Synthesis of Silver Nano Particles Using Myricetin and the In-Vitro Assessment of Anti-Colorectal Cancer Activity: In-Silico Integration
Abstract
1. Introduction
2. Results
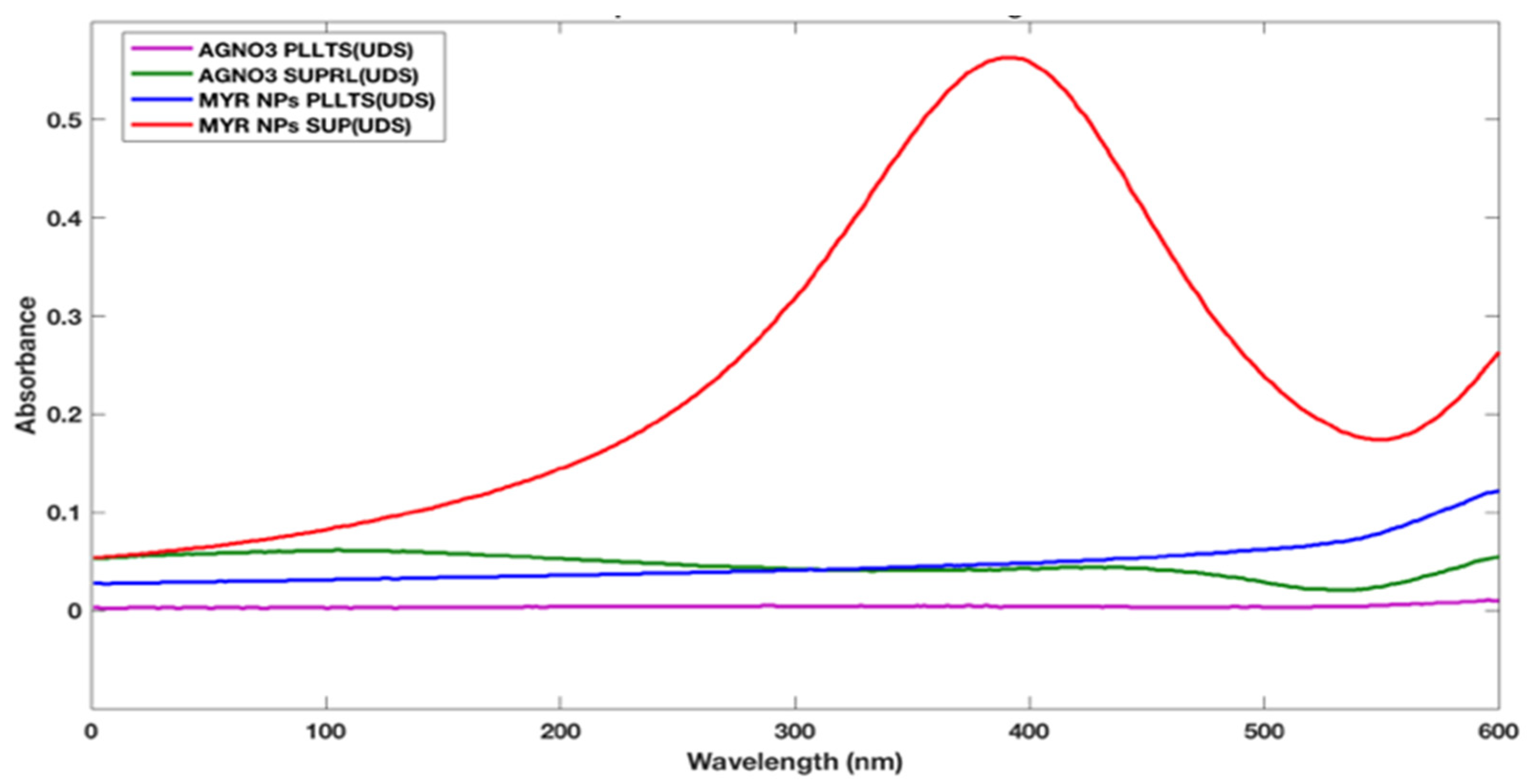
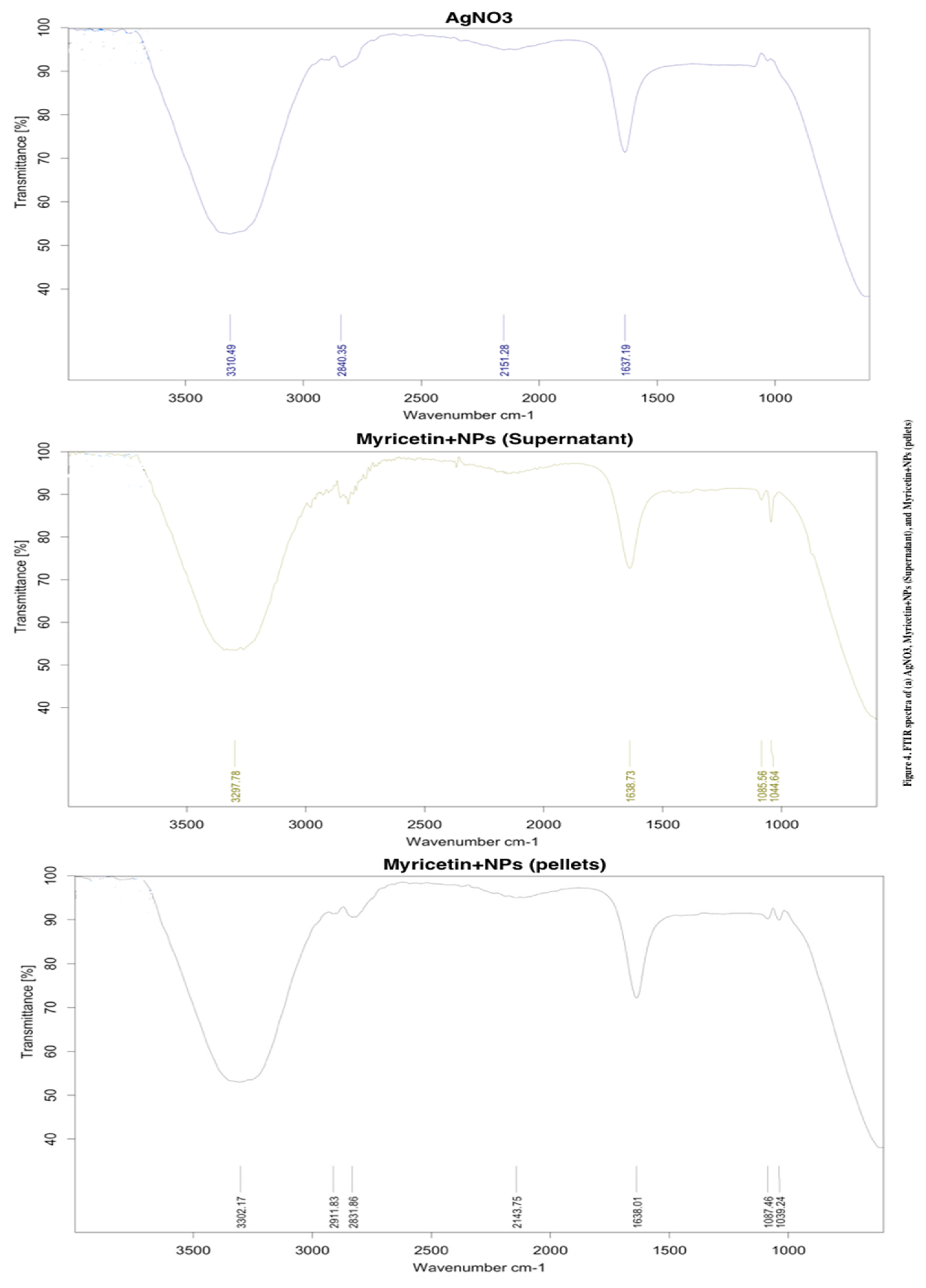
2.1. AgNPs Synthesis and Characterization
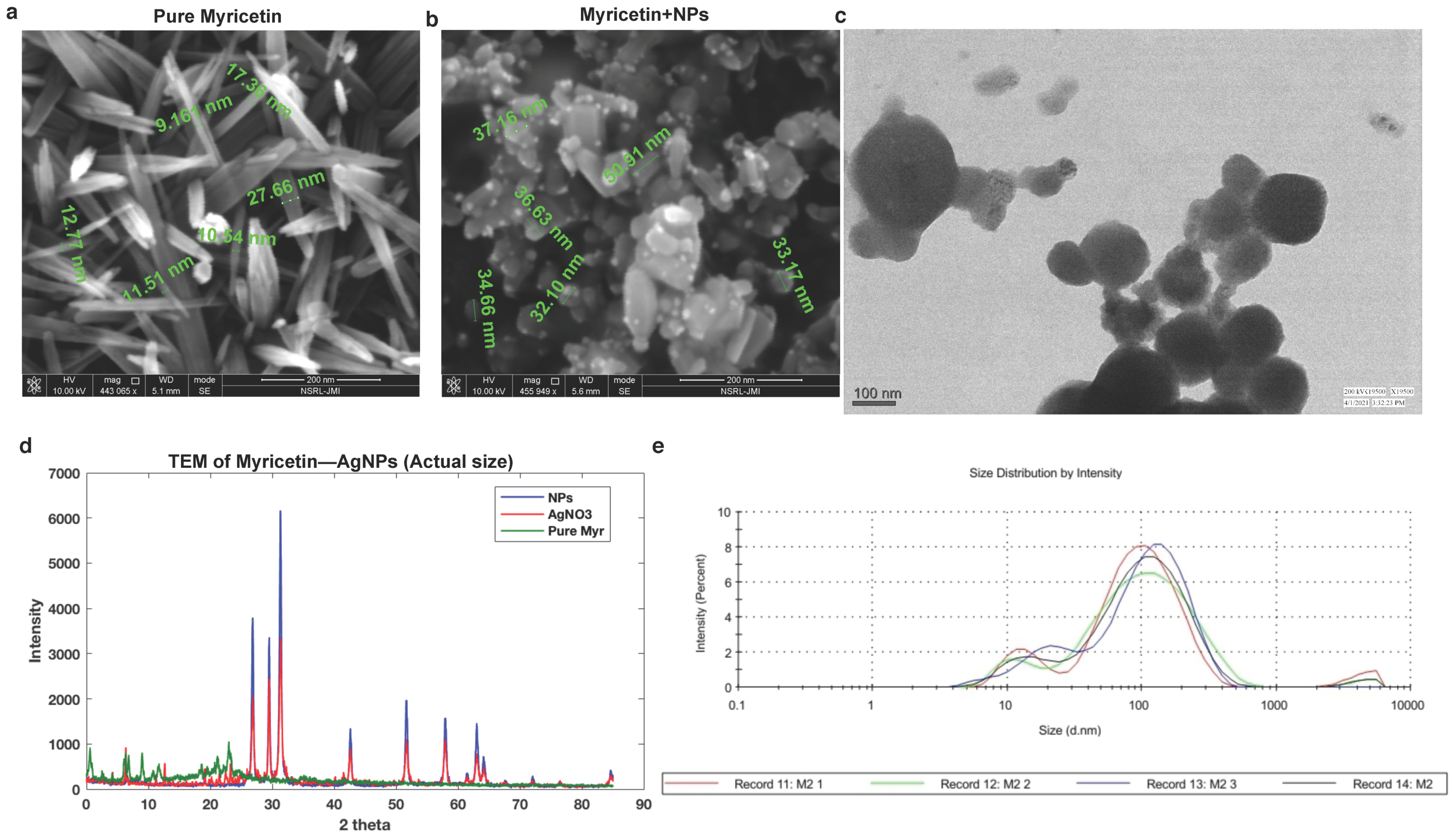
2.2. Morphological Characteristics of BioAgNPs
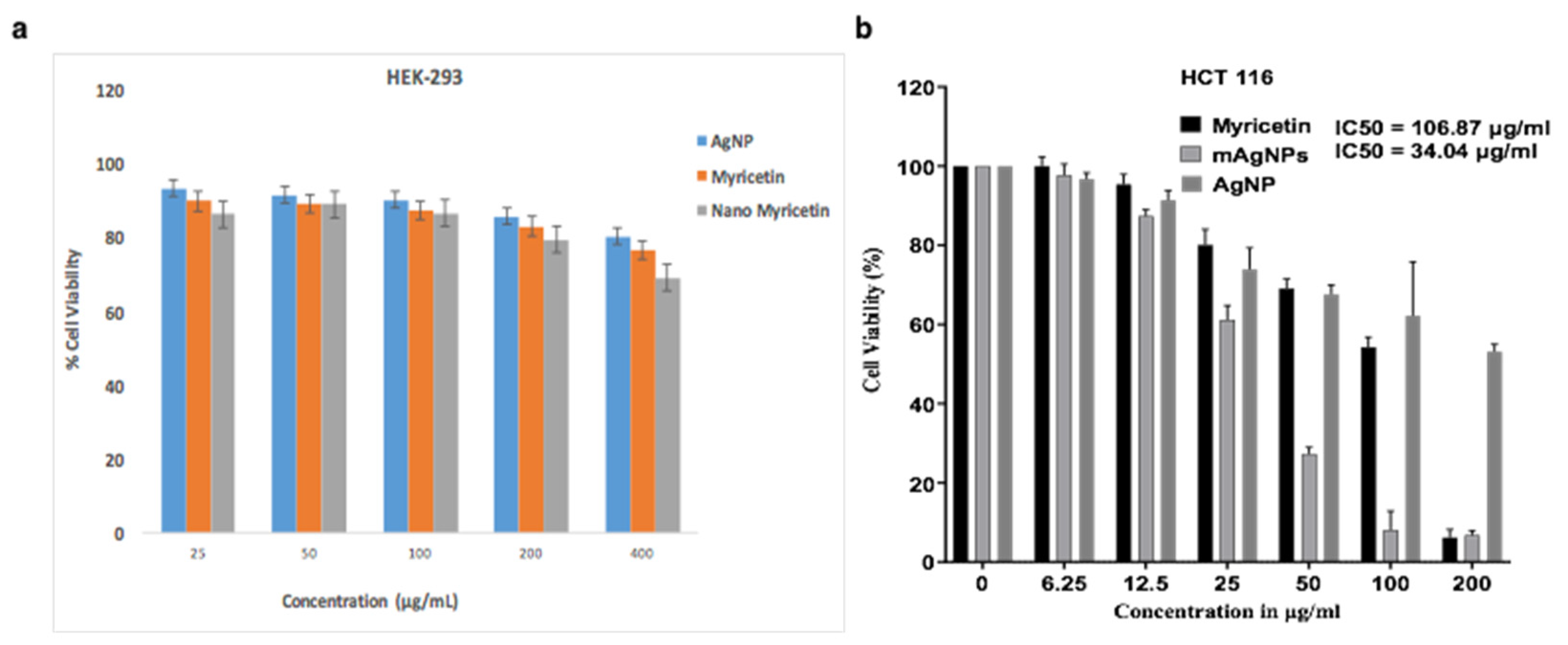
2.3. Human Colorectal Cancer Cells’ Vitality Was Decreased by Myricetin
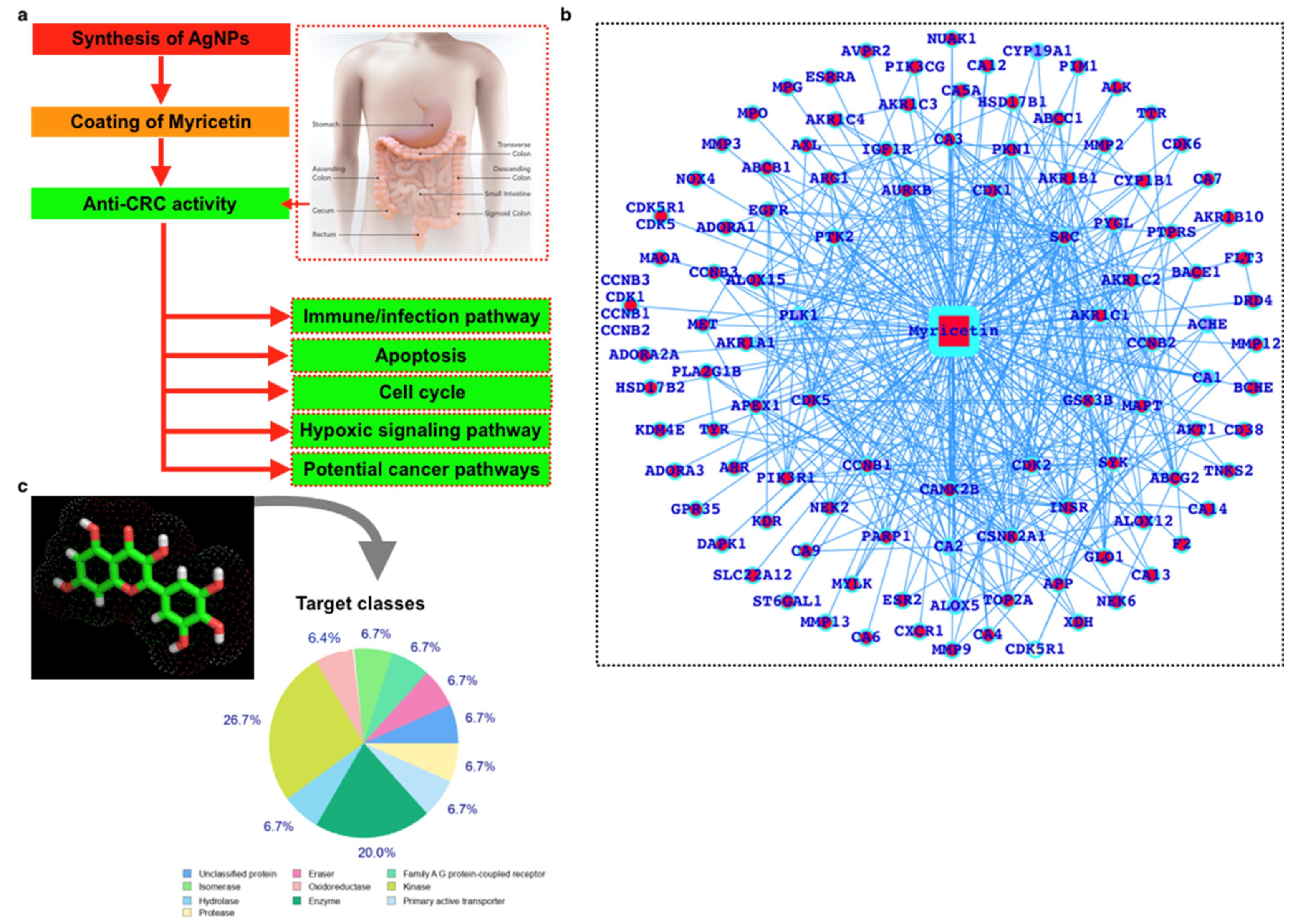
2.4. CRC Gene Expression Profiling and Functional Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Collection and Preparation of Plant Extract (Myricetin)
4.2. Biosynthesis of Silver Nanoparticles (AgNPs)
4.3. Characterization of Silver Nanoparticles
4.4. Cell Culture and Treatment
4.5. Cell Viability Assay
4.6. Transmission Electron Microscopy
4.7. Statistical Analysis
4.8. Gene Expression and Pathway Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, L.; Mooney, D.J. Biomaterials and emerging anticancer therapeutics: Engineering the microenvironment. Nat. Rev. Cancer 2016, 16, 56–66. [Google Scholar] [CrossRef]
- Li, Z.; Ali, I.; Qiu, J.; Zhao, H.; Ma, W.; Bai, A.; Wang, D.; Li, J. Eco-Friendly and Facile Synthesis of Antioxidant, Antibacterial and Anticancer Dihydromyricetin-Mediated Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 481–492. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Benakashani, F.; Allafchian, A.R.; Jalali, S.A.H. Biosynthesis of silver nanoparticles using Capparis spinosa L. leaf extract and their antibacterial activity. Karbala Int. J. Mod. Sci. 2016, 2, 251–258. [Google Scholar]
- Cicha, I.; Unterweger, H.; Lyer, S.; Janko, C.; Friedrich, R.P.; Pöttler, M.; Alexiou, C. Nanomedicine for cardiovascular disorders. Nanomedicine 2019, 14, 3007–3012. [Google Scholar] [CrossRef]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostructure Chem. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Torres, A.J.; Wu, M.; Holowka, D.; Baird, B. Nanobiotechnology and Cell Biology: Micro- and Nanofabricated Surfaces to Investigate Receptor-Mediated Signaling. Annu. Rev. Biophys. 2008, 37, 265–288. [Google Scholar] [CrossRef]
- Fatimah, I. Green synthesis of silver nanoparticles using extract of. J. Adv. Res. 2016, 7, 961–969. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Sahu, N.; Soni, D.; Chandrashekhar, B.; Satpute, D.B.; Saravanadevi, S.; Sarangi, B.K.; Pandey, R.A. Synthesis of silver nanoparticles using flavonoids: Hesperidin, naringin and diosmin, and their antibacterial effects and cytotoxicity. Int. Nano Lett. 2016, 6, 173–181. [Google Scholar] [CrossRef]
- Kilit, A.C.; Kose, E.O.; Imir, N.G.; Aydemir, E. Anticancer and antimicrobial activities of diosmin. Genet. Mol. Res. 2021, 20, 1–18. [Google Scholar] [CrossRef]
- Gomes, H.I.O.; Martins, C.S.M.; Prior, J.A.V. Silver Nanoparticles as Carriers of Anticancer Drugs for Efficient Target Treatment of Cancer Cells. Nanomaterials 2021, 11, 964. [Google Scholar] [CrossRef]
