Metal Cluster Triggered-Assembling Heterogeneous Au-Ag Nanoclusters with Highly Loading Performance and Biocompatible Capability
Abstract
:1. Introduction
2. Result and Discussion
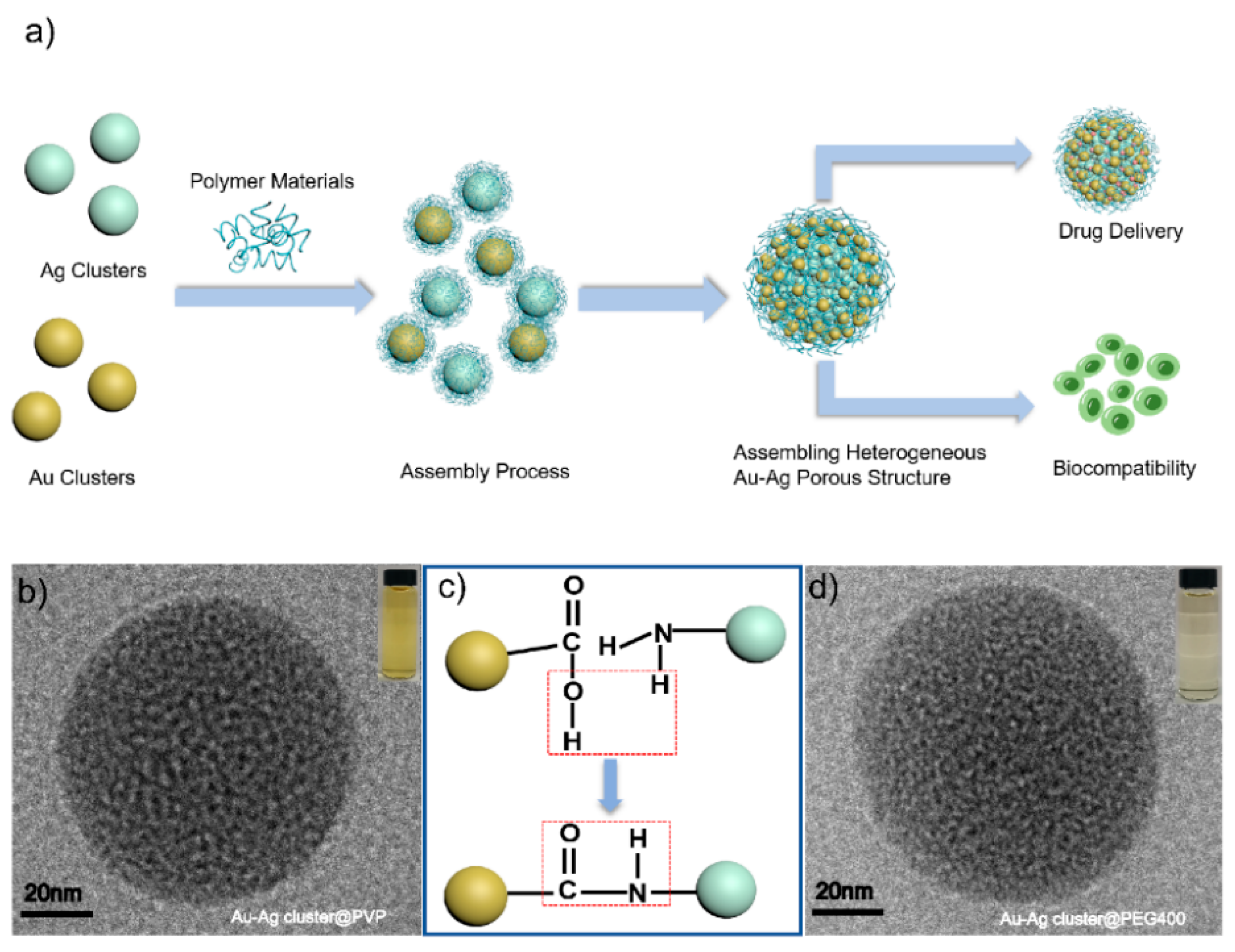
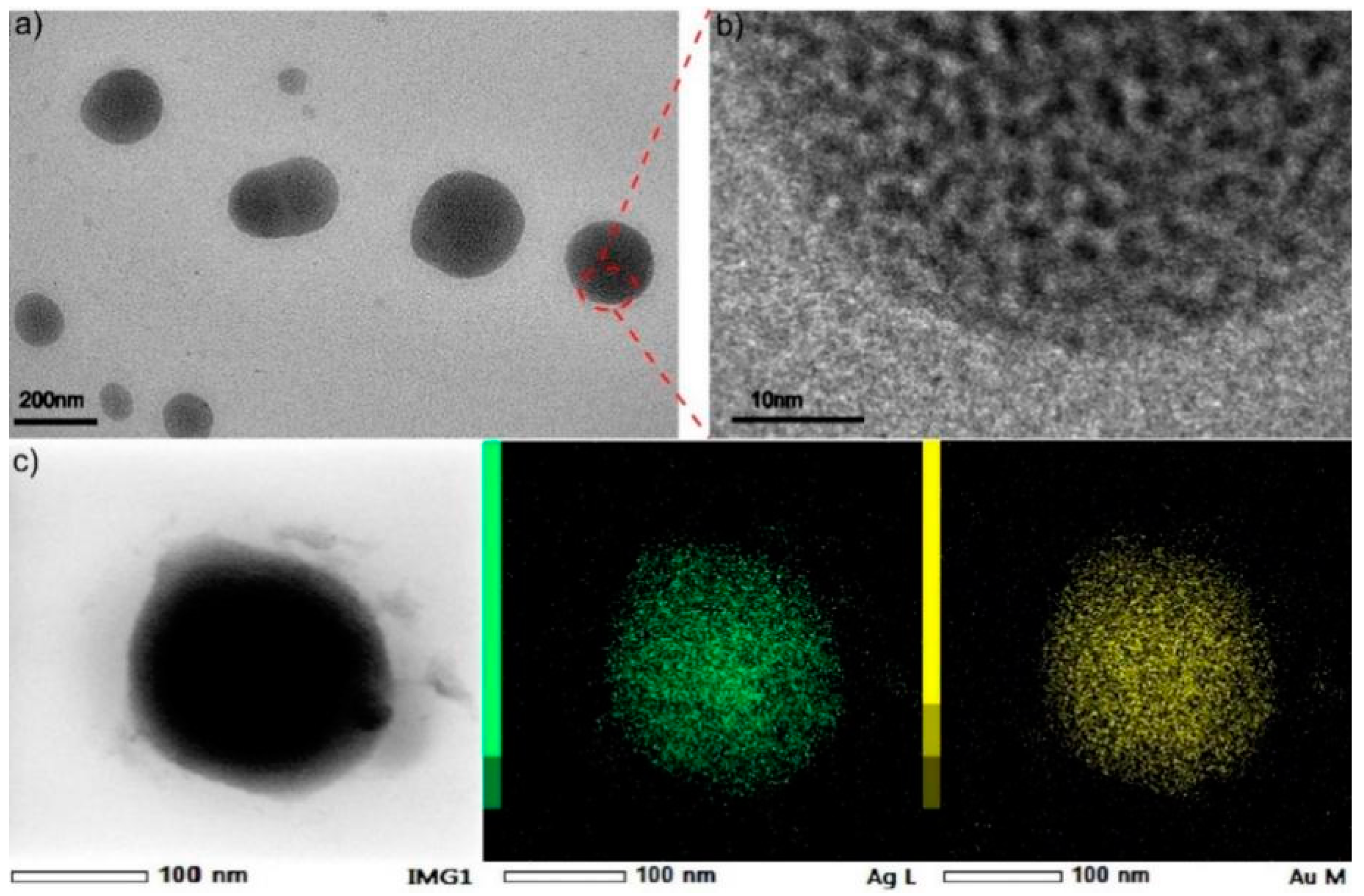
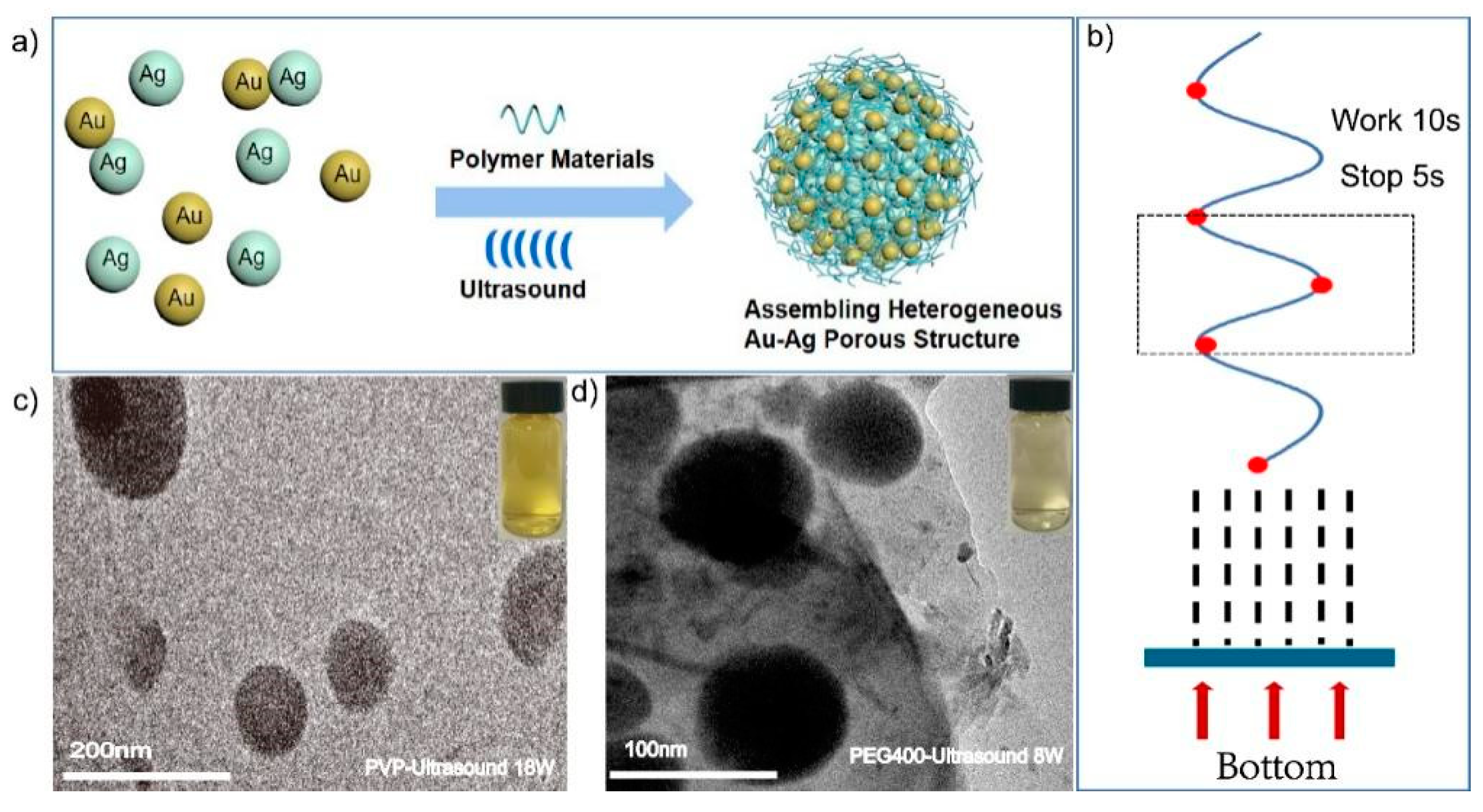
2.1. Synthesis and Characterization of Assembled Heterogeneous Au-Ag NCs
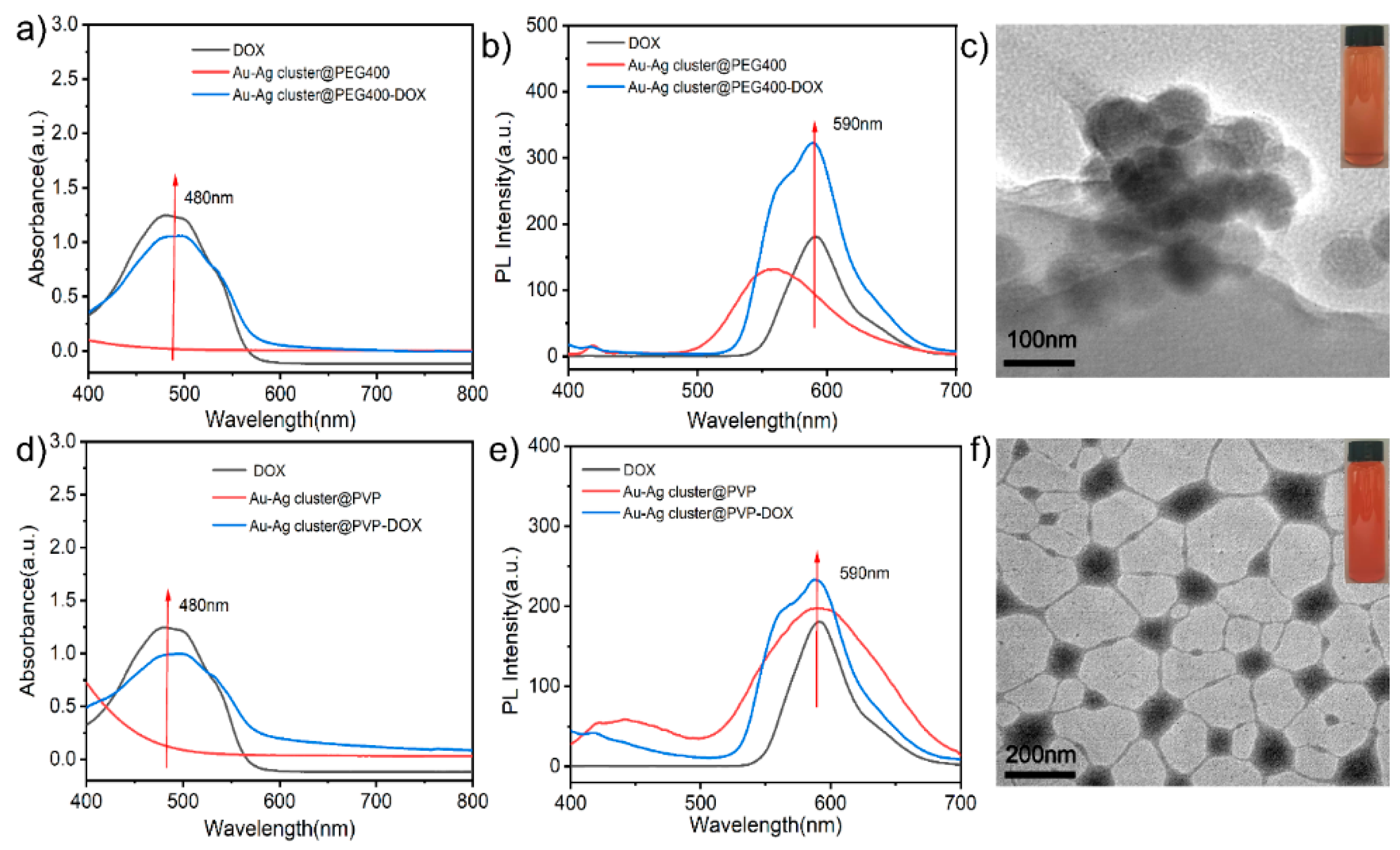
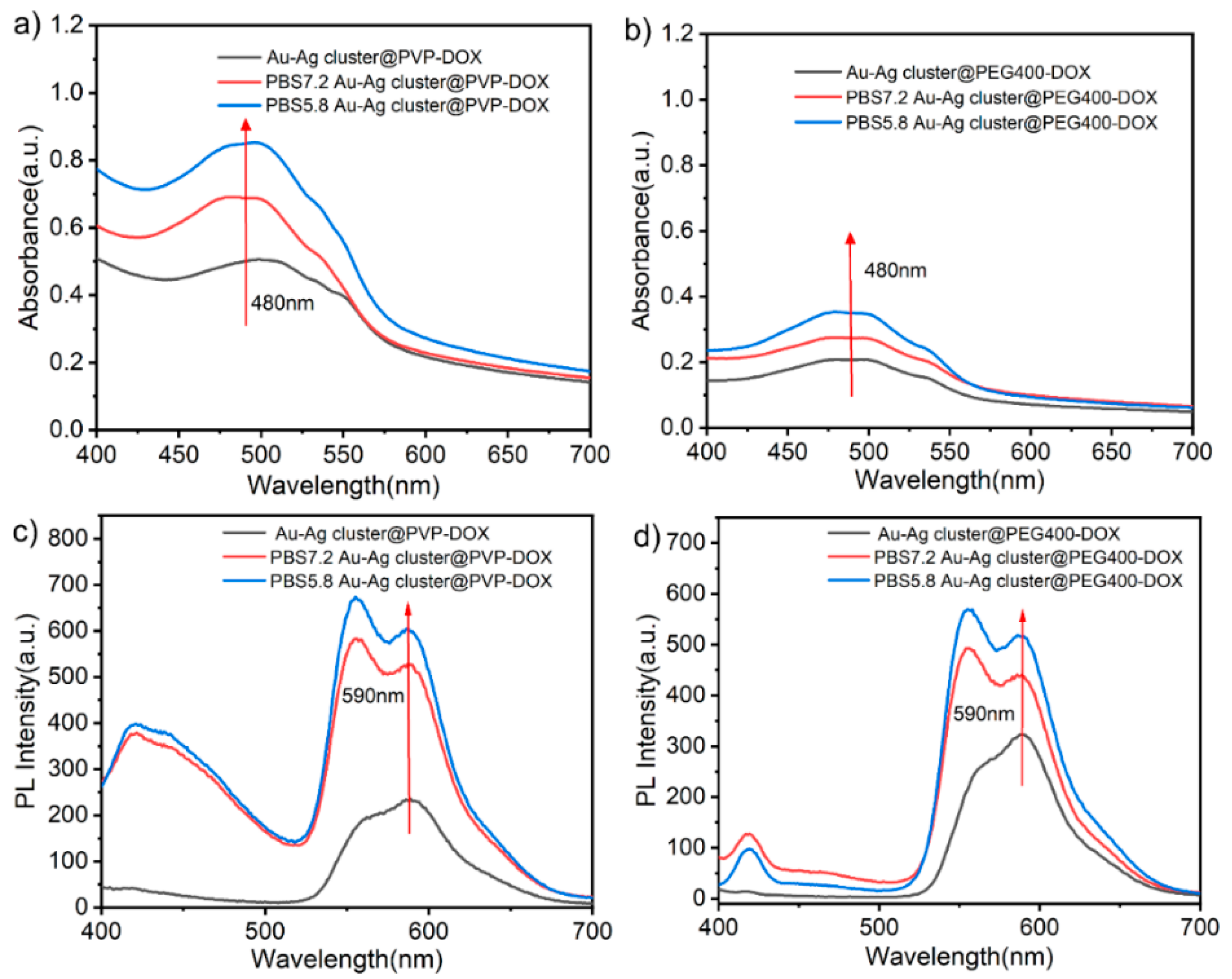
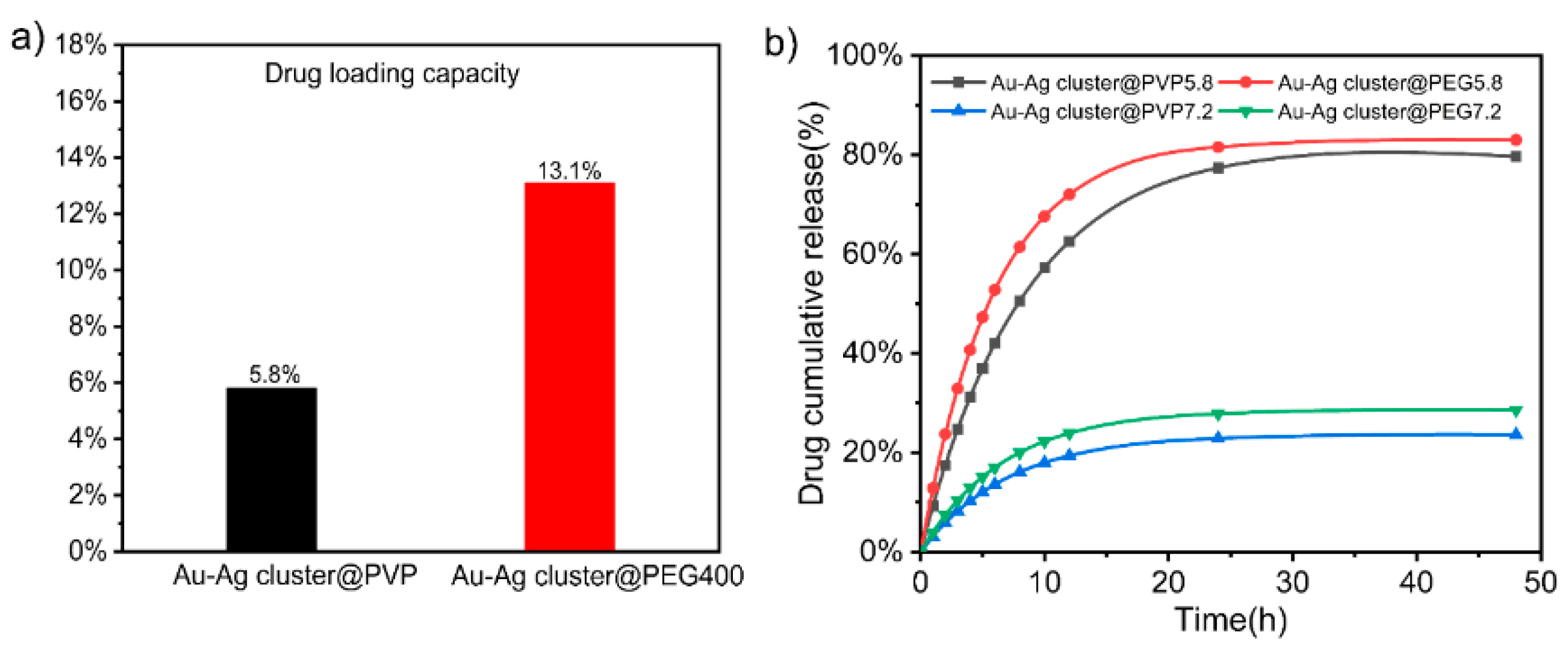
2.2. Drug Loading and Releasing Performance of Assembled Au-Ag NCs
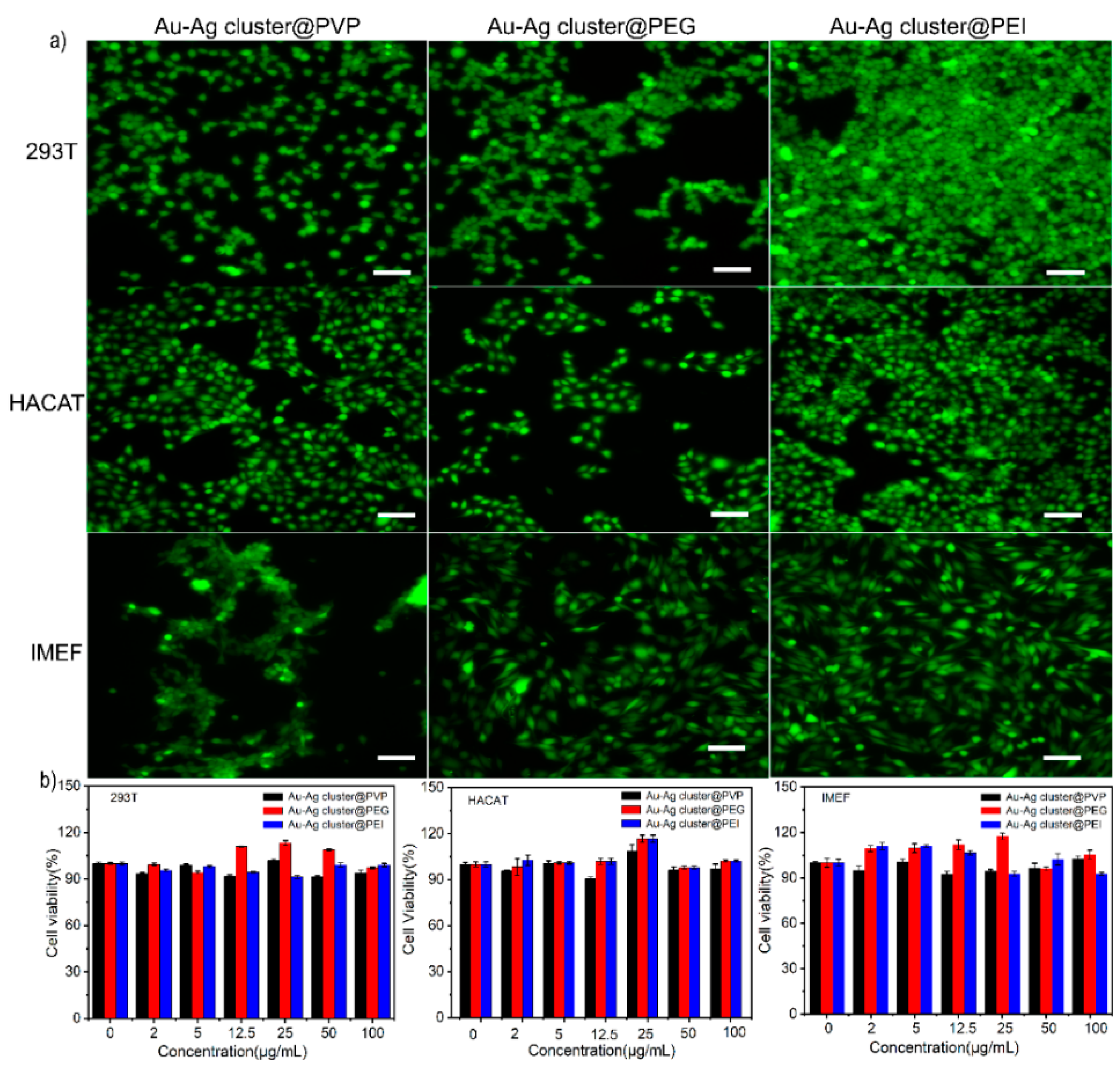
2.3. Biocompatibility of Assembled Heterogeneous Au-Ag NCs
2.4. Synthesis and Characterization of Ultrasonically Induced Assembly of Heterogeneous Au-Ag NCs
3. Materials and Methods
3.1. Materials
3.2. The Fabrication of Assembled Au-Ag NCs
3.2.1. Synthesis of Au and Ag Clusters
3.2.2. Synthesis of Assembling Heterogeneous Au-Ag NCs (Au-Ag Cluster @PVP, Au-Ag Cluster @PEG400 and Au-Ag Cluster @PEI)
3.3. Drug Loading and Releasing Characteristics Measurements
3.4. Biocompatibility Measurement and Related Experiments
3.5. Heterogeneous Au-Ag NCs via Assembly Process with Ultrasound Field Function
3.6. Characterization of Heterogeneous NCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Lin, L.; Sun, D.; Chen, H.; Yang, D.; Li, Q. Bio-inspired synthesis of metal nanomaterials and applications. Chem. Soc. Rev. 2015, 44, 6330–6374. [Google Scholar] [CrossRef]
- Sainio, S.; Leppänen, E.; Mynttinen, E.; Palomäki, T.; Wester, N.; Etula, J.; Isoaho, N.; Peltola, E.; Koehne, J.; Meyyappan, M.; et al. Integrating Carbon Nanomaterials with Metals for Bio-sensing Applications. Mol. Neurobiol. 2020, 57, 179–190. [Google Scholar] [CrossRef]
- Yan, Z.; Yuan, H.; Zhao, Q.; Xing, L.; Zheng, X.; Wang, W.; Zhao, Y.; Yu, Y.; Hu, L.; Yao, W. Recent developments of nanoenzyme-based colorimetric sensors for heavy metal detection and the interaction mechanism. Analyst 2020, 145, 3173–3187. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Park, B.; Padhi, D.K.; Ibrahim, I.A.M.; Kim, S.; Kim, K.H.; Lee, K.-S.; Lee, C.-L.; Han, J.W.; Oh, S.H.; et al. Disordered-Layer-Mediated Reverse Metal-Oxide Interactions for Enhanced Photocatalytic Water Splitting. Nano Lett. 2021, 21, 5247–5253. [Google Scholar] [CrossRef]
