Chronic Exposure to Endocrine Disruptor Vinclozolin Leads to Lung Damage via Nrf2–Nf-kb Pathway Alterations
Abstract
1. Introduction
2. Results
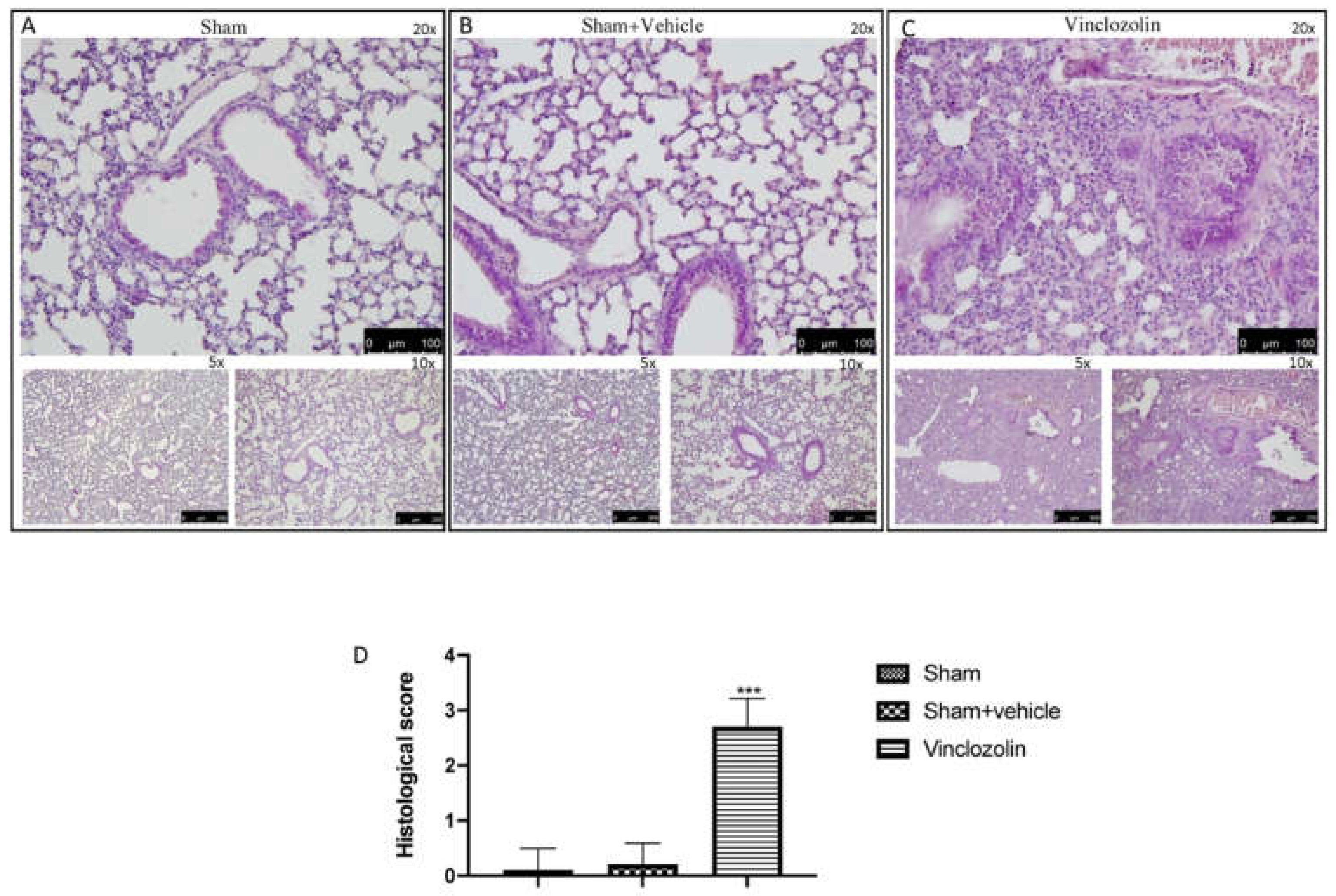
2.1. Exposure to Vinclozolin Induced Lung Alterations
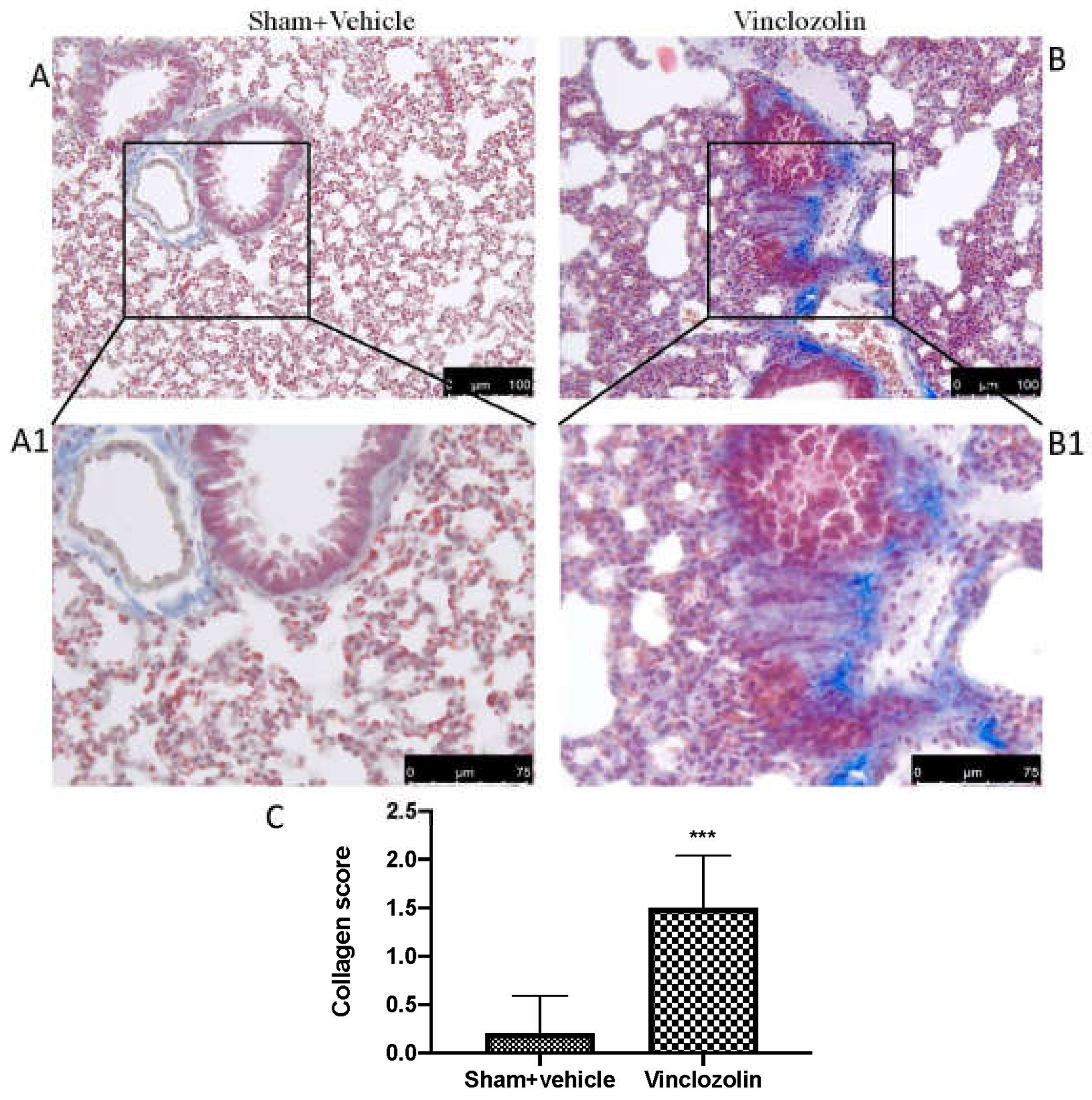
2.2. Exposure to Vinclozolin Induced Lung Fibrosis
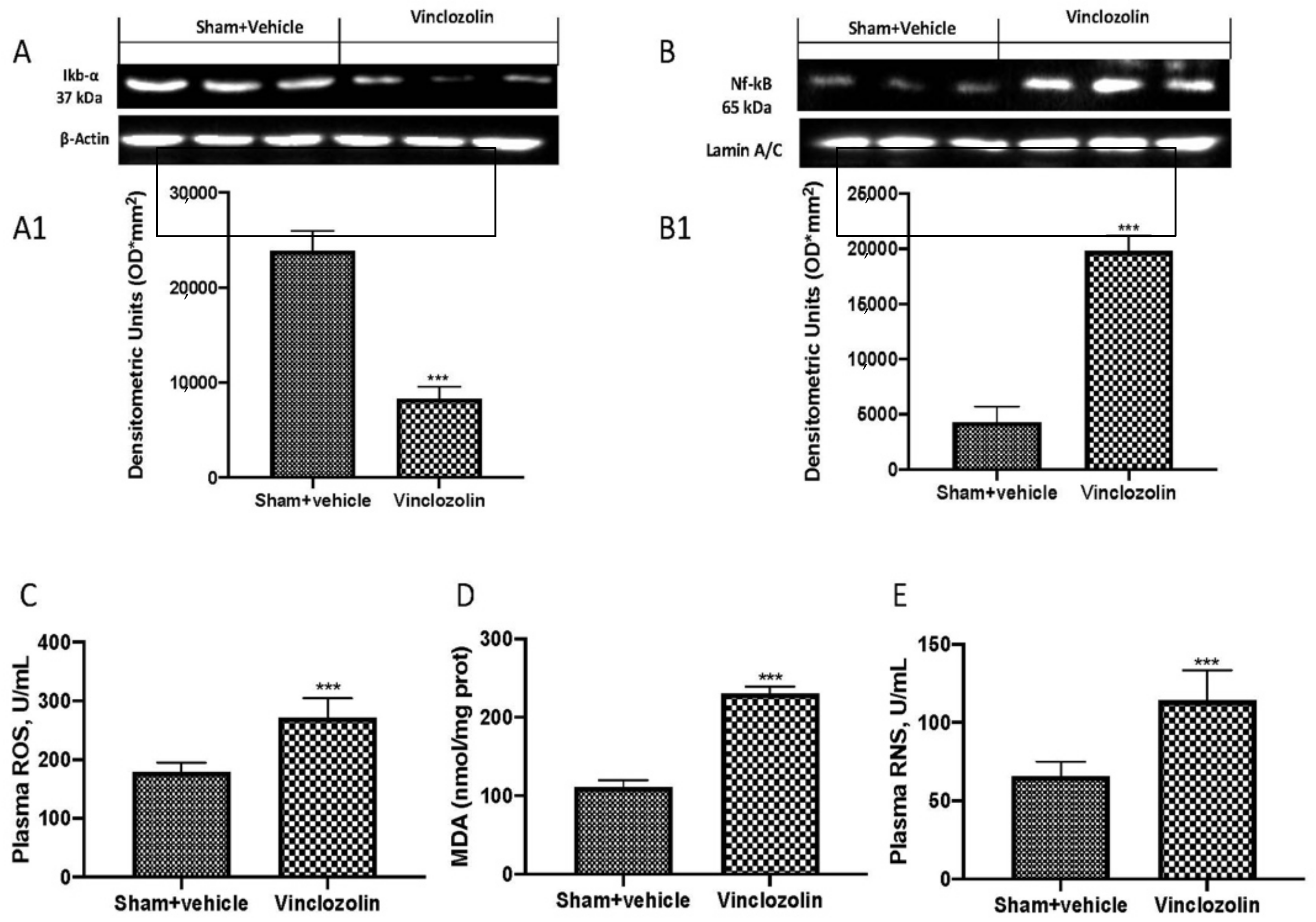
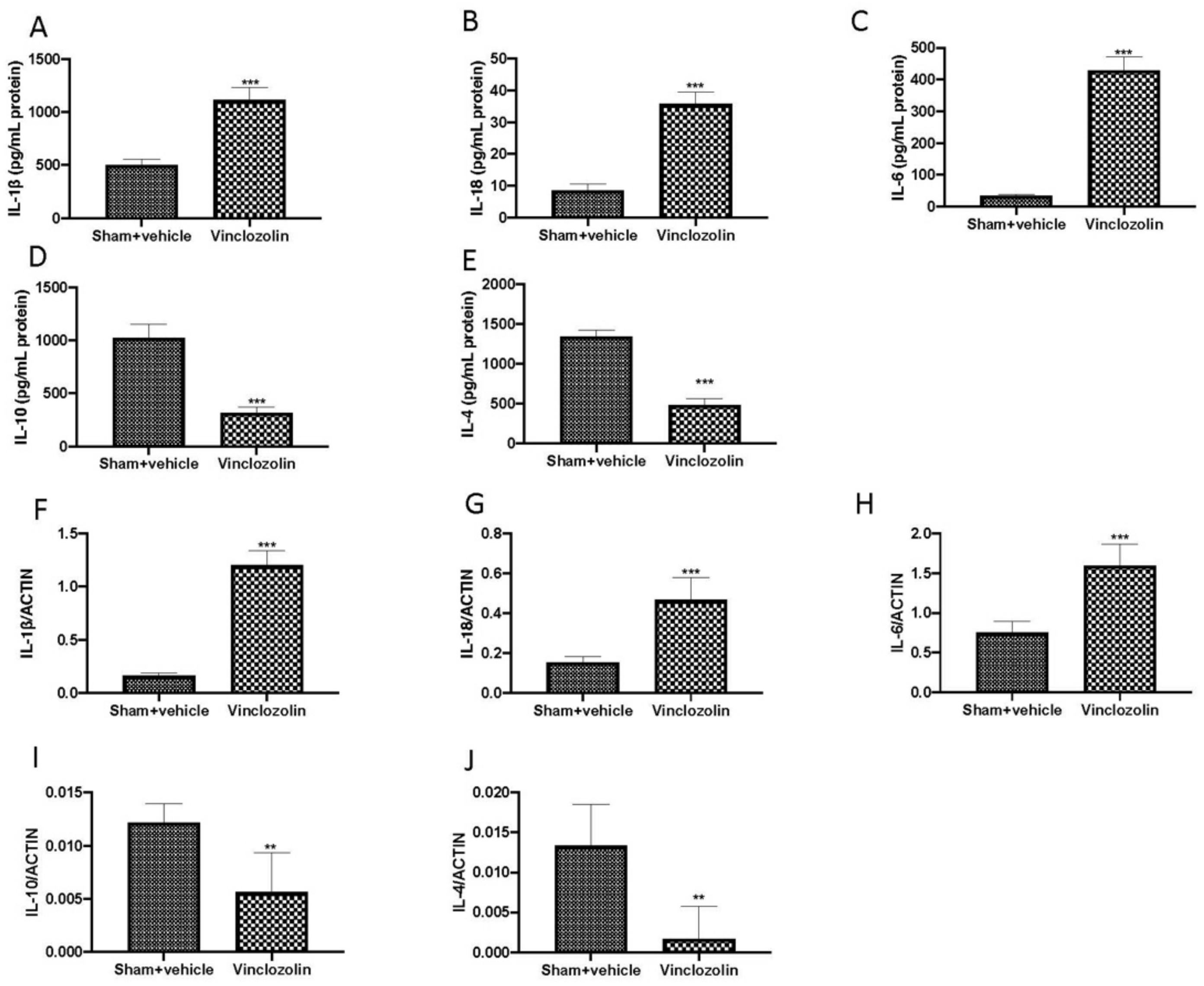
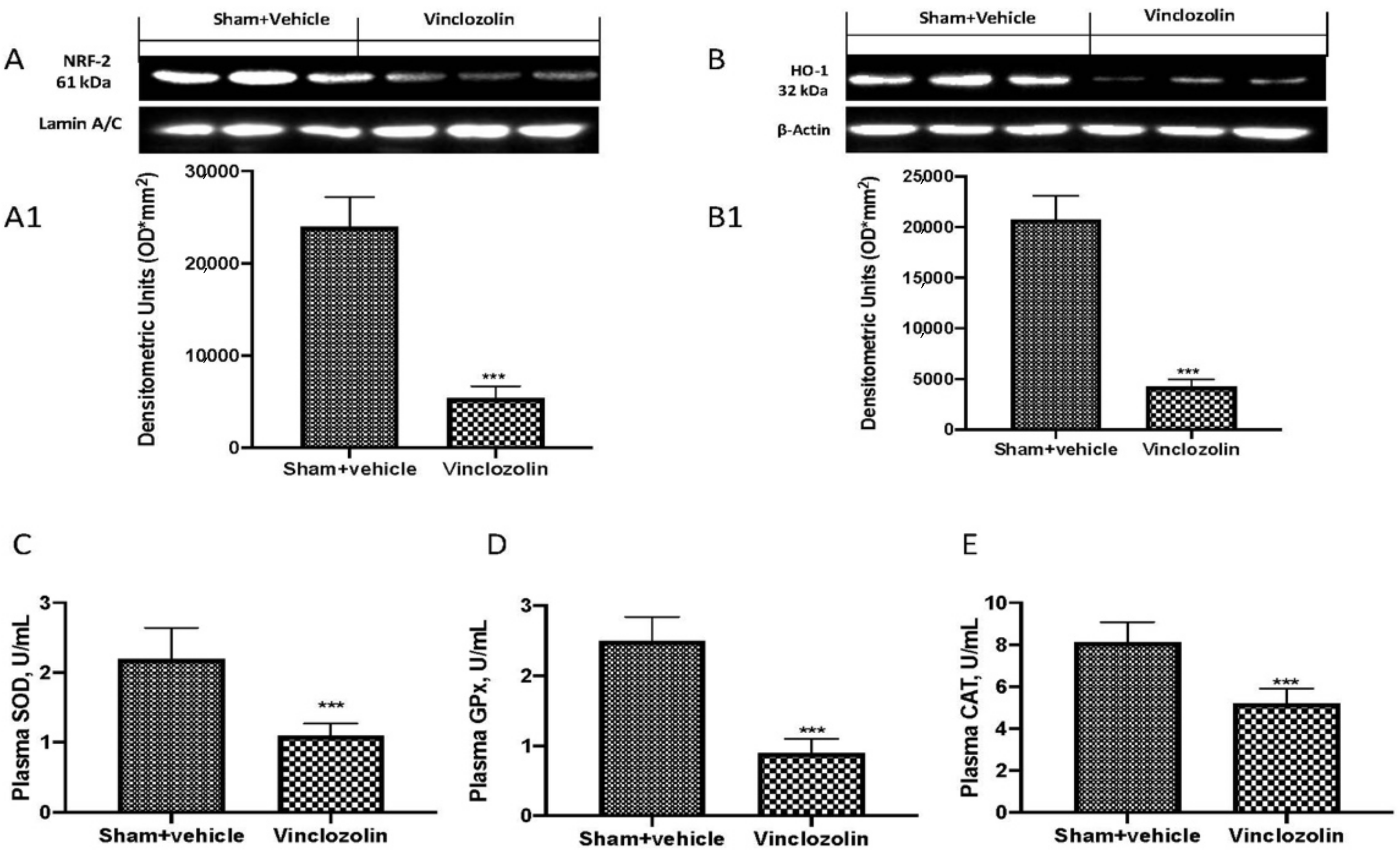
2.3. Exposure to Vinclozolin Induced Inflammation and Oxidative Stress
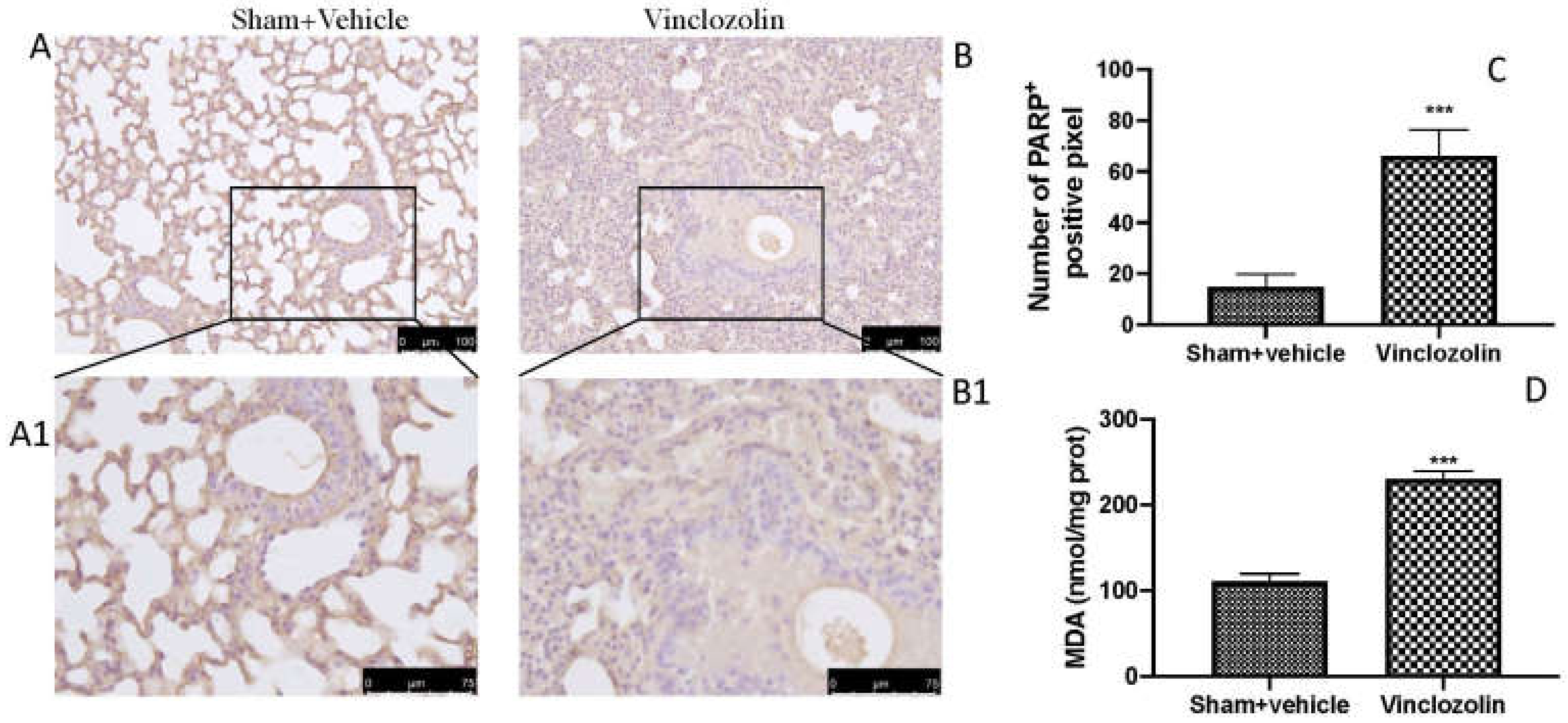
2.4. Exposure to Vinclozolin Induced DNA Damage and Lipid Peroxidation
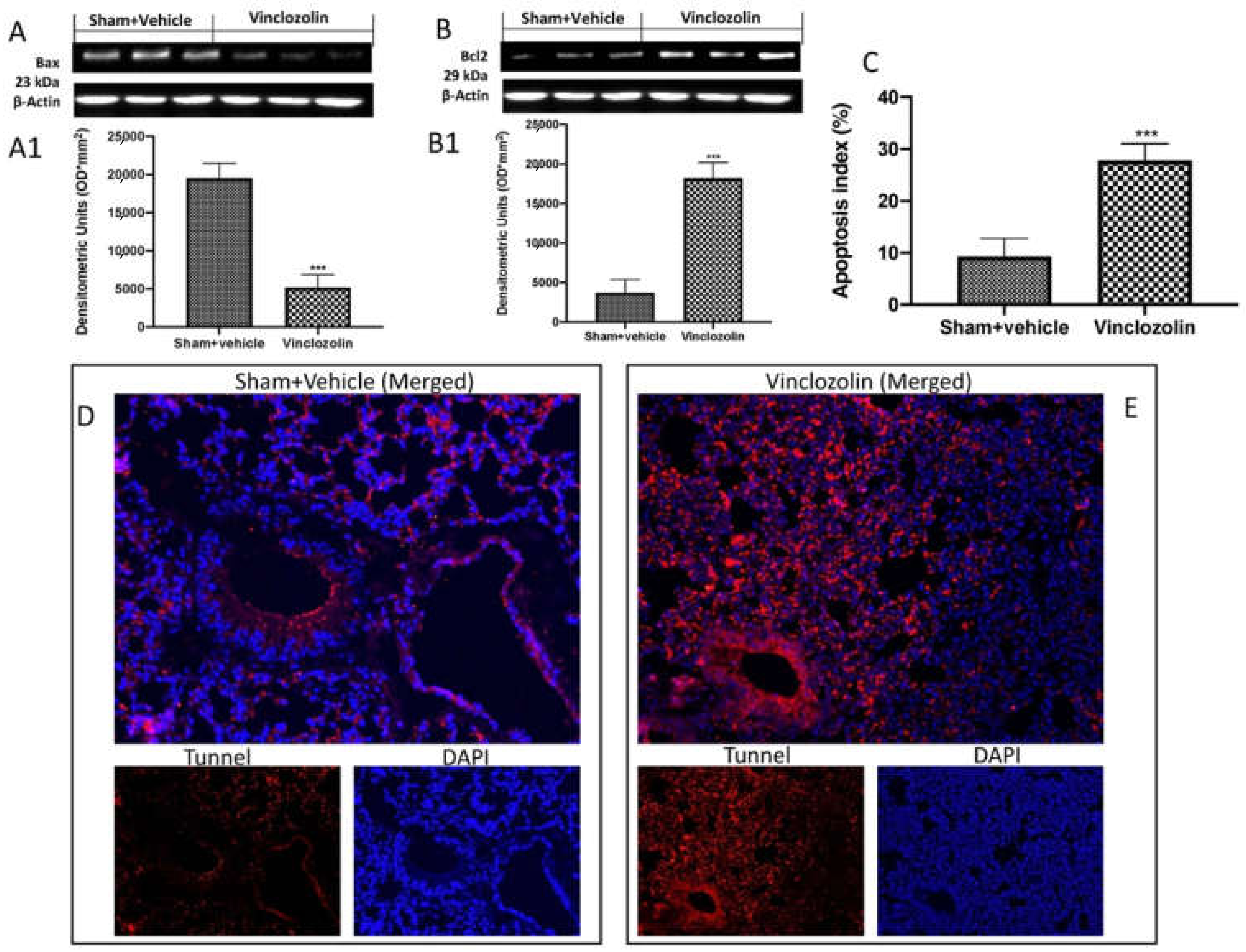
2.5. Exposure to Vinclozolin Induced Lung Apoptosis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design and Groups
- Sham: animals that were untreated (shown only in the histology);
- Sham+vehicle: animals that were orally administered with corn oil;
- Vinclozolin: animals that were orally administered 100 mg of vinclozolin daily for 28 days.
4.3. Western Blot Analysis of Cytosolic and Nuclear Extracts
4.4. Histopathological Evaluation
4.5. Immunohistochemical Evaluation
4.6. Evaluation of Tissue Lipid Peroxidation
4.7. Assessment of the Antioxidant System
4.8. Assessment of Cytokine Production
4.9. Terminal Deoxynucleotidyl Nick-End Labeling (TUNEL) Assay
4.10. Real-Time Quantitative Polymerase Chain Reaction Analysis (RT qPCR)
4.11. Materials
4.12. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcoccia, D.; Smeriglio, A.; Mantovani, A.; Trombetta, D.; Lorenzetti, S. Intracellular distribution of vinclozolin and its metabolites differently affects 5alpha-dihydrotestosterone (DHT)-induced PSA secretion in LNCaP cells. Reprod. Toxicol. 2022, 111, 83–91. [Google Scholar] [CrossRef]
- Kang, C.Q.; Meng, Q.Y.; Dang, W.; Shao, Y.J.; Lu, H.L. Effects of chronic exposure to the fungicide vinclozolin on gut microbiota community in an aquatic turtle. Ecotoxicol. Environ. Saf. 2022, 239, 113621. [Google Scholar] [CrossRef]
- Lu, H.L.; Kang, C.Q.; Meng, Q.Y.; Hu, J.R.; Melvin, S.D. Functional and hepatic metabolite changes in aquatic turtle hatchlings exposed to the anti-androgenic fungicide vinclozolin. Ecotoxicol. Environ. Saf. 2022, 231, 113220. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, J.; Zhang, Y.; Fu, H.; Yan, Z.; Zhu, Y. Vinclozolin-induced mouse penile malformation and “small testis” via miR132, miR195a together with the Hippo signaling pathway. Toxicology 2021, 460, 152842. [Google Scholar] [CrossRef]
