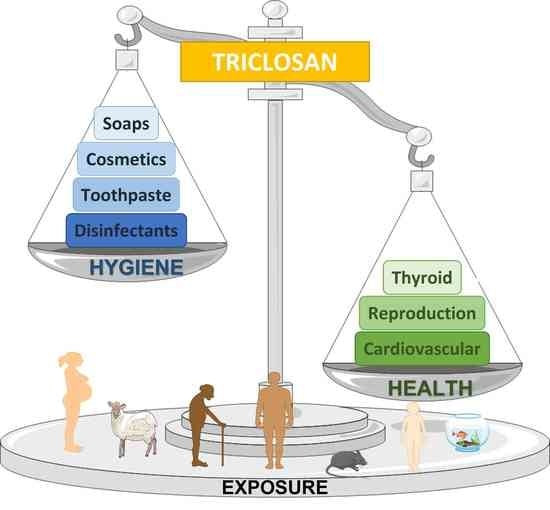
Triclosan and Its Consequences on the Reproductive, Cardiovascular and Thyroid Levels
Abstract
1. Introduction
2. Physical and Chemical Properties of Triclosan
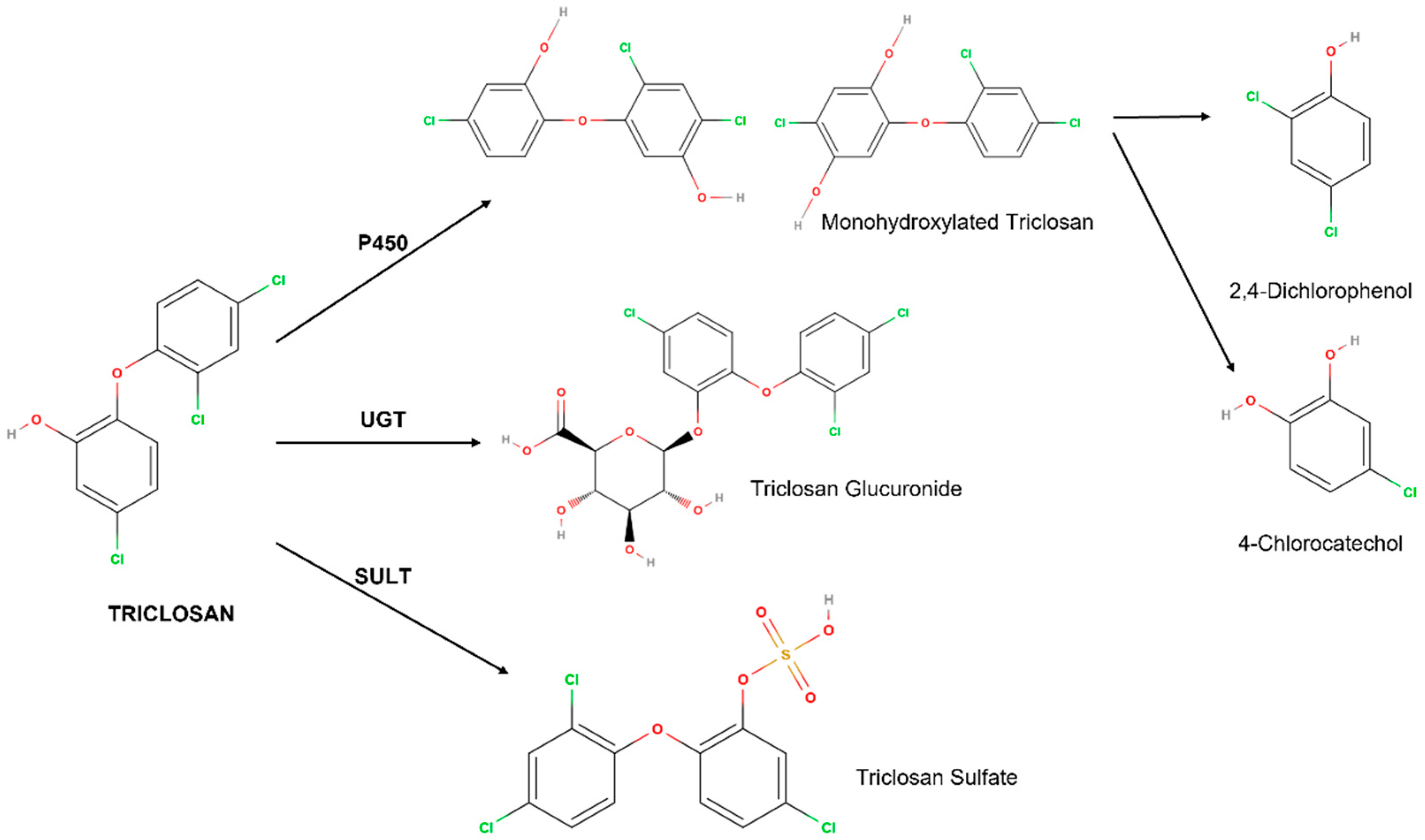
3. Metabolism
4. Effects on the Reproductive System
4.1. Animals
4.2. Humans
5. Effects on the Cardiovascular System
5.1. Animals
5.2. Humans
6. Effects on the Thyroid
6.1. Animals
6.2. Humans
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bedoux, G.; Roig, B.; Thomas, O.; Dupont, V.; Le Bot, B. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ. Sci. Pollut. Res. 2012, 19, 1044–1065. [Google Scholar] [CrossRef]
- Yueh, M.F.; Tukey, R.H. Triclosan: A Widespread Environmental Toxicant with Many Biological Effects. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 251–272. [Google Scholar] [CrossRef]
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health Part B Crit. Rev. 2017, 20, 447–469. [Google Scholar] [CrossRef]
- Shrestha, P.; Zhang, Y.M.; Chen, W.J.; Wong, T.Y. Triclosan: Antimicrobial mechanisms, antibiotics interactions, clinical applications, and human health. J. Environ. Sci. Health Part C Toxicol. Carcinog. 2020, 38, 245–268. [Google Scholar] [CrossRef]
- Tamura, I.; Saito, M.; Nishimura, Y.; Satoh, M.; Yamamoto, H.; Oyama, Y. Elevation of Intracellular Ca2+ Level by Triclosan in Rat Thymic Lymphocytes: Increase in Membrane Ca2+ Permeability and Induction of Intracellular Ca2+ Release. J. Health Sci. 2011, 57, 540–546. [Google Scholar] [CrossRef]
- EC. Commission Regulation (EU) No 358/2014 Amending Annexes II and V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products. 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32014R0358 (accessed on 31 May 2022).
