Modulation of ATP Production Influences Inorganic Polyphosphate Levels in Non-Athletes’ Platelets at the Resting State
Abstract
:1. Introduction
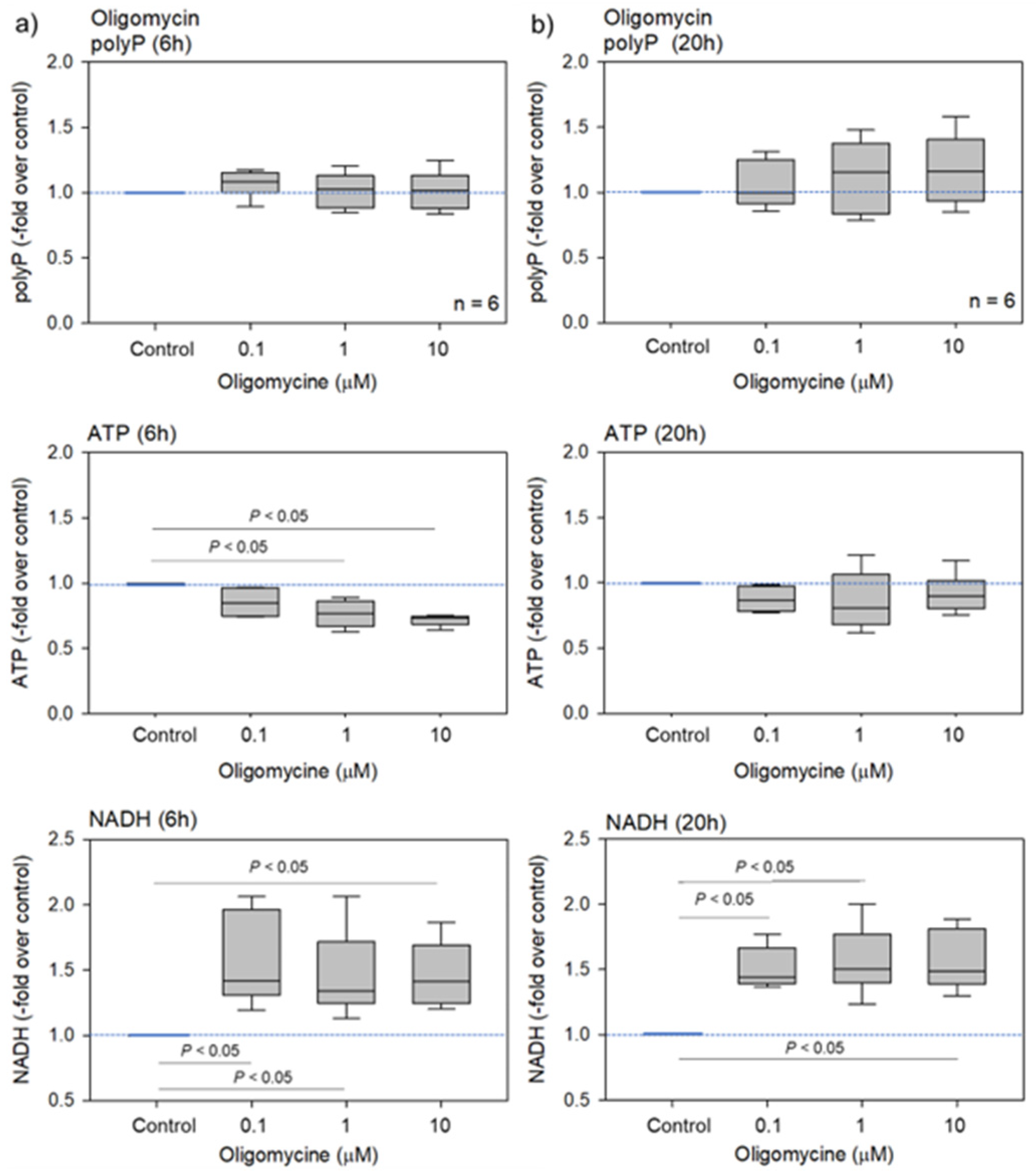
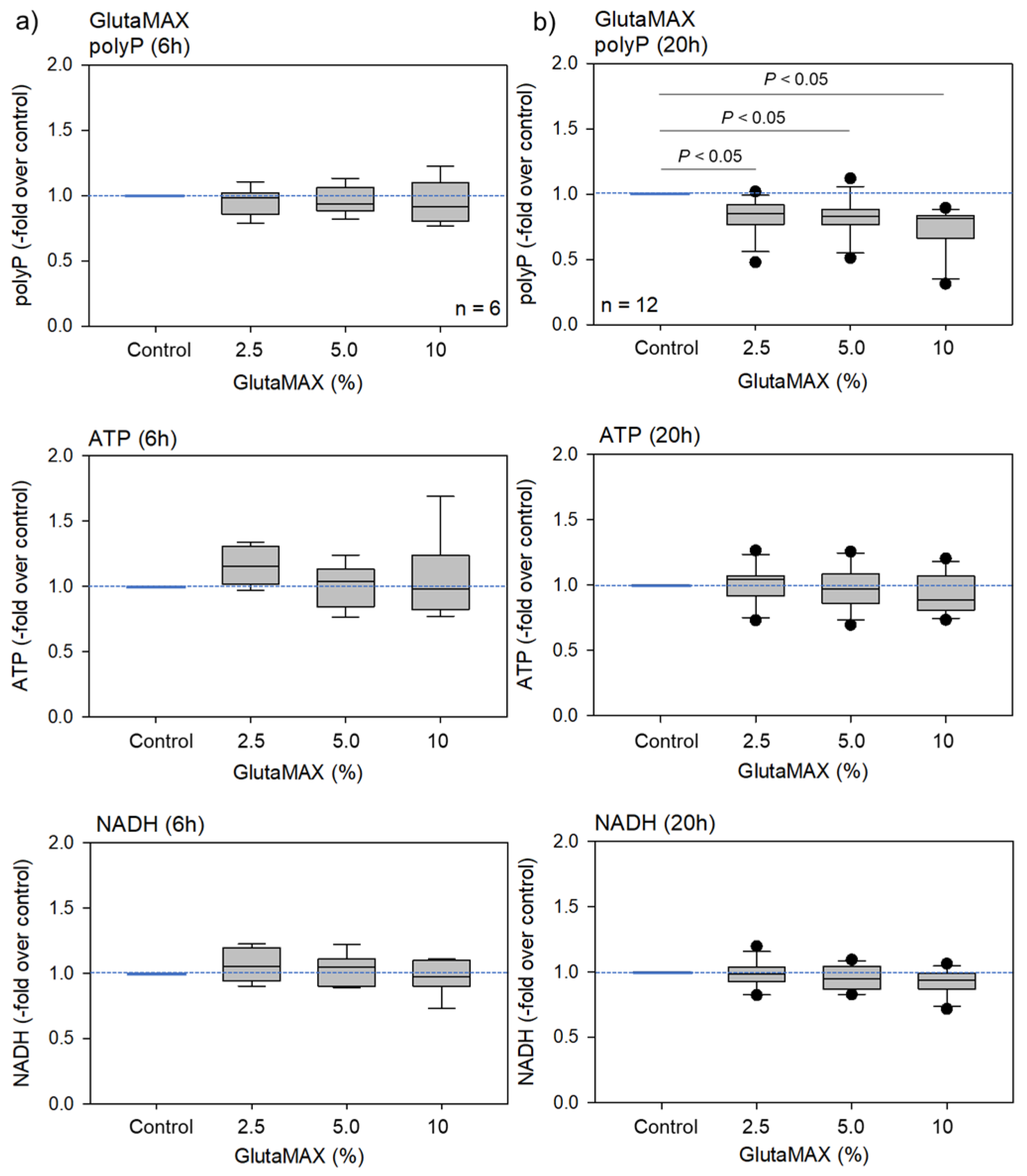
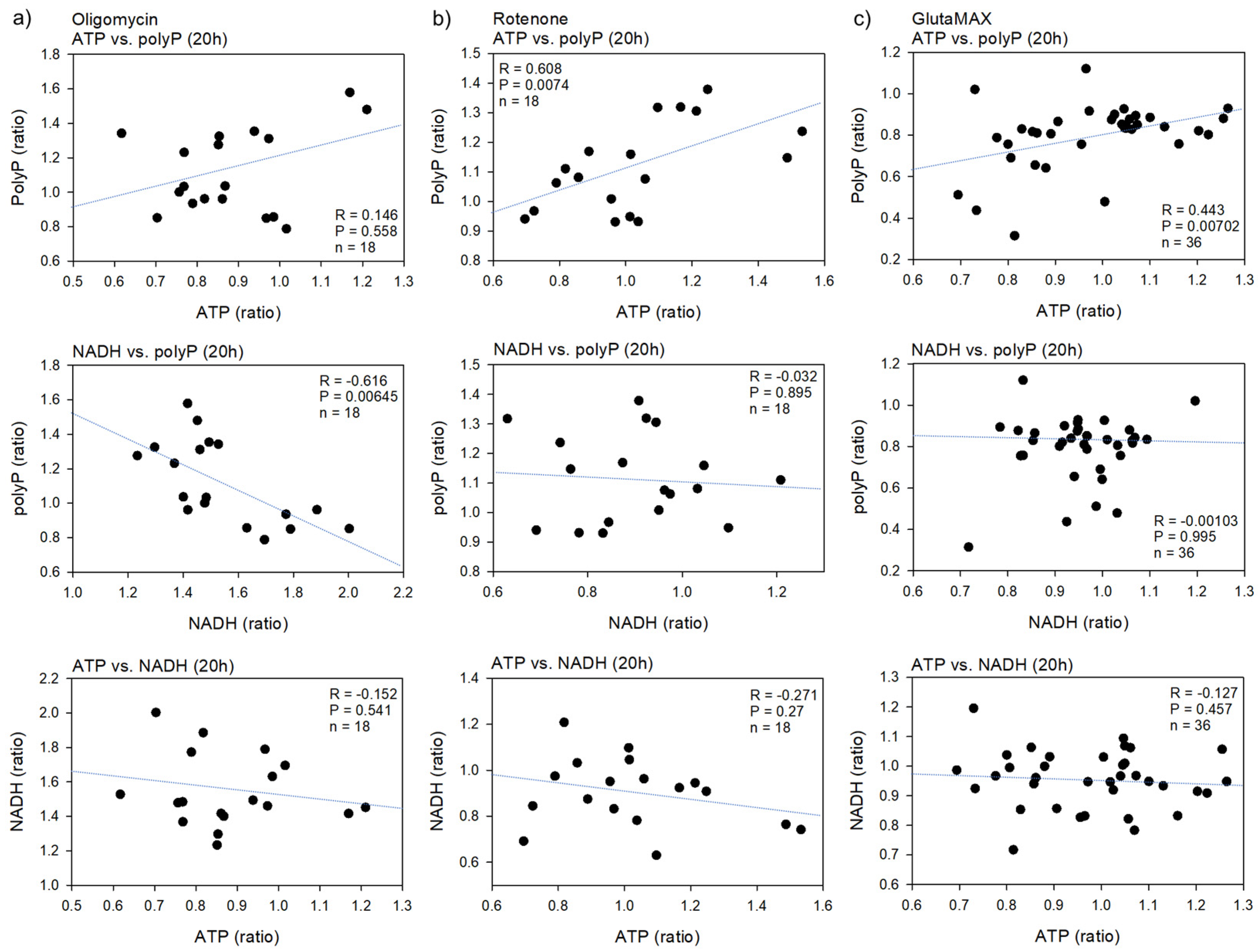
2. Results
2.1. Biochemical and Fluorometric Quantification of Platelet PolyP
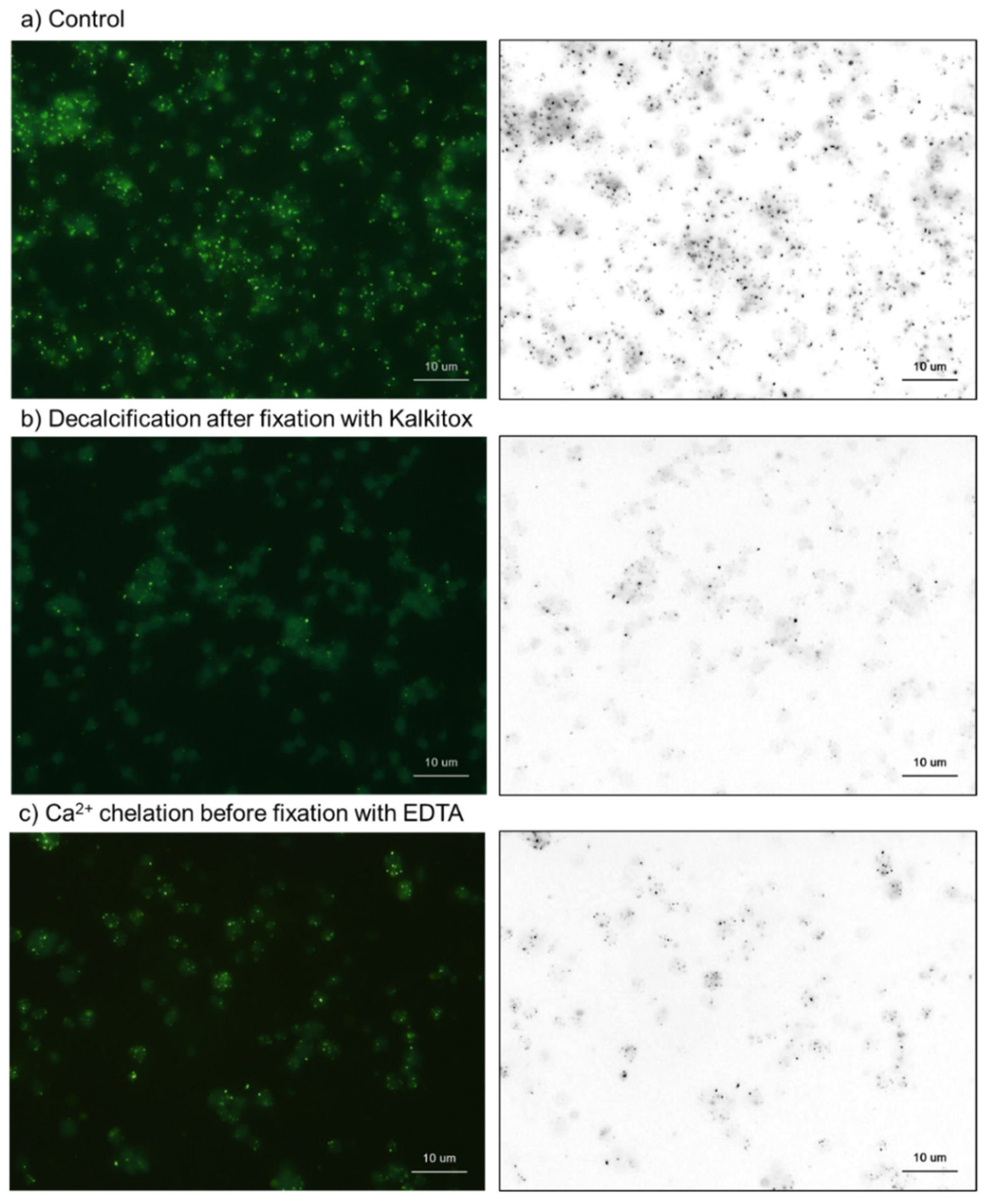
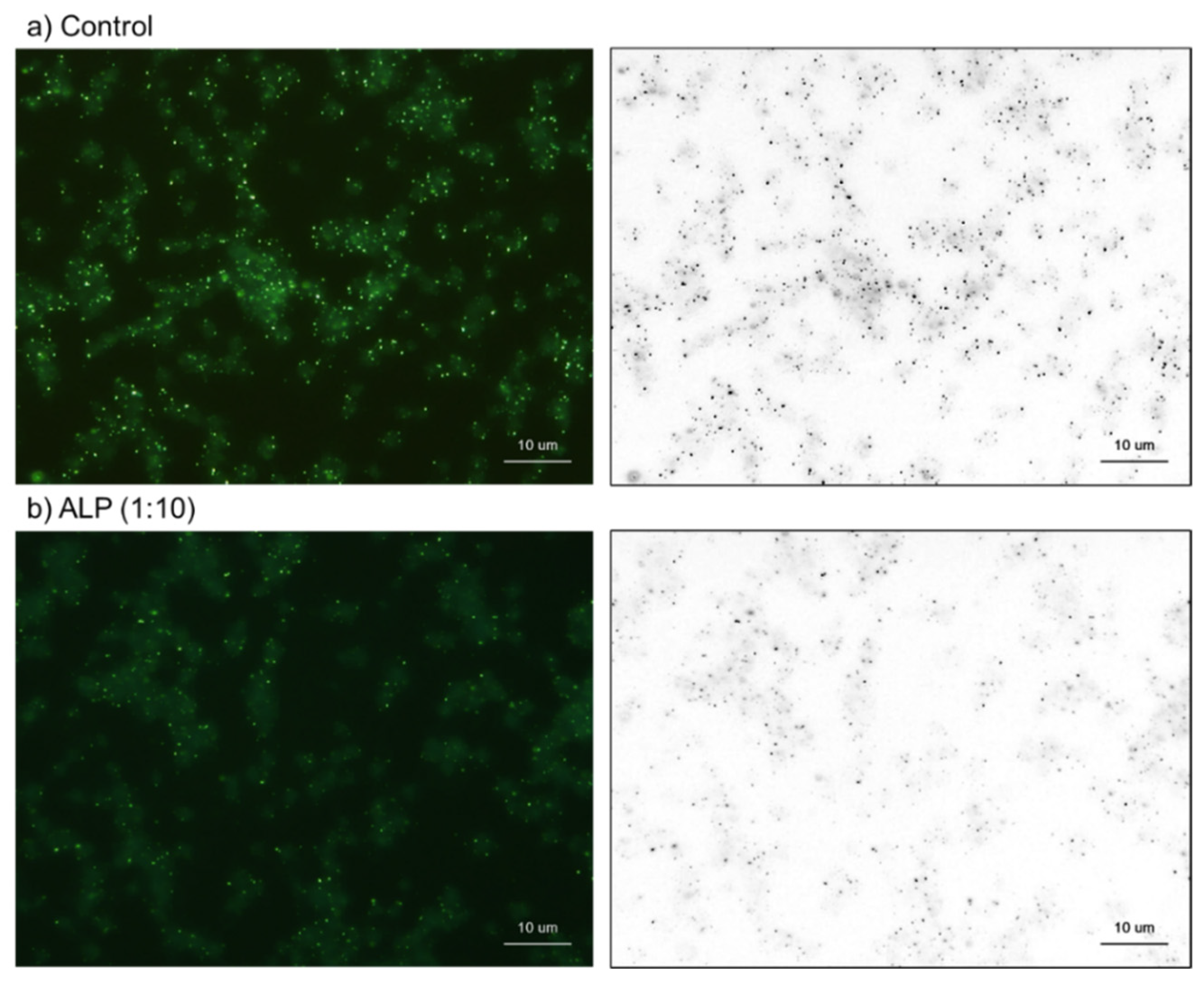
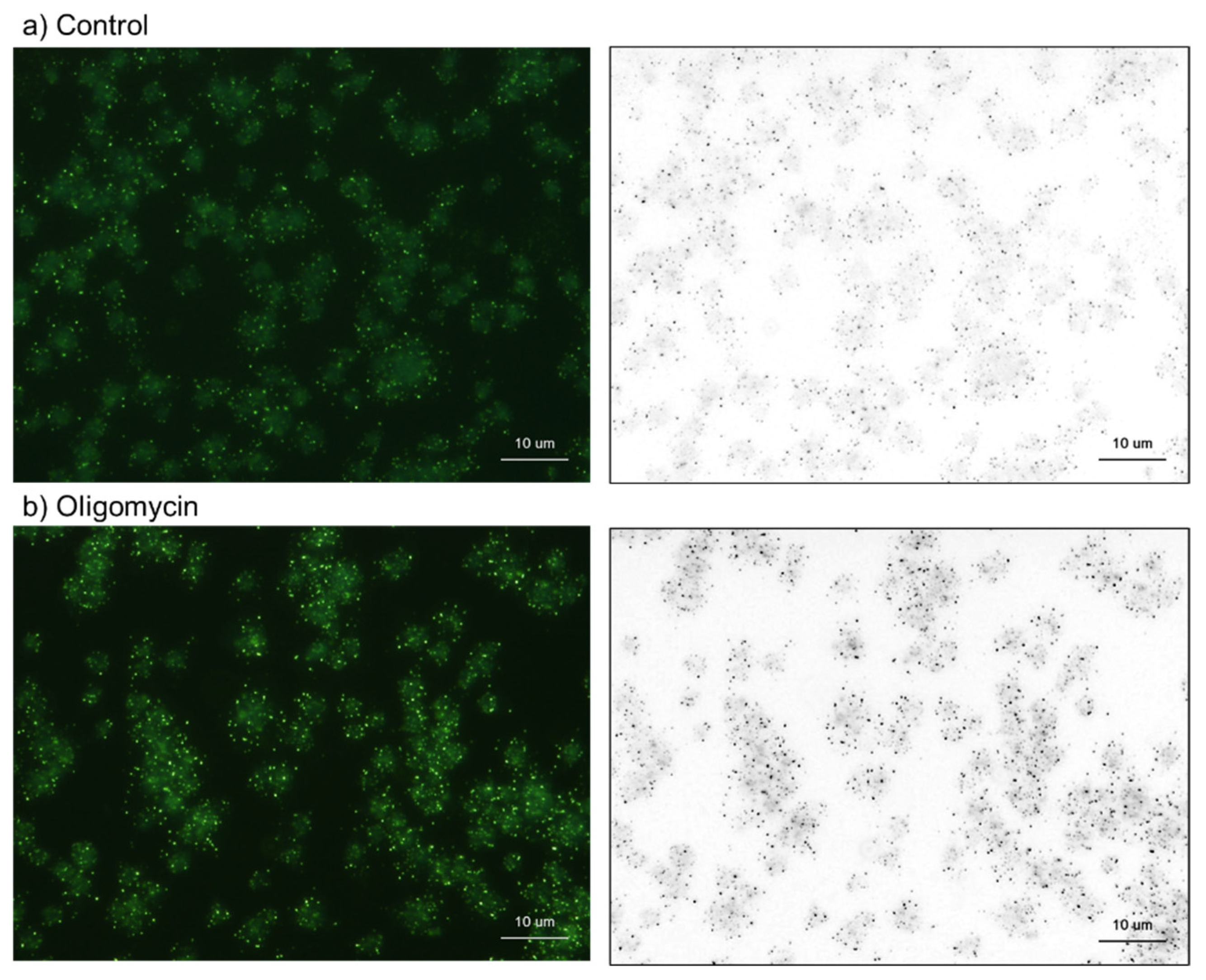
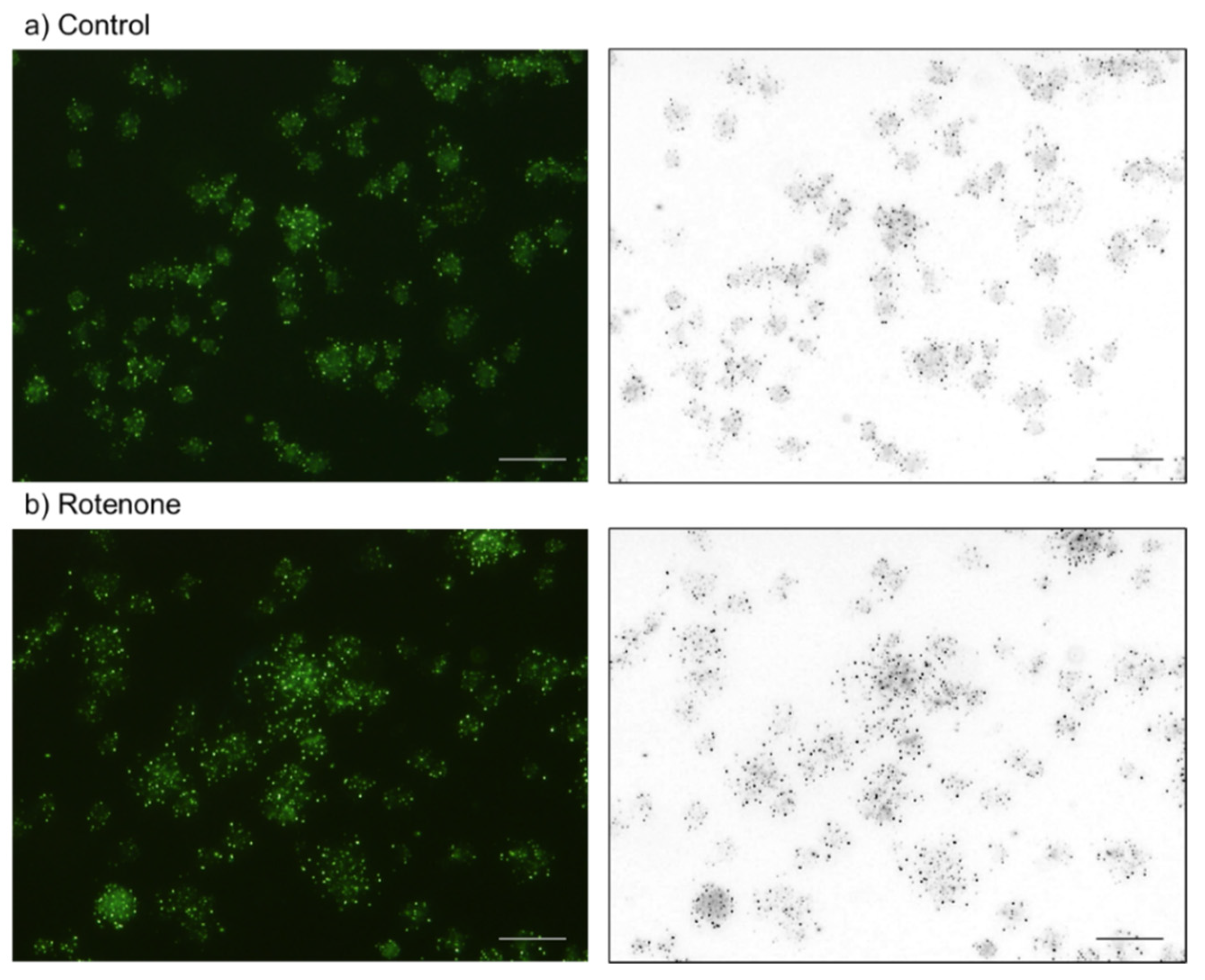
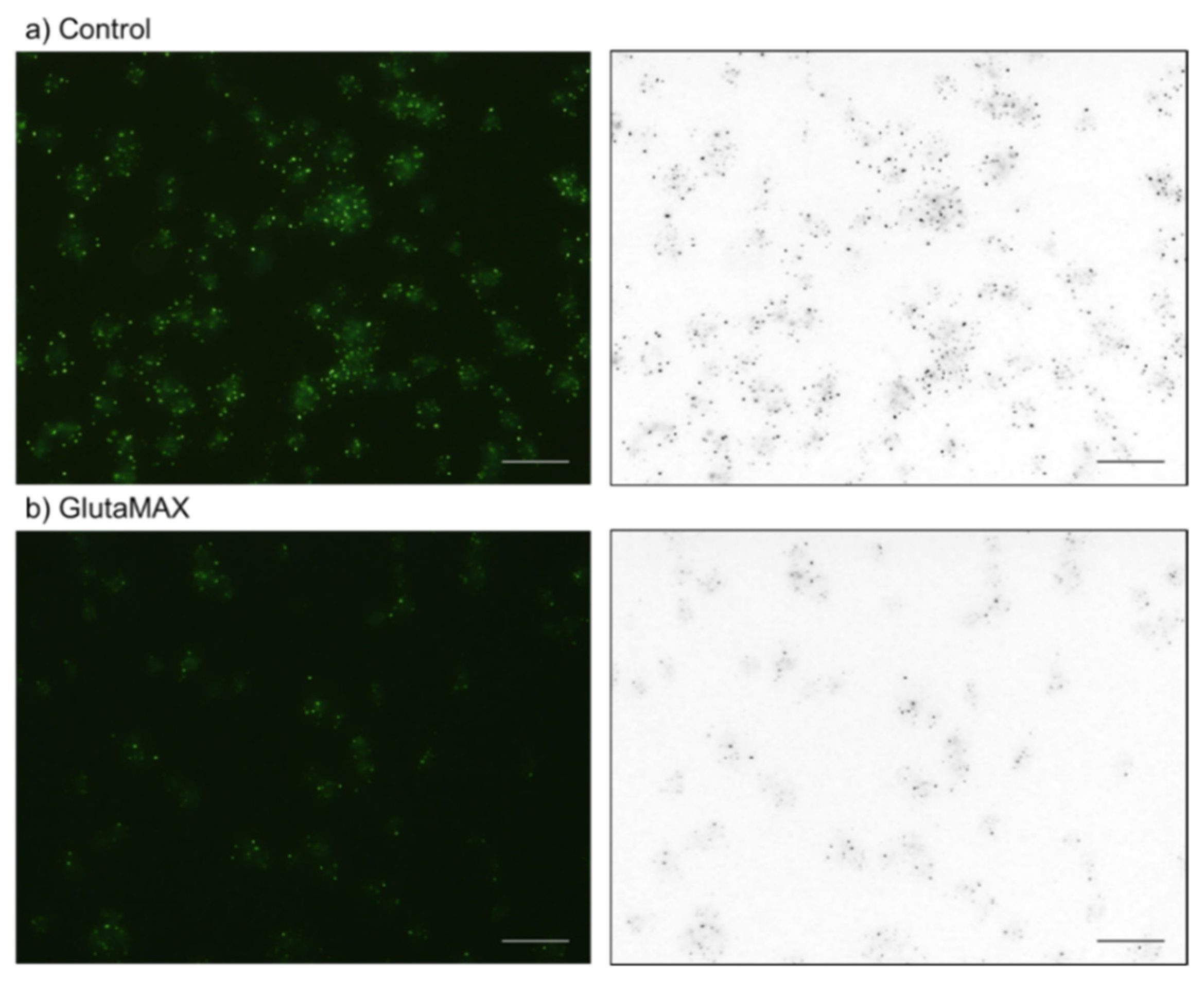
2.2. Cytochemical Visualization of Platelet PolyP
3. Discussion
3.1. DAPI-Stained Microparticles
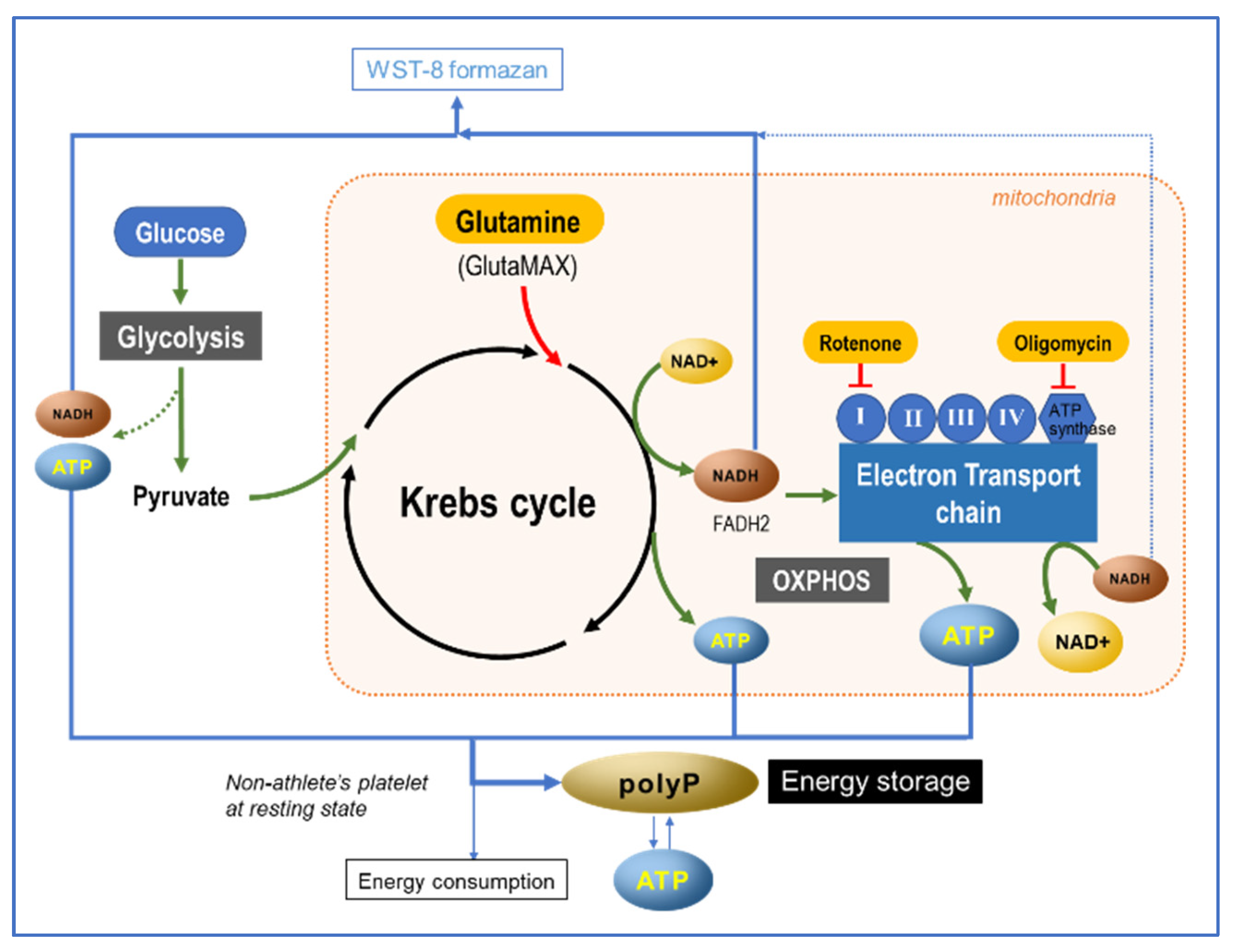
3.2. Energy Metabolism and PolyP Turnover at Cellular Levels
3.3. Possible Involvement of ATP in PolyP Production
3.4. Clinical Relevance
4. Materials and Methods
4.1. Reagents
4.2. Blood Cell Counting
4.3. Preparation of Pure Platelet-Rich Plasma and Treatments
4.4. Determination of PolyP Levels
4.5. Determination of NADH and ATP Levels
4.6. Cytochemical Visualization of Platelet PolyP