- Wu, K.; Li, Y.; Pan, P.; Li, Z.; Yu, Y.; Huang, J.; Ma, F.; Tian, L.; Fang, Y.; Wang, Y.; et al. Gestational vinclozolin exposure suppresses fetal testis development in rats. Ecotoxicol. Environ. Saf. 2020, 203, 111053. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, G.; Wartalski, K.; Tabarowski, Z.; Duda, M. Effects of vinclozolin exposure on the expression and activity of SIRT1 and SIRT6 in the porcine ovary. J. Physiol. Pharmacol. 2019, 70, 153–165. [Google Scholar] [CrossRef]
- Aquilino, M.; Sanchez-Arguello, P.; Novo, M.; Martinez-Guitarte, J.L. Effects on tadpole snail gene expression after exposure to vinclozolin. Ecotoxicol. Environ. Saf. 2019, 170, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Gazo, I.; Linhartova, P.; Shaliutina, A.; Hulak, M. Influence of environmentally relevant concentrations of vinclozolin on quality, DNA integrity, and antioxidant responses of sterlet Acipenser ruthenus spermatozoa. Chem. Biol. Interact. 2013, 203, 377–385. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Gugliandolo, E.; Siracusa, R.; Cordaro, M.; Genovese, T.; Peritore, A.F.; Crupi, R.; Interdonato, L.; Di Paola, D.; Cuzzocrea, S.; et al. Toxic Exposure to Endocrine Disruptors Worsens Parkinson’s Disease Progression through NRF2/HO-1 Alteration. Biomedicines 2022, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Gugliandolo, E.; Cordaro, M.; Fusco, R.; Genovese, T.; Peritore, A.F.; Crupi, R.; Interdonato, L.; Di Paola, D.; Cuzzocrea, S.; et al. Toxic Effects of Endocrine Disruptor Exposure on Collagen-Induced Arthritis. Biomolecules 2022, 12, 564. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; King, S.E.; McBirney, M.; Kubsad, D.; Pappalardo, M.; Beck, D.; Sadler-Riggleman, I.; Skinner, M.K. Vinclozolin induced epigenetic transgenerational inheritance of pathologies and sperm epimutation biomarkers for specific diseases. PLoS ONE 2018, 13, e0202662. [Google Scholar] [CrossRef] [PubMed]
- Aquilino, M.; Sanchez-Arguello, P.; Martinez-Guitarte, J.L. Genotoxic effects of vinclozolin on the aquatic insect Chironomus riparius (Diptera, Chironomidae). Environ. Pollut. 2018, 232, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, S.; Roediger, B.; Abtin, A.; Shklovskaya, E.; Fazekas de St Groth, B.; Yamane, H.; Weninger, W.; Le Gros, G.; Ronchese, F. CD326(lo)CD103(lo)CD11b(lo) dermal dendritic cells are activated by thymic stromal lymphopoietin during contact sensitization in mice. J. Immunol. 2014, 193, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef]
