Arrangement of Hydrogen Bonds in Aqueous Solutions of Different Globular Proteins
Abstract
1. Introduction
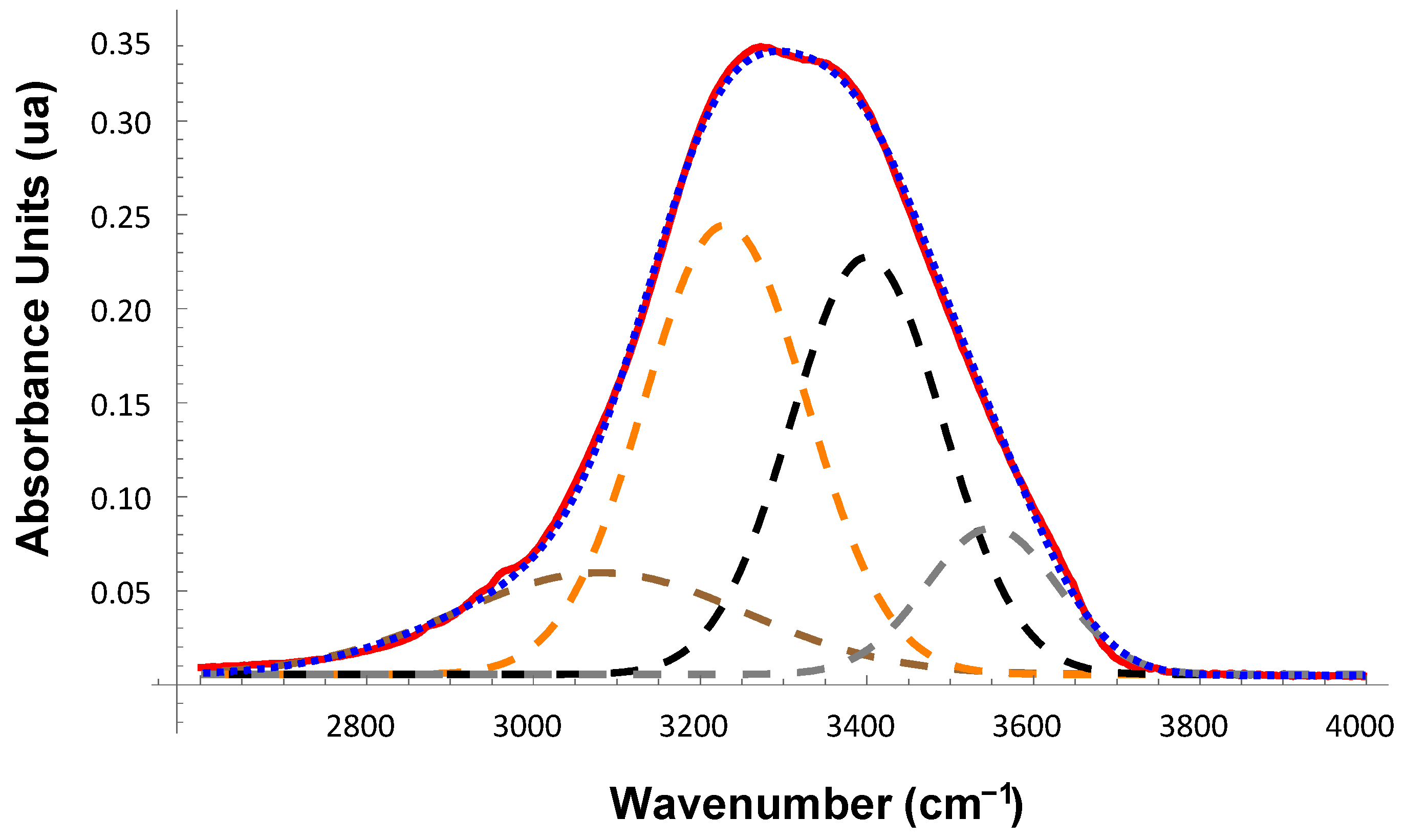
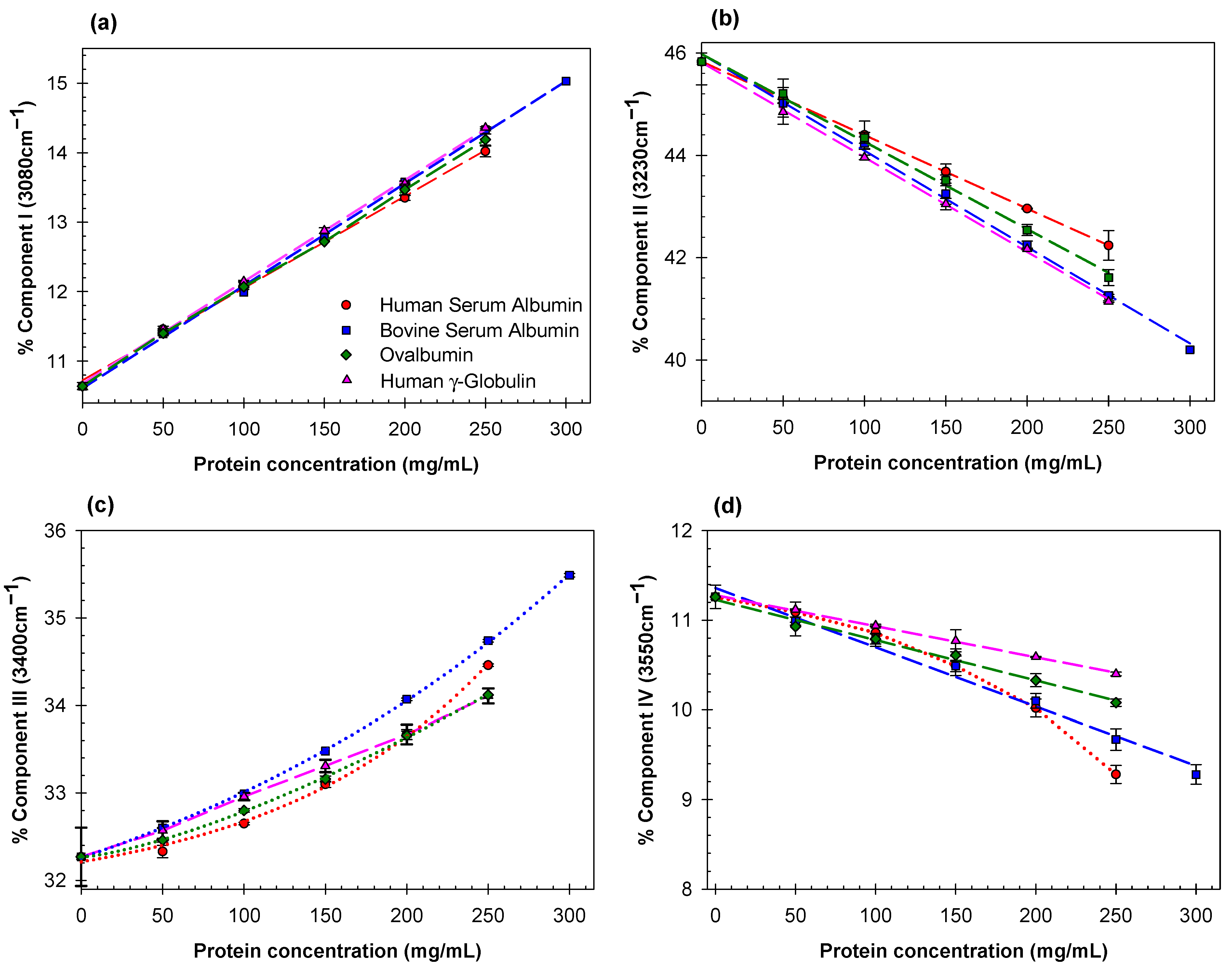