4.7. Treatments with Decalcification Agents or ALP
4.8. Image Analysis for DAPI-Stained Particles
4.9. Visualization of Mitochondrial Membrane Potential
4.10. Image Analysis for Mitochondrial Membrane Potential
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simbulan-Rosenthal, C.M.; Carney, B.C.; Gaur, A.; Moghe, M.; Crooke, E.; Moffatt, L.T.; Shupp, J.W.; Rosenthal, D.S. Inorganic Polyphosphates are Important for Cell Survival and Motility of Human Skin Keratinocytes and Play a Role in Wound Healing. In Contemporary Topics about Phosphorus in Biology and Materials; Churchill, D., Sikirić, M., Čolović, B., Milhofer, H., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Solesio, M.E.; McIntyre, B. Mitochondrial inorganic polyphosphate (polyP): The missing link of mammalian bioenergetics. Neural Regen. Res. 2021, 16, 2227–2228. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.H.; Choi, S.H.; Smith, S.A. Polyphosphate: An ancient molecule that links platelets, coagulation, and inflammation. Blood 2012, 119, 5972–5979. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Souza, L.F.; Oliveira, M.F. Mitochondria: Biological roles in platelet physiology and pathology. Int. J. Biochem. Cell Biol. 2014, 50, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Melchinger, H.; Jain, K.; Tyagi, T.; Hwa, J. Role of Platelet Mitochondria: Life in a Nucleus-Free Zone. Front. Cardiovasc. Med. 2019, 6, 153. [Google Scholar] [CrossRef]
- Ravera, S.; Signorello, M.G.; Bartolucci, M.; Ferrando, S.; Manni, L.; Caicci, F.; Calzia, D.; Panfoli, I.; Morelli, A.; Leoncini, G. Extramitochondrial energy production in platelets. Biol. Cell 2018, 110, 97–108. [Google Scholar] [CrossRef]
- Sato, A.; Aizawa, H.; Tsujino, T.; Isobe, K.; Watanabe, T.; Kitamura, Y.; Kawase, T. Fluorescent Cytochemical Detection of Polyphosphates Associated with Human Platelets. Int. J. Mol. Sci. 2021, 22, 1040. [Google Scholar] [CrossRef]
- Watanabe, T.; Kitamura, Y.; Aizawa, H.; Masuki, H.; Tsujino, T.; Sato, A.; Kawabata, H.; Isobe, K.; Nakata, K.; Kawase, T. Fluorometric Quantification of Human Platelet