The Utilization of Physiologically Active Molecular Components of Grape Seeds and Grape Marc
Abstract
1. Introduction
Polyphenols
2. Review Methodology
3. Basic Physical and Chemical Properties of Polyphenols
3.1. Physical Properties
3.2. Chemical and Biochemical Properties of Polyphenols
3.3. Analysis of Polyphenols
4. The Beneficial Effects of Polyphenols on Health and Its Molecular Mechanisms
4.1. Antioxidant and Free Radical Scavenging Activity
4.2. Anti-Atherosclerosis and Cardioprotective Effects
4.3. Neuroprotective Effects
4.4. Anti-Inflammatory Effect
4.5. Mutation Reduction and Anti-Cancer Effect
4.6. Influencing Signal Transduction
4.7. Effects on the Vascular Wall and Choroidal Cells
4.8. Effects on Diabetes
4.9. Effects on the Cell Cycle
4.10. Other Impacts
4.10.1. Anti-Caries Effect
4.10.2. Antihyperlipidemic Effect
4.10.3. Antibacterial and Antifungal Effect
4.10.4. Anti-HIV Effect
4.10.5. Sensory Effect
4.10.6. Hepatoprotective Effect
4.11. Anti-SARS-CoV-2 Effect
4.12. Risks Associated with Polyphenols
5. In Vivo Investigations of Grape Seed Extract and Its Components
6. Clinical Studies of Grape Seed Extracts
7. Grapeseed Oil and Polyphenols
7.1. The Composition of Grape Seeds
7.2. Location of Polyphenols in Grape Seed Cells
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Extraction of Polyphenols from Grape Seeds
Appendix A.1. Oxidative Polymerisation of Polyphenols during Separation
Appendix A.1.1. Oxidative Polymerization of Polyphenols Can Occur
Appendix A.1.2. Depolymerization of Polyphenols Can Also Occur
- spontaneously, e.g., in acid-butanol [181]. Under highly acidic conditions, proanthocyanidins are converted to anthocyanidins by cleavage of the C-C interflavanil bond;
- and may also degrade enzymatically during storage [182]. If they are composed of only catechol and epicatechol subunits, the products of hydrolysis are only cyanidins, then proanthocyanidins are called procyanidins. Procyanidins are the most abundant proanthocyanidins in plant-derived food [147,183].
Appendix A.2. Extraction of Grape Seed Oil from Grape Seed Flour
- (a)
- extraction Grape seed oil is obtained from grapeseed flour by extraction with petroleum oil at 60–70 °C for 6 h. De-oiled flour can be obtained by removing the residual oil (acetone: water: acetic acid, followed by methanol: water: acetic acid extraction at 90:9.5:0.5 for 8 h) [123].
- (b)
- pressing at 60–68 °C.
Appendix A.2.1. Direct Extraction of Polyphenols (CO2, Ethanol)
Appendix A.2.2. Direct Extraction of Vitamin E from Grape Seed Flour
Appendix A.3. Extraction of Polyphenols from Grape Seed Oil
Appendix A.3.1. Preliminary Removal of the Carboxylic Acid Fraction
Appendix A.3.2. Separation of Polyphenols from Grape Seed Oil
Appendix A.3.3. Enzymatic Pretreatment Effect
Appendix A.4. Methods for the Determination of Polyphenol Content
| Seed | Peel | |||
|---|---|---|---|---|
| Catechin | Epicatechin | Resveratrol | Rutin | Quercitine |
| 60–205 mg/100 g | 47–205 mg/100 g | 0.6–25 mg/100 g | 41–169 mg/100 g | 0–1.07 mg/100 g |
Appendix A.5. Alternative Polyphenol Sources
| Grape Seeds | Grape Seed Flour after Pressing | |
|---|---|---|
| Catechin | 31.5% | 47.0% |
| Procyanidin B1 | 14.0% | 15.4% |
| Procyanidin B2 | 18.5% | 10.5% |
| Epicatechin | 22.4% | 24.9% |
| Epicatechin gallate | 13.4% | 1.9% |
| Quercetin 3-O-glucuronide | 0.2% | 0.3% |
References
- Troilo, M.; Difonzo, G.; Paradiso, V.; Summo, C.; Caponio, F. Bioactive Compounds from Vine Shoots, Grape Stalks, and Wine Lees: Their Potential Use in Agro-Food Chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Ghendov-Mosanu, A.; Cojocari, D.; Balan, G.; Patras, A.; Lung, I.; Soran, M.-L.; Opriş, O.; Cristea, E.; Sturza, R. Chemometric Optimization of Biologically Active Compounds Extraction from Grape Marc: Composition and Antimicrobial Activity. Molecules 2022, 27, 1610. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mejía, E.; Roriz, C.L.; Heleno, S.A.; Calhelha, R.; Dias, M.I.; Pinela, J.; Rosales-Conrado, N.; León-González, M.E.; Ferreira, I.C.; Barros, L. Valorisation of black mulberry and grape seeds: Chemical characterization and bioactive potential. Food Chem. 2020, 337, 127998. [Google Scholar] [CrossRef]
- Jin, Q.; Neilson, A.P.; Stewart, A.C.; O’Keefe, S.F.; Kim, Y.-T.; McGuire, M.; Wilder, G.; Huang, H. Integrated Approach for the Valorization of Red Grape Pomace: Production of Oil, Polyphenols, and Acetone–Butanol–Ethanol. ACS Sustain. Chem. Eng. 2018, 6, 16279–16286. [Google Scholar] [CrossRef]
- Khan, N.; Fahad, S.; Naushad, M.; Faisal, S. Grape production critical review in the world. SSRN Electron. J. 2020. Available online: http://dx.doi.org/10.2139/ssrn.359584 (accessed on 24 July 2022).