- Shah, M.Z.; Guan, Z.-H.; Din, A.U.; Ali, A.; Rehman, A.U.; Jan, K.; Faisal, S.; Saud, S.; Adnan, M.; Wahid, F.; et al. Synthesis of silver nanoparticles using Plantago lanceolata extract and assessing their antibacterial and antioxidant activities. Sci. Rep. 2021, 11, 20754. [Google Scholar] [CrossRef]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. ScienceDirect. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef]
- Zhu, M.-L.; Zhang, P.-M.; Jiang, M.; Yu, S.-W.; Wang, L. Myricetin induces apoptosis and autophagy by inhibiting PI3K/Akt/mTOR signalling in human colon cancer cells. BMC Complementary Med. Ther. 2020, 20, 209. [Google Scholar] [CrossRef]
- Kim, M.E.; Ha, T.K.; Yoon, J.H.; Lee, J.S. Myricetin induces cell death of human colon cancer cells via BAX/BCL2-dependent pathway. AR 2014, 34, 701–706. [Google Scholar]
- Huang, H.; Chen, A.Y.; Ye, X.; Li, B.; Rojanasakul, Y.; Rankin, G.O.; Chen, Y.C. Myricetin inhibits proliferation of cisplatin-resistant cancer cells through a p53-dependent apoptotic pathway. Int. J. Oncol. 2015, 47, 1494–1502. [Google Scholar] [CrossRef]
- Ma, L.; Cao, X.; Wang, H.; Lu, K.; Wang, Y.; Tu, C.; Dai, Y.; Meng, Y.; Li, Y.; Yu, P.; et al. Discovery of Myricetin as a Potent Inhibitor of Human Flap Endonuclease 1, Which Potentially Can Be Used as Sensitizing Agent against HT-29 Human Colon Cancer Cells. J. Agric. Food Chem. 2019, 67, 1656–1665. [Google Scholar] [CrossRef]
- Kuntz, S.; Wenzel, U.; Daniel, H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999, 38, 133–142. [Google Scholar] [CrossRef]
- Wang, C.; Niimi, M.; Watanabe, T.; Wang, Y.; Liang, J.; Fan, J. Treatment of atherosclerosis by traditional Chinese medicine: Questions and quandaries. Atherosclerosis 2018, 277, 136–144. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Modulation of signaling pathways in prostate cancer by green tea polyphenols. Biochem. Pharmacol. 2013, 85, 667–672. [Google Scholar] [CrossRef]
- Di Nicolantonio, F.; Vitiello, P.P.; Marsoni, S.; Siena, S.; Tabernero, J.; Trusolino, L.; Bernards, R.; Bardelli, A. Precision oncology in metastatic colorectal cancer—From biology to medicine. Nat. Rev. Clin. Oncol. 2021, 8, 506–525. [Google Scholar] [CrossRef]
- Swanton, C. Cancer evolution: The final frontier of precision medicine? Ann. Oncol. 2014, 25, 549–551. [Google Scholar] [CrossRef]
- Hansen, J.; Iyengar, R. Computation as the Mechanistic Bridge Between Precision Medicine and Systems Therapeutics. Clin. Pharmacol. Ther. 2012, 93, 117–128. [Google Scholar] [CrossRef]
- Barbolosi, D.; Ciccolini, J.; Lacarelle, B.; Barlési, F.; André, N. Computational oncology—mathematical modelling of drugregimens for precision medicine. Nat. Rev. Clin. Oncol. 2015, 13, 242–254. [Google Scholar] [CrossRef]
- Bodaghi, S.; Yamanegi, K.; Xiao, S.-Y.; Da Costa, M.; Palefsky, J.M.; Zheng, Z.-M. Colorectal papillomavirus infection in patients with colorectal cancer. Clin. Cancer Res. 2005, 11, 2862–2867. [Google Scholar] [CrossRef]
- Yaeger, R.; Chatila, W.K.; Lipsyc, M.D.; Hechtman, J.F.; Cercek, A.; Sanchez-Vega, F.; Jayakumaran, G.; Middha, S.; Zehir, A.; Donoghue, M.T.A.; et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 2018, 33, 125–136.e3. [Google Scholar] [CrossRef]
