The Stimulation of Macrophages by Systematical Administration of GM-CSF Can Accelerate Adult Wound Healing Process
Abstract
1. Introduction
2. Results
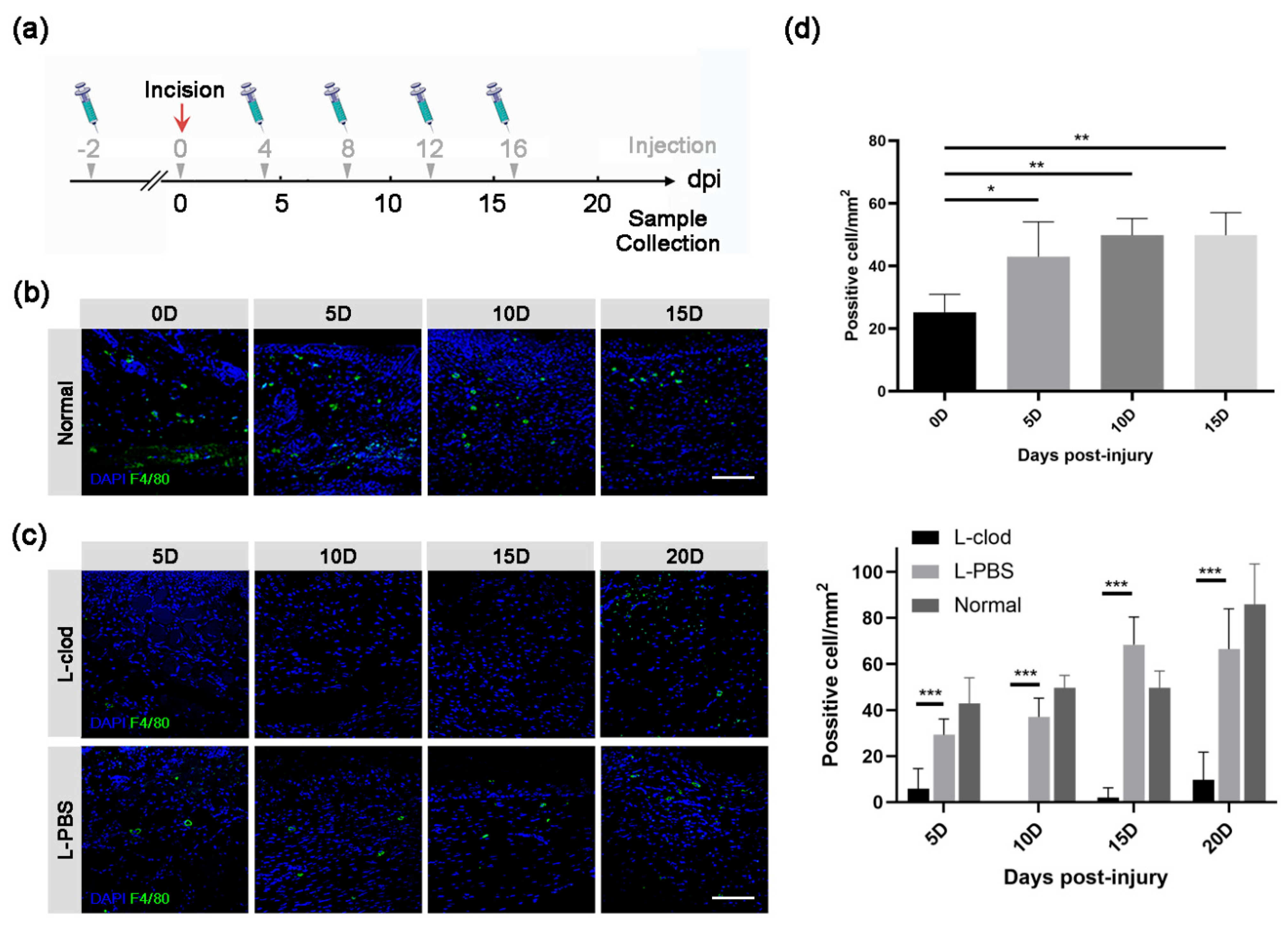
2.1. Macrophages Presented throughout the Whole Process of a Full-Thickness Incision Skin Defect
2.2. The Population of Macrophages Was Decreased after the Injection of Clodronate Liposomes
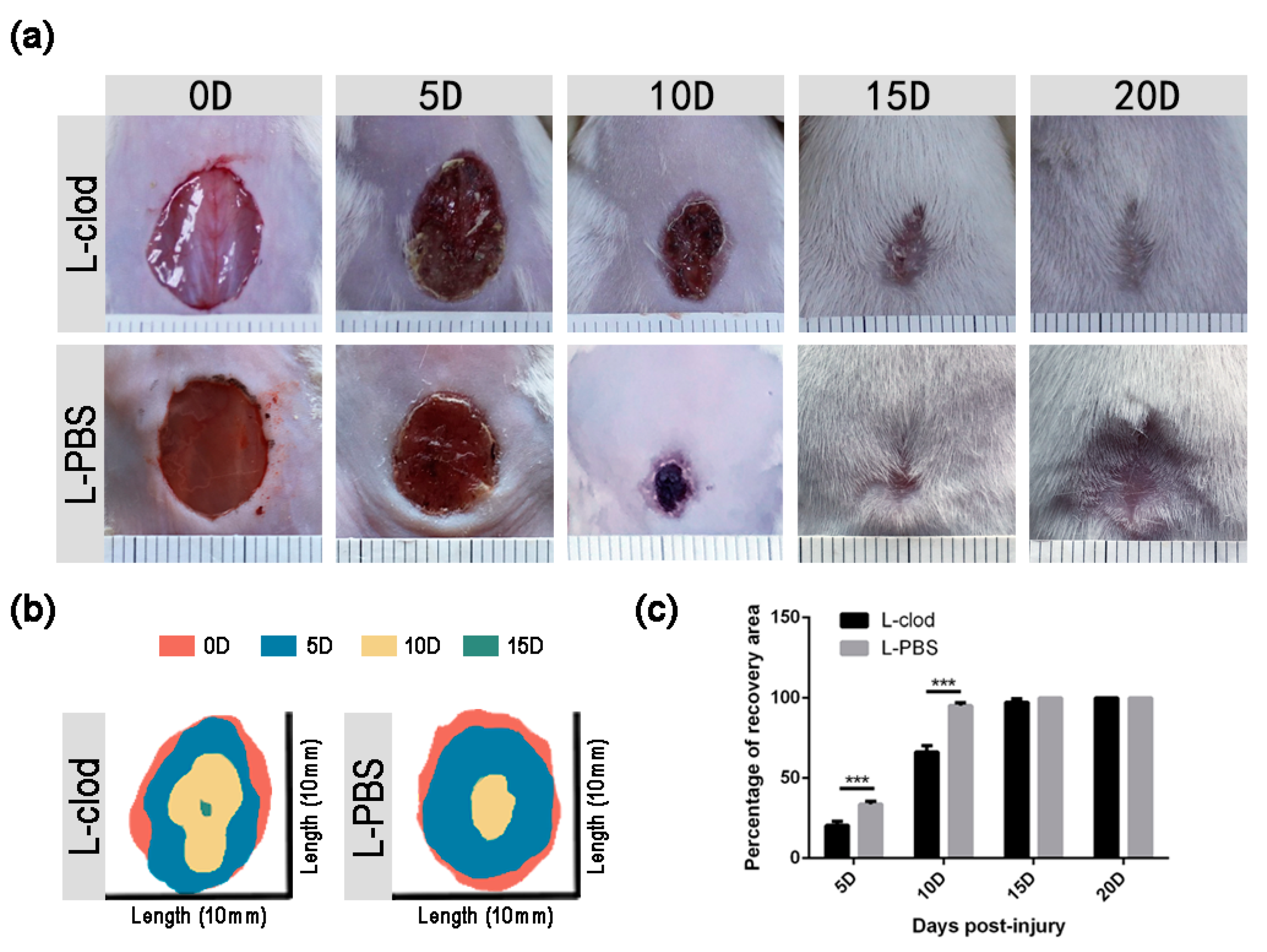
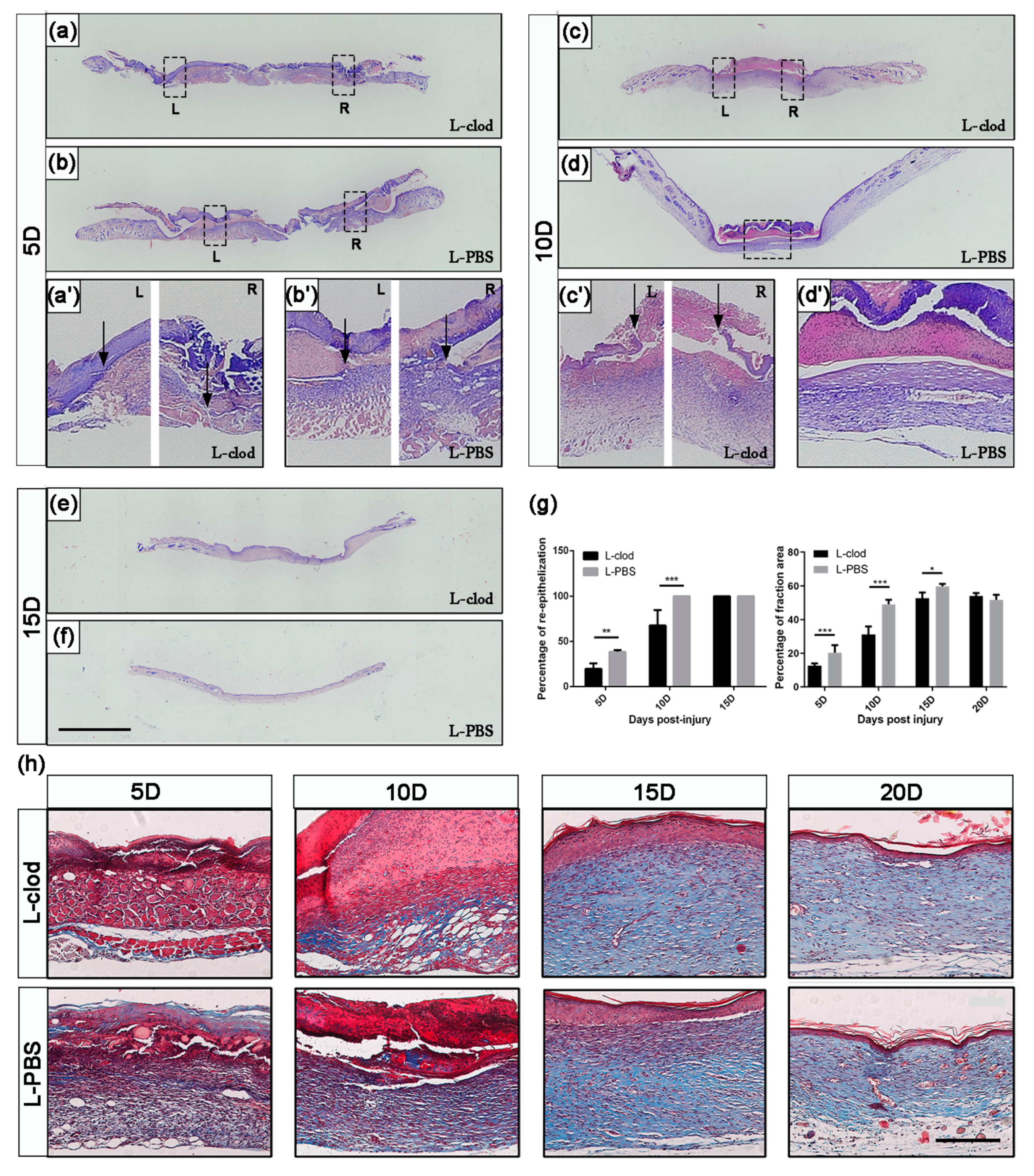
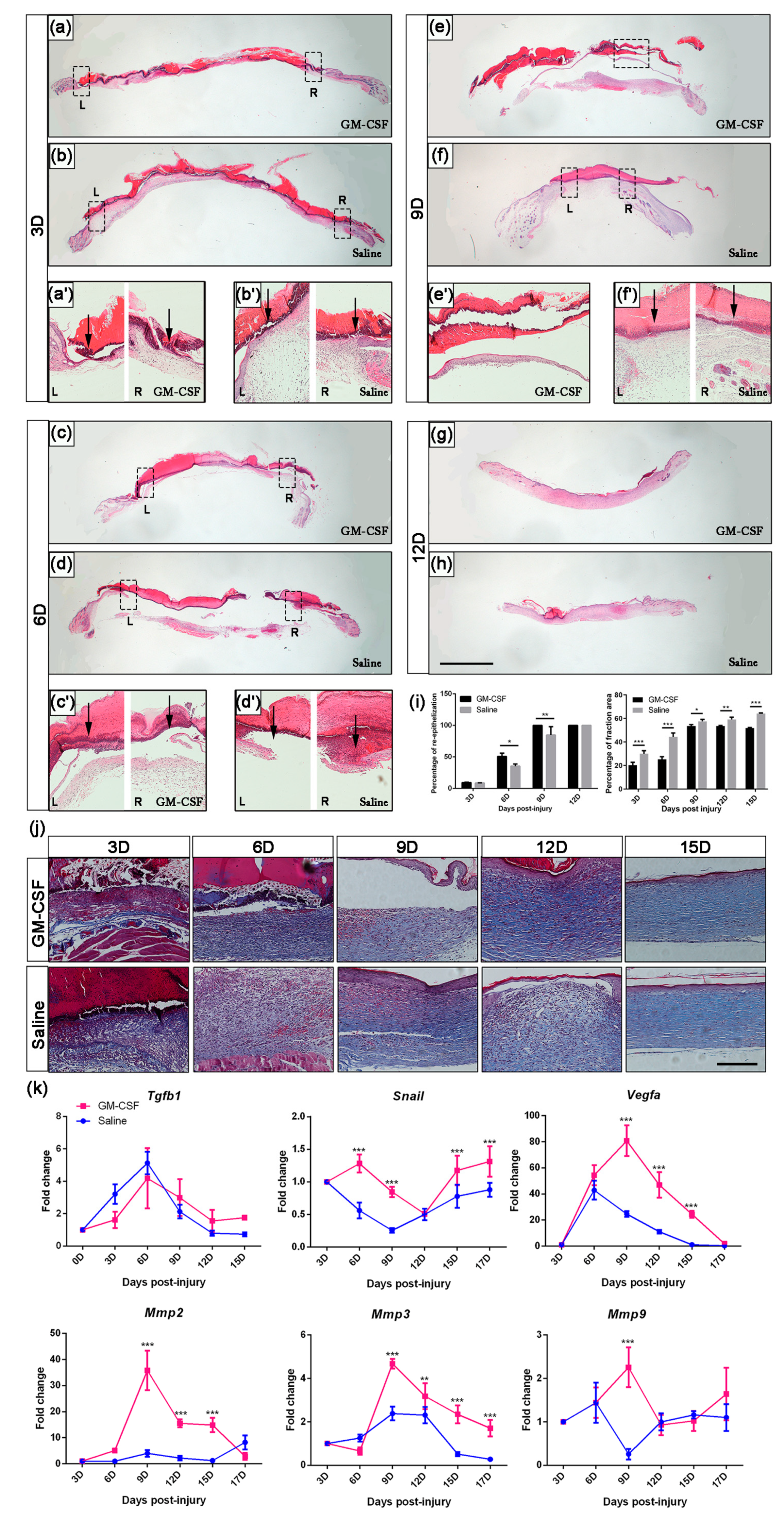
2.3. The Depletion of Macrophages Resulted in Delayed Wound Closure and Prolonged Re-Epithelialization
2.4. The Expression Levels of Regeneration-Associated Genes and Proteins after Macrophage Depletion
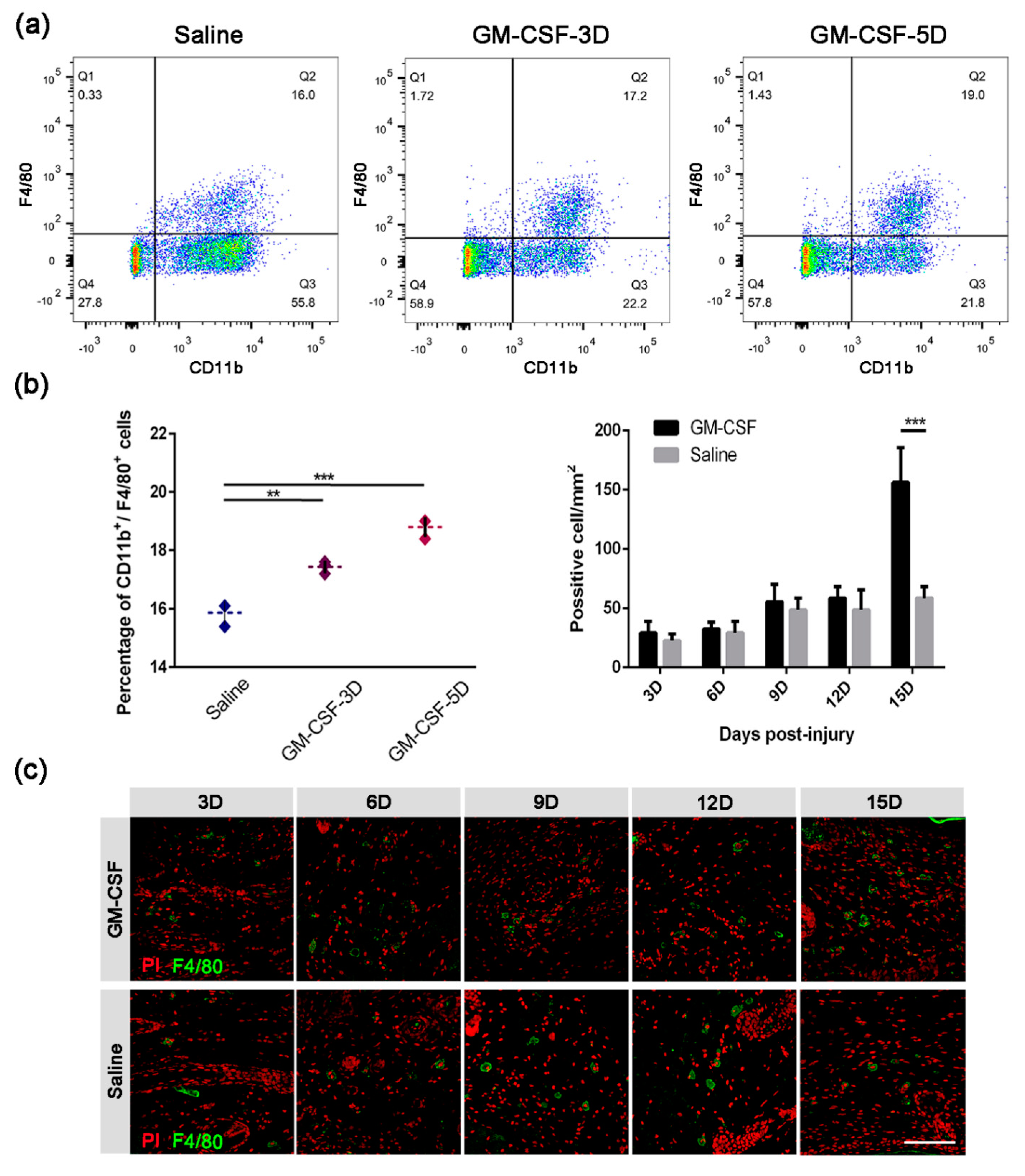
2.5. The Injection of GM-CSF Resulted in an Upregulation of the Quantity of Macrophages in Both Peripheral Blood and Skin Tissue
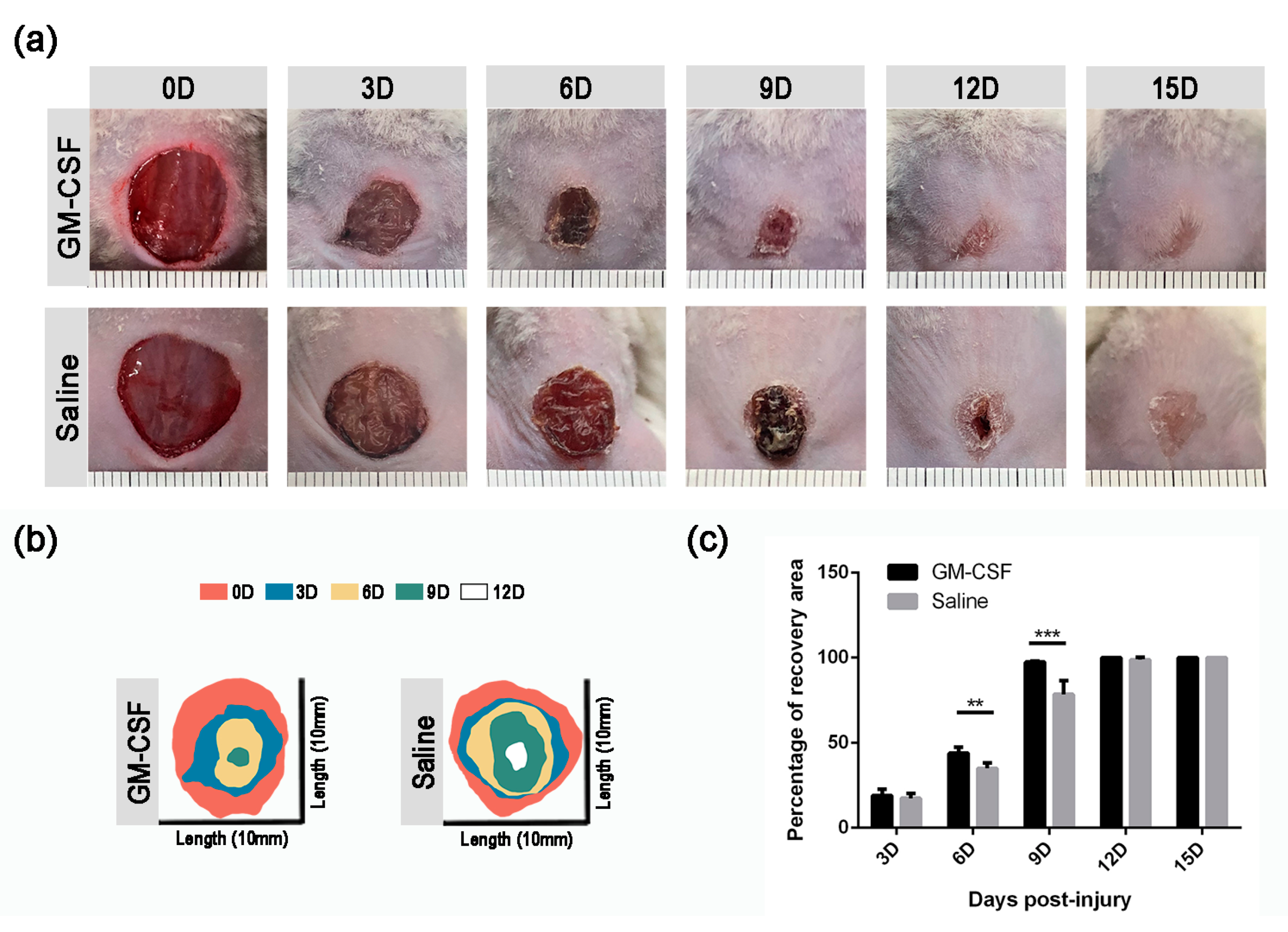
2.6. The Treatment of GM-CSF Injection Accelerated Wound Healing Process by Upregulating Regeneration-Associated Genes
3. Discussion
4. Materials and Methods
4.1. Animal Maintenance and Full-Thickness Skin Defect Study
4.2. Histology and Immunohistochemistry (IHC)/Immunofluorescent (IF) Staining
4.3. Macrophage Depletion
4.4. RNA Isolation and Quantitative Gene Expression
4.5. Protein Isolation and Quantification
4.6. Macrophage Mobilization
4.7. FACS Analysis
4.8. Antibodies and Cytokine Detection