- Pombo, M.; Castro-Feijoo, L. Endocrine disruptors. J. Pediatric. Endocrinol. Metab. 2005, 18, 1145–1155. [Google Scholar] [CrossRef]
- Benvenga, S.; Elia, G.; Ragusa, F.; Paparo, S.R.; Sturniolo, M.M.; Ferrari, S.M.; Antonelli, A.; Fallahi, P. Endocrine disruptors and thyroid autoimmunity. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 11. [Google Scholar] [CrossRef]
- Monneret, C. What is an endocrine disruptor? Comptes Rendus Biol. 2017, 340, 403–405. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Axelstad, M.; Boberg, J.; Vinggaard, A.M.; Christiansen, S.; Hass, U. Triclosan exposure reduces thyroxine levels in pregnant and lactating rat dams and in directly exposed offspring. Food Chem. Toxicol. 2013, 59, 534–540. [Google Scholar] [CrossRef]
- Barbolt, T.A. Chemistry and Safety of Triclosan, and Its Use as an Antimicrobial Coating on Coated VICRYL* Plus Antibacterial Suture (Coated Polyglactin 910 Suture with Triclosan). Surg. Infect. 2002, 3, s45–s53. [Google Scholar] [CrossRef]
- Dann, A.B.; Hontela, A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2011, 31, 285–311. [Google Scholar] [CrossRef]
- Infarmed. Análise Laboratorial de Produtos de Higiene Oral. 2017. Available online: https://www.infarmed.pt/documents/15786/2215138/Produtos+de+Higiene+Oral/56215a29-68cb-48d5-b32f-650ecbcf0196 (accessed on 31 May 2022).
- FDA. Safety and Effectiveness of Consumer Antiseptic Rubs; Topical Antimicrobial Drug Products for over-the-Counter Human Use; Food and Drug Administration: Silver Spring, MD, USA, 2019; Volume 84.
- ECHA. Decision on Substance Evaluation Pursuant to Article 46(1) of Regulation (EC) No 1907/2006. 2014. Available online: https://echa.europa.eu/documents/10162/d2148173-ecbf-3f1b-988d-89ba9d06680d (accessed on 31 May 2022).