- Guerrero-Bosagna, C.; Settles, M.; Lucker, B.; Skinner, M.K. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE 2010, 5, e13100. [Google Scholar] [CrossRef]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Kavlock, R.J.; Daston, G.P.; DeRosa, C.; Fenner-Crisp, P.; Gray, L.E.; Kaattari, S.; Lucier, G.; Luster, M.; Mac, M.J.; Maczka, C.; et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: A report of the U.S. EPA-sponsored workshop. Environ. Health Perspect. 1996, 104 (Suppl. 4), 715–740. [Google Scholar] [CrossRef]
- Derghal, A.; Djelloul, M.; Trouslard, J.; Mounien, L. An Emerging Role of micro-RNA in the Effect of the Endocrine Disruptors. Front. Neurosci. 2016, 10, 318. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Wolf, C.J.; LeBlanc, G.A.; Ostby, J.S.; Gray, L.E., Jr. Characterization of the period of sensitivity of fetal male sexual development to vinclozolin. Toxicol. Sci. 2000, 55, 152–161. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Nilsson, E.; Klukovich, R.; Sadler-Riggleman, I.; Beck, D.; Xie, Y.; Yan, W.; Skinner, M.K. Environmental toxicant induced epigenetic transgenerational inheritance of ovarian pathology and granulosa cell epigenome and transcriptome alterations: Ancestral origins of polycystic ovarian syndrome and primary ovarian insufiency. Epigenetics 2018, 13, 875–895. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Historical insights into cytokines. Eur. J. Immunol. 2007, 37 (Suppl. 1), S34–S45. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Shono, T.; Taguchi, T. Short-time exposure to mono-n-butyl phthalate (MBP)-induced oxidative stress associated with DNA damage and the atrophy of the testis in pubertal rats. Environ. Sci. Pollut. Res. Int. 2014, 21, 3187–3190. [Google Scholar] [CrossRef] [PubMed]
- Neier, K.; Marchlewicz, E.H.; Dolinoy, D.C.; Padmanabhan, V. Assessing Human Health Risk to Endocrine Disrupting Chemicals: A Focus on Prenatal Exposures and Oxidative Stress. Endocr. Disruptors 2015, 3, e1069916. [Google Scholar] [CrossRef]
- Radice, S.; Marabini, L.; Gervasoni, M.; Ferraris, M.; Chiesara, E. Adaptation to oxidative stress: Effects of vinclozolin and iprodione on the HepG2 cell line. Toxicology 1998, 129, 183–191. [Google Scholar] [CrossRef]
- Campolo, M.; Esposito, E.; Ahmad, A.; Di Paola, R.; Paterniti, I.; Cordaro, M.; Bruschetta, G.; Wallace, J.L.; Cuzzocrea, S. Hydrogen sulfide-releasing cyclooxygenase inhibitor ATB-346 enhances motor function and reduces cortical lesion volume following traumatic brain injury in mice. J. Neuroinflamm. 2014, 11, 196. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Talero, E.; Siracusa, R.; Alcaide, A.; Cordaro, M.; Maria Zubelia, J.; Bruschetta, G.; Crupi, R.; Esposito, E.; Cuzzocrea, S.; et al. Protective effect of polyphenols in an inflammatory process associated with experimental pulmonary fibrosis in mice. Br. J. Nutr. 2015, 114, 853–865. [Google Scholar] [CrossRef]