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMP | bone morphogenetic protein |
| CVF | collagen volume fraction |
| ECM | extra-cellular matrix |
| ELISA | enzyme-linked immunosorbent assay |
| EMT | epithelial-mesenchymal transition |
| FGF | fibroblast growth factor |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| IF | immunofluorescent |
| IHC | immunohistochemistry |
| L-clod | clodronate liposome |
| L-PBS | PBS liposome |
| MMPs | matrix Metalloproteinases |
| RT-qPCR | real-time quantitative PCR |
| TGF | transforming growth factor |
| VEGF | vascular endothelial growth factor |
| WBC | white blood cells |
References
- Başağaoğlu, B.; Bhadkamkar, M.; Hollier, P.; Reece, E. Approach to Reconstruction of Cheek Defects. Semin. Plast. Surg. 2018, 32, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Santema, T.B.; Poyck, P.P.; Ubbink, D.T. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst. Rev. 2016, 2, Cd011255. [Google Scholar] [CrossRef] [PubMed]
- Rheinwatd, J.G.; Green, H. Seria cultivation of strains of human epidemal keratinocytes: The formation keratinizin colonies from single cell is. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef]
- Pearson, R.G.; Bhandari, R.; Quirk, R.A.; Shakesheff, K.M. Recent advances in tissue engineering: An invited review. J. Long-Term Eff. Med. Implant. 2002, 12, 1–33. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Sharma, V.; Kang, N.V.; Garcia-Gareta, E. A Universal Classification System of Skin Substitutes Inspired by Factorial Design. Tissue Eng. Part B Rev. 2018, 24, 279–288. [Google Scholar] [CrossRef]