- Russo, M.; Siravegna, G.; Blaszkowsky, L.S.; Corti, G.; Crisafulli, G.; Ahronian, L.G.; Mussolin, B.; Kwak, E.L.; Buscarino, M.; Lazzari, L.; et al. Tumor Heterogeneity and Lesion-Specific Response to Targeted Therapy in Colorectal Cancer. Cancer Discov. 2016, 6, 147–153. [Google Scholar] [CrossRef]
- Zhang, J. Crosstalk Between the MSI Status and Tumor Microenvironment in Colorectal Cancer. Front. Immunol. 2020, 11, 2039. [Google Scholar]
- Schürch, C.M.; Bhate, S.S.; Barlow, G.L.; Phillips, D.J.; Noti, L.; Zlobec, I.; Chu, P.; Black, S.; Demeter, J.; McIlwain, D.R.; et al. Coordinated Cellular Neighborhoods Orchestrate Antitumoral Immunity at the Colorectal Cancer Invasive Front. Cell 2020, 182, 1341–1359.e19. [Google Scholar] [CrossRef]
- Markowitz, S.D.; Bertagnolli, M.M. Molecular origins of cancer: Molecular basis of colorectal cancer. N. Engl. J. Med. 2009, 361, 2449–2460. [Google Scholar] [CrossRef]
- Michor, F.; Iwasa, Y.; Lengauer, C.; Nowak, M. Dynamics of colorectal cancer. Semin. Cancer Biol. 2005, 15, 484–493. [Google Scholar] [CrossRef]
- DeVita, V.T.; Eggermont, A.M.M.; Hellman, S.; Kerr, D.J. Clinical cancer research: The past, present and the future. Nat. Rev. Clin. Oncol. 2014, 11, 663–669. [Google Scholar] [CrossRef]
- Moradi, A.; Pourseif, M.M.; Jafari, B.; Parvizpour, S.; Omidi, Y. Pharmacological Research. Pharmacol. Res. 2020, 156, 104790. [Google Scholar] [CrossRef]
- Hayes, D.N.; Kim, W.Y. The next steps in next-gen sequencing of cancer genomes. J. Clin. Investig. 2015, 125, 462–468. [Google Scholar] [CrossRef][Green Version]
- Newton, K.F.; Newman, W.; Hill, J. Review of biomarkers in colorectal cancer. Colorectal Dis. 2011, 14, 3–17. [Google Scholar] [CrossRef]
- Cheung, K.J.; Ewald, A.J. A collective route to metastasis: Seeding by tumor cell clusters. Science 2016, 352, 167–169. [Google Scholar] [CrossRef]
- Bansal, M.; Yang, J.; Karan, C.; Menden, M.P.; Costello, J.C.; Tang, H.; Xiao, G.; Li, Y.; Allen, J.; Zhong, R.; et al. A community computational challenge to predict the activity of pairs of compounds. Nat. Biotechnol. 2014, 32, 1213–1222. [Google Scholar] [CrossRef]
- Barderas, R.; Babel, I.; Díaz-Uriarte, R.; Moreno, V.; Suárez, A.; Bonilla, F.; Villar-Vázquez, R.; Capella, G.; Casal, J.I. An optimized predictor panel for colorectal cancer diagnosis based on the combination of tumor-associated antigens obtained from protein and phage microarrays. J. Proteom. 2012, 75, 4647–4655. [Google Scholar] [CrossRef]
- Garnis, C.; Buys, T.P.; Lam, W.L. Genetic alteration and gene expression modulation during cancer progression. Mol. Cancer 2004, 3, 9. [Google Scholar] [CrossRef]
- Maley, C.; Reid, B. Natural selection in neoplastic progression of Barrett’s esophagus. Semin. Cancer Biol. 2005, 15, 474–483. [Google Scholar] [CrossRef]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.e6. [Google Scholar] [CrossRef]
- Lapointe, J.; Li, C.; Higgins, J.P.; van de Rijn, M.; Bair, E.; Montgomery, K.; Ferrari, M.; Egevad, L.; Rayford, W.; Bergerheim, U.; et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc. Natl. Acad. Sci. USA 2004, 101, 811–816. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Lee, A.J.X.; Swanton, C. Tumour heterogeneity and drug resistance: Personalising cancer medicine through functional genomics. Biochem. Pharmacol. 2012, 83, 1013–1020. [Google Scholar] [CrossRef]