- Genovese, T.; Duranti, A.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Peritore, A.F.; Crupi, R.; Gugliandolo, E.; Cuzzocrea, S.; Di Paola, R.; et al. Fatty Acid Amide Hydrolase (FAAH) Inhibition Plays a Key Role in Counteracting Acute Lung Injury. Int. J. Mol. Sci. 2022, 23, 2781. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Scuto, M.; Cuzzocrea, S.; Di Paola, R.; et al. Modulation of NLRP3 Inflammasome through Formyl Peptide Receptor 1 (Fpr-1) Pathway as a New Therapeutic Target in Bronchiolitis Obliterans Syndrome. Int. J. Mol. Sci. 2020, 21, 2144. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Peritore, A.F.; Crupi, R.; Interdonato, L.; Gugliandolo, E.; Cuzzocrea, S.; Paola, R.D.; et al. Acai (Euterpe Oleraceae Mart.) Seeds Regulate NF-kappaB and Nrf2/ARE Pathways Protecting Lung against Acute and Chronic Inflammation. Cell. Physiol. Biochem. 2022, 56, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. Consumption of Anacardium occidentale L. (Cashew Nuts) Inhibits Oxidative Stress through Modulation of the Nrf2/HO-1 and NF-kB Pathways. Molecules 2020, 25, 4426. [Google Scholar] [CrossRef] [PubMed]
- Knet, M.; Wartalski, K.; Hoja-Lukowicz, D.; Tabarowski, Z.; Slomczynska, M.; Duda, M. Analysis of porcine granulosa cell death signaling pathways induced by vinclozolin. Theriogenology 2015, 84, 927–939. [Google Scholar] [CrossRef]
- Knet, M.; Tabarowski, Z.; Slomczynska, M.; Duda, M. The effects of the environmental antiandrogen vinclozolin on the induction of granulosa cell apoptosis during follicular atresia in pigs. Theriogenology 2014, 81, 1239–1247. [Google Scholar] [CrossRef]
- Lin, J.; Xia, J.; Zhao, H.S.; Hou, R.; Talukder, M.; Yu, L.; Guo, J.Y.; Li, J.L. Lycopene Triggers Nrf2-AMPK Cross Talk to Alleviate Atrazine-Induced Nephrotoxicity in Mice. J. Agric. Food Chem. 2018, 66, 12385–12394. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Bi, H.; Ma, K.; Li, B. Developmental Exposure to Atrazine Impairs Spatial Memory and Downregulates the Hippocampal D1 Dopamine Receptor and cAMP-Dependent Signaling Pathway in Rats. Int. J. Mol. Sci. 2018, 19, 2241. [Google Scholar] [CrossRef]
- Cordaro, M.; Paterniti, I.; Siracusa, R.; Impellizzeri, D.; Esposito, E.; Cuzzocrea, S. KU0063794, a Dual mTORC1 and mTORC2 Inhibitor, Reduces Neural Tissue Damage and Locomotor Impairment After Spinal Cord Injury in Mice. Mol. Neurobiol. 2017, 54, 2415–2427. [Google Scholar] [CrossRef]