- Aguilar, S.; Nye, E.; Chan, J.; Loebinger, M.; Spencer-Dene, B.; Fisk, N.; Stamp, G.; Bonnet, D.; Janes, S.M. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells 2007, 25, 1586–1594. [Google Scholar] [CrossRef]
- Bello, Y.M.; Falabella, A.F. The role of graftskin (Apligraf) in difficult-to-heal venous leg ulcers. J. Wound Care 2002, 11, 182–183. [Google Scholar] [CrossRef]
- Hongmin, L.; Wei, Z.; Xingrong, Y.; Jing, W.; Wenxin, G.; Jihong, C.; Xin, X.; Fulin, C. Osteoinductive nanohydroxyapatite bone substitute prepared via in situ hydrothermal transformation of cuttlefish bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 816–824. [Google Scholar] [CrossRef]
- Sun, M.; Jiang, M.; Cui, J.; Liu, W.; Yin, L.; Xu, C.; Wei, Q.; Yan, X.; Chen, F. A novel approach for the cryodesiccated preservation of tissue-engineered skin substitutes with trehalose. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 60–66. [Google Scholar] [CrossRef]
- Gainza, G.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1551–1573. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef] [PubMed]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Börner, M.G.; et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Jt. Surg. Am. Vol. 2002, 84, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Czyz, M. Fibroblast growth factor receptor signaling in skin cancers. Cells 2019, 8, 540. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Macrophages are required for adult salamander limb regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9415–9420. [Google Scholar] [CrossRef]
- Raggatt, L.J.; Wullschleger, M.E.; Alexander, K.A.; Wu, A.C.; Millard, S.M.; Kaur, S.; Maugham, M.L.; Gregory, L.S.; Steck, R.; Pettit, A.R. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am. J. Pathol. 2014, 184, 3192–3204. [Google Scholar] [CrossRef]
- Liu, C.; Wu, C.; Yang, Q.; Gao, J.; Li, L.; Yang, D.; Luo, L. Macrophages Mediate the Repair of Brain Vascular Rupture through Direct Physical Adhesion and Mechanical Traction. Immunity 2016, 44, 1162–1176. [Google Scholar] [CrossRef]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Calavia, N.G.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef]
- Xiong, M.; Elson, G.; Legarda, D.; Leibovich, A.J. Production of vascular endothelial growth factor by murine macrophages. Am. J. Pathol. 1998, 153, 587–598. [Google Scholar] [CrossRef]
- McLaren, J.; Prentice, A.; Charnock-Jones, D.S.; Millican, S.A.; Muller, K.H.; Sharkey, A.M.; Smith, S.K. Vegf is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J. Clin. Investig. 1996, 98, 482–489. [Google Scholar] [CrossRef]