- Cullinan, M.P.; Palmer, J.E.; Carle, A.D.; West, M.J.; Westerman, B.; Seymour, G.J. The influence of a triclosan toothpaste on adverse events in patients with cardiovascular disease over 5-years. Sci. Total Environ. 2015, 508, 546–552. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Wnuk, A.; Kajta, M.; Wójtowicz, A.K. Triclosan activates aryl hydrocarbon receptor (AhR)-dependent apoptosis and affects Cyp1a1 and Cyp1b1 expression in mouse neocortical neurons. Environ. Res. 2016, 151, 106–114. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skóra, B.; Wójtowicz, A.K. Triclosan affects the expression of nitric oxide synthases (NOSs), peroxisome proliferator-activated receptor gamma (PPARγ), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in mouse neocortical neurons in vitro. Toxicol. In Vitro 2021, 73, 105143. [Google Scholar] [CrossRef]
- Li, J.; Quan, X.; Zhang, Y.; Yu, T.; Lei, S.; Huang, Z.; Wang, Q.; Song, W.; Yang, X.; Xu, P. PPARγ Regulates Triclosan Induced Placental Dysfunction. Cells 2021, 11, 86. [Google Scholar] [CrossRef]
- Ahn, K.C.; Zhao, B.; Chen, J.; Cherednichenko, G.; Sanmarti, E.; Denison, M.S.; Lasley, B.; Pessah, I.N.; Kultz, D.; Chang, D.P.Y.; et al. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens. Environ. Health Perspect. 2008, 116, 1203–1210. [Google Scholar] [CrossRef]
- Ohtake, F.; Baba, A.; Fujii-Kuriyama, Y.; Kato, S. Intrinsic AhR function underlies cross-talk of dioxins with sex hormone signalings. Biochem. Biophys. Res. Commun. 2008, 370, 541–546. [Google Scholar] [CrossRef]
- Bhargava, H.N.; Leonard, P.A. Triclosan: Applications and safety. Am. J. Infect. Control 1996, 24, 209–218. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledon, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current Status, Occurrence, Environmental Risks and Bioaccumulation Potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684. [Google Scholar] [CrossRef]
- Olaniyan, L.W.B.; Mkwetshana, N.; Okoh, A.I. Triclosan in water, implications for human and environmental health. Springerplus 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.L.; Stingley, R.L.; Beland, F.A.; Harrouk, W.; Lumpkins, D.L.; Howard, P. Occurrence, Efficacy, Metabolism, and Toxicity of Triclosan. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2010, 28, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Lee, J.Y.; Kwack, S.J.; Shin, C.Y.; Jang, H.J.; Kim, H.Y.; Kim, M.K.; Seo, D.W.; Lee, B.M.; Kim, K.B. Risk Assessment of Triclosan, a Cosmetic Preservative. Toxicol. Res. 2019, 35, 137–154. [Google Scholar] [CrossRef] [PubMed]
- SCCS. Scientific Advice on the Safety of Triclocarban and Triclosan as Substances with Potential Endocrine Disrupting Properties in Cosmetic Products. 2022. Available online: https://health.ec.europa.eu/publications/safety-triclocarban-and-triclosan-substances-potential-endocrine-disrupting-properties-cosmetic_en (accessed on 31 May 2022).
- Sigma-Aldrich. Triclosan Product Specification; Sigma-Aldrich: St. Louis, MO, USA, 2022; Available online: https://www.sigmaaldrich.com/PT/en/sds/sial/phr1338 (accessed on 10 January 2022).