- Fusco, R.; D’Amico, R.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Absence of formyl peptide receptor 1 causes endometriotic lesion regression in a mouse model of surgically-induced endometriosis. Oncotarget 2018, 9, 31355–31366. [Google Scholar] [CrossRef][Green Version]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Crupi, R.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Petrosino, S.; Evangelista, M.; Di Paola, R.; et al. N-Palmitoylethanolamine-oxazoline (PEA-OXA): A new therapeutic strategy to reduce neuroinflammation, oxidative stress associated to vascular dementia in an experimental model of repeated bilateral common carotid arteries occlusion. Neurobiol. Dis. 2019, 125, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Peritore, A.F.; D’Amico, R.; Gugliandolo, E.; Di Paola, R.; Cuzzocrea, S. 2-Pentadecyl-2-Oxazoline Reduces Neuroinflammatory Environment in the MPTP Model of Parkinson Disease. Mol. Neurobiol. 2018, 55, 9251–9266. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Cordaro, M.; Bruschetta, G.; Crupi, R.; Pascali, J.; Alfonsi, D.; Marcolongo, G.; Cuzzocrea, S. 2-pentadecyl-2-oxazoline: Identification in coffee, synthesis and activity in a rat model of carrageenan-induced hindpaw inflammation. Pharmacol. Res. 2016, 108, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Fusco, R.; Impellizzeri, D.; Cordaro, M.; Britti, D.; Morittu, V.M.; Evangelista, M.; Cuzzocrea, S. Adelmidrol, in combination with hyaluronic acid, displays increased anti-inflammatory and analgesic effects against monosodium iodoacetate-induced osteoarthritis in rats. Arthritis Res. Ther. 2016, 18, 291. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cordaro, M.; Genovese, T.; Impellizzeri, D.; Siracusa, R.; Gugliandolo, E.; Peritore, A.F.; D’Amico, R.; Crupi, R.; Cuzzocrea, S.; et al. Adelmidrol: A New Promising Antioxidant and Anti-Inflammatory Therapeutic Tool in Pulmonary Fibrosis. Antioxidants 2020, 9, 601. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cordaro, M.; Siracusa, R.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Biochemical Evaluation of the Antioxidant Effects of Hydroxytyrosol on Pancreatitis-Associated Gut Injury. Antioxidants 2020, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Siracusa, R.; Fusco, R.; D’Amico, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Scuto, M.; Crupi, R.; Mandalari, G.; et al. Cashew (Anacardium occidentale L.) Nuts Counteract Oxidative Stress and Inflammation in an Acute Experimental Model of Carrageenan-Induced Paw Edema. Antioxidants 2020, 9, 660. [Google Scholar] [CrossRef]