- Wang, E. Understanding genomic alterations in cancer genomes using an integrative network approach. Cancer Lett. 2013, 340, 261–269. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Roepman, P.; Schlicker, A.; Tabernero, J.; Majewski, I.; Tian, S.; Moreno, V.; Snel, M.H.; Chresta, C.M.; Rosenberg, R.; Nitsche, U.; et al. Colorectal cancer intrinsic subtypes predict chemotherapy benefit, deficient mismatch repair and epithelial-to-mesenchymal transition. Int. J. Cancer 2013, 134, 552–562. [Google Scholar] [CrossRef]
- Alhopuro, P.; Sammalkorpi, H.; Niittymäki, I.; Biström, M.; Raitila, A.; Saharinen, J.; Nousiainen, K.; Lehtonen, H.J.; Heliövaara, E.; Puhakka, J.; et al. Candidate driver genes in microsatellite-unstable colorectal cancer. Int. J. Cancer 2011, 130, 1558–1566. [Google Scholar] [CrossRef]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in Colorectal Cancer: Current State of Knowledge including Clinical Trials and Molecular Mechanism of Action. BioMed Res. Int. 2018, 2018, 4154185. [Google Scholar] [CrossRef]
- Koosha, S.; Alshawsh, M.A.; Looi, C.Y.; Seyedan, A.; Mohamed, Z. An Association Map on the Effect of Flavonoids on the Signaling Pathways in Colorectal Cancer. Int. J. Med. Sci. 2016, 13, 374–385. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Mosaddeghi, P.; Eslami, M.; Farahmandnejad, M.; Akhavein, M.; Ranjbarfarrokhi, R.; Khorraminejad-Shirazi, M.; Shahabinezhad, F.; Taghipour, M.; Dorvash, M.; Sakhteman, A.; et al. A systems pharmacology approach to identify the autophagy-inducing effects of Traditional Persian medicinal plants. Sci. Rep. 2020, 11, 336. [Google Scholar] [CrossRef]
- Dai, T.; He, W.; Yao, C.; Ma, X.; Ren, W.; Mai, Y.; Wu, A. Applications of inorganic nanoparticles in the diagnosis and therapy of atherosclerosis. Biomater. Sci. 2020, 8, 3784–3799. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, B.; Kanwar, S.S.; Kumar, A. Lead Phytochemicals for Anticancer Drug Development. Front. Plant Sci. 2016, 7, 1667. [Google Scholar] [CrossRef]
- LaFoya, B.; Munroe, J.A.; Albig, A.R. A comparison of resveratrol and other polyphenolic compounds on Notch activation and endothelial cell activity. PLoS ONE 2019, 14, e0210607. [Google Scholar] [CrossRef]
- Caillon, L.; Hoffmann, A.R.F.; Botz, A.; Khemtemourian, L. Molecular Structure, Membrane Interactions, and Toxicity of the Islet Amyloid Polypeptide in Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 5639875. [Google Scholar] [CrossRef]
- El-Gayar, K.E.; Al Abboud, M.A.; Essa, A.M.M. Characterization of Thermophilic Bacteria Isolatedfrom two Hot Springs in Jazan, Saudi Arabia. J. Pure Appl. Microbiol. 2017, 11, 743–752. [Google Scholar] [CrossRef]
- Linch, M.; Sanz-Garcia, M.; Soriano, E.; Zhang, Y.; Riou, P.; Rosse, C.; Cameron, A.; Knowles, P.; Purkiss, A.; Kjaer, S.; et al. A Cancer-Associated Mutation in Atypical Protein Kinase C Occurs in a Substrate-Specific Recruitment Motif. Sci. Signal. 2013, 6, ra82. [Google Scholar] [CrossRef]