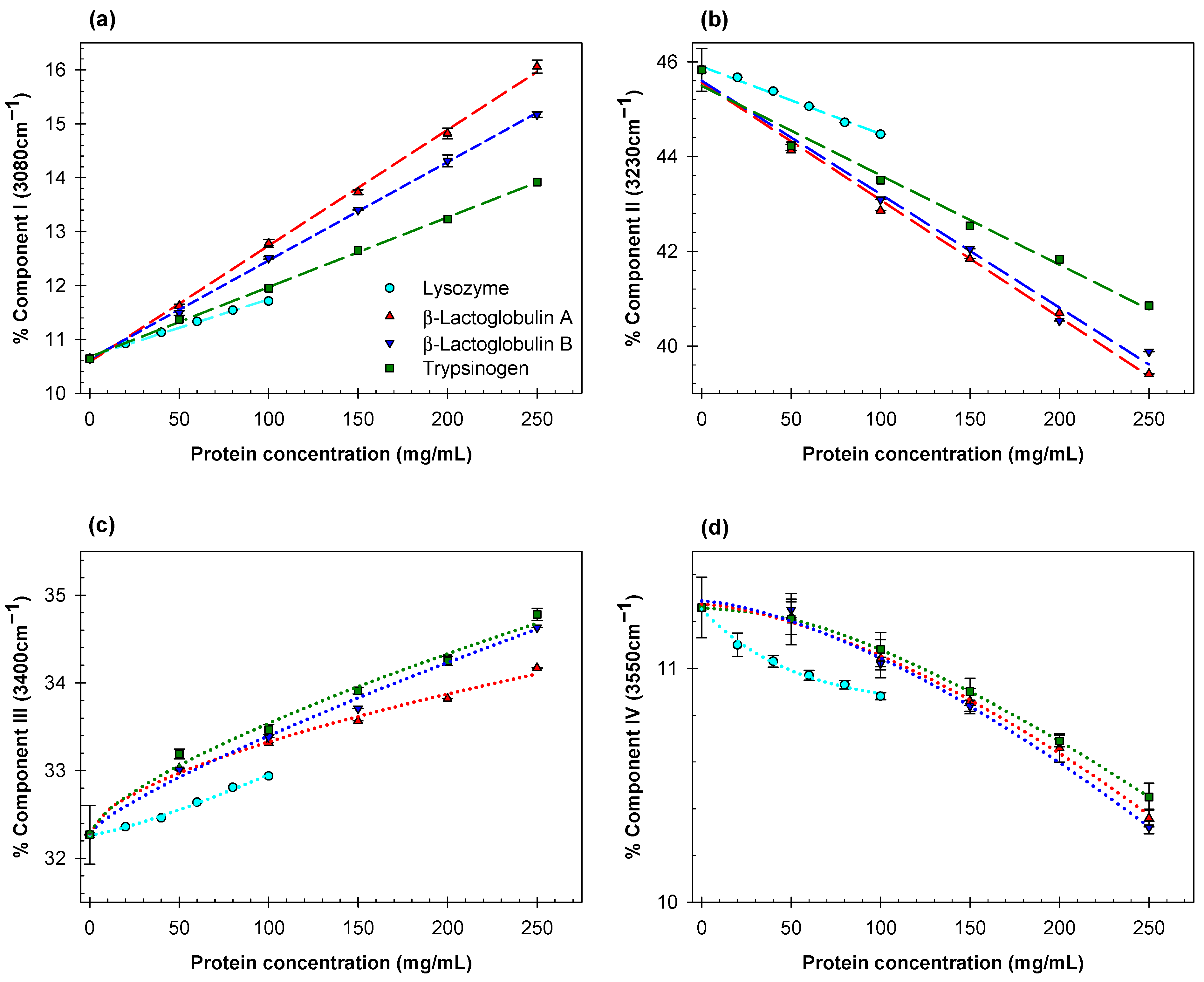
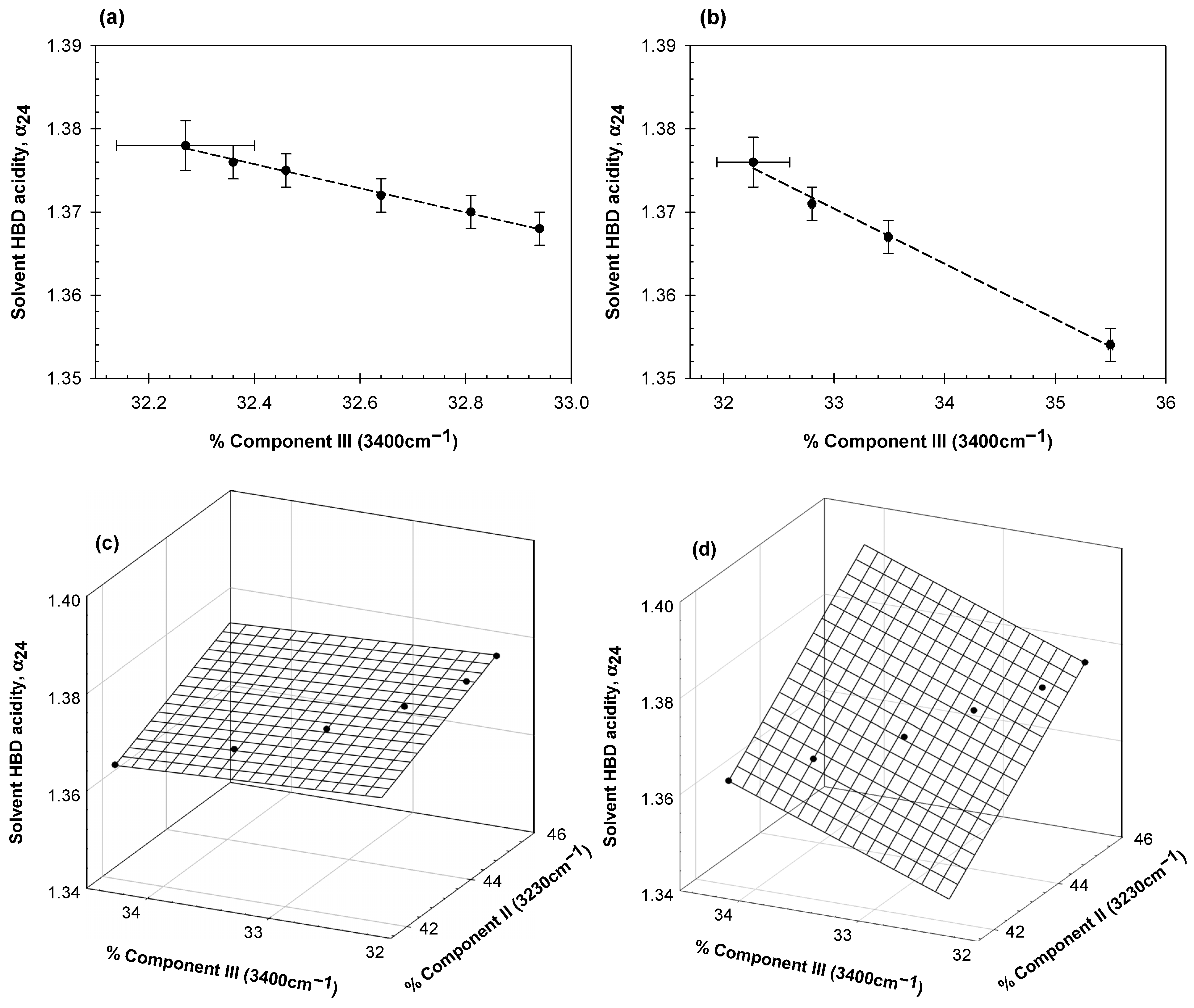
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. ATR-FTIR Measurements
3.2.2. Analysis of ATR-FTIR Spectra
3.2.3. NMR Measurements
4. Conclusions
- The earlier model of four different structures for water represented by exactly four Gaussian spectral components and describing the OH-stretch band in the ATR-FTIR spectra of solutions of non-ionic polymers and inorganic salts [20], is also applicable to protein solutions.