- Modesti, M.; Macaluso, M.; Taglieri, I.; Bellincontro, A.; Sanmartin, C. Ozone and Bioactive Compounds in Grapes and Wine. Foods 2021, 10, 2934. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Rajasekar, N.; Sivanantham, A.; Ravikumar, V.; Rajasekaran, S. An overview on the role of plant-derived tannins for the treatment of lung cancer. Phytochemistry 2021, 188, 112799. [Google Scholar] [CrossRef]
- Noce, A.; Di Daniele, F.; Campo, M.; Di Lauro, M.; Zaitseva, A.P.; Di Daniele, N.; Marrone, G.; Romani, A. Effect of Hydrolysable Tannins and Anthocyanins on Recurrent Urinary Tract Infections in Nephropathic Patients: Preliminary Data. Nutrients 2021, 13, 591. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Shu, F.; Jiang, B.; Yuan, Y.; Li, M.; Wu, W.; Jin, Y.; Xiao, H. Biological Activities and Emerging Roles of Lignin and Lignin-Based Products—A Review. Biomacromolecules 2021, 22, 4905–4918. [Google Scholar] [CrossRef]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How far is Lignin from being a biomedical material? Bioact. Mater. 2022, 8, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Chou, I.-W.; Hung, M.-C. Natural tannins as anti-SARS-CoV-2 compounds. Int. J. Biol. Sci. 2022, 18, 3818–3826. [Google Scholar] [CrossRef] [PubMed]
- Canon, F.; Caillé, S.; Sarni-Manchado, P.; Cheynier, V. Wine taste and mouthfeel. In Managing Wine Quality, 2nd ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing Ltd.: Cambridge, UK, 2022; pp. 41–95. [Google Scholar]
- Di Stefano, V.; Buzzanca, C.; Melilli, M.G.; Indelicato, S.; Mauro, M.; Vazzana, M.; Arizza, V.; Lucarini, M.; Durazzo, A.; Bongiorno, D. Polyphenol Characterization and Antioxidant Activity of Grape Seeds and Skins from Sicily: A Preliminary Study. Sustainability 2022, 14, 6702. [Google Scholar] [CrossRef]
- El Kersh, D.M.; Hammad, G.; Donia, M.S.; Farag, M.A. A Comprehensive Review on Grape Juice Beverage in Context to Its Processing and Composition with Future Perspectives to Maximize Its Value. Food Bioprocess Technol. 2022, 1–23. [Google Scholar] [CrossRef]
- Garrido-Bañuelos, G.; Buica, A.; du Toit, W. Relationship between anthocyanins, proanthocyanidins, and cell wall polysaccharides in grapes and red wines. A current state-of-art review. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Padilla-González, G.F.; Grosskopf, E.; Sadgrove, N.J.; Simmonds, M.S.J. Chemical Diversity of Flavan-3-Ols in Grape Seeds: Modulating Factors and Quality Requirements. Plants 2022, 11, 809. [Google Scholar] [CrossRef]
- Kandaswami, C.; Middleton, E., Jr. Free Radical Scavenging and Antioxidant Activity of Plant Flavonoids. Adv. Exp. Med. Biol. 1994, 366, 351–376. [Google Scholar]
- Esparza, I.; Cimminelli, M.J.; Moler, J.A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Stability of Phenolic Compounds in Grape Stem Extracts. Antioxidants 2020, 9, 720. [Google Scholar] [CrossRef]
- Adrar, N.S.; Madani, K.; Adrar, S. Impact of the inhibition of proteins activities and the chemical aspect of polyphenols-proteins interactions. PharmaNutrition 2019, 7, 100142. [Google Scholar] [CrossRef]
- Bódi, Z. Genetic Polymorphism, Heraldic Elements and Some Qualitative Traits in Maize Genotypes; University of Debrecen: Debrecen, Hungary, 2007. [Google Scholar]
- Balga, I.; Kiss, A.; Gál, L.; Leskó, A.; Kállay, M. Evaluating the correlation between chemical and sensory compounds in Blaufränkisch and Cabernet Franc wines. Wine Stud. 2014, 3, 16–18. [Google Scholar] [CrossRef]
- Pompei, C.; Peri, C. Determination of catechins in wines. VITIS J. Grapevine Res. 2017, 9, 312. [Google Scholar]
- Kállay, M.; Torok, Z. Determination of Resveratrol Isomers in Hungarian Wines. Hortic. Sci. Kerteszeti Tudomany 1997, 29, 78–82. [Google Scholar]
- Bélafi-Bakó, K.; Boór, A. Concentration of Cornelian cherry fruit juice by membrane osmotic distillation. Desalin. Water Treat. 2011, 35, 271–274. [Google Scholar] [CrossRef]
- Silva, T.M.S.; Camara, C.A.; da Silva Lins, A.C.; Barbosa-Filho, J.M.; da Silva, E.M.S.; Freitas, B.M.; de Assis Ribeiro dos Santos, F. Chemical composition and free radical scavenging activity of pollen loads from stingless bee Melipona subnitida Ducke. J. Food Compos. Anal. 2006, 19, 507–511. [Google Scholar] [CrossRef]
- Szabo, L.; Molnar, R.; Tomesz, A.; Deutsch, A.; Darago, R.; Nowrasteh, G.; Varjas, T.; Nemeth, B.; Budan, F.; Kiss, I. The effects of flavonoids, green tea polyphenols and coffee on DMBA induced LINE-1 DNA hypomethylation. PLoS ONE 2021, 16, e0250157. [Google Scholar] [CrossRef]
- Molnar, R.; Szabo, L.; Tomesz, A.; Deutsch, A.; Darago, R.; Ghodratollah, N.; Varjas, T.; Nemeth, B.; Budan, F.; Kiss, I. In vivo effects of olive oil and trans-fatty acids on miR-134, miR-132, miR-124-1, miR-9-3 and mTORC1 gene expression in a DMBA-treated mouse model. PLoS ONE 2021, 16, e0246022. [Google Scholar] [CrossRef]
- Molnar, R.; Szabo, L.; Tomesz, A.; Deutsch, A.; Darago, R.; Raposa, B.L.; Ghodratollah, N.; Varjas, T.; Nemeth, B.; Orsos, Z.; et al. The Chemopreventive Effects of Polyphenols and Coffee, Based upon a DMBA Mouse Model with microRNA and mTOR Gene Expression Biomarkers. Cells 2022, 11, 1300. [Google Scholar] [CrossRef]
- Szabo, L.; Molnar, R.; Tomesz, A.; Deutsch, A.; Darago, R.; Varjas, T.; Ritter, Z.; Szentpeteri, J.L.; Andreidesz, K.; Mathe, D.; et al. Olive Oil Improves While Trans Fatty Acids Further Aggravate the Hypomethylation of LINE-1 Retrotransposon DNA in an Environmental Carcinogen Model. Nutrients 2022, 14, 908. [Google Scholar]
