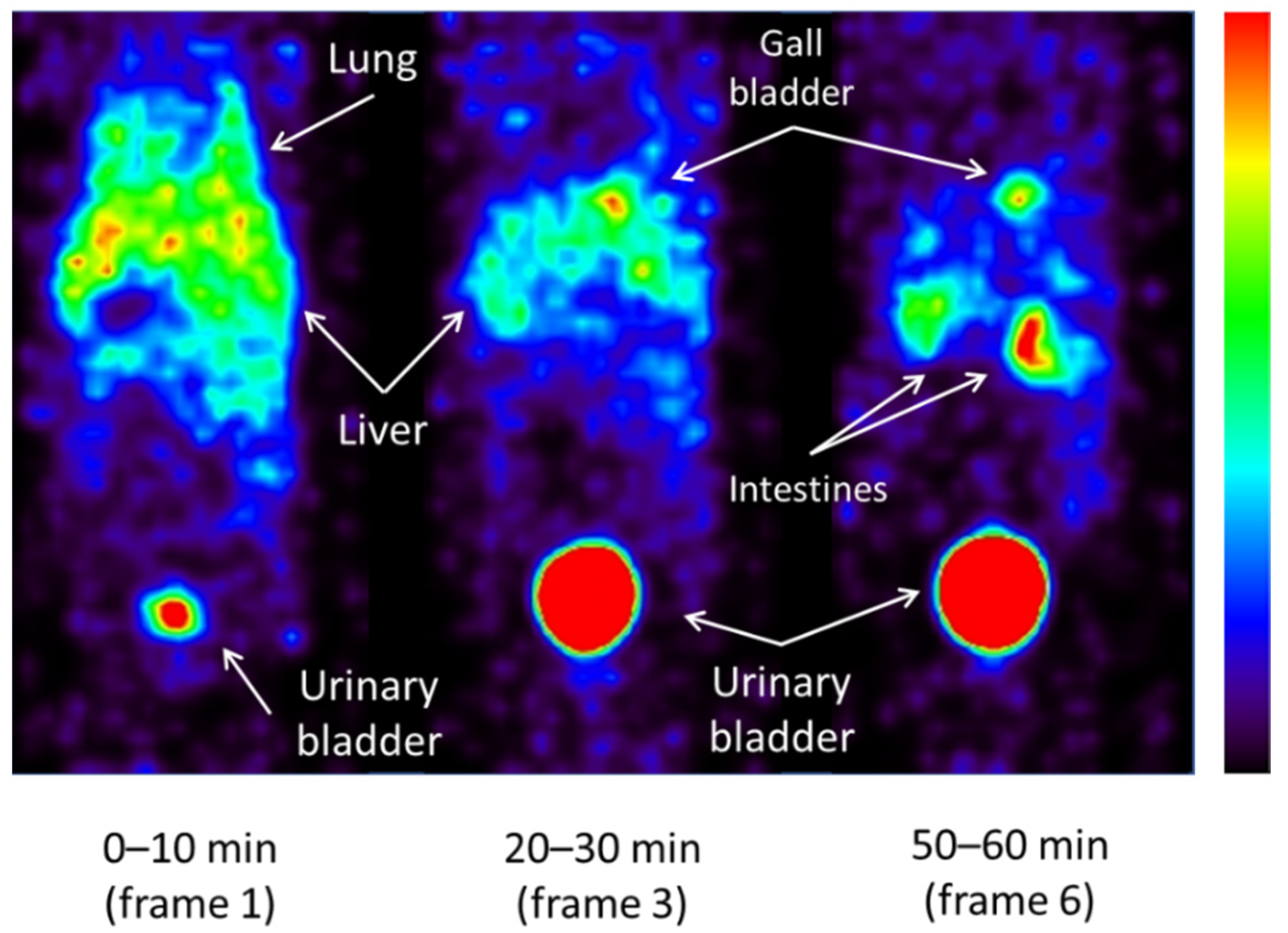
2.3. Whole-Body Imaging of 123I-ODV and Metabolite of 123I-ODV in Normal Mice
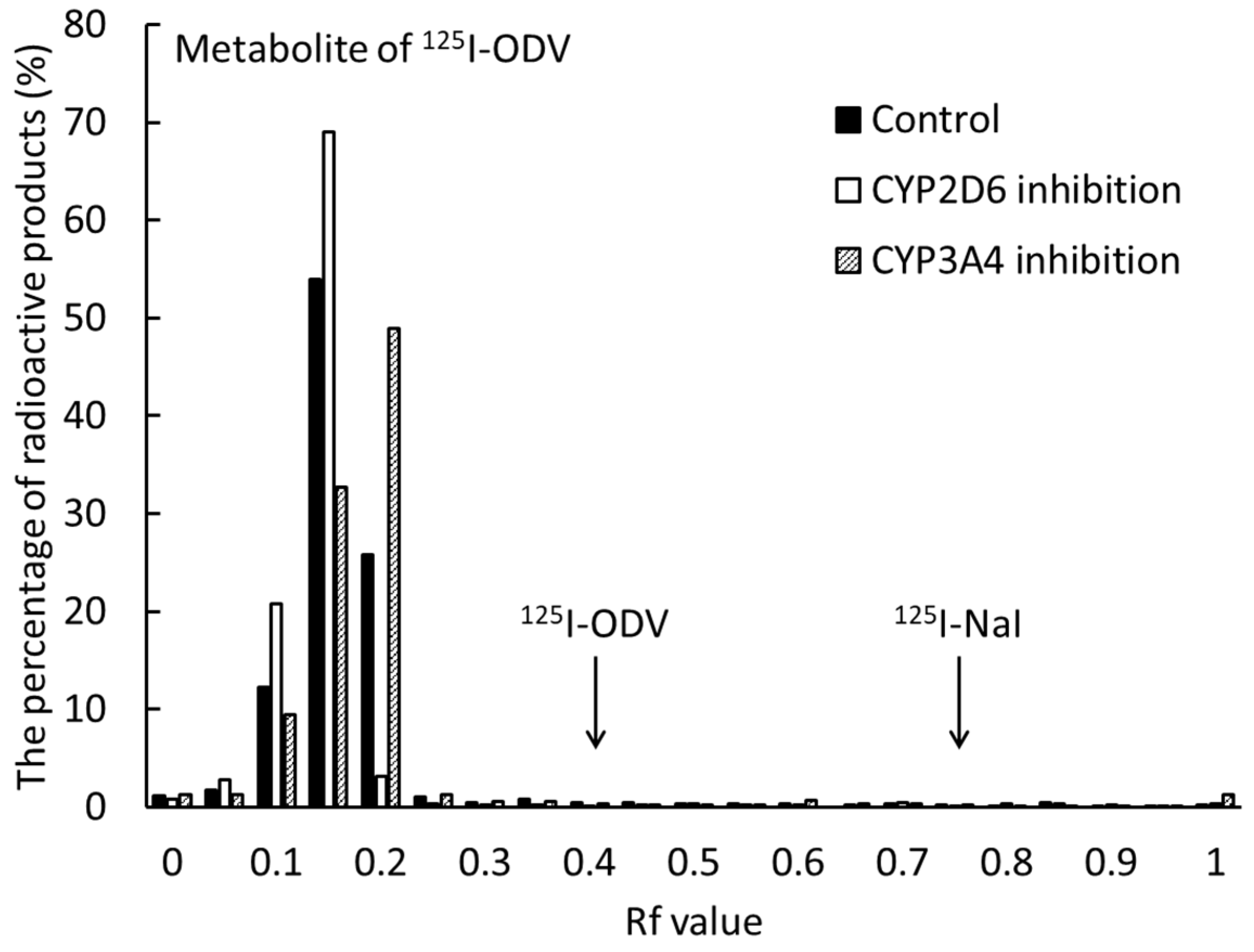
2.4. Metabolism of 125I-ODV in Bile of Normal and CYP-Inhibited Mice
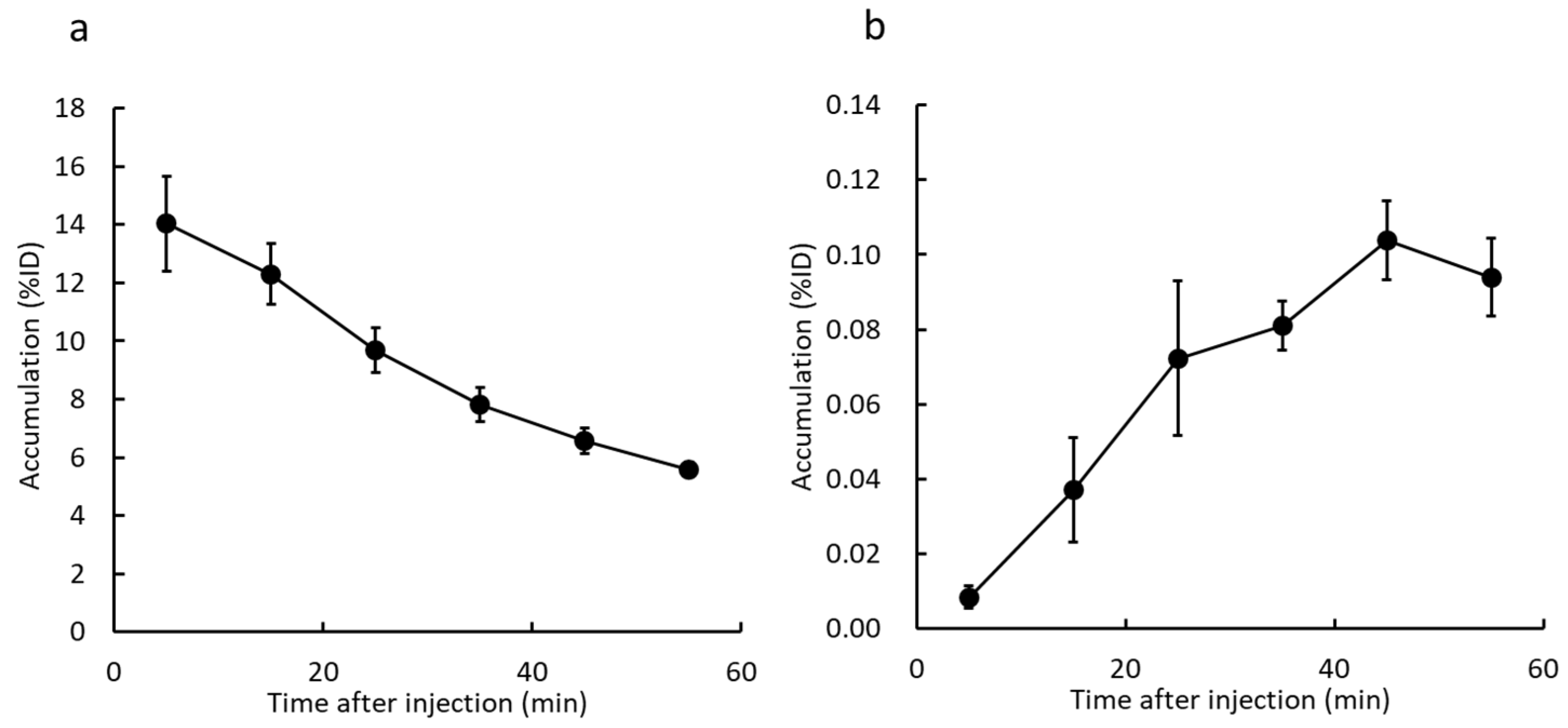
2.5. Biological Distribution of 125I-ODV and Metabolite of 125I-ODV in Normal and CYP-Inhibited Mice
3. Discussion
4. Materials and Method
4.1. Materials
4.2. Labeling of 125I-ODV
4.3. Metabolism of 125I-ODV In Vitro
4.4. Whole-Body Imaging of 123I-ODV in Normal Mice
4.5. Metabolism of 125I-ODV in Bile of Normal and CYP-Inhibited Mice
4.6. Biological Distribution of 125I-ODV in Normal and CYP-Inhibited Mice
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingelman-Sundberg, M.; Oscarson, M.; McLellan, R.A. Polymorphic human cytochrome P450 enzymes: An opportunity for individualized drug treatment. Trends Pharmacol. Sci. 1999, 20, 342–349. [Google Scholar] [CrossRef]
- Rogers, J.F.; Nafziger, A.N.; Bertino, J.S., Jr. Pharmacogenetics affects dosing, efficacy, and toxicity of cytochrome P450-metabolized drugs. Am. J. Med. 2002, 113, 746–750. [Google Scholar] [CrossRef]
- Laizure, S.C.; Herring, V.; Hu, Z.; Witbrodt, K.; Parker, R.B. The role of human carboxylesterases in drug metabolism: Have we overlooked their importance? Pharmacotherapy 2013, 33, 210–222. [Google Scholar] [CrossRef]