Polyphosphate Using 4′,6-Diamidine-2-phenylindole Dihydrochloride: Applications in the Japanese Population. Int. J. Mol. Sci. 2021, 22, 7257. [Google Scholar] [CrossRef]
- Uematsu, T.; Sato, A.; Aizawa, H.; Tsujino, T.; Watanabe, T.; Isobe, K.; Kawabata, H.; Kitamura, Y.; Tanaka, T.; Kawase, T. Effects of SARS-CoV-2 mRNA vaccines on platelet polyphosphate levels and inflammation: A pilot study. Biomed. Rep. 2022, 16, 21. [Google Scholar] [CrossRef]
- Ushiki, T.; Mochizuki, T.; Suzuki, K.; Kamimura, M.; Ishiguro, H.; Watanabe, S.; Omori, G.; Yamamoto, N.; Kawase, T. Platelet polyphosphate and energy metabolism in professional male athletes (soccer players): A cross-sectional pilot study. Physiol. Rep. 2022, 10, e15409. [Google Scholar] [CrossRef]
- Borden, E.A.; Furey, M.; Gattone, N.J.; Hambardikar, V.D.; Liang, X.H.; Scoma, E.R.; Samra, A.A.; D-Gary, L.R.; Dennis, D.J.; Fricker, D.; et al. Is there a link between inorganic polyphosphate (polyP), mitochondria, and neurodegeneration? Pharmacol. Res. 2021, 163, 105211. [Google Scholar] [CrossRef]