- Belosludtsev, K.N.; Belosludtseva, N.V.; Tenkov, K.S.; Penkov, N.V.; Agafonov, A.V.; Pavlik, L.L.; Yashin, V.A.; Samartsev, V.N.; Dubinin, M.V. Study of the mechanism of permeabilization of lecithin lipossomes and rat liver mitochondria by the antimicrobial drug triclosan. BBA Biomembr. 2018, 1860, 264–271. [Google Scholar] [CrossRef]
- Lygre, H.; Moe, G.; Skalevik, R.; Holmsen, H. Interaction of triclosan with eukaryotic membrane lipids. Eur. J. Oral Sci. 2003, 111, 216–222. [Google Scholar] [CrossRef]
- Wu, J.L.; Liu, J.; Cai, Z.W. Determination of triclosan metabolites by using in-source fragmentation from high-performance liquid chromatography/negative atmospheric pressure chemical ionization ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 1828–1834. [Google Scholar] [CrossRef]
- Adolfsson-Erici, M.; Pettersson, M.; Parkkonen, J.; Sturve, J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere 2002, 46, 1485–1489. [Google Scholar] [CrossRef]
- Guillén, J.; Bernabeu, A.; Shapiro, S.; José, V. Location and orientation of Triclosan in phospholipid model membranes. Eur. Biophys. J. 2004, 33, 448–453. [Google Scholar] [CrossRef]
- Allmyr, M.; Adolfsson-Erici, M.; McLachlan, M.S.; Sandborgh-Englund, G. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci. Total Environ. 2006, 372, 87–93. [Google Scholar] [CrossRef]
- Lin, Y.J. Buccal absortin of triclosan following topical mouthrinse application. Am. J. Dent. 2000, 13, 215–217. [Google Scholar]
- Aiello, A.E.; Larson, E.L.; Levy, S.B. Consumer Antibacterial Soaps: Effective or Just Risky? Clin. Infect. Dis. 2007, 45, S137–S147. [Google Scholar] [CrossRef] [PubMed]
- Queckenberg, C.; Meins, J.; Wachall, B.; Doroshyenko, O.; Tomalik-Scharte, D.; Bastian, B.; Abdel-Tawab, M.; Fuhr, U. Absorption, Pharmacokinetics, and Safety of Triclosan after Dermal Administration. Antimicrob. Agents Chemother. 2010, 54, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Kanetoshi, A.; Ogawa, H.; Katsura, E.; Okui, T.; Kaneshima, H. Disposition and excretion of irgasan dp300 and its chlorinated derivatives in mice. Arch. Environ. Contam. Toxicol. 1988, 17, 637–644. [Google Scholar] [CrossRef]
- Moss, T.; Howes, D.; Williams, F.M. Percutaneous penetration and dermal metabolism of triclosan (2,4,4′-trichloro-2′-hydroxydiphenyl ether). Food Chem. Toxicol. 2000, 38, 361–370. [Google Scholar] [CrossRef]
- Fang, J.L.; Vanlandingham, M.; da Costa, G.G.; Beland, F.A. Absorption and metabolism of triclosan after application to the skin of B6C3F1 mice. Environ. Toxicol. 2016, 31, 609–623. [Google Scholar] [CrossRef]
- Geens, T.; Neels, H.; Covaci, A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 2012, 87, 796–802. [Google Scholar] [CrossRef]
- Wu, Y.F.; Chitranshi, P.; Loukotkova, L.; da Costa, G.G.; Beland, F.A.; Zhang, J.; Fang, J.L. Cytochrome P450-mediated metabolism of triclosan attenuates its cytotoxicity in hepatic cells. Arch. Toxicol. 2017, 91, 2405–2423. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Shao, Y.; Xiao, H.X.; Santiago-Schubel, B.; Meyer-Alert, H.; Schiwy, S.; Yin, D.Q.; Hollert, H.; Kuppers, S. Electrochemical simulation of triclosan metabolism and toxicological evaluation. Sci. Total Environ. 2018, 622, 1193–1201. [Google Scholar] [CrossRef]
- Rodricks, J.V.; Swenberg, J.A.; Borzelleca, J.F.; Maronpot, R.R.; Shipp, A.M. Triclosan: A critical review of the experimental data and development of margins of safety for consumer products. Crit. Rev. Toxicol. 2010, 40, 422–484. [Google Scholar] [CrossRef]