- Singh, D.; Kashyap, A.; Pandey, R.V.; Saini, K.S. Novel advances in cytochrome P450 research. Drug Discov. Today 2011, 16, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.R.; Backes, W.L. Formation of P450·P450 complexes and their effect on P450 function. Pharmacol. Ther. 2012, 133, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Rendic, S.; Guengerich, F.P. Update information on drug metabolism systems—2009, part II: Summary of information on the effects of diseases and environmental factors on human cytochrome P450 (CYP) enzymes and transporters. Curr. Drug Metab. 2010, 11, 4–84. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.C.; Lee, S.C.; Wang, L.Z.; Fan, L.; Guo, J.Y.; Lamba, J.; Schuetz, E.; Lim, R.; Lim, H.L.; Ong, A.B.; et al. Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J. Clin. Oncol. 2002, 20, 3683–3690. [Google Scholar] [CrossRef]
- Rivory, L.P.; Slaviero, K.A.; Hoskins, J.M.; Clarke, S.J. The erythromycin breath test for the prediction of drug clearance. Clin. Pharmacokinet. 2001, 40, 151–158. [Google Scholar] [CrossRef]
- Mizutani, A.; Kobayashi, M.; Fujita, K.; Takahashi, K.; Hokama, T.; Takasu, H.; Nishi, K.; Nishii, R.; Shikano, N.; Fukuchi, K.; et al. 123I-iomazenil whole-body imaging to detect hepatic carboxylesterase drug-metabolizing enzyme activity. Nucl. Med. Commun. 2018, 39, 825–833. [Google Scholar] [CrossRef]
- Williams, J.A.; Hyland, R.; Jones, B.C.; Smith, D.A.; Hurst, S.; Goosen, T.C.; Peterkin, V.; Koup, J.R.; Ball, S.E. Drug-drug interactions for UDP-glucuronosyltransferase substrates: A pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug. Metab. Dispos. 2004, 32, 1201–1208. [Google Scholar] [CrossRef]