- Sharma, S.D.; Meeran, S.M.; Katiyar, S.K. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-κB signaling in in vivo SKH-1 hairless mice. Mol. Cancer Ther. 2007, 6, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K. Grape seed proanthocyanidines and skin cancer prevention: Inhibition of oxidative stress and protection of immune system. Mol. Nutr. Food Res. 2008, 52 (Suppl. S1), S71–S76. [Google Scholar] [CrossRef]
- Assunção, M.; de Freitas, V.; Paula-Barbosa, M. Grape seed flavanols, but not Port wine, prevent ethanol-induced neuronal lipofuscin formation. Brain Res. 2007, 1129, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Balu, M.; Sangeetha, P.; Murali, G.; Panneerselvam, C. Modulatory role of grape seed extract on age-related oxidative DNA damage in central nervous system of rats. Brain Res. Bull. 2006, 68, 469–473. [Google Scholar] [CrossRef]
- Kim, S.-R.; Seong, K.-J.; Kim, W.-J.; Jung, J.-Y. Epigallocatechin Gallate Protects against Hypoxia-Induced Inflammation in Microglia via NF-κB Suppression and Nrf-2/HO-1 Activation. Int. J. Mol. Sci. 2022, 23, 4004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, J.; Zheng, Y.; Guan, X.; Lai, C.; Gao, H.; Ho, C.-T.; Lin, B. Selenium-enriched oolong tea (Camellia sinensis) extract exerts anti-inflammatory potential via targeting NF-κB and MAPK pathways in macrophages. Food Sci. Hum. Wellness 2022, 11, 635–642. [Google Scholar] [CrossRef]
- Taranu, I.; Gras, M.A.; Habeanu, M.; Pistol, G.C.; Lefter, N.; Palade, M.L.; Ropota, M.; Chedea, V.S.; Marin, D.E. Active ingredients from oil by-products modulate spleen inflammatory and antioxidant response in pigs. Arch. Zootech. 2020, 23, 81–97. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Graier, W.F. Manipulation of Mitochondrial Function by Polyphenols for New Treatment Strategies. In Polyphenols: Mechanisms of Action in Human Health and Disease; Academic Press: New York, NY, USA, 2018; pp. 277–292. [Google Scholar]
- Parrado, C.; Philips, N.; Gilaberte, Y.; Juarranz, A.; González, S. Oral Photoprotection: Effective Agents and Potential Candidates. Front. Med. 2018, 5, 188. [Google Scholar] [CrossRef]
- Bezerra, M.S.; Gouveia, B.B.; Barberino, R.S.; Menezes, V.G.; Macedo, T.J.S.; Cavalcante, A.Y.P.; Monte, A.P.O.; Santos, J.M.S.; Matos, M.H.T. Resveratrol promotes in vitro activation of ovine primordial follicles by reducing DNA damage and enhancing granulosa cell proliferation via phosphatidylinositol 3-kinase pathway. Reprod. Domest. Anim. 2018, 53, 1298–1305. [Google Scholar] [CrossRef]
- Al-Mutary, M.G.; Al-Ghadi, M.Q.; Ammari, A.A.; Al-Himadi, A.R.; Al-Jolimeed, A.H.; Arafah, M.W.; Amran, R.A.; Aleissa, M.S.; Swelum, A.A.-A. Effect of different concentrations of resveratrol on the quality and in vitro fertilizing ability of ram semen stored at 5 °C for up to 168 h. Theriogenology 2020, 152, 139–146. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Sarabi, M.M.; Sayahi, A.; Zal, F. Protective effects of quercetin against hyperglycemia-induced oxidative stress in hepatic HepG2 cell line. Avic. J. Phytomed. 2020, 11, 269–280. [Google Scholar]
- Appiah, M.O.; Li, W.; Zhao, J.; Liu, H.; Dong, Y.; Xiang, J.; Wang, J.; Lu, W. Quercetin supplemented casein-based extender improves the post-thaw quality of rooster semen. Cryobiology 2020, 94, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Mostafavi-Pour, Z.; Amiri, A.; Keshavarzi, F.; Nejabat, N.; Ramezani, F.; Sardarian, A.; Zal, F. Chemoprevention of Prostate Cancer Cells by Vitamin C plus Quercetin: Role of Nrf2 in Inducing Oxidative Stress. Nutr. Cancer 2020, 73, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Liu, X.L.; Kong, L.; Zhang, M.Y.; Chen, Y.J.; Zhu, X.; Hao, Y.C. Neuroprotection of quercetin on central neurons against chronic high glucose through enhancement of Nrf2/ARE/glyoxalase-1 pathway mediated by phosphorylation regulation. Biomed. Pharmacother. 2019, 109, 2145–2154. [Google Scholar] [CrossRef]
- Gao, W.; Pu, L.; Chen, M.; Wei, J.; Xin, Z.; Wang, Y.; Yao, Z.; Shi, T.; Guo, C. Glutathione homeostasis is significantly altered by quercetin via the Keap1/Nrf2 and MAPK signaling pathways in rats. J. Clin. Biochem. Nutr. 2018, 62, 56–62. [Google Scholar] [CrossRef]
- Bellamri, M.; Turesky, R.J. Dietary Carcinogens and DNA Adducts in Prostate Cancer. Prostate Cancer 2019, 1210, 29–55. [Google Scholar]
- Li, X.; He, X.; Chen, S.; Le, Y.; Bryant, M.S.; Guo, L.; Witt, K.L.; Mei, N. The genotoxicity potential of luteolin is enhanced by CYP1A1 and CYP1A2 in human lymphoblastoid TK6 cells. Toxicol. Lett. 2021, 344, 58–68. [Google Scholar] [CrossRef]
- Vissenaekens, H.; Smagghe, G.; Criel, H.; Grootaert, C.; Raes, K.; Rajkovic, A.; Goeminne, G.; Boon, N.; De Schutter, K.; Van Camp, J. Intracellular quercetin accumulation and its impact on mitochondrial dysfunction in intestinal Caco-2 cells. Food Res. Int. 2021, 145, 110430. [Google Scholar] [CrossRef]
- Chang, W.S.; Tsai, C.W.; Yang, J.S.; Hsu, Y.M.; Shih, L.C.; Chiu, H.Y.; Bau, D.T.; Tsai, F.J. Resveratrol inhibited the metastatic behaviors of cisplatin-resistant human oral cancer cells via phosphorylation of ERK/p-38 and suppression of MMP-2/9. J. Food Biochem. 2021, 45, e13666. [Google Scholar] [CrossRef]
- Li, T.; Tan, Y.; Ouyang, S.; He, J.; Liu, L. Resveratrol protects against myocardial ischemia-reperfusion injury via attenuating ferroptosis. Gene 2021, 808, 145968. [Google Scholar] [CrossRef]