- Niemuth, N.J.; Curtis, B.J.; Hang, M.N.; Gallagher, M.J.; Fairbrother, D.H.; Hamers, R.J.; Klaper, R.D. Next-Generation Complex Metal Oxide Nanomaterials Negatively Impact Growth and Development in the Benthic Invertebrate Chironomus riparius upon Settling. Environ. Sci. Technol. 2019, 53, 3860–3870. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Z.; Xie, Y.; Jiang, X. Modulating the catalytic activity of gold nanoparticles using amine-terminated ligands. Chem. Sci. 2022, 13, 1080–1087. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Kutsevol, N.V.; Tomchuk, A.V.; Khort, P.S.; Virych, P.A.; Chumachenko, V.A.; Kuziv, Y.I.; Naumenko, A.P.; Marinin, A.I. Plasmonic enhancement of the antibacterial photodynamic efficiency of a zinc tetraphenylporphyrin photosensitizer/dextran polyacrylamide anionic copolymer/Au nanoparticles hybrid nanosystem. RSC Adv. 2021, 12, 11–23. [Google Scholar] [CrossRef]
- El-Gendi, A.; Ghanem, A.F.; Yassin, M.A.; Abdel Rehim, M.H. Antifouling and antimicrobial polyethersulfone/hyperbranched polyester-amide/Ag composite. RSC Adv. 2020, 10, 24169–24175. [Google Scholar] [CrossRef]
- Chen, J.; Gong, M.; Fan, Y.; Feng, J.; Han, L.; Xin, H.L.; Cao, M.; Zhang, Q.; Zhang, D.; Lei, D.; et al. Collective Plasmon Coupling in Gold Nanoparticle Clusters for Highly Efficient Photothermal Therapy. ACS Nano 2022, 16, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Suciu, M.; Mirescu, C.; Crăciunescu, I.; Macavei, S.G.; Leoștean, C.; Ştefan, R.; Olar, L.E.; Tripon, S.-C.; Ciorîță, A.; Barbu-Tudoran, L. In Vivo Distribution of Poly(ethylene glycol) Functionalized Iron Oxide Nanoclusters: An Ultrastructural Study. Nanomaterials 2021, 11, 2184. [Google Scholar] [CrossRef]
- Fereja, S.L.; Fang, Z.; Li, P.; Guo, J.; Fereja, T.H.; Chen, W. “Turn-off” sensing probe based on fluorescent gold nanoclusters for the sensitive detection of hemin. Anal. Bioanal. Chem. 2021, 413, 1639–1649. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Miyasaka, T.; Ando, N. Application of noble metal cluster to PMMA resin and influence on mechanical properties and color. Dent. Mater. J. 2015, 34, 781–788. [Google Scholar] [CrossRef]