- Ushach, I.; Zlotnik, A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J. Leukoc. Biol. 2016, 100, 481–489. [Google Scholar] [CrossRef]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and physiology of wound healing. Facial Plast. Surg. Clin. N. Am. 2011, 19, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef] [PubMed]
- Van, R.N.; Sanders, A. Kupffer cell depletion by liposome-delivered drugs. Hepatology 1996, 23, 1239–1243. [Google Scholar]
- Duffield, J.S.; Forbes, S.J.; Constandinou, C.M.; Clay, S.; Partolina, M.; Vuthoori, S.; Wu, S.; Lang, R.; Iredale, J.P. Selective depletion of macrophages reveals distinct opposing roles during liver injury and repair. J. Clin. Investig. 2005, 115, 56–65. [Google Scholar] [CrossRef]
- Kasuya, A.; Tokura, Y. Attempts to accelerate wound healing. J. Dermatol. Sci. 2014, 76, 169–172. [Google Scholar] [CrossRef]
- Gailit, J.; Welch, M.P.; Clark, R.A. TGF-β1 Stimulates Expression of Keratinocyte Integrins During Re-Epithelialization of Cutaneous Wounds. J. Investig. Dermatol. 1994, 103, 221–227. [Google Scholar] [CrossRef]
- Massague, J.; Blain, S.W.; Lo, R.S. Tgfβ signaling in growth control, cancer, and heritable disorders. Cell 2000, 103, 295–309. [Google Scholar] [CrossRef]
- Levesque, M.; Gatien, S.; Finnson, K.; Desmeules, S.; Villiard, E.; Pilote, M.; Philip, A.; Roy, S. Transforming growth factor: Beta signaling is essential for limb regeneration in axolotls. PLoS ONE 2007, 2, e1227. [Google Scholar] [CrossRef]