- Kolahdouz-Mohammadi, R.; Shidfar, F.; Khodaverdi, S.; Arablou, T.; Heidari, S.; Rashidi, N.; Delbandi, A.A. Resveratrol treatment reduces expression of MCP-1, IL-6, IL-8 and RANTES in endometriotic stromal cells. J. Cell. Mol. Med. 2021, 25, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, C. Research Progress of ANP, NPRA, and Cx43 in Gastric Cancer. Open J. Pathol. 2022, 12, 52–63. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Si, H. Synergistic anti-inflammatory effects and mechanisms of the combination of resveratrol and curcumin in human vascular endothelial cells and rodent aorta. J. Nutr. Biochem. 2022, 108. [Google Scholar] [CrossRef] [PubMed]
- Rossin, D.; Barbosa-Pereira, L.; Iaia, N.; Sottero, B.; Danzero, A.; Poli, G.; Zeppa, G.; Biasi, F. Protective Effect of Cocoa Bean Shell against Intestinal Damage: An Example of Byproduct Valorization. Antioxidants 2021, 10, 280. [Google Scholar] [CrossRef]
- Aboufarrag, H.T.; Needs, P.W.; Rimbach, G.; Kroon, P.A. The Effects of Anthocyanins and Their Microbial Metabolites on the Expression and Enzyme Activities of Paraoxonase 1, an Important Marker of HDL Function. Nutrients 2019, 11, 2872. [Google Scholar] [CrossRef]
- Tanaka, Y.; Furuta, A.; Asano, K.; Kobayashi, H. Modulation of Th1/Th2 cytokine balance by quercetin in vitro. Medicines 2020, 7, 46. [Google Scholar] [CrossRef]
- Le, B.; Ngoc, A.; Yang, S. Synbiotic fermented soymilk with Weissella cibaria FB069 and xylooligosaccharides prevents proliferation in human colon cancer cells. J. Appl. Microbiol. 2019, 128, 1486–1496. [Google Scholar] [CrossRef]
- Li, Z.; Feng, C.; Dong, H.; Jin, W.; Zhang, W.; Zhan, J.; Wang, S. Health promoting activities and corresponding mechanism of (–)-epicatechin-3-gallate. Food Sci. Hum. Wellness 2022, 11, 568–578. [Google Scholar] [CrossRef]
- López-Fernández-Sobrino, R.; Soliz-Rueda, J.R.; Ávila-Román, J.; Arola-Arnal, A.; Suárez, M.; Muguerza, B.; Bravo, F.I. Blood pressure-lowering effect of wine lees phenolic compounds is mediated by endothelial-derived factors: Role of sirtuin 1. Antioxidants 2021, 10, 1073. [Google Scholar] [CrossRef]
- Shao, D.; Di, Y.; Lian, Z.; Zhu, B.; Xu, X.; Guo, D.; Huang, Q.; Jiang, C.; Kong, J.; Shi, J. Grape seed proanthocyanidins suppressed macrophage foam cell formation by miRNA-9 via targeting ACAT1 in THP-1 cells. Food Funct. 2020, 11, 1258–1269. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Yan, H.; Jiao, G.; Wang, H.; Chi, H.; Zhou, H.; Chen, L.; Shan, Y.; Chen, Y. Resveratrol Protects Osteoblasts Against Dexamethasone-Induced Cytotoxicity Through Activation of AMP-Activated Protein Kinase. Drug Des. Dev. Ther. 2020, 14, 4451–4463. [Google Scholar] [CrossRef]
- Posadino, A.M.; Giordo, R.; Cossu, A.; Nasrallah, G.K.; Shaito, A.; Abou-Saleh, H.; Eid, A.H.; Pintus, G. Flavin Oxidase-Induced ROS Generation Modulates PKC Biphasic Effect of Resveratrol on Endothelial Cell Survival. Biomolecules 2019, 9, 209. [Google Scholar] [CrossRef]
- Yu, H.; Pan, W.; Huang, H.; Chen, J.; Sun, B.; Yang, L.; Zhu, P. Screening Analysis of Sirtuins Family Expression on Anti-Inflammation of Resveratrol in Endothelial Cells. Med Sci. Monit. 2019, 25, 4137–4148. [Google Scholar] [CrossRef]
- Kim, S.J.; Jeong, H.J.; Lee, K.M.; Myung, N.Y.; An, N.H.; Yang, W.M.; Park, S.K.; Lee, H.-J.; Hong, S.-H.; Kim, H.-M.; et al. Epigallocatechin-3-gallate suppresses NF-κB activation and phosphorylation of p38 MAPK and JNK in human astrocytoma U373MG cells. J. Nutr. Biochem. 2007, 18, 587–596. [Google Scholar] [CrossRef]
- He, M.; Xia, L.; Li, J. Potential Mechanisms of Plant-Derived Natural Products in the Treatment of Cervical Cancer. Biomolecules 2021, 11, 1539. [Google Scholar] [CrossRef]
- Schroeter, H.; Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem. J. 2001, 358, 547–557. [Google Scholar] [CrossRef]
- Cho, M.-L.; Heo, Y.-J.; Park, M.-K.; Oh, H.-J.; Park, J.-S.; Woo, Y.-J.; Ju, J.-H.; Park, S.-H.; Kim, H.-Y.; Min, J.-K. Grape seed proanthocyanidin extract (GSPE) attenuates collagen-induced arthritis. Immunol. Lett. 2009, 124, 102–110. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Health aspects of functional grape seed constituents. Trends Food Sci. Technol. 2004, 15, 422–433. [Google Scholar] [CrossRef]
- Li, H.; Li, R.; Wang, L.; Liao, D.; Zhang, W.; Wang, J. Proanthocyanidins attenuate the high glucose-induced damage of retinal pigment epithelial cells by attenuating oxidative stress and inhibiting activation of the NLRP3 inflammasome. J. Biochem. Mol. Toxicol. 2021, 35, e22845. [Google Scholar] [CrossRef]
- Zhu, W.; Li, M.C.; Wang, F.R.; Mackenzie, G.G.; Oteiza, P.I. The inhibitory effect of ECG and EGCG dimeric procyanidins on colorectal cancer cells growth is associated with their actions at lipid rafts and the inhibition of the epidermal growth factor receptor signaling. Biochem. Pharmacol. 2020, 175, 113923. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.-C.; Li, S.; Zhan, J.; Ho, C.-T. Immunomodulatory Effects of Green Tea Polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef]
- Akyuva, Y.; Nazıroğlu, M. Resveratrol attenuates hypoxia-induced neuronal cell death, inflammation and mitochondrial oxidative stress by modulation of TRPM2 channel. Sci. Rep. 2020, 10, 6449. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, Y.; Mi, Y.; Wang, J.; Zhang, H.; Xu, J.; Yang, Y.; Liu, J.; Ding, L.; Yang, J.; et al. A Novel Quinolyl-Substituted Analogue of Resveratrol Inhibits LPS-Induced Inflammatory Responses in Microglial Cells by Blocking the NF-κB/MAPK Signaling Pathways. Mol. Nutr. Food res. 2019, 63, 1801380. [Google Scholar] [CrossRef]