- Chen, T.; Lin, H.; Cao, Y.; Yao, Q.; Xie, J. Interactions of Metal Nanoclusters with Light: Fundamentals and Applications. Adv. Mater. 2021, 34, e2103918. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, H.; Li, T.; Liu, J.; Yin, J.; Mohammed, O.F.; Bakr, O.M.; Liu, Y.; Yang, B.; Zhang, H. Contribution of Metal Defects in the Assembly Induced Emission of Cu Nanoclusters. J. Am. Chem. Soc. 2017, 139, 4318–4321. [Google Scholar] [CrossRef]
- Wang, J.; Lin, X.; Shu, T.; Su, L.; Liang, F.; Zhang, X. Self-Assembly of Metal Nanoclusters for Aggregation-Induced Emission. Int. J. Mol. Sci. 2019, 20, 1891. [Google Scholar] [CrossRef]
- Yao, Q.; Yuan, X.; Yu, Y.; Yu, Y.; Xie, J.; Lee, J.Y. Introducing amphiphilicity to noble metal nanoclusters via phase-transfer driven ion-pairing reaction. J. Am. Chem. Soc. 2015, 137, 2128–2136. [Google Scholar] [CrossRef]
- Yao, Q.; Luo, Z.; Yuan, X.; Yu, Y.; Zhang, C.; Xie, J.; Lee, J.Y. Assembly of nanoions via electrostatic interactions: Ion-like behavior of charged noble metal nanoclusters. Sci. Rep. 2014, 4, 3848. [Google Scholar] [CrossRef]
- Fernández-Ujados, M.; Trapiella-Alfonso, L.; Costa-Fernández, J.M.; Pereiro, R.; Sanz-Medel, A. One-step aqueous synthesis of fluorescent copper nanoclusters by direct metal reduction. Nanotechnology 2013, 24, 495601. [Google Scholar] [CrossRef]
- Shen, H.; Deng, G.; Kaappa, S.; Tan, T.; Han, Y.-Z.; Malola, S.; Lin, S.-C.; Teo, B.K.; Häkkinen, H.; Zheng, N. Highly Robust but Surface-Active: An N-Heterocyclic Carbene-Stabilized Au Nanocluster. Angew. Chem. 2019, 58, 17731–17735. [Google Scholar] [CrossRef]
- Richtsmeier, S.C.; Parks, E.K.; Liu, K.; Pobo, L.G.; Riley, S.J. Gas phase reactions of iron clusters with hydrogen. I. Kinetics. J. Chem. Sci. 1985, 82, 3659–3665. [Google Scholar] [CrossRef]
- Fayet, P.; Granzer, F.; Hegenbart, G.; Moisar, E.; Pischel, B.; Woste, L. Latent-image generation by deposition of monodisperse silver clusters. Phys. Rev. Lett. 1985, 55, 3002–3004. [Google Scholar] [CrossRef]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, T.; Kong, C.; Hao, M.; Wang, Y.; Li, J.; Liu, B.; Gao, Y.; Jiang, J. Photothermal exposure of polydopamine-coated branched Au-Ag nanoparticles induces cell cycle arrest, apoptosis, and autophagy in human bladder cancer cells. Int. J. Nanomed. 2018, 13, 6413–6428. [Google Scholar] [CrossRef]