- Underwood, E.; Redell, J.B.; Zhao, J.; Moore, A.N.; Dash, P.K. A method for assessing tissue respiration in anatomically defined brain regions. Sci. Rep. 2020, 10, 13179. [Google Scholar] [CrossRef] [PubMed]
- Won, J.-H.; Park, S.; Hong, S.; Son, S.; Yu, J.-W. Rotenone-induced Impairment of Mitochondrial Electron Transport Chain Confers a Selective Priming Signal for NLRP3 Inflammasome Activation. J. Biol. Chem. 2015, 290, 27425–27437. [Google Scholar] [CrossRef]
- Zhao, M.-H.; Kim, N.-H.; Cui, X.-S. GlutaMAX prolongs the shelf life of the culture medium for porcine parthenotes. Theriogenology 2016, 85, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Kamphorst, J.J.; Mathew, R.; Chung, M.K.; White, E.; Shlomi, T.; Rabinowitz, J.D. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol. Syst. Biol. 2013, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.; Schröder, H.C.; Wang, X. Inorganic Polyphosphates as Storage for and Generator of Metabolic Energy in the Extracellular Matrix. Chem. Rev. 2019, 119, 12337–12374. [Google Scholar] [CrossRef]
- Pavlov, E.; Aschar-Sobbi, R.; Campanella, M.; Turner, R.J.; Gómez-García, M.R.; Abramov, A.Y. Inorganic Polyphosphate and Energy Metabolism in Mammalian Cells. J. Biol. Chem. 2010, 285, 9420–9428. [Google Scholar] [CrossRef]
- Boyineni, J.; Sredni, S.T.; Margaryan, N.V.; Demirkhanyan, L.; Tye, M.; Johnson, R.; Gonzalez-Nilo, F.; Hendrix, M.J.; Pavlov, E.; Soares, M.B.; et al. Inorganic polyphosphate as an energy source in tumorigenesis. Oncotarget 2020, 11, 4613–4624. [Google Scholar] [CrossRef]
- Baev, A.Y.; Angelova, P.R.; Abramov, A.Y. Inorganic polyphosphate is produced and hydrolyzed in F0F1-ATP synthase of mammalian mitochondria. Biochem. J. 2020, 477, 1515–1524. [Google Scholar] [CrossRef]
- Guitart-Mampel, M.; Urquiza, P.; Neto, F.C.; Anderson, J.R.; Hambardikar, V.; Scoma, E.R.; Merrihew, G.E.; Wang, L.; MacCoss, M.J.; Raftery, D.; et al. Mitochondrial Inorganic Polyphosphate (polyP) Is a Potent Regulator of Mammalian Bioenergetics in SH-SY5Y Cells: A Proteomics and Metabolomics Study. Front. Cell Dev. Biol. 2022, 10, 833127. [Google Scholar] [CrossRef]