- Gordon, D.J.; Resio, B.; Pellman, D. Causes and consequences ofaneuploidy in cancer. Nature Reviews Genetics 2012, 13, 189–203. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, S.Y.; Lee, J.-Y.; Park, J.H.Y. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef]
- Azizi, M.; Ghourchian, H.; Yazdian, F.; Dashtestani, F.; AlizadehZeinabad, H. Cytotoxic effect of albumin coated copper nanoparticle on human breast cancer cells of MDA-MB 231. PLoS ONE 2017, 12, e0188639. [Google Scholar] [CrossRef]
- Soares, J.M.; Faria, B.M.D.; Ascari, L.M.; Souza, J.M.D.; Soares, A.G.; Cordeiro, Y.; Romão, L.F. Diosmin induces caspase-dependent apoptosis in human glioblastoma cells. An. Acad. Bras. Cienc. 2019, 91, e20191031. [Google Scholar] [CrossRef]
- Tong, N.; Zhang, Z.; Zhang, W.; Qiu, Y.; Gong, Y.; Yin, L.; Qiu, Q.; Wu, X. Diosmin Alleviates Retinal Edema by Protecting the Blood-Retinal Barrier and Reducing Retinal Vascular Permeability during Ischemia/Reperfusion Injury. PLoS ONE 2013, 8, e61794. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545. [Google Scholar] [CrossRef]
- Glaab, E.; Baudot, A.; Krasnogor, N.; Schneider, R.; Valencia, A. EnrichNet: Network-based gene set enrichment analysis. Bioinformatics 2012, 28, i451–i457. [Google Scholar] [CrossRef]
- Ahmed, S.; Mobashir, M.; Al-Keridis, L.A.; Alshammari, N.; Adnan, M.; Abid, M.; Hassan, M.I. A Network-Guided Approach to Discover Phytochemical-Based Anticancer Therapy: Targeting MARK4 for Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 914032. [Google Scholar] [CrossRef]
- Abatangelo, L.; Maglietta, R.; Distaso, A.; D’Addabbo, A.; Creanza, T.; Mukherjee, S.; Ancona, N. Comparative study of gene set enrichment methods. BMC Bioinform. 2009, 10, 275. [Google Scholar] [CrossRef]
- Mi, H.; Poudel, S.; Muruganujan, A.; Casagrande, J.T.; Thomas, P.D. PANTHER version 10: Expanded protein families and functions, and analysis tools. Nucleic Acids Res. 2016, 44, D336–D342. [Google Scholar] [CrossRef]
- Reimers, M. Making Informed Choices about Microarray Data Analysis. PLoS Comput. Biol. 2010, 6, e1000786. [Google Scholar] [CrossRef]
- Chen, K.-H.; Wang, K.-J.; Tsai, M.-L.; Wang, K.-M.; Adrian, A.M.; Cheng, W.-C.; Yang, T.-S.; Teng, N.-C.; Tan, K.-P.; Chang, K.-S. Gene selection for cancer identification: A decision tree model empowered by particle swarm optimization algorithm. BMC Bioinform. 2014, 15, 49. [Google Scholar] [CrossRef]
- Bild, A.H.; Parker, J.S.; Gustafson, A.M.; Acharya, C.R.; Hoadley, K.A.; Anders, C.; Marcom, P.K.; Carey, L.A.; Potti, A.; Nevins, J.R.; et al. An integration of complementary strategies for gene-expression analysis to reveal novel therapeutic opportunities for breast cancer. Breast Cancer Res. 2009, 11, R55. [Google Scholar] [CrossRef]
- Salomonis, N.; Hanspers, K.; Zambon, A.C.; Vranizan, K.; Lawlor, S.C.; Dahlquist, K.D.; Doniger, S.W.; Stuart, J.; Conklin, B.R.; Pico, A.R. GenMAPP 2: New features and resources for pathway analysis. BMC Bioinform. 2007, 8, 217. [Google Scholar] [CrossRef]
- Girke, T. Microarray Analysis. 2012, pp. 1–42. Available online: http://faculty.ucr.edu/~tgirke/HTML_Presentations/Manuals/Workshop_Dec_6_10_2012/Rmicroarray/arrayBasics.pdf (accessed on 14 September 2022).