- Miao, Z.; Chen, S.; Xu, C.Y.; Ma, Y.; Qian, H.; Xu, Y.; Chen, H.; Wang, X.; He, G.; Lu, Y.; et al. PEGylated rhenium nanoclusters: A degradable metal photothermal nanoagent for cancer therapy. Chem. Sci. 2019, 10, 5435–5443. [Google Scholar] [CrossRef]
- Gu, W.; Zhang, Q.; Zhang, T.; Li, Y.; Xiang, J.; Peng, R.; Liu, J. Hybrid polymeric nano-capsules loaded with gold nanoclusters and indocyanine green for dual-modal imaging and photothermal therapy. J. Mater. Chem. B 2016, 4, 910–919. [Google Scholar] [CrossRef]
- Katla, S.K.; Zhang, J.; Castro, E.; Bernal, R.A.; Li, X. Atomically Precise Au25(SG)18 Nanoclusters: Rapid Single-Step Synthesis and Application in Photothermal Therapy. ACS. Appl. Mater. Interfaces 2018, 10, 75–82. [Google Scholar] [CrossRef]
- Su, X.; Liu, J. pH-Guided Self-Assembly of Copper Nanoclusters with Aggregation-Induced Emission. ACS. Appl. Mater. Interfaces 2017, 9, 3902–3910. [Google Scholar] [CrossRef]
- Li, D.; Chen, Z.; Mei, X. Fluorescence enhancement for noble metal nanoclusters. Adv. Colloid Interface Sci. 2017, 250, 25–39. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, H.; Zhang, S.; Xu, J. The synthesis of metal nanoclusters and their applications in bio-sensing and imaging. Methods Appl. Fluoresc. 2019, 8, 012001. [Google Scholar] [CrossRef]
- Triulzi, R.C.; Micic, M.; Giordani, S.; Serry, M.; Chiou, W.A.; Leblanc, R.M. Immunoasssay based on the antibody-conjugated PAMAM-dendrimer-gold quantum dot complex. Chem. Commun. 2006, 48, 5068–5070. [Google Scholar] [CrossRef]
- Han, C.; Guo, W. Fluorescent Noble Metal Nanoclusters Loaded Protein Hydrogel Exhibiting Anti-Biofouling and Self-Healing Properties for Electrochemiluminescence Biosensing Applications. Small 2020, 16, e2002621. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, Z.; Liu, Z.; Lin, Y.; Yuan, X.; Xie, J. Molecular reactivity of thiolate-protected noble metal nanoclusters: Synthesis, self-assembly, and applications. Chem. Sci. 2020, 12, 99–127. [Google Scholar] [CrossRef]
- Kang, X.; Li, Y.; Zhu, M.; Jin, R. Atomically precise alloy nanoclusters: Syntheses, structures, and properties. Chem. Soc. Rev. 2020, 49, 6443–6514. [Google Scholar] [CrossRef]
- Kwak, K.; Lee, D. Electrochemistry of Atomically Precise Metal Nanoclusters. Acc. Chem. Res. 2019, 52, 12–22. [Google Scholar] [CrossRef]