- Godoy, A.L.; Rocha, A.; da Silva Souza, C.; Lanchote, V.L. Pharmacokinetics of venlafaxine enantiomers and their metabolites in psoriasis patients. J. Clin. Pharmacol. 2016, 56, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.; Cha, I.J.; Park, J.S.; Shon, J.H.; Liu, K.H.; Shin, J.G. High-throughput screening of inhibitory potential of nine cytochrome P450 enzymes in vitro using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, D.; Raghavan, N.; Yao, M.; Ma, L.; Frost, C.E.; Maxwell, B.D.; Chen, S.Y.; He, K.; Goosen, T.C.; et al. In Vitro Assessment of Metabolic Drug-Drug Interaction Potential of Apixaban through Cytochrome P450 Phenotyping, Inhibition, and Induction Studies. Drug Metab. Dispos. 2010, 38, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Turpeinen, M.; Uusitalo, J.; Jalonen, J.; Pelkonen, O. Multiple P450 substrates in a single run: Rapid and comprehensive in vitro interaction assay. Eur. J. Pharm. Sci. 2005, 24, 123–132. [Google Scholar] [CrossRef]
- Katoh, M.; Sawada, T.; Soeno, Y.; Nakajima, M.; Tateno, C.; Yoshizato, K.; Yokoi, T. In vivo drug metabolism model for human cytochrome P450 enzyme using chimeric mice with humanized liver. J. Pharm. Sci. 2007, 96, 428–437. [Google Scholar] [CrossRef]
- Cho, D.Y.; Bae, S.H.; Lee, J.K.; Kim, Y.W.; Kim, B.T.; Bae, S.K. Selective inhibition of cytochrome P450 2D6 by sarpogrelate and its active metabolite, M-1, in human liver microsomes. Drug Metab. Dispos. 2014, 42, 33–39. [Google Scholar] [CrossRef]
- Weissenstein, U.; Kunz, M.; Oufir, M.; Wang, J.T.; Hamburger, M.; Urech, K.; Regueiro, U.; Baumgartner, S. Absence of herb-drug interactions of mistletoe with the tamoxifen metabolite (E/Z)-endoxifen and cytochrome P450 3A4/5 and 2D6 in vitro. BMC Complement. Altern. Med. 2019, 19, 23. [Google Scholar] [CrossRef]