- The effects of different proteins on the structure of water depend strongly on the nature and spatial arrangement of the solvent-exposed protein groups.
- The solvent hydrogen bond donor acidity in aqueous protein solutions (α24) may be estimated by the NMR technique described in [27], which has not previously been applied to protein solutions.
- For the globular proteins in the aqueous solutions examined here, the contributions of Gaussian components II and III are strongly correlated with the hydrogen bond donor acidity of water.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ball, P. Water as an active constituent in cell biology. Chem. Rev. 2008, 108, 74–108. [Google Scholar] [CrossRef]
- Ball, P. Water is an active matrix of life for cell and molecular biology. Proc. Natl. Acad. Sci. USA 2017, 114, 13327–13335. [Google Scholar] [CrossRef]
- Ball, P. Biophysics: More than a bystander. Nature 2011, 478, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. Concluding remarks: Cum grano salis. Faraday Discuss. 2013, 160, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Templeton, C.; Elber, R. Why Does RNA Collapse? The Importance of Water in a Simulation Study of Helix-Junction-Helix Systems. J. Am. Chem. Soc. 2018, 140, 16948–16951. [Google Scholar] [CrossRef] [PubMed]
- Zaslavsky, B.Y.; Uversky, V.N. In Aqua Veritas: The Indispensable yet Mostly Ignored Role of Water in Phase Separation and Membrane-less Organelles. Biochemistry 2018, 57, 2437–2451. [Google Scholar] [CrossRef]
- Bellissent-Funel, M.C.; Hassanali, A.; Havenith, M.; Henchman, R.; Pohl, P.; Sterpone, F.; van der Spoel, D.; Xu, Y.; Garcia, A.E. Water Determines the Structure and Dynamics of Proteins. Chem. Rev. 2016, 116, 7673–7697. [Google Scholar] [CrossRef]
- You, X.; Shirley, J.C.; Lee, E.; Baiz, C.R. Short-and long-range crowding effects on water’s hydrogen bond networks. Cell Rep. Phys. Sci. 2021, 2, 100419. [Google Scholar] [CrossRef]
- Ferreira, L.A.; Gusev, N.B.; Uversky, V.N.; Zaslavsky, B.Y. Effect of human heat shock protein HspB6 on the solvent features of water in aqueous solutions. J. Biomol. Struct. Dyn. 2018, 36, 1520–1528. [Google Scholar] [CrossRef]
- Ferreira, L.A.; Walczyk Mooradally, A.; Zaslavsky, B.; Uversky, V.N.; Graether, S.P. Effect of an Intrinsically Disordered Plant Stress Protein on the Properties of Water. Biophys. J. 2018, 115, 1696–1706. [Google Scholar] [CrossRef]
- Ferreira, L.A.; Loureiro, J.A.; Gomes, J.; Uversky, V.N.; Madeira, P.P.; Zaslavsky, B.Y. Why physicochemical properties of aqueous solutions of various compounds are linearly interrelated. J. Mol. Liq. 2016, 221, 116–123. [Google Scholar] [CrossRef]
- Ferreira, L.A.; Uversky, V.N.; Zaslavsky, B.Y. Role of solvent properties of water in crowding effects induced by macromolecular agents and osmolytes. Mol. Biosyst. 2017, 13, 2551–2563. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.A.; Madeira, P.P.; Breydo, L.; Reichardt, C.; Uversky, V.N.; Zaslavsky, B.Y. Role of solvent properties of aqueous media in macromolecular crowding effects. J. Biomol. Struct. Dyn. 2016, 34, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Madeira, P.P.; Reis, C.A.; Rodrigues, A.E.; Mikheeva, L.M.; Zaslavsky, B.Y. Solvent properties governing solute partitioning in polymer/polymer aqueous two-phase systems: Nonionic compounds. J. Phys. Chem. B 2010, 114, 457–462. [Google Scholar] [CrossRef]