- Huang, F.; You, M.; Chen, T.; Zhu, G.; Liang, H.; Tan, W. Self-assembled hybrid nanoparticles for targeted co-delivery of two drugs into cancer cells. Chem. Commun. 2014, 50, 3103–3105. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, Z.; Li, T.; Wang, H.; Liu, Y.; Stein, H.S.; Mao, Y.; Gao, J.; Jiao, M.; Dong, Q.; et al. High-throughput, combinatorial synthesis of multimetallic nanoclusters. Proc. Natl. Acad. Sci. USA 2020, 117, 6316–6322. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, E. Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today 2014, 9, 132–157. [Google Scholar] [CrossRef]
- Mirzaei-Kalar, Z.; Yavari, A.; Jouyban, A. Increasing DNA binding affinity of doxorubicin by loading on Fe3O4 nanoparticles: A multi-spectroscopic study. Spectrochim. Acta Part A 2020, 229, 117985. [Google Scholar] [CrossRef]
- Fang, X.; Lui, K.H.; Li, S.; Lo, W.S.; Li, X.; Gu, Y.; Wong, W.T. Multifunctional Nanotheranostic Gold Nanocage/Selenium Core-Shell for PAI-Guided Chemo-Photothermal Synergistic Therapy In vivo. Int. J. Nanomed. 2020, 15, 10271–10284. [Google Scholar] [CrossRef]
- Guo, Q.; Kuang, L.; Cao, H.; Li, W.; Wei, J. Self-assembled mPEG-PCL-g-PEI micelles for multifunctional nanoprobes of doxorubicin delivery and magnetic resonance imaging and optical imaging. Colloids Surf. B 2015, 136, 687–693. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial Gold Nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Liu, L.; Bai, Q.; Sui, N.; Zhu, Z. Self-assembled ultrasmall silver nanoclusters on liposome for topical antimicrobial delivery. Colloids Surf. B 2021, 200, 111618. [Google Scholar] [CrossRef]
- Chen, Y.; Phipps, M.L.; Werner, J.H.; Chakraborty, S.; Martinez, J.S. DNA Templated Metal Nanoclusters: From Emergent Properties to Unique Applications. Acc. Chem. Res. 2018, 51, 2756–2763. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Peng, Y.; Shi, Z.; Zhang, D.; Jia, Q. Copper nanocluster composites for analytical (bio)-sensing and imaging: A review. Mikrochim. Acta 2021, 188, 384. [Google Scholar] [CrossRef]