- Martínez-Martínez, D.; Soto, A.; Gil de Araujo, B.; Gallego, B.; Chiloeches, A.; Lasa, M. Resveratrol promotes apoptosis through the induction of dual specificity phosphatase 1 and sensitizes prostate cancer cells to cisplatin. Food Chem. Toxicol. 2018, 124, 273–279. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Zhu, G.; Liu, H.; Chen, J.; Wang, Y.; He, X. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-κB and AP-1 signaling pathway in vitro. Medicine 2020, 99, e22241. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Wu, Y.-H.; Huang, W.-C.; Pang, J.-H.S.; Huang, T.-H.; Cheng, C.-Y. Anti-inflammatory property of quercetin through downregulation of ICAM-1 and MMP-9 in TNF-α-activated retinal pigment epithelial cells. Cytokine 2019, 116, 48–60. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Lee, H.; Sung, J. Relative protective activities of quercetin, quercetin-3-glucoside, and rutin in alcohol-induced liver injury. J. Food Biochem. 2019, 43, e13002. [Google Scholar] [CrossRef]
- Wu, T.; Grootaert, C.; Pitart, J.; Vidovic, N.K.; Kamiloglu, S.; Possemiers, S.; Glibetic, M.; Smagghe, G.; Raes, K.; Van de Wiele, T.; et al. Aronia (Aronia melanocarpa) polyphenols modulate the microbial community in a Simulator of the Human Intestinal Microbial Ecosystem (SHIME) and decrease secretion of proinflammatory markers in a Caco-2/endothelial cell coculture model. Mol. Nutr. Food Res. 2018, 62, 1800607. [Google Scholar] [CrossRef]
- Raina, K.; Singh, R.P.; Agarwal, R.; Agarwal, C. Oral Grape Seed Extract Inhibits Prostate Tumor Growth and Progression in TRAMP Mice. Cancer Res. 2007, 67, 5976–5982. [Google Scholar] [CrossRef]
- Veluri, R.; Singh, R.P.; Liu, Z.; Thompson, J.A.; Agarwal, R.; Agarwal, C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis 2006, 27, 1445–1453. [Google Scholar] [CrossRef]
- Mantena, S.K.; Baliga, M.S.; Katiyar, S.K. Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis 2005, 27, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Mandair, R.; Agarwal, R.; Agarwal, C. Grape Seed Extract Induces Cell Cycle Arrest and Apoptosis in Human Colon Carcinoma Cells. Nutr. Cancer 2008, 60, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Dutta, P.; Austin, D.; Wang, P.; Awad, A.; Vadgama, J.V. Combination of resveratrol and 5-flurouracil enhanced anti-telomerase activity and apoptosis by inhibiting STAT3 and Akt signaling pathways in human colorectal cancer cells. Oncotarget 2018, 9, 32943–32957. [Google Scholar] [CrossRef]
- Fan, Y.; Li, J.; Yang, Y.; Zhao, X.; Liu, Y.; Zhou, L.; Feng, Y.; Yu, Y.; Cheng, Y. Resveratrol modulates the apoptosis and autophagic death of human lung adenocarcinoma A549 cells via a p53-dependent pathway: Integrated bioinformatics analysis and experimental validation. Int. J. Oncol. 2020, 57, 925–938. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Y.; Gao, S.; Wang, M.; Ge, M.; Yang, Q.; Liao, M.; Xu, L.; Chen, J.; Zeng, Z.; et al. Norlichexanthone purified from plant endophyte prevents postmenopausal osteoporosis by targeting ERα to inhibit RANKL signaling. Acta Pharm. Sin. B 2021, 11, 442–455. [Google Scholar] [CrossRef]
- Kim, S.Y.; Hassan, A.H.; Chung, K.S.; Kim, S.Y.; Han, H.S.; Lee, H.H.; Jung, S.H.; Lee, K.Y.; Shin, J.S.; Jang, E.; et al. Mosloflavone-Resveratrol Hybrid TMS-HDMF-5z Exhibits Potent In Vitro and In Vivo Anti-Inflammatory Effects Through NF-κB, AP-1, and JAK/STAT Inactivation. Front. Pharmacol. 2022, 13, 857789. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2020, 73, 310–320. [Google Scholar] [CrossRef]
- da Costa, P.S.; Ramos, P.S.; Ferreira, C.; Silva, J.L.; El-Bacha, T.; Fialho, E. Pro-Oxidant Effect of Resveratrol on Human Breast Cancer MCF-7 Cells is Associated with CK2 Inhibition. Nutr. Cancer 2021, 74, 2142–2151. [Google Scholar] [CrossRef]
- Fang, X.-S.; Zhang, M.-H.; Zhang, X.-Z.; Guo, J.-Y.; Jin, Z. Insulin-like growth factor-1 inhibits the apoptosis of rat gastric smooth muscle cells cultured under high glucose condition through PI3K-Akt-PKC-Ca2+ pathway. Biotechnol. Biotechnol. Equip. 2019, 33, 456–464. [Google Scholar] [CrossRef]
- Li, H.; Cheng, Y.; Wang, H.; Sun, H.; Liu, Y.; Liu, K.; Peng, S. Inhibition of nitrobenzene-induced DNA and hemoglobin adductions by dietary constituents. Appl. Radiat. Isot. 2003, 58, 291–298. [Google Scholar] [CrossRef]
- Huang, K.-Y.; Wang, T.-H.; Chen, C.-C.; Leu, Y.-L.; Li, H.-J.; Jhong, C.-L.; Chen, C.-Y. Growth Suppression in Lung Cancer Cells Harboring EGFR-C797S Mutation by Quercetin. Biomolecules 2021, 11, 1271. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yuan, S.; Zhao, Q.; Wang, B.; Wang, X.; Li, K. Quercetin enhances chemotherapeutic effect of doxorubicin against human breast cancer cells while reducing toxic side effects of it. Biomed. Pharmacother. 2018, 100, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Lu, J.-L.; Liang, Y.-R.; Li, Q.-S. Suppressive Effects of EGCG on Cervical Cancer. Molecules 2018, 23, 2334. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, Y.D.G.; Rochetti, A.L.; Souza, V.C.; da Silva, E.R.; Scatolini, A.M.; Genovese, M.I.; Yasui, G.S.; Fukumasu, H. Antineoplastic Effect of Procyanidin-rich Extract of Lafoensia Pacari in Lung Carcinoma Cells. Braz. Arch. Biol. Technol. 2019, 62, 1–13. Available online: http://dx.doi.org/10.1590/1678-4324-2019160638 (accessed on 24 July 2022).