- Fusco, R.; Gugliandolo, E.; Siracusa, R.; Scuto, M.; Cordaro, M.; D’Amico, R.; Evangelista, M.; Peli, A.; Peritore, A.F.; Impellizzeri, D.; et al. Formyl Peptide Receptor 1 Signaling in Acute Inflammation and Neural Differentiation Induced by Traumatic Brain Injury. Biology 2020, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Luo, D.; Wu, D.; Liu, H.; Li, M.; Sun, Q.; Jia, L. Lung inflammation induced by exposure to Bisphenol-A is associated with mTOR-mediated autophagy in adolescent mice. Chemosphere 2020, 248, 126035. [Google Scholar] [CrossRef]
- Cordaro, M.; Impellizzeri, D.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a Palmitoylethanolamide Analogue, as a New Pharmacological Treatment for the Management of Inflammatory Bowel Disease. Mol. Pharmacol. 2016, 90, 549–561. [Google Scholar] [CrossRef]
- Crupi, R.; Marino, A.; Cuzzocrea, S. n-3 fatty acids: Role in neurogenesis and neuroplasticity. Curr. Med. Chem. 2013, 20, 2953–2963. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Cordaro, M.; Crupi, R.; Mandalari, G.; Impellizzeri, D.; et al. The Role of Cashew (Anacardium occidentale L.) Nuts on an Experimental Model of Painful Degenerative Joint Disease. Antioxidants 2020, 9, 511. [Google Scholar] [CrossRef]
- Siracusa, R.; Paterniti, I.; Cordaro, M.; Crupi, R.; Bruschetta, G.; Campolo, M.; Cuzzocrea, S.; Esposito, E. Neuroprotective Effects of Temsirolimus in Animal Models of Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 2403–2419. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Mazzon, E.; Esposito, E.; Muia, C.; Abdelrahman, M.; Di Paola, R.; Crisafulli, C.; Bramanti, P.; Thiemermann, C. Glycogen synthase kinase-3beta inhibition attenuates the development of ischaemia/reperfusion injury of the gut. Intensive Care Med. 2007, 33, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, D.; Natale, S.; Gugliandolo, E.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Assessment of 2-Pentadecyl-2-oxazoline Role on Lipopolysaccharide-Induced Inflammation on Early Stage Development of Zebrafish (Danio rerio). Life 2022, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Siracusa, R.; D’Amico, R.; Genovese, T.; Franco, G.; Marino, Y.; Di Paola, D.; Cuzzocrea, S.; Impellizzeri, D.; Di Paola, R.; et al. Role of Etanercept and Infliximab on Nociceptive Changes Induced by the Experimental Model of Fibromyalgia. Int. J. Mol. Sci. 2022, 23, 6139. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, D.; Abbate, J.M.; Iaria, C.