- da Silva, N.R.; Ferreira, L.A.; Teixeira, J.A.; Uversky, V.N.; Zaslavsky, B.Y. Effects of sodium chloride and sodium perchlorate on properties and partition behavior of solutes in aqueous dextran-polyethylene glycol and polyethylene glycol-sodium sulfate two-phase systems. J. Chromatogr. A 2019, 1583, 28–38. [Google Scholar] [CrossRef]
- Titus, A.R.; Ferreira, L.A.; Belgovskiy, A.I.; Kooijman, E.E.; Mann, E.K.; Mann, J.A., Jr.; Meyer, W.V.; Smart, A.E.; Uversky, V.N.; Zaslavsky, B.Y. Interfacial tension and mechanism of liquid-liquid phase separation in aqueous media. Phys. Chem. Chem. Phys. 2020, 22, 4574–4580. [Google Scholar] [CrossRef]
- Madeira, P.P.; Titus, A.R.; Ferreira, L.A.; Belgovskiy, A.I.; Mann, E.K.; Mann, J.A., Jr.; Meyer, W.V.; Smart, A.E.; Uversky, V.N.; Zaslavsky, B.Y. Hydrogen Bond Arrangement Is Shown to Differ in Coexisting Phases of Aqueous Two-Phase Systems. Biomolecules 2021, 11, 1787. [Google Scholar] [CrossRef]
- Alexandrova, A.N.; Jorgensen, W.L. Why urea eliminates ammonia rather than hydrolyzes in aqueous solution. J. Phys. Chem. B 2007, 111, 720–730. [Google Scholar] [CrossRef]
- Masuda, K.; Haramaki, T.; Nakashima, S.; Habert, B.; Martinez, I.; Kashiwabara, S. Structural change of water with solutes and temperature up to 100 degrees C in aqueous solutions as revealed by attenuated total reflectance infrared spectroscopy. Appl. Spectrosc. 2003, 57, 274–281. [Google Scholar] [CrossRef]
- da Silva, N.; Ferreira, L.A.; Belgovskiy, A.I.; Madeira, P.P.; Teixeira, J.A.; Mann, E.K.; Mann, J.A., Jr.; Meyer, W.V.; Smart, A.E.; Chernyak, V.Y. Effects of different solutes on the physical chemical properties of aqueous solutions via rearrangement of hydrogen bonds in water. J. Mol. Liq. 2021, 335, 116288. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.-H.; Zhao, L.-J. ATR-FTIR spectroscopic studies on aqueous LiClO4, NaClO4, and Mg(ClO4)2 solutions. Phys. Chem. Chem. Phys. 2004, 6, 537–542. [Google Scholar] [CrossRef]
- Kitadai, N.; Sawai, T.; Tonoue, R.; Nakashima, S.; Katsura, M.; Fukushi, K. Effects of ions on the OH stretching band of water as revealed by ATR-IR spectroscopy. J. Solut. Chem. 2014, 43, 1055–1077. [Google Scholar] [CrossRef]
- Miklos, A.C.; Sarkar, M.; Wang, Y.; Pielak, G.J. Protein crowding tunes protein stability. J. Am. Chem. Soc. 2011, 133, 7116–7120. [Google Scholar] [CrossRef] [PubMed]
- Benton, L.A.; Smith, A.E.; Young, G.B.; Pielak, G.J. Unexpected effects of macromolecular crowding on protein stability. Biochemistry 2012, 51, 9773–9775. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sarkar, M.; Smith, A.E.; Krois, A.S.; Pielak, G.J. Macromolecular crowding and protein stability. J. Am. Chem. Soc. 2012, 134, 16614–16618. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Lu, J.; Pielak, G.J. Protein crowder charge and protein stability. Biochemistry 2014, 53, 1601–1606. [Google Scholar] [CrossRef]