- Kurtoglu Ozdes, E.; Altunkaynak, B.Z.; Deniz, O.G.; Ilkaya, F.; Guzel, H.; Kokcu, A. A stereological study of the effects of antidepressants on postmenopausal rat kidney. Biotech. Histochem. 2020, 95, 262–267. [Google Scholar] [CrossRef]
- Seneca, N.; Zoghbi, S.S.; Shetty, H.U.; Tuan, E.; Kannan, P.; Taku, A.; Innis, R.B.; Pike, V.W. Effects of ketoconazole on the biodistribution and metabolism of [11C]loperamide and [11C]-N-desmethyl-loperamide in wild-type and P-gp knockout mice. Nucl. Med. Biol. 2010, 37, 335–345. [Google Scholar] [CrossRef]
- MacLeod, A.K.; McLaughlin, L.A.; Henderson, C.J.; Wolf, C.R. Application of Mice Humanized for CYP2D6 to the Study of Tamoxifen Metabolism and Drug-Drug Interaction with Antidepressants. Drug Metab. Dispos. 2017, 45, 17–22. [Google Scholar] [CrossRef] [PubMed]
| Organ (%ID/g) | 5 min | 15 min | 30 min | 60 min | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood | 1.89 | ± | 0.03 | 1.31 | ± | 0.12 | 1.34 | ± | 0.29 | 0.74 | ± | 0.22 |
| Brain | 0.80 | ± | 0.12 | 0.75 | ± | 0.05 | 0.73 | ± | 0.08 | 0.60 | ± | 0.05 |
| Heart | 5.64 | ± | 0.50 | 2.71 | ± | 0.18 | 2.04 | ± | 0.12 | 1.00 | ± | 0.24 |
| Lung | 27.04 | ± | 1.59 | 16.75 | ± | 2.02 | 13.30 | ± | 2.64 | 5.01 | ± | 1.14 |
| Thyroid * | 0.06 | ± | 0.01 | 0.03 | ± | 0.02 | 0.04 | ± | 0.00 | 0.02 | ± | 0.00 |
| Gallbladder | 4.31 | ± | 0.39 | 5.26 | ± | 1.58 | 24.23 | ± | 3.53 | 33.05 | ± | 7.75 |