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Environmental Risk Assessment of Dexamethasone Sodium Phosphate and Tocilizumab Mixture in Zebrafish Early Life Stage (Danio rerio). Toxics 2022, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, D.; Capparucci, F.; Abbate, J.M.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Genovese, T.; Impellizzeri, D.; et al. Environmental Risk Assessment of Oxaliplatin Exposure on Early Life Stages of Zebrafish (Danio rerio). Toxics 2022, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Salinaro, A.T.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cordaro, M.; Cuzzocrea, S. Hidrox® Counteracts Cyclophosphamide-Induced Male Infertility through NRF2 Pathways in a Mouse Model. Antioxidants 2021, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, D.; Capparucci, F.; Lanteri, G.; Crupi, R.; Marino, Y.; Franco, G.A.; Cuzzocrea, S.; Spano, N.; Gugliandolo, E.; Peritore, A.F. Environmental Toxicity Assessment of Sodium Fluoride and Platinum-Derived Drugs Co-Exposure on Aquatic Organisms. Toxics 2022, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, D.; Natale, S.; Iaria, C.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Intestinal Disorder in Zebrafish Larvae (Danio rerio): The Protective Action of N-Palmitoylethanolamide-oxazoline. Life 2022, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; Impellizzeri, D.; D’Amico, R.; Fusco, R.; Peritore, A.F.; Di Paola, D.; Interdonato, L.; Gugliandolo, E.; Crupi, R.; Di Paola, R.; et al. Role of Bevacizumab on Vascular Endothelial Growth Factor in Apolipoprotein E Deficient Mice after Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 4162. [Google Scholar] [CrossRef]
- An, X.; Sun, X.; Hou, Y.; Yang, X.; Chen, H.; Zhang, P.; Wu, J. Protective effect of oxytocin on LPS-induced acute lung injury in mice. Sci. Rep. 2019, 9, 2836. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Gugliandolo, E.; Crupi, R.; Cuzzocrea, S. A new co-micronized composite containing palmitoylethanolamide and polydatin shows superior oral efficacy compared to their association in a rat paw model of carrageenan-induced inflammation. Eur. J. Pharmacol. 2016, 782, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; Mancuso, G.; Midiri, A.; Di Paola, R.; Cuzzocrea, S. Therapeutic potential of dinitrobenzene sulfonic acid (DNBS)-induced colitis in mice by targeting IL-1beta and IL-18. Biochem. Pharmacol. 2018, 155, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Cuzzocrea, S.; Crupi, R. An Update of Palmitoylethanolamide and Luteolin Effects in Preclinical and Clinical Studies of Neuroinflammatory Events. Antioxidants 2020, 9, 216. [Google Scholar] [CrossRef]