- Quackenbush, J. Microarray data normalization and transformation. Nat. Genet. 2002, 32, 496–501. [Google Scholar] [CrossRef]
- Simon, R. Microarray-based expression profiling and informatics. Curr. Opin. Biotechnol. 2008, 19, 26–29. [Google Scholar] [CrossRef]
- Ideker, T.; Thorsson, V.; Siegel, A.F.; Hood, L.E. Testing for differentially-expressed genes by maximum-likelihood analysis of microarray data. J. Comput. Biol. 2000, 7, 805–817. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2011, 40, D109–D114. [Google Scholar] [CrossRef]
- Mobashir, M. Mathematical Modeling and Evolution of Signal Transduction Pathways and Networks. Ph.D. Thesis, Otto-von-Guericke Universität Magdeburg, Magdeburg, Germany, 2013. [Google Scholar]
- Mobashir, M.; Madhusudhan, T.; Isermann, B.; Beyer, T.; Schraven, B. Negative Interactions and Feedback Regulations Are Required for Transient Cellular Response. Sci. Rep. 2014, 4, 3718. [Google Scholar] [CrossRef]
- Mobashir, M.; Schraven, B.; Beyer, T. Simulated evolution of signal transduction networks. PLoS ONE 2012, 7, e50905. [Google Scholar] [CrossRef]
- Warsi, M.K.; Kamal, M.A.; Baeshen, M.N.; Izhari, M.A.; Mobashir, A.F.A.M. Comparative Study of Gene Expression Profiling Unravels Functions associated with Pathogenesis of Dengue Infection. Curr. Pharm. Des. 2020, 26, 5293–5299. [Google Scholar] [CrossRef]
- Kamal, M.A.; Warsi, M.K.; Alnajeebi, A.; Ali, H.A.; Helmi, N.; Izhari, M.A.; Mustafa, S.; Mobashir, M. Gene expression profiling and clinical relevance unravel the role hypoxia and immune signaling genes and pathways in breast cancer: Role of hypoxia and immune signaling genes in breast cancer. J. Intern. Med. Sci. Art 2020, 1, 2–10. [Google Scholar] [CrossRef]
- Bajrai, L.; Sohrab, S.S.; Alandijany, T.A.; Mobashir, M.; Parveen, S.; Kamal, M.A.; Azhar, E.I. Gene expression profiling of early acute febrile stage of dengue infection and its comparative analysis with Streptococcus pneumoniae infection. Front. Cell. Infect. Microbiol. 2021, 1018. [Google Scholar] [CrossRef]
- Eldakhakhny, B.M.; Al Sadoun, H.; Choudhry, H.; Mobashir, M. In-Silico Study of Immune System Associated Genes in Case of Type-2 Diabetes With Insulin Action and Resistance, and/or Obesity. Front. Endocrinol. 2021, 12, 641888. [Google Scholar]
- Kumar, P.P.; Kamal, M.A.; Warsi, M.K.; Alnajeebi, A.; Ali, H.A.; Helmi, N.; Izhari, M.A.; Mustafa, S.; Firoz, A.; Mobashir, M. In-silico study reveals immunological signaling pathways, their genes, and potential herbal drug targets in ovarian cancer. Inform. Med. Unlocked 2020, 20, 100422. [Google Scholar]
- Helmi, N.; Alammari, D.; Mobashir, M. Role of Potential COVID-19 Immune System Associated Genes and the Potential Pathways linkage with Type-2 Diabetes. Comb. Chem. High Throughput Screen. 2021, 24, 1–11. [Google Scholar] [CrossRef]
- Alexeyenko, A.; Sonnhammer, E.L.L. Global networks of functional coupling in eukaryotes from comprehensive data integration. Genome Res. 2009, 19, 1107–1116. [Google Scholar] [CrossRef]