| Liver | 6.91 | ± | 1.05 | 6.04 | ± | 0.94 | 5.45 | ± | 1.03 | 2.89 | ± | 0.61 |
| Spleen | 9.22 | ± | 2.16 | 4.79 | ± | 0.49 | 3.45 | ± | 0.32 | 1.75 | ± | 0.41 |
| Pancreas | 7.87 | ± | 0.60 | 6.04 | ± | 0.90 | 5.02 | ± | 0.73 | 2.92 | ± | 0.73 |
| Stomach * | 2.44 | ± | 0.31 | 3.19 | ± | 0.42 | 2.75 | ± | 0.70 | 1.88 | ± | 0.29 |
| Small intestines * | 6.85 | ± | 1.35 | 7.70 | ± | 0.85 | 8.47 | ± | 0.87 | 11.03 | ± | 3.03 |
| Large intestines * | 1.32 | ± | 0.16 | 1.18 | ± | 0.46 | 0.68 | ± | 0.15 | 0.47 | ± | 0.05 |
| Kidney | 22.95 | ± | 0.43 | 16.37 | ± | 0.63 | 14.27 | ± | 1.20 | 9.42 | ± | 2.23 |
| Urine * | 0.63 | ± | 0.89 | 3.37 | ± | 3.92 | 3.69 | ± | 2.23 | 7.31 | ± | 2.80 |
| Organ (%ID/g) | 5 min | 15 min | 30 min | 60 min | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood | 1.91 | ± | 0.40 | 1.71 | ± | 0.14 ‡ | 1.44 | ± | 0.15 | 0.65 | ± | 0.02 |
| Brain | 0.71 | ± | 0.03 | 0.80 | ± | 0.09 | 0.74 | ± | 0.05 | 0.52 | ± | 0.03 |
| Heart | 5.73 | ± | 0.81 | 3.93 | ± | 0.37 ‡ | 2.34 | ± | 0.27 | 0.96 | ± | 0.03 |
| Lung | 29.72 | ± | 1.47 | 21.72 | ± | 3.69 | 11.31 | ± | 2.60 | 5.81 | ± | 0.35 |
| Thyroid * | 0.05 | ± | 0.01 | 0.05 | ± | 0.01 | 0.09 | ± | 0.01 ‡ | 0.11 | ± | 0.01 ‡ |
| Gallbladder | 2.39 | ± | 1.90 | 5.43 | ± | 1.63 | 9.16 | ± | 5.20 ‡ | 20.14 | ± | 1.72 ‡ |
| Liver | 5.66 | ± | 0.93 | 4.72 | ± | 1.40 | 5.03 | ± | 0.47 | 2.12 | ± | 0.75 |
| Spleen | 6.61 | ± | 0.70 | 6.35 | ± | 0.86 † | 4.03 | ± | 0.74 | 1.94 | ± | 0.46 |
| Pancreas | 8.93 | ± | 0.62 † | 5.94 | ± | 0.95 | 23.69 | ± | 37.81 | 2.77 | ± | 0.02 |
| Stomach * | 2.37 | ± | 0.32 | 2.34 | ± | 0.53 | 2.24 | ± | 0.34 | 1.90 | ± | 0.37 |