- Lim, H.-J.; Kang, S.-H.; Song, Y.-J.; Jeon, Y.-D.; Jin, J.-S. Inhibitory Effect of Quercetin on Propionibacterium acnes-induced Skin Inflammation. Int. Immunopharmacol. 2021, 96, 107557. [Google Scholar] [CrossRef] [PubMed]
- Zubčić, K.; Radovanović, V.; Vlainić, J.; Hof, P.R.; Oršolić, N.; Šimić, G.; Jembrek, M.J. PI3K/Akt and ERK1/2 signalling are involved in quercetin-mediated neuroprotection against copper-induced injury. Oxid. Med. Cell. Longev. 2020, 2020, 9834742. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chang, C.-C.; Yang, Y.; Yuan, L.; Xu, L.; Ho, C.-T.; Li, S. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Ong’Achwa, M.J.; Ge, L.; Qian, Y.; Chen, L.; Hu, X.; Li, F.; Wei, H.; Zhang, C.; et al. Resveratrol Inhibits the TGF-β1-Induced Proliferation of Cardiac Fibroblasts and Collagen Secretion by Downregulating miR-17 in Rat. BioMed Res. Int. 2018, 2018, 8730593. [Google Scholar] [CrossRef]
- Hammad, A.; Namani, A.; Elshaer, M.; Wang, X.J.; Tang, X. “NRF2 addiction” in lung cancer cells and its impact on cancer therapy. Cancer Lett. 2019, 467, 40–49. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Kumari, A.; Gulati, A.; Padwad, Y.; Sharma, R. Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology 2018, 20, 171–189. [Google Scholar] [CrossRef]
- Das, M.; Devi, K.P.; Belwal, T.; Devkota, H.P.; Tewari, D.; Sahebnasagh, A.; Nabavi, S.F.; Kashani, H.R.K.; Rasekhian, M.; Xu, S.; et al. Harnessing polyphenol power by targeting eNOS for vascular diseases. Crit. Rev. Food Sci. Nutr. 2021, 1–26. [Google Scholar] [CrossRef]
- Cerezo López, A.B.; Hornedo Ortega, R.; García Parrilla, M.D.C.; Troncoso González, A.M.; Labrador, M.; Gutiérrez, A. Anti-VEGF Signalling Mechanism in HUVECs by Melatonin, Serotonin, Hydroxytyrosol and Other Bioactive Compounds. Nutrients 2019, 11, 2421. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Cires, M.J.; Gotteland, M. Quercetin and Epigallocatechin Gallate in the Prevention and Treatment of Obesity: From Molecular to Clinical Studies. J. Med. Food 2019, 22, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.-L.; Wei, W.-H.; Vuthijumnonk, J. Evaluation of anti-angiogenic activities of aqueous extracts of regular and selenium-rich green teas using chick chorioallantoic membrane as an experimental model. Am. J. Life Sci. Res. 2019, 7, 1–8. [Google Scholar]
- Giglio, R.V.; Patti, A.M.; Cicero, A.F.G.; Lippi, G.; Rizzo, M.; Toth, P.P.; Banach, M. Polyphenols: Potential Use in the Prevention and Treatment of Cardiovascular Diseases. Curr. Pharm. Des. 2018, 24, 239–258. [Google Scholar] [CrossRef]
- Ni, D.; Ai, Z.; Munoz-Sandoval, D.; Suresh, R.; Ellis, P.R.; Yuqiong, C.; Sharp, P.A.; Butterworth, P.J.; Yu, Z.; Corpe, C.P. Inhibition of the facilitative sugar transporters (GLUTs) by tea extracts and catechins. FASEB J. 2020, 34, 9995–10010. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Failla, M.L. Intestinal cell models for investigating the uptake, metabolism and absorption of dietary nutrients and bioactive compounds. Curr. Opin. Food Sci. 2021, 41, 169–179. [Google Scholar] [CrossRef]
- Olvera-Sandoval, C.; Fabela-Illescas, H.E.; Fernández-Martínez, E.; Ortiz-Rodríguez, M.A.; Cariño-Cortés, R.; Ariza-Ortega, J.A.; Hernández-González, J.C.; Olivo, D.; Valadez-Vega, C.; Belefant-Miller, H.; et al. Potential Mechanisms of the Improvement of Glucose Homeostasis in Type 2 Diabetes by Pomegranate Juice. Antioxidants 2022, 11, 553. [Google Scholar] [CrossRef]
- Heo, J.R.; Kim, S.M.; Hwang, K.A.; Kang, J.H.; Choi, K.C. Resveratrol induced reactive oxygen species and endoplasmic reticulum stress mediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Int. J. Mol. Med. 2018, 42, 1427–1435. [Google Scholar] [CrossRef]
- Radapong, S.; Chan, K.; Sarker, S.D.; Ritchie, K.J. Oxyresveratrol Modulates Gene Expression of Apoptosis, Cell Cycle Control and DNA Repair in MCF7 Cells. Front. Pharmacol. 2021, 12, 694562. [Google Scholar] [CrossRef]
- Nivelle, L.; Aires, V.; Rioult, D.; Martiny, L.; Tarpin, M.; Delmas, D. Molecular analysis of differential antiproliferative activity of resveratrol, epsilon viniferin and labruscol on melanoma cells and normal dermal cells. Food Chem. Toxicol. 2018, 116, 323–334. [Google Scholar] [CrossRef]
- Gu, J.; Fan, Y.-Q.; Zhang, H.-L.; Pan, J.-A.; Yu, J.-Y.; Zhang, J.-F.; Wang, C.-Q. Resveratrol suppresses doxorubicin-induced cardiotoxicity by disrupting E2F1 mediated autophagy inhibition and apoptosis promotion. Biochem. Pharmacol. 2018, 150, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Razak, S.; Afsar, T.; Ullah, A.; Almajwal, A.; Alkholief, M.; Alshamsan, A.; Jahan, S. Taxifolin, a natural flavonoid interacts with cell cycle regulators causes cell cycle arrest and causes tumor regression by activating Wnt/β-catenin signaling pathway. BMC Cancer 2018, 18, 1043. [Google Scholar] [CrossRef]
- Rummun, N.; Rondeau, P.; Bourdon, E.; Pires, E.; McCullagh, J.; Claridge, T.D.W.; Bahorun, T.; Li, W.-W.; Neergheen, V.S. Terminalia bentzoe, a mascarene endemic plant, inhibits human hepatocellular carcinoma cells growth in vitro via G0/G1 phase cell cycle arrest. Pharmaceuticals 2020, 13, 303. [Google Scholar] [CrossRef]
- Xie, Q.; Bedran-Russo, A.K.; Wu, C.D. In vitro remineralization effects of grape seed extract on artificial root caries. J. Dent. 2008, 36, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Vogels, N.; Nijs, I.M.T.; Westerterp-Plantenga, M.S. The effect of grape-seed extract on 24 h energy intake in humans. Eur. J. Clin. Nutr. 2004, 58, 667–673. [Google Scholar] [CrossRef][Green Version]