- Okawa, S.; Angarica, V.E.; Lemischka, I.; Moore, K.; del Sol, A. A differential network analysis approach for lineagespeci. Nat. Publ. Group 2015, 1, 15012. [Google Scholar]

| Pathway | Present in |
|---|---|
| KEGG_04151_PI3K-Akt_signaling | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma, Healthy versus Adjacent cells |
| KEGG_04510_Focal_adhesion | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma, Healthy versus Adjacent cells |
| KEGG_04512_ECM-receptor_interaction | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma, Healthy versus Adjacent cells |
| KEGG_00040_Pentose_and_glucuronate_interconversions | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00071_Fatty_acid_metabolism | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00120_Primary_bile_acid_biosynthesis | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00140_Steroid_hormone_biosynthesis | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00230_Purine_metabolism | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00260_Glycine__serine_and_threonine_metabolism | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00350_Tyrosine_metabolism | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00500_Starch_and_sucrose_metabolism | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00561_Glycerolipid_metabolism | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00564_Glycerophospholipid_metabolism | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00590_Arachidonic_acid_metabolism | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00830_Retinol_metabolism | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00860_Porphyrin_and_chlorophyll_metabolism | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00910_Nitrogen_metabolism | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00980_Metabolism_of_xenobiotics_by_cytochrome_P450 | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00982_Drug_metabolism_-_cytochrome_P450 | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_00983_Drug_metabolism_-_other_enzymes | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_03320_PPAR_signaling_pathway | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04010_MAPK_signaling_pathway | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04014_Ras_signaling | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04015_Rap1_signaling | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04020_Calcium_signaling_pathway | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04022_cGMP-PKG_signaling | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04024_cAMP_signaling | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04060_Cytokine-cytokine_receptor_interaction | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04064_NF-kappa_B_signaling | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04068_FoxO_signaling | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04080_Neuroactive_ligand-receptor_interaction | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04110_Cell_cycle | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04152_AMPK_signaling | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04270_Vascular_smooth_muscle_contraction | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04310_Wnt_signaling_pathway | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04350_TGF-beta_signaling_pathway | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04360_Axon_guidance | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04371_Apelin_signaling | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04392_Hippo_Signaling_Pathway | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04514_Cell_adhesion_molecules_(CAMs) | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04530_Tight_junction | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04550_Signaling_pathways_regulating_pluripotency_of_stem_cells | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04610_Complement_and_coagulation_cascades | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04611_Platelet_activation | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04630_Jak-STAT_signaling_pathway | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04640_Hematopoietic_cell_lineage | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04668_TNF_signaling | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04670_Leukocyte_transendothelial_migration | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04810_Regulation_of_actin_cytoskeleton | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04916_Melanogenesis | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04919_Thyroid_hormone_signaling_pathway | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04920_Adipocytokine_signaling_pathway | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
| KEGG_04924_Renin_secretion | Healthy versus Primary Adenocarcinoma, Adjacent cells versus Primary Adenocarcinoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwer, S.T.; Mobashir, M.; Fantoukh, O.I.; Khan, B.; Imtiyaz, K.; Naqvi, I.H.; Rizvi, M.M.A. Synthesis of Silver Nano Particles Using Myricetin and the In-Vitro Assessment of Anti-Colorectal Cancer Activity: In-Silico Integration. Int. J. Mol. Sci. 2022, 23, 11024. https://doi.org/10.3390/ijms231911024
Anwer ST, Mobashir M, Fantoukh OI, Khan B, Imtiyaz K, Naqvi IH, Rizvi MMA. Synthesis of Silver Nano Particles Using Myricetin and the In-Vitro Assessment of Anti-Colorectal Cancer Activity: In-Silico Integration. International Journal of Molecular Sciences. 2022; 23(19):11024. https://doi.org/10.3390/ijms231911024
Chicago/Turabian StyleAnwer, Syed Tauqeer, Mohammad Mobashir, Omer I. Fantoukh, Bushra Khan, Khalid Imtiyaz, Irshad Hussain Naqvi, and M. Moshahid Alam Rizvi. 2022. "Synthesis of Silver Nano Particles Using Myricetin and the In-Vitro Assessment of Anti-Colorectal Cancer Activity: In-Silico Integration" International Journal of Molecular Sciences 23, no. 19: 11024. https://doi.org/10.3390/ijms231911024
APA StyleAnwer, S. T., Mobashir, M., Fantoukh, O. I., Khan, B., Imtiyaz, K., Naqvi, I. H., & Rizvi, M. M. A. (2022). Synthesis of Silver Nano Particles Using Myricetin and the In-Vitro Assessment of Anti-Colorectal Cancer Activity: In-Silico Integration. International Journal of Molecular Sciences, 23(19), 11024. https://doi.org/10.3390/ijms231911024