| Small intestines * | 6.06 | ± | 0.29 | 6.37 | ± | 1.47 | 7.71 | ± | 3.01 | 10.07 | ± | 3.63 |
| Large intestines * | 1.19 | ± | 0.12 | 1.00 | ± | 0.30 | 1.00 | ± | 0.25 | 0.68 | ± | 0.02 ‡ |
| Kidney | 21.72 | ± | 0.85 | 19.74 | ± | 2.36 | 15.65 | ± | 1.34 | 9.60 | ± | 3.05 |
| Urine * | 2.10 | ± | 0.37 | 5.25 | ± | 2.28 | 5.63 | ± | 2.03 | 2.01 | ± | 2.27 |
| Organ (%ID/g) | 5 min | 15 min | 30 min | 60 min | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood | 1.83 | ± | 0.47 | 1.59 | ± | 0.33 | 1.12 | ± | 0.03 | 0.67 | ± | 0.30 |
| Brain | 0.79 | ± | 0.04 | 0.82 | ± | 0.18 | 0.71 | ± | 0.11 | 0.58 | ± | 0.18 |
| Heart | 5.19 | ± | 0.74 | 2.87 | ± | 0.42 | 1.47 | ± | 0.08 ‡ | 0.62 | ± | 0.25 |
| Lung | 22.13 | ± | 2.64 | 11.96 | ± | 0.51 † | 7.77 | ± | 2.29 † | 3.15 | ± | 0.82 |
| Thyroid * | 0.05 | ± | 0.01 | 0.04 | ± | 0.01 | 0.05 | ± | 0.04 | 0.10 | ± | 0.09 |
| Gallbladder | 1.59 | ± | 1.08 ‡ | 10.66 | ± | 1.56 ‡ | 21.66 | ± | 2.30 | 26.70 | ± | 10.19 |
| Liver | 7.19 | ± | 1.14 | 5.56 | ± | 1.20 | 5.08 | ± | 1.32 | 1.75 | ± | 0.39 † |
| Spleen | 4.62 | ± | 1.25 † | 4.97 | ± | 0.92 | 2.88 | ± | 0.59 | 1.46 | ± | 0.41 |
| Pancreas | 6.02 | ± | 1.68 | 5.71 | ± | 1.20 | 3.94 | ± | 0.83 | 2.51 | ± | 0.65 |
| Stomach * | 1.77 | ± | 0.54 | 2.40 | ± | 0.38 | 3.20 | ± | 0.70 | 2.20 | ± | 0.81 |
| Small intestines * | 7.24 | ± | 1.56 | 5.64 | ± | 0.45 | 10.30 | ± | 1.59 | 10.85 | ± | 2.01 |
| Large intestines * | 1.12 | ± | 0.16 | 0.93 | ± | 0.06 | 0.74 | ± | 0.25 | 0.67 | ± | 0.23 |