- A Moreno, D.; Ilic, N.; Poulev, A.; Brasaemle, D.L.; Fried, S.K.; Raskin, I. Inhibitory effects of grape seed extract on lipases. Nutrition 2003, 19, 876–879. [Google Scholar] [CrossRef]
- Mittal, A.; Elmets, C.A.; Katiyar, S.K. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: Relationship to decreased fat and lipid peroxidation. Carcinogenesis 2003, 24, 1379–1388. [Google Scholar] [CrossRef]
- Leifert, W.R.; Abeywardena, M.Y. Grape seed and red wine polyphenol extracts inhibit cellular cholesterol uptake, cell proliferation, and 5-lipoxygenase activity. Nutr. Res. 2008, 28, 842–850. [Google Scholar] [CrossRef]
- Jayaprakasha, G.; Selvi, T.; Sakariah, K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Cornebise, C.; Courtaut, F.; Taillandier-Coindard, M.; Valls-Fonayet, J.; Richard, T.; Monchaud, D.; Aires, V.; Delmas, D. Red Wine Extract Inhibits VEGF Secretion and Its Signaling Pathway in Retinal ARPE-19 Cells to Potentially Disrupt AMD. Molecules 2020, 25, 5564. [Google Scholar] [CrossRef]
- Iraci, N.; Tabarrini, O.; Santi, C.; Sancineto, L. NCp7: Targeting a multitask protein for next-generation anti-HIV drug development part 2. Noncovalent inhibitors and nucleic acid binders. Drug Discov. Today 2018, 23, 687–695. [Google Scholar] [CrossRef]
- Hsu, Y.-A.; Chen, C.-S.; Wang, Y.-C.; Lin, E.-S.; Chang, C.-Y.; Chen, J.; Wu, M.-Y.; Lin, H.-J.; Wan, L. Anti-Inflammatory Effects of Resveratrol on Human Retinal Pigment Cells and a Myopia Animal Model. Curr. Issues Mol. Biol. 2021, 43, 716–727. [Google Scholar] [CrossRef]
- Lee, J.-H.; Baek, S.Y.; Jang, E.J.; Ku, S.K.; Kim, K.M.; Ki, S.H.; Kim, C.-E.; Park, K.I.; Kim, S.C.; Kim, Y.W. Oxyresveratrol ameliorates nonalcoholic fatty liver disease by regulating hepatic lipogenesis and fatty acid oxidation through liver kinase B1 and AMP-activated protein kinase. Chem. Interact. 2018, 289, 68–74. [Google Scholar] [CrossRef]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of flavonoids against coronavirus infection. Chem. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef]
- Islam, F.; Khadija, J.F.; Islam, R.; Shohag, S.; Mitra, S.; Alghamdi, S.; Babalghith, A.O.; Theyab, A.; Rahman, M.T.; Akter, A.; et al. Investigating Polyphenol Nanoformulations for Therapeutic Targets against Diabetes Mellitus. Evid.-Based Complement. Altern. Med. 2022, 2022, 5649156. [Google Scholar] [CrossRef]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326S–329S. [Google Scholar] [CrossRef]
- Ray, S.; Bagchi, D.; Lim, P.M.; Bagchi, M.; Gross, S.M.; Kothari, S.C.; Preuss, H.G.; Stohs, S.J. Acute and long-term safety evaluation of a novel IH636 grape seed proanthocyanidin extract. Res. Commun. Mol. Pathol. Pharmacol. 2001, 109, 165–197. [Google Scholar]
- Cerdá, B.; Cerón, J.J.; Tomás-Barberán, A.F.A.; Espín, J.C. Repeated Oral Administration of High Doses of the Pomegranate Ellagitannin Punicalagin to Rats for 37 Days Is Not Toxic. J. Agric. Food Chem. 2003, 51, 3493–3501. [Google Scholar] [CrossRef]
- Dunnick, J.K.; Halley, J.R. Toxicity and carcinogenicity studies of quercetin, a natural component of foods. Toxicol. Sci. 1992, 19, 423–431. [Google Scholar] [CrossRef]
- Jones, E.; Hughes, R. Quercetin, flavonoids and the life-span of mice. Exp. Gerontol. 1982, 17, 213–217. [Google Scholar] [CrossRef]
- Sinha, M.; Sachan, D.K.; Bhattacharya, R.; Singh, P.; Parthasarathi, R. ToxDP2 Database: Toxicity prediction of dietary polyphenols. Food Chem. 2021, 370, 131350. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Hirose, M.; Takahashi, S.; Ogawa, K.; Shirai, T.; Ito, N. Forestomach and kidney carcinogenicity of caffeic acid in F344 rats and C57BL/6N x C3H/HeN F1 mice. Cancer Res. 1991, 51, 5655–5660. [Google Scholar] [PubMed]
- Zhu, B.T.; Liehr, J.G. Inhibition of Catechol O-Methyltransferase-catalyzed O-Methylation of 2-and 4-Hydroxyestradiol by Quercetin: Possible Role in Estradiol-Induced Tumorigenesis. J. Biol. Chem. 1996, 271, 1357–1363. [Google Scholar] [CrossRef]
- Hirose, M.; Hoshiya, T.; Mizoguchi, Y.; Nakamura, A.; Akagi, K.; Shirai, T. Green tea catechins enhance tumor development in the colon without effects in the lung or thyroid after pretreatment with 1,2-Dimethylhydrazine or 2,2′-dihydroxy-di-n-propylnitrosamine in male F344 rats. Cancer Lett. 2001, 168, 23–29. [Google Scholar] [CrossRef]
- Van Der Woude, H.; Gliszczyńska-Świgło, A.; Struijs, K.; Smeets, A.; Alink, G.M.; Rietjens, I.M. Biphasic modulation of cell proliferation by quercetin at concentrations physiologically relevant in humans. Cancer Lett. 2003, 200, 41–47. [Google Scholar] [CrossRef]
- Aron, P.M. Composition of flavonoid Phenolic Polymers Isolated from Red Wine during Maceration and Significance of Flavan-3-Ols in Foods Pertaining to Biological Activity. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2007. [Google Scholar]
- Ferreira, A.; Lisboa, P.; Oliveira, K.; Lima, L.; Barros, I.; Carvalho, D. Inhibition of thyroid type 1 deiodinase activity by flavonoids. Food Chem. Toxicol. 2002, 40, 913–917. [Google Scholar] [CrossRef]
- Doerge, D.R.; Sheehan, D.M. Goitrogenic and estrogenic activity of soy isoflavones. Environ. Health Perspect. 2002, 110, 349–353. [Google Scholar] [CrossRef]
- Chang, H.C.; Doerge, D.R. Dietary Genistein Inactivates Rat Thyroid Peroxidase in Vivo without an Apparent Hypothyroid Effect. Toxicol. Appl. Pharmacol. 2000, 168, 244–252. [Google Scholar] [CrossRef]