- Wang, L.Q.; Falany, C.N.; James, M.O. Triclosan as a substrate and inhibitor of 3′-phosphoadenosine-5′-phosphosulfate-sulfotransferase and UDP-glucuronosyl transferase in human liver fractions. Drug Metab. Dispos. 2004, 32, 1162–1169. [Google Scholar] [CrossRef]
- Provencher, G.; Berube, R.; Dumas, P.; Bienvenu, J.F.; Gaudreau, E.; Belanger, P.; Ayotte, P. Determination of bisphenol A, triclosan and their metabolites in human urine using isotope-dilution liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1348, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Sandborgh-Englund, G.; Adolfsson-Erici, M.; Odham, G.; Ekstrand, J. Pharmacokinetics of triclosan following oral ingestion in humans. J. Toxicol. Environ. Health Part A Curr. Issues 2006, 69, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Lorigo, M.; Cairrao, E. Fetoplacental vasculature as a model to study human cardiovascular endocrine disruption. Mol. Asp. Med. 2022, 87, 38. [Google Scholar] [CrossRef] [PubMed]
- Feiteiro, J.; Mariana, M.; Cairrão, E. Health toxicity of brominated flame retardants: From environmental to human exposure. Environ. Pollut. 2021, 285, 117475. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, I.; Lorigo, M.; Cairrao, E. Update about the disrupting-effects of phthalates on the human reproductive system. Mol. Reprod. Dev. 2021, 88, 650–672. [Google Scholar] [CrossRef]
- Azzouz, A.; Rascon, A.J.; Ballesteros, E. Simultaneous determination of parabens, alkylphenols, phenylphenols, bisphenol A and triclosan in human urine, blood and breast milk by continuous solid-phase extraction and gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2016, 119, 16–26. [Google Scholar] [CrossRef]
- Allmyr, M.; Harden, F.; Toms, L.M.L.; Mueller, J.F.; McLachlan, M.S.; Adolfsson-Erici, M.; Sandborgh-Englund, G. The influence of age and gender on triclosan concentrations in Australian human blood serum. Sci. Total Environ. 2008, 393, 162–167. [Google Scholar] [CrossRef]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Urinary concentrations of triclosan in the U.S. population: 2003-2004. Env. Health Perspect. 2008, 116, 303–307. [Google Scholar] [CrossRef]
- Jin, C.; Chen, Y.; Zhang, P.; Xiong, Z.; Wang, C.; Tian, Y. Advances on research of human exposure to triclosan. Chin. J. Prev. Med. 2016, 50, 285–288. [Google Scholar] [CrossRef]
- Macisaac, J.K.; Gerona, R.R.; Blanc, P.D.; Apatira, L.; Friesen, M.W.; Coppolino, M.; Janssen, S. Health Care Worker Exposures to the Antibacterial Agent Triclosan. J. Occup. Environ. Med. 2014, 56, 834–839. [Google Scholar] [CrossRef]
- Ishibashi, H.; Matsumura, N.; Hirano, M.; Matsuoka, M.; Shiratsuchi, H.; Ishibashi, Y.; Takao, Y.; Arizono, K. Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat. Toxicol. 2004, 67, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ai, W.M.; Sun, L.M.; Fang, F.; Wang, X.D.; Chen, S.B.; Wang, H.L. Triclosan-induced liver injury in zebrafish (Danio rerio) via regulating MAPK/p53 signaling pathway. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 222, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Yingjie, Q.; Jiayi, H.; Ping, H.; Jiangbo, Q.; Xubo, W.; Jun, W. Long-term exposure to environmental relevant triclosan induces reproductive toxicity on adult zebrafish and its potential mechanism. Sci. Total Environ. 2022, 826, 154026. [Google Scholar]
- Kumar, V.; Chakraborty, A.; Kural, M.R.; Roy, P. Alteration of testicular steroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reprod. Toxicol. 2009, 27, 177–185. [Google Scholar] [CrossRef]
- Stoker, T.E.; Gibson, E.K.; Zorrilla, L.M. Triclosan Exposure Modulates Estrogen-Dependent Responses in the Female Wistar Rat. Toxicol. Sci. 2010, 117, 45–53. [Google Scholar] [CrossRef]