- Clemetson, K.J. The role of platelets in defence against pathogens. Hamostaseologie 2011, 31, 264–268. [Google Scholar] [CrossRef]
- Kawase, T.; Mubarak, S.; Mourão, C.F. The Platelet Concentrates Therapy: From the Biased Past to the Anticipated Future. Bioengineering 2020, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Kawase, T. A Strategic and Worldwide Cooperative Challenge Required for the Next Generation of Platelet Concentrates. Int. J. Mol. Sci. 2022, 23, 3437. [Google Scholar] [CrossRef] [PubMed]
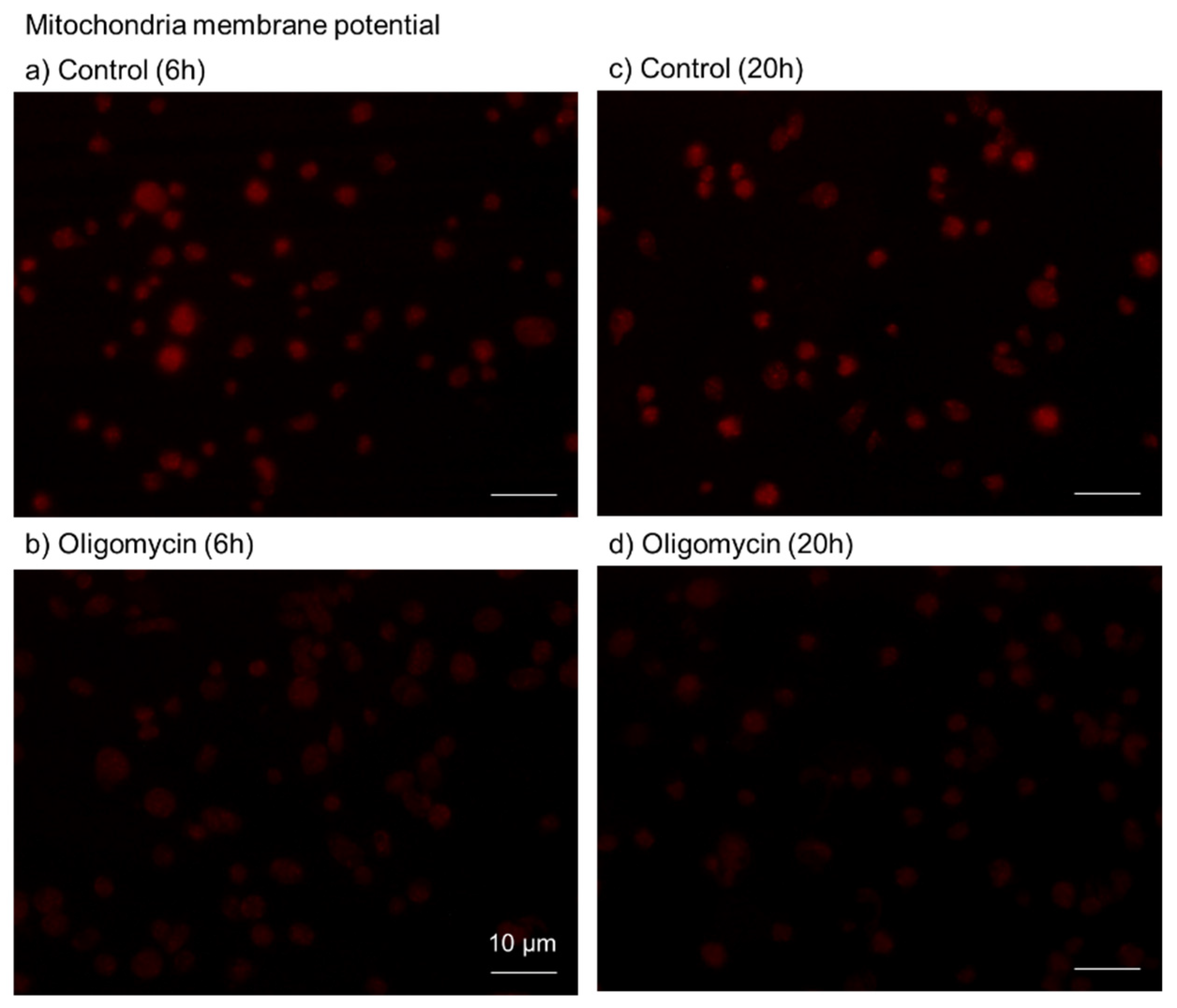

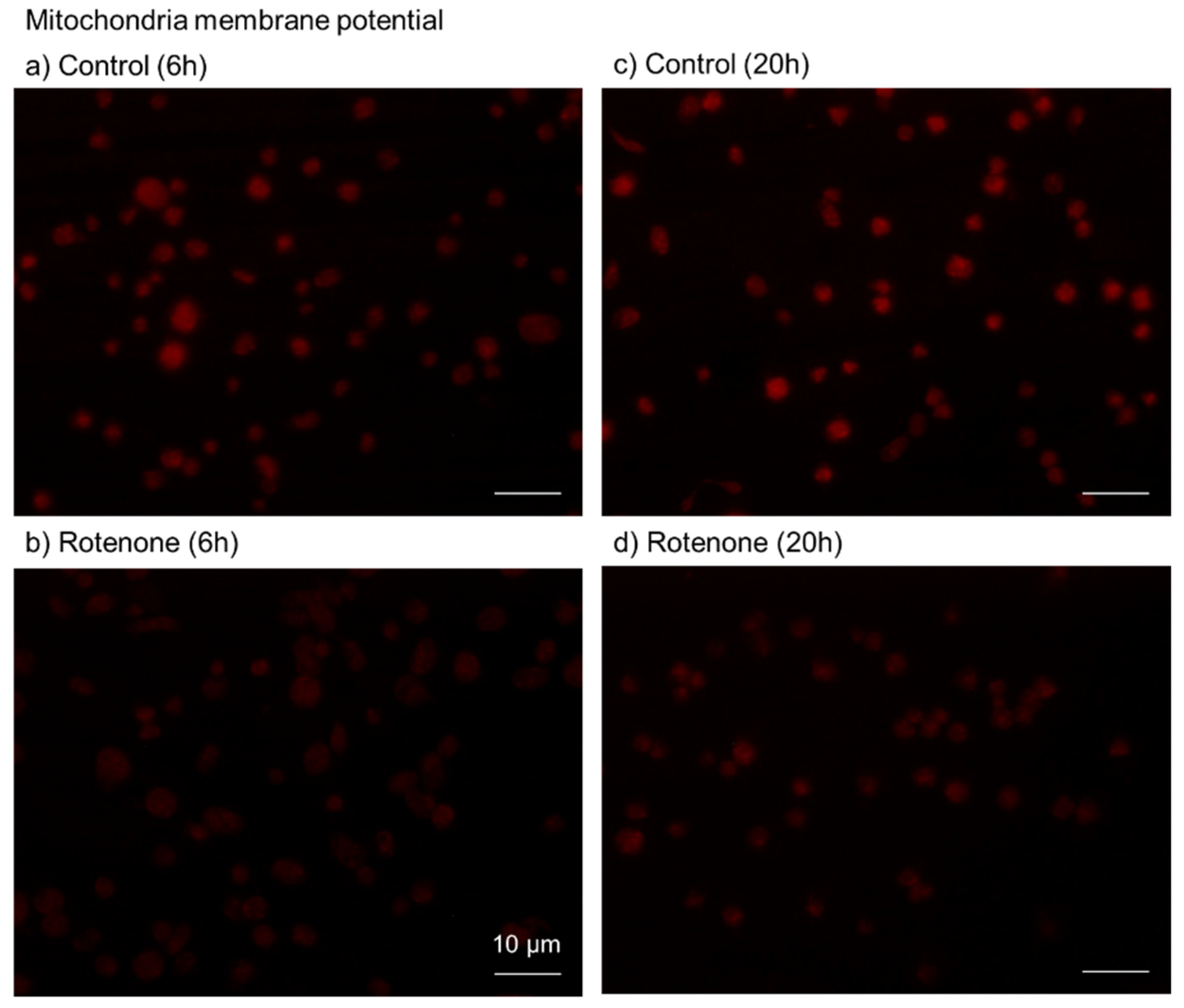
| 6 h | 20 h | |
|---|---|---|
| Control | 27.79 ± 0.64 | 27.19 ± 1.02 |
| Oligomycin | 14.87 ± 1.83 * | 16.42 ± 1.04 * |
| Rotenone | 18.33 ± 2.36 | 20.02 ± 2.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ushiki, T.; Mochizuki, T.; Suzuki, K.; Kamimura, M.; Ishiguro, H.; Suwabe, T.; Kawase, T. Modulation of ATP Production Influences Inorganic Polyphosphate Levels in Non-Athletes’ Platelets at the Resting State. Int. J. Mol. Sci. 2022, 23, 11293. https://doi.org/10.3390/ijms231911293
Ushiki T, Mochizuki T, Suzuki K, Kamimura M, Ishiguro H, Suwabe T, Kawase T. Modulation of ATP Production Influences Inorganic Polyphosphate Levels in Non-Athletes’ Platelets at the Resting State. International Journal of Molecular Sciences. 2022; 23(19):11293. https://doi.org/10.3390/ijms231911293
Chicago/Turabian StyleUshiki, Takashi, Tomoharu Mochizuki, Katsuya Suzuki, Masami Kamimura, Hajime Ishiguro, Tatsuya Suwabe, and Tomoyuki Kawase. 2022. "Modulation of ATP Production Influences Inorganic Polyphosphate Levels in Non-Athletes’ Platelets at the Resting State" International Journal of Molecular Sciences 23, no. 19: 11293. https://doi.org/10.3390/ijms231911293
APA StyleUshiki, T., Mochizuki, T., Suzuki, K., Kamimura, M., Ishiguro, H., Suwabe, T., & Kawase, T. (2022). Modulation of ATP Production Influences Inorganic Polyphosphate Levels in Non-Athletes’ Platelets at the Resting State. International Journal of Molecular Sciences, 23(19), 11293. https://doi.org/10.3390/ijms231911293