- Bennetau-Pelissero, C.; Breton, B.; Bennetau, B.; Corraze, G.; Le Menn, F.; Davail-Cuisset, B.; Helou, C.; Kaushik, S.J. Effect of genistein-enriched diets on the endocrine process of gametogenesis and on reproduction efficiency of the rainbow trout Oncorhynchus mykiss. Gen. Comp. Endocrinol. 2001, 121, 173–187. [Google Scholar] [CrossRef]
- Temme, E.; Van Hoydonck, P. Tea consumption and iron status. Eur. J. Clin. Nutr. 2002, 56, 379–386. [Google Scholar] [CrossRef]
- Zijp, I.M.; Korver, O.; Tijburg, L.B.M. Effect of Tea and Other Dietary Factors on Iron Absorption. Crit. Rev. Food Sci. Nutr. 2000, 40, 371–398. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds—Nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef]
- Veronese, M.L.; Gillen, L.P.; Burke, J.P.; Dorval, E.P.; Hauck, W.W.; Pequignot, E.; Waldman, S.A.; Greenberg, H.E. Exposure-dependent inhibition of intestinal and hepatic CYP3A4 in vivo by grapefruit juice. J. Clin. Pharmacol. 2003, 43, 831–839. [Google Scholar] [CrossRef]
- Chen, M.; Yu, S.-J. Lipophilic Grape Seed Proanthocyanidin Exerts Anti-Proliferative and Pro-Apoptotic Effects on PC3 Human Prostate Cancer Cells and Suppresses PC3 Xenograft Tumor Growth in Vivo. J. Agric. Food Chem. 2018, 67, 229–235. [Google Scholar] [CrossRef]
- Wang, L.; Huang, W.; Zhan, J. Grape Seed Proanthocyanidins Induce Autophagy and Modulate Survivin in HepG2 Cells and Inhibit Xenograft Tumor Growth in Vivo. Nutrients 2019, 11, 2983. [Google Scholar] [CrossRef]
- Liu, J.; Hu, S.; Zhu, B.; Shao, S.; Yuan, L. Grape seed procyanidin suppresses inflammation in cigarette smoke-exposed pulmonary arterial hypertension rats by the PPAR-γ/COX-2 pathway. Nutr. Metab. Cardiovasc. Dis. 2019, 30, 347–354. [Google Scholar] [CrossRef]
- Chen, F.; Wang, H.; Zhao, J.; Yan, J.; Meng, H.; Zhan, H.; Chen, L.; Yuan, L. Grape seed proanthocyanidin inhibits monocrotaline-induced pulmonary arterial hypertension via attenuating inflammation: In vivo and in vitro studies. J. Nutr. Biochem. 2019, 67, 72–77. [Google Scholar] [CrossRef]
- Liu, W.Z.; Ma, Z.J.; Kang, J.H.; Lin, A.X.; Wang, Z.H.; Chen, H.W.; Guo, X.D.; He, X.G.; Kang, X.W. Grape Seed Proanthocyanidins Exert a Neuroprotective Effect by Regulating Microglial M1/M2 Polarisation in Rats with Spinal Cord Injury. Med. Inflamm. 2022, 2022, 2579003. [Google Scholar] [CrossRef]
- Ben Youssef, S.; Brisson, G.; Doucet-Beaupré, H.; Castonguay, A.M.; Gora, C.; Amri, M.; Lévesque, M. Neuroprotective benefits of grape seed and skin extract in a mouse model of Parkinson’s disease. Nutr. Neurosci. 2021, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Elhakeim, E.H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin. Rheumatol. 2018, 37, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Nazarian, B.; Reiner, Ž.; Amirani, E.; Kolahdooz, F.; Chamani, M.; Asemi, Z. The effects of grape seed extract on glycemic control, serum lipoproteins, inflammation, and body weight: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2020, 34, 239–253. [Google Scholar] [CrossRef]
- Izadpanah, A.; Soorgi, S.; Geraminejad, N.; Hosseini, M. Effect of grape seed extract ointment on cesarean section wound healing: A double-blind, randomized, controlled clinical trial. Complement. Ther. Clin. Pract. 2019, 35, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.T.; Lu, Q.Y.; Xue, B.; Neis, P.; Zamora, F.D.; Lundmark, L.; Qualls, C.; Massie, L. A Pilot Study of a Grape Seed Procyanidin Extract for Lung Cancer Chemoprevention Grape Seed Extract for Lung Cancer Chemoprevention. Cancer Prev. Res. 2019, 12, 557–566. [Google Scholar] [CrossRef]
- Mao, J.T.; Xue, B.; Fan, S.; Neis, P.; Qualls, C.; Massie, L.; Fiehn, O. Leucoselect Phytosome Modulates Serum Eicosapentaenoic Acid, Docosahexaenoic Acid, and Prostaglandin E3 in a Phase I Lung Cancer Chemoprevention Study Effects of Grape Seed Extract on Complex Lipid Metabolomics. Cancer Prev. Res. 2021, 14, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, R.; Parandoosh, M.; Khorsandi, H.; Hosseinzadeh, N.; Madani Tonekaboni, M.; Saidpour, A.; Babaei, H.; Ghorbani, A. Grape seed extract supplementation along with a restricted-calorie diet improves cardiovascular risk factors in obese or overweight adult individuals: A randomized, placebo-controlled trial. Phytother. Res. 2021, 35, 987–995. [Google Scholar] [CrossRef]
- Mohammad, A.; Shahnaz, T.; Sorayya, K. Effect of 8 weeks’ supplementation grape seed extract on insulin resistance in iranian adolescents with metabolic syndrome: A randomized controlled trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 15, 197–203. [Google Scholar]
- Argani, H.; Ghorbanihaghjo, A.; Vatankhahan, H.; Rashtchizadeh, N.; Raeisi, S.; Ilghami, H. The effect of red grape seed extract on serum paraoxonase activity in patients with mild to moderate hyperlipidemia. Sao Paulo Med. J. 2016, 134, 234–239. [Google Scholar] [CrossRef]
- Nakazono, M.; Ma, L.; Zaitsu, K. Synthesis of poly (3,4,5-trihydroxybenzoate ester) dendrimers and their chemiluminescence. Tetrahedron Lett. 2002, 43, 8185–8189. [Google Scholar] [CrossRef]
- Moshawih, S.; Mydin, R.B.S.; Kalakotla, S.; Jarrar, Q.B. Potential application of resveratrol in nanocarriers against cancer: Overview and future trends. J. Drug Deliv. Sci. Technol. 2019, 53, 101187. [Google Scholar]
- Sanz del Olmo, N.; Peña González, C.E.; Rojas, J.D.; Gómez, R.; Ortega, P.; Escarpa, A.; de la Mata, F.J. Antioxidant and antibacterial properties of carbosilane dendrimers