- Jung, E.M.; An, B.S.; Choi, K.C.; Jeung, E.B. Potential estrogenic activity of triclosan in the uterus of immature rats and rat pituitary GH3 cells. Toxicol. Lett. 2012, 208, 142–148. [Google Scholar] [CrossRef]
- Louis, G.W.; Hallinger, D.R.; Stoker, T.E. The effect of triclosan on the uterotrophic response to extended doses of ethinyl estradiol in the weanling rat. Reprod. Toxicol. 2013, 36, 71–77. [Google Scholar] [CrossRef]
- Crawford, B.R.; deCatanzaro, D. Disruption of blastocyst implantation by triclosan in mice: Impacts of repeated and acute doses and combination with bisphenol-A. Reprod. Toxicol. 2012, 34, 607–613. [Google Scholar] [CrossRef]
- Wang, X.L.; Chen, X.J.; Feng, X.J.; Chang, F.; Chen, M.J.; Xia, Y.K.; Chen, L. Triclosan causes spontaneous abortion accompanied by decline of estrogen sulfotransferase activity in humans and mice. Sci. Rep. 2015, 5, 11. [Google Scholar] [CrossRef]
- Feng, Y.X.; Zhang, P.; Zhang, Z.B.; Shi, J.C.; Jiao, Z.H.; Shao, B. Endocrine Disrupting Effects of Triclosan on the Placenta in Pregnant Rats. PLoS ONE 2016, 11, 14. [Google Scholar] [CrossRef]
- Montagnini, B.G.; Forcato, S.; Pernoncine, K.V.; Monteiro, M.C.; Pereira, M.R.F.; Costa, N.O.; Moreira, E.G.; Anselmo-Franci, J.A.; Gerardin, D.C.C. Developmental and Reproductive Outcomes in Male Rats Exposed to Triclosan: Two-Generation Study. Front. Endocrinol. 2021, 12, 738980. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Sen Singh, S.; Singh, S.P.; Singh, P. Evaluation of Triclosan-induced reproductive impairments in the accessory reproductive organs and sperm indices in the mice. Acta Histochem. 2021, 123, 9. [Google Scholar] [CrossRef] [PubMed]
- James, M.O.; Li, W.J.; Summerlot, D.P.; Rowland-Faux, L.; Wood, C.E. Triclosan is a potent inhibitor of estradiol and estrone sulfonation in sheep placenta. Environ. Int. 2010, 36, 942–949. [Google Scholar] [CrossRef]
- Wang, C.F.; Tian, Y. Reproductive endocrine-disrupting effects of triclosan: Population exposure, present evidence and potential mechanisms. Env. Pollut. 2015, 206, 195–201. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, W.S.; Li, W.J.; Liu, C.; Chen, Y.Y.; Yang, Q.L.; Wang, Y.; Sun, K. Inhibition of 11 beta-HSD2 Expression by Triclosan via Induction of Apoptosis in Human Placental Syncytiotrophoblasts. J. Clin. Endocrinol. Metab. 2015, 100, E542–E549. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Etzel, T.M.; Calafat, A.M.; Ye, X.Y.; Chen, A.M.; Lanphear, B.P.; Savitz, D.A.; Yolton, K.; Braun, J.M. Urinary triclosan concentrations during pregnancy and birth outcomes. Environ. Res. 2017, 156, 505–511. [Google Scholar] [CrossRef]
- Jurewicz, J.; Wielgomas, B.; Radwan, M.; Karwacka, A.; Klimowska, A.; Dziewirska, E.; Korczak, K.; Zajdel, R.; Radwan, P.; Hanke, W. Triclosan exposure and ovarian reserve. Reprod. Toxicol. 2019, 89, 168–172. [Google Scholar] [CrossRef]
- Yuan, G.X.; Ma, Y.; Zeng, Y.X.; Pan, H.B.; Liu, P.Y.; Liu, Y.; Liu, G.H.; Cheng, J.Q.; Guo, Y.S. Associations between low-dose triclosan exposure and semen quality in a Chinese population. Environ. Pollut. 2022, 299, 7. [Google Scholar] [CrossRef]
- Saley, A.; Hess, M.; Miller, K.; Howard, D.; King-Heiden, T.C. Cardiac Toxicity of Triclosan in Developing Zebrafish. Zebrafish 2016, 13, 399–404. [Google Scholar] [CrossRef]
- Wang, D.T.; Zhang, Y.H.; Li, J.Y.; Dahlgren, R.A.; Wang, X.D.; Huang, H.S.; Wang, H.L. Risk assessment of cardiotoxicity to zebrafish (Danio rerio) by environmental exposure to triclosan and its derivatives. Environ. Pollut. 2020, 265, 12. [Google Scholar] [CrossRef]