- Liu, B.; Jin, L.; Zhong, W.; Lopes, A.; Suib, S.L.; He, J. Ultrafine and Ligand-Free Precious Metal (Ru, Ag, Au, Rh and Pd) Nanoclusters Supported on Phosphorus-Doped Carbon. Chemistry 2018, 24, 2565–2569. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhu, L.; Guo, J.; Zhang, L.; Shi, Y.; Zhang, Y.; Hou, K.; Zheng, Y.; Zhu, Y.; Lv, J.; et al. Self-Assembly of Chiral Gold Clusters into Crystalline Nanocubes of Exceptional Optical Activity. Angew. Chem. Int. Ed. Engl. 2017, 56, 15397–15401. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Q.; Wang, Z.; Xu, C.; Han, H.; Li, A.; Ma, G.; Li, J.; Lu, C.; Chen, H.; et al. Qualitative and Quantitative Analysis of Tumor Cell Invasion Using Au Clusters. Nanomaterials 2021, 12, 145. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, H.C. Simultaneous synthesis and assembly of noble metal nanoclusters with variable micellar templates. J. Am. Chem. Soc. 2014, 136, 13805–13817. [Google Scholar] [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef]
- Yahia-Ammar, A.; Sierra, D.; Merola, F.; Hildebrandt, N.; Le Guevel, X. Self-Assembled Gold Nanoclusters for Bright Fluorescence Imaging and Enhanced Drug Delivery. ACS Nano 2016, 10, 2591–2599. [Google Scholar] [CrossRef]
- Chakraborty, A.; Fernandez, A.C.; Som, A.; Mondal, B.; Natarajan, G.; Paramasivam, G.; Lahtinen, T.; Hakkinen, H.; Nonappa; Pradeep, T. Atomically Precise Nanocluster Assemblies Encapsulating Plasmonic Gold Nanorods. Angew. Chem. Int. Ed. Engl. 2018, 57, 6522–6526. [Google Scholar] [CrossRef]
- Yonesato, K.; Ito, H.; Itakura, H.; Yokogawa, D.; Kikuchi, T.; Mizuno, N.; Yamaguchi, K.; Suzuki, K. Controlled Assembly Synthesis of Atomically Precise Ultrastable Silver Nanoclusters with Polyoxometalates. J. Am. Chem. Soc. 2019, 141, 19550–19554. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, Z.; Sun, D.; Bai, H.; Zhu, Z.; Bi, Y.; Zhao, T.; Xin, X. pH-guided self-assembly of silver nanoclusters with aggregation-induced emission for rewritable fluorescent platform and white light emitting diode application. J. Colloid Interface Sci. 2020, 567, 235–242. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Dong, S.; Deng, Y.; Hao, J. Nanocapsules of Magnetic Au Self-Assembly for DNA Migration and Secondary Self-Assembly. ACS Appl. Mater. Interfaces 2018, 10, 5348–5357. [Google Scholar] [CrossRef]