- Di Paola, D.; Iaria, C.; Capparucci, F.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Aflatoxin B1 Toxicity in Zebrafish Larva (Danio rerio): Protective Role of Hericium erinaceus. Toxins 2021, 13, 710. [Google Scholar] [CrossRef] [PubMed]
- Paola, D.D.; Capparucci, F.; Natale, S.; Crupi, R.; Cuzzocrea, S.; Spano, N.; Gugliandolo, E.; Peritore, A.F. Combined Effects of Potassium Perchlorate and a Neonicotinoid on Zebrafish Larvae (Danio rerio). Toxics 2022, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, D.; Capparucci, F.; Lanteri, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Combined Toxicity of Xenobiotics Bisphenol A and Heavy Metals on Zebrafish Embryos (Danio rerio). Toxics 2021, 9, 344. [Google Scholar] [CrossRef]
- Di Paola, D.; Natale, S.; Iaria, C.; Crupi, R.; Cuzzocrea, S.; Spano, N.; Gugliandolo, E.; Peritore, A.F. Environmental Co-Exposure to Potassium Perchlorate and Cd Caused Toxicity and Thyroid Endocrine Disruption in Zebrafish Embryos and Larvae (Danio rerio). Toxics 2022, 10, 198. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amico, R.; Di Paola, D.; Impellizzeri, D.; Genovese, T.; Fusco, R.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Interdonato, L.; Cuzzocrea, S.; et al. Chronic Exposure to Endocrine Disruptor Vinclozolin Leads to Lung Damage via Nrf2–Nf-kb Pathway Alterations. Int. J. Mol. Sci. 2022, 23, 11320. https://doi.org/10.3390/ijms231911320
D’Amico R, Di Paola D, Impellizzeri D, Genovese T, Fusco R, Peritore AF, Gugliandolo E, Crupi R, Interdonato L, Cuzzocrea S, et al. Chronic Exposure to Endocrine Disruptor Vinclozolin Leads to Lung Damage via Nrf2–Nf-kb Pathway Alterations. International Journal of Molecular Sciences. 2022; 23(19):11320. https://doi.org/10.3390/ijms231911320
Chicago/Turabian StyleD’Amico, Ramona, Davide Di Paola, Daniela Impellizzeri, Tiziana Genovese, Roberta Fusco, Alessio Filippo Peritore, Enrico Gugliandolo, Rosalia Crupi, Livia Interdonato, Salvatore Cuzzocrea, and et al. 2022. "Chronic Exposure to Endocrine Disruptor Vinclozolin Leads to Lung Damage via Nrf2–Nf-kb Pathway Alterations" International Journal of Molecular Sciences 23, no. 19: 11320. https://doi.org/10.3390/ijms231911320
APA StyleD’Amico, R., Di Paola, D., Impellizzeri, D., Genovese, T., Fusco, R., Peritore, A. F., Gugliandolo, E., Crupi, R., Interdonato, L., Cuzzocrea, S., Di Paola, R., Siracusa, R., & Cordaro, M. (2022). Chronic Exposure to Endocrine Disruptor Vinclozolin Leads to Lung Damage via Nrf2–Nf-kb Pathway Alterations. International Journal of Molecular Sciences, 23(19), 11320. https://doi.org/10.3390/ijms231911320