- Ma, Y.; Zang, L.X.; Wang, D.T.; Jiang, J.H.; Wang, C.H.; Wang, X.D.; Fang, F.; Wang, H.L. Effects of miR-181a-5p abnormal expression on zebrafish (Danio rerio) vascular development following triclosan exposure. Chemosphere 2019, 223, 523–535. [Google Scholar] [CrossRef]
- Cherednichenko, G.; Zhang, R.; Bannister, R.A.; Timofeyev, V.; Li, N.; Fritsch, E.B.; Feng, W.; Barrientos, G.C.; Schebb, N.H.; Hammock, B.D.; et al. Triclosan impairs excitation-contraction coupling and Ca2+ dynamics in striated muscle. Proc. Natl. Acad. Sci. USA 2012, 109, 14158–14163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhang, X.Z.; Zhang, Y.Q.; Liu, M.Y.; Jin, J.; Yan, J.; Shen, X.; Hu, N.; Dong, D.L. Mitochondrial uncoupler triclosan induces vasorelaxation of rat arteries. Acta Pharm. Sin. B 2017, 7, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, M.P.; Palmer, J.E.; Faddy, M.J.; Westerman, B.; Carle, A.D.; West, M.J.; Seymour, G.J. The Influence of Triclosan on Biomarkers of Cardiovascular Risk in Patients in the Cardiovascular and Periodontal Study (CAPS): A Randomized Controlled Trial. J. Periodontol. 2015, 86, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Du, G.Z.; Yu, M.M.; Wang, L.L.; Hu, W.Y.; Song, L.; Lu, C.C.; Wang, X.R. Transcriptome and DNA Methylome Dynamics during Triclosan- Induced Cardiomyocyte Differentiation Toxicity. Stem Cells Int. 2018, 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, R.R.; Zhang, L.B. Triclosan stimulates human vascular endothelial cell injury via repression of the PI3K/Akt/mTOR axis. Chemosphere 2020, 241, 7. [Google Scholar] [CrossRef]
- Crofton, K.M.; Paul, K.B.; De Vito, M.J.; Hedge, J.M. Short-term in vivo exposure to the water contaminant triclosan: Evidence for disruption of thyroxine. Environ. Toxicol. Pharmacol. 2007, 24, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Marie, A.M.; Ahmed-Farid, O.A. Combined approaches for evaluation of xenoestrogen neural toxicity and thyroid dysfunction: Screening of oxido-nitrosative markers, DNA fragmentation, and biogenic amine degradation. J. Biochem. Mol. Toxicol. 2020, 34, 10. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, M.; Zeng, L.; Liu, C.J. P38/TRHr-Dependent Regulation of TPO in Thyroid Cells Contributes to the Hypothyroidism of Triclosan-Treated Rats. Cell. Physiol. Biochem. 2018, 45, 1303–1315. [Google Scholar] [CrossRef]
- Schnitzler, J.G.; Frederich, B.; Dussenne, M.; Klaren, P.H.M.; Silvestre, F.; Das, K. Triclosan exposure results in alterations of thyroid hormone status and retarded early development and metamorphosis in Cyprinodon variegatus. Aquat. Toxicol. 2016, 181, 1–10. [Google Scholar] [CrossRef]
- Cullinan, M.P.; Palmer, J.E.; Carle, A.D.; West, M.J.; Seymour, G.J. Long term use of triclosan toothpaste and thyroid function. Sci. Total Environ. 2012, 416, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Chen, A.M.; Hoofnagle, A.; Papandonatos, G.D.; Jackson-Browne, M.; Hauser, R.; Romano, M.E.; Karagas, M.R.; Yolton, K.; Zoeller, R.T.; et al. Associations of early life urinary triclosan concentrations with maternal, neonatal, and child thyroid hormone levels. Horm. Behav. 2018, 101, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Koeppe, E.S.; Ferguson, K.K.; Colacino, J.A.; Meeker, J.D. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci. Total Environ. 2013, 445, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Gunier, R.B.; Chevrier, J.; Calafat, A.M.; Ye, X.Y.; Eskenazi, B.; Harley, K.G. Associations of maternal exposure to triclosan, parabens, and other phenols with prenatal maternal and neonatal thyroid hormone levels. Environ. Res. 2018, 165, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, A.; Shu, H.; Peeters, R.P.; Kortenkamp, A.; Lindh, C.H.; Demeneix, B.; Bornehag, C.G.; Korevaar, T.I.M. Association of urinary bisphenols and triclosan with thyroid function during early pregnancy. Environ. Int. 2019, 133, 7. [Google Scholar] [CrossRef]