- Madeira, P.P.; Passos, H.; Gomes, J.; Coutinho, J.A.; Freire, M.G. Alternative probe for the determination of the hydrogen-bond acidity of ionic liquids and their aqueous solutions. Phys. Chem. Chem. Phys. 2017, 19, 11011–11016. [Google Scholar] [CrossRef]
- Sharp, K. Entropy-enthalpy compensation: Fact or artifact? Protein Sci. 2001, 10, 661–667. [Google Scholar] [CrossRef]
- Dunitz, J.D. Win some, lose some: Enthalpy-entropy compensation in weak intermolecular interactions. Chem. Biol. 1995, 2, 709–712. [Google Scholar] [CrossRef]
- Batchelor, J.D.; Olteanu, A.; Tripathy, A.; Pielak, G.J. Impact of protein denaturants and stabilizers on water structure. J. Am. Chem. Soc. 2004, 126, 1958–1961. [Google Scholar] [CrossRef]
- Sharp, K.A.; Vanderkooi, J.M. Water in the half shell: Structure of water, focusing on angular structure and solvation. Acc. Chem. Res. 2010, 43, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cremer, P.S. Chemistry of Hofmeister anions and osmolytes. Annu. Rev. Phys. Chem. 2010, 61, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Salis, A.; Ninham, B.W. Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. [Google Scholar] [CrossRef] [PubMed]
- Narang, P.; Vepuri, S.B.; Venkatesu, P.; Soliman, M.E. An unexplored remarkable PNIPAM-osmolyte interaction study: An integrated experimental and simulation approach. J. Colloid Interface Sci. 2017, 504, 417–428. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titus, A.R.; Madeira, P.P.; Ferreira, L.A.; Belgovskiy, A.I.; Mann, E.K.; Mann, J.A., Jr.; Meyer, W.V.; Smart, A.E.; Uversky, V.N.; Zaslavsky, B.Y. Arrangement of Hydrogen Bonds in Aqueous Solutions of Different Globular Proteins. Int. J. Mol. Sci. 2022, 23, 11381. https://doi.org/10.3390/ijms231911381
Titus AR, Madeira PP, Ferreira LA, Belgovskiy AI, Mann EK, Mann JA Jr., Meyer WV, Smart AE, Uversky VN, Zaslavsky BY. Arrangement of Hydrogen Bonds in Aqueous Solutions of Different Globular Proteins. International Journal of Molecular Sciences. 2022; 23(19):11381. https://doi.org/10.3390/ijms231911381
Chicago/Turabian StyleTitus, Amber R., Pedro P. Madeira, Luisa A. Ferreira, Alexander I. Belgovskiy, Elizabeth K. Mann, Jay Adin Mann, Jr., William V. Meyer, Anthony E. Smart, Vladimir N. Uversky, and Boris Y. Zaslavsky. 2022. "Arrangement of Hydrogen Bonds in Aqueous Solutions of Different Globular Proteins" International Journal of Molecular Sciences 23, no. 19: 11381. https://doi.org/10.3390/ijms231911381
APA StyleTitus, A. R., Madeira, P. P., Ferreira, L. A., Belgovskiy, A. I., Mann, E. K., Mann, J. A., Jr., Meyer, W. V., Smart, A. E., Uversky, V. N., & Zaslavsky, B. Y. (2022). Arrangement of Hydrogen Bonds in Aqueous Solutions of Different Globular Proteins. International Journal of Molecular Sciences, 23(19), 11381. https://doi.org/10.3390/ijms231911381