- Bu, X.; Fu, Y.; Jiang, X.; Jin, H.; Gui, R. Self-assembly of DNA-templated copper nanoclusters and carbon dots for ratiometric fluorometric and visual determination of arginine and acetaminophen with a logic-gate operation. Microchim. Acta 2020, 187, 154. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, D.; Ning, X.; Zhang, D.; Zhang, S.; Zhang, Z.; Shan, D.; Wang, Z.; Liu, D.; Mao, X.; et al. Self-Assembly of Biocompatible FeSe Hollow Nanostructures and 2D CuFeSe Nanosheets with One- and Two-Photon Luminescence Properties. Small 2019, 15, e1900627. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, X.; Song, Q.; Yin, Z. A facile route to the synthesis copper oxide/reduced graphene oxide nanocomposites and electrochemical detection of catechol organic pollutant. CrystEngComm 2012, 14, 6710–6719. [Google Scholar] [CrossRef]
- Chen, T.; Gu, T.; Cheng, L.; Li, X.; Han, G.; Liu, Z. Porous Pt nanoparticles loaded with doxorubicin to enable synergistic Chemo-/Electrodynamic Therapy. Biomaterials 2020, 255, 120202. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Dun, Y.; Xie, L.; Jiang, W.; Sun, X.; Hu, P.; Zheng, S.; Yu, Y. Preparation of doxorubicin-loaded porous iron Oxide@ polydopamine nanocomposites for MR imaging and synergistic photothermal-chemotherapy of cancer. Colloids Surf. B 2021, 208, 112107. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Kasprzak, A.; Poplawska, M.; Ruzycka, M.; Grudzinski, I.P.; Nowicka, A.M. Controlled Drug Release and Cytotoxicity Studies of Beta-Lapachone and Doxorubicin Loaded into Cyclodextrins Attached to a Polyethyleneimine Matrix. Int. J. Mol. Sci. 2020, 21, 5832. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, A.; James, H.P.; Jadhav, S. PEG-grafted phospholipids in vesicles: Effect of PEG chain length and concentration on mechanical properties. Chem. Phys. Lipids 2019, 218, 47–56. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.; Gu, X.; Gong, H.; Cheng, L.; Shi, X.; Feng, L.; Sun, B.; Liu, Z. Drug delivery with PEGylated MoS2 nano-sheets for combined photothermal and chemotherapy of cancer. Adv. Mater. 2014, 26, 3433–3440. [Google Scholar] [CrossRef] [PubMed]
- Alijagic, A.; Barbero, F.; Gaglio, D.; Napodano, E.; Benada, O.; Kofronova, O.; Puntes, V.F.; Bastus, N.G.; Pinsino, A. Gold nanoparticles coated with polyvinylpyrrolidone and sea urchin extracellular molecules induce transient immune activation. J. Hazard. Mater. 2021, 402, 123793. [Google Scholar] [CrossRef]