- Ha, N.Y.; Kim, D.H.; Ryu, J.Y. Relationship between triclosan exposure and thyroid hormones: The Second Korean National Environmental Health Survey (2012–2014). Ann. Occup. Environ. Med. 2019, 31, 9. [Google Scholar] [CrossRef]
- Skarha, J.; Minguez-Alarcon, L.; Williams, P.L.; Korevaar, T.I.M.; de Poortere, R.A.; Broeren, M.A.C.; Ford, J.B.; Eliot, M.; Hauser, R.; Braun, J.M. Cross-sectional associations between urinary triclosan and serum thyroid function biomarker concentrations in women. Environ. Int. 2019, 122, 256–262. [Google Scholar] [CrossRef]
| CAS No. | 3380-34-5 |
| Chemical structure | 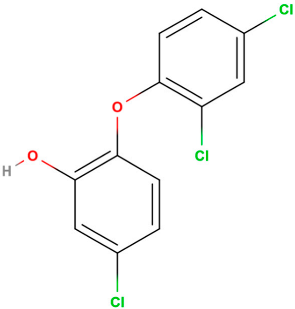 |
| Molecular formula | C12H7Cl3O2 |
| Commercial name | Irgasan DP 300, FAT 80′023, CH 3565, GP41-353, Irgacare MP, Lexol 300, Cloxifenolum e Ster-Zac |
| Form | Powder or crystalline powder |
| Colour | White |
| Applications | Antimicrobial, antiseptic, and preservative |
| Nature | Hydrophobic/lipophilic |
| Molecular weight | 289.5 g/mol |
| Density | 1.49 g/cm3 |
| Dissociation constant (pKa) (20 °C) | 7.9 |
| Henry constant (Hc) (atm mol−1·m−3) | 1.5 × 10−7 (25 °C) |
| Octanol-water partition coefficient (log Kow) | 4.8 |
| Sorption coefficient (Koc) | 18,408 |
| Vapour pressure | 4 × 10−6 Pa (mm Hg a 20 °C) |
| Triclosan degradation products | Methyl-TCS, dioxins, chlorophenol, chloroform |
| Solvent | Solubilities at 25 °C (g Triclosan/100 g Solvent) |
|---|---|
| Distilled water (20 °C) | 0.001 |
| Acetone | >100 |
| Ethanol 70% or 95% | >100 |
| Isopropanol | >100 |
| Propylene glycol | >100 |
| Hexane | 8.5 |
| Tween 20 | >100 |
| Glycerine | 0.15 |
| Type of Product and Category | Triclosan Concentration (%) | References |
|---|---|---|
| Oral hygiene | ||
| Toothpaste | 0.3 | [6] |
| Mouthwash | 0.03 | [36] |
| Skin washing products | ||
| Liquid soap | 0.1 to 0.45 | [37] |
| Shower gel | 0.3 | [28] |
| Dishwasher | 0.1 | [3] |
| Products applied to the skin | ||
| Body lotion | 0.3 | [28] |
| Facial moisturizer | 0.3 | |
| Deodorant | 0.3 | |
| Fluid | Concentration (nM) | Country | Reference |
|---|---|---|---|
| Serum | 4.1–41.4 | Spain | [52] |
| Plasma | 0.0035–1200 | Australia, Sweden | [35,53] |
| Urine | 8.3–13090 | USA | [53,54] |
| 0.56 ± 1.8 (non-obese) | India | [55] | |
| 0.16 ± 0.27 (obese) | |||
| 1.1–7.3 | Spain | [52] | |
| 0.51 ± 0.53 | USA | [56] | |
| Breast milk | 0.86–7.3 | Spain | [52] |
| 0.062–252 | USA, Australia, Sweden | [33,53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, A.C.; Mariana, M.; Cairrao, E. Triclosan and Its Consequences on the Reproductive, Cardiovascular and Thyroid Levels. Int. J. Mol. Sci. 2022, 23, 11427. https://doi.org/10.3390/ijms231911427
Marques AC, Mariana M, Cairrao E. Triclosan and Its Consequences on the Reproductive, Cardiovascular and Thyroid Levels. International Journal of Molecular Sciences. 2022; 23(19):11427. https://doi.org/10.3390/ijms231911427
Chicago/Turabian StyleMarques, Ana C., Melissa Mariana, and Elisa Cairrao. 2022. "Triclosan and Its Consequences on the Reproductive, Cardiovascular and Thyroid Levels" International Journal of Molecular Sciences 23, no. 19: 11427. https://doi.org/10.3390/ijms231911427
APA StyleMarques, A. C., Mariana, M., & Cairrao, E. (2022). Triclosan and Its Consequences on the Reproductive, Cardiovascular and Thyroid Levels. International Journal of Molecular Sciences, 23(19), 11427. https://doi.org/10.3390/ijms231911427