- Pilcher, B.K.; Wang, M.; Qin, X.; Parks, W.C.; Senior, R.M.; Welgus, H.G. Role of MMPs and their inhibition in cutaneous wound healing and allergic contact hypersensitivity. Ann. N. Y. Acad. Sci. 1999, 30, 15–24. [Google Scholar]
- Breen, E.C. VEGF in biological control. J. Cell. Biochem. 2007, 102, 1358–1367. [Google Scholar] [CrossRef]
- Ciarlillo, D.; Celeste, C.; Carmeliet, P.; Boerboom, D.; Theoret, C. A hypoxia response element in the Vegfa promoter is required for basal Vegfa expression in skin and for optimal granulation tissue formation during wound healing in mice. PLoS ONE 2017, 12, e0180586. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Ferreira, A.M.; Oberyszyn, T.M.; Bergdall, V.K.; Dipietro, L.A. Regulation of scar formation by vascular endothelial growth factor. Lab. Investig. 2008, 88, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The role of snail in EMT and tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J.; et al. Silk Fibroin Biomaterial Shows Safe and Effective Wound Healing in Animal Models and a Randomized Controlled Clinical Trial. Adv. Healthc. Mater. 2017, 6, 1700121. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, X.; Shen, Y.I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981. [Google Scholar] [CrossRef]

| Time Points | Groups | p Value | |
|---|---|---|---|
| L-Clod-Injected | L-PBS-Injected | ||
| 5D | 20.43 ± 2.49% | 33.71 ± 1.89% | <0.001 |
| 10D | 66.31 ± 4.04% | 95.24 ± 1.66% | <0.001 |
| 15D | 97.34 ± 2.13% | 100.00 ± 0.00% | 0.13 |