- Rahme, K.; Dagher, N. Chemistry Routes for Copolymer Synthesis Containing PEG for Targeting, Imaging, and Drug Delivery Purposes. Pharmaceutics 2019, 11, 327. [Google Scholar] [CrossRef]
- Jia, X.; Li, J.; Zhang, X.; Wang, E. Controlling the synthesis and assembly of fluorescent Au/Ag alloy nanoclusters. Chem Commun. 2015, 51, 17417–17419. [Google Scholar] [CrossRef]
- Fuentes-Garcia, J.A.; Santoyo-Salzar, J.; Rangel-Cortes, E.; Goya, G.F.; Cardozo-Mata, V.; Pescador-Rojas, J.A. Effect of ultrasonic irradiation power on sonochemical synthesis of gold nanoparticles. Ultrason. Sonochem. 2021, 70, 105274. [Google Scholar] [CrossRef]
- Wang, S.; Yang, B.; Zhang, Z.; Xu, X.; Li, H.; Cheng, G.; Yang, Z.; Du, H.; Yang, Y.; Yang, X. Au nanoclusters/porous silica particles nanocomposites as fluorescence enhanced sensors for sensing and mapping of copper(II) in cells. Nanotechnology 2019, 30, 475701. [Google Scholar] [CrossRef]
- Kumar, S.; Bolan, M.D.; Bigioni, T.P. Glutathione-stabilized magic-number silver cluster compounds. J. Am. Chem. Soc. 2010, 132, 13141–13143. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Ma, X.; Yang, Y.; Hu, X.; Wang, T.; Chen, S.; Mao, X. Metal Cluster Triggered-Assembling Heterogeneous Au-Ag Nanoclusters with Highly Loading Performance and Biocompatible Capability. Int. J. Mol. Sci. 2022, 23, 11197. https://doi.org/10.3390/ijms231911197
He X, Ma X, Yang Y, Hu X, Wang T, Chen S, Mao X. Metal Cluster Triggered-Assembling Heterogeneous Au-Ag Nanoclusters with Highly Loading Performance and Biocompatible Capability. International Journal of Molecular Sciences. 2022; 23(19):11197. https://doi.org/10.3390/ijms231911197
Chicago/Turabian StyleHe, Xiaoxiao, Xiaohong Ma, Yujun Yang, Xi Hu, Teng Wang, Shiyue Chen, and Xiang Mao. 2022. "Metal Cluster Triggered-Assembling Heterogeneous Au-Ag Nanoclusters with Highly Loading Performance and Biocompatible Capability" International Journal of Molecular Sciences 23, no. 19: 11197. https://doi.org/10.3390/ijms231911197
APA StyleHe, X., Ma, X., Yang, Y., Hu, X., Wang, T., Chen, S., & Mao, X. (2022). Metal Cluster Triggered-Assembling Heterogeneous Au-Ag Nanoclusters with Highly Loading Performance and Biocompatible Capability. International Journal of Molecular Sciences, 23(19), 11197. https://doi.org/10.3390/ijms231911197