| Kidney | 19.94 | ± | 1.82 | 17.13 | ± | 3.30 | 15.90 | ± | 1.45 | 8.33 | ± | 2.33 |
| Urine * | 0.33 | ± | 0.11 | 6.18 | ± | 0.48 | 22.96 | ± | 2.22 | 33.92 | ± | 21.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizutani, A.; Kobayashi, M.; Aibe, R.; Muranaka, Y.; Nishi, K.; Kitamura, M.; Suzuki, C.; Nishii, R.; Shikano, N.; Magata, Y.; et al. Measurement of Hepatic CYP3A4 and 2D6 Activity Using Radioiodine-Labeled O-Desmethylvenlafaxine. Int. J. Mol. Sci. 2022, 23, 11458. https://doi.org/10.3390/ijms231911458
Mizutani A, Kobayashi M, Aibe R, Muranaka Y, Nishi K, Kitamura M, Suzuki C, Nishii R, Shikano N, Magata Y, et al. Measurement of Hepatic CYP3A4 and 2D6 Activity Using Radioiodine-Labeled O-Desmethylvenlafaxine. International Journal of Molecular Sciences. 2022; 23(19):11458. https://doi.org/10.3390/ijms231911458
Chicago/Turabian StyleMizutani, Asuka, Masato Kobayashi, Riku Aibe, Yuka Muranaka, Kodai Nishi, Masanori Kitamura, Chie Suzuki, Ryuichi Nishii, Naoto Shikano, Yasuhiro Magata, and et al. 2022. "Measurement of Hepatic CYP3A4 and 2D6 Activity Using Radioiodine-Labeled O-Desmethylvenlafaxine" International Journal of Molecular Sciences 23, no. 19: 11458. https://doi.org/10.3390/ijms231911458
APA StyleMizutani, A., Kobayashi, M., Aibe, R., Muranaka, Y., Nishi, K., Kitamura, M., Suzuki, C., Nishii, R., Shikano, N., Magata, Y., Ishida, Y., Kunishima, M., & Kawai, K. (2022). Measurement of Hepatic CYP3A4 and 2D6 Activity Using Radioiodine-Labeled O-Desmethylvenlafaxine. International Journal of Molecular Sciences, 23(19), 11458. https://doi.org/10.3390/ijms231911458