| 20D | 100.00 ± 0.00% | 100.00 ± 0.00% | >0.05 |
| Time Points | Groups | p Value | |
|---|---|---|---|
| Saline-Injected | GM-CSF-Injected | ||
| 3D | 17.44 ± 2.92% | 19.05 ± 3.67% | 0.56 |
| 6D | 35.00 ± 1.93% | 43.98 ± 2.01% | <0.001 |
| 9D | 78.40 ± 4.64% | 97.38 ± 0.42% | <0.001 |
| 12D | 98.83 ± 1.53% | 100.00 ± 0.00% | 0.67 |
| 15D | 100.00 ± 0.00% | 100.00 ± 0.00% | >0.05 |
| Gene Product | ||
|---|---|---|
| CSF1R | Forward | 5′-GAAGGTGGCTGTGAAGATGCTAA-3′ |
| Reverse | 5′-AGGTTGACTATATTCTCGTGCTGTC-3′ | |
| VEGFA | Forward | 5′-AGGCTGCTGTAACGATGAAG-3′ |
| Reverse | 5′-TCTCCTATGTGCTGGCTTTG-3′ | |
| TGFβ1 | Forward | 5′-CTGAACCAAGGAGACGGAATAC-3′ |
| Reverse | 5′-GGGCTGATCCCGTTGATTT-3′ | |
| IL6 | Forward | 5′-CTTCCATCCAGTTGCCTTCT-3′ |
| Reverse | 5′-CTCCGACTTGTGAAGTGGTATAG-3′ | |
| SNAIL | Forward | 5′-GAGAAGCCATTCTCCTGCTC-3′ |
| Reverse | 5′-GCACTGGTATCTCTTCACATCC-3′ | |
| CAMP | Forward | 5′-TCCCTAGACACCAATCTCTACC-3′ |
| Reverse | 5′-GCCACATACAGTCTCCTTCAC-3′ | |
| MMP3 | Forward | 5′-ATGTCACTGGTACCAACCTATTC-3′ |
| Reverse | 5′-CAAGTCTGTGGAGGACTTGTAG-3′ | |
| MMP9 | Forward | 5′-TGCACTGGGCTTAGATCATTC-3′ |
| Reverse | 5′-TGCCGTCTATGTCGTCTTTATTC-3′ | |
| MMP2 | Forward | 5′-CTGGAATGCCATCCCTGATAA-3′ |
| Reverse | 5′-GGTTCTCCAGCTTCAGGTAATAA-3′ | |
| FGF2 | Forward | 5′-CTTACCGGTCACGGAAATACTC-3′ |
| Reverse | 5′-AGCTCTTAGCAGACATTGGAAG-3′ | |
| TNFα | Forward | 5′-CGATGGGTTGTACCTTGTCTAC-3′ |
| Reverse | 5′-GCAGAGAGGAGGTTGACTTTC-3′ | |
| IL8 | Forward | 5′-TCCTGCTTGAATGGCTTGAATACTA-3′ |
| Reverse | 5′-CGGTGTCCTGATTATCGTCCTC-3′ | |
| α-ACTIN | Forward | 5′-CTCCCTGGAGAAGAGCTATGA-3′ |
| Reverse | 5′-CCAAGAAGGAAGGCTGGAAA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Jia, L.; Zheng, H.; Feng, J.; Wei, S.; Li, J.; Cui, J.; Chen, F. The Stimulation of Macrophages by Systematical Administration of GM-CSF Can Accelerate Adult Wound Healing Process. Int. J. Mol. Sci. 2022, 23, 11287. https://doi.org/10.3390/ijms231911287
Zhang J, Jia L, Zheng H, Feng J, Wei S, Li J, Cui J, Chen F. The Stimulation of Macrophages by Systematical Administration of GM-CSF Can Accelerate Adult Wound Healing Process. International Journal of Molecular Sciences. 2022; 23(19):11287. https://doi.org/10.3390/ijms231911287
Chicago/Turabian StyleZhang, Jing, Liyuan Jia, Hanxue Zheng, Juantao Feng, Sili Wei, Juan Li, Jihong Cui, and Fulin Chen. 2022. "The Stimulation of Macrophages by Systematical Administration of GM-CSF Can Accelerate Adult Wound Healing Process" International Journal of Molecular Sciences 23, no. 19: 11287. https://doi.org/10.3390/ijms231911287
APA StyleZhang, J., Jia, L., Zheng, H., Feng, J., Wei, S., Li, J., Cui, J., & Chen, F. (2022). The Stimulation of Macrophages by Systematical Administration of GM-CSF Can Accelerate Adult Wound Healing Process. International Journal of Molecular Sciences, 23(19), 11287. https://doi.org/10.3390/ijms231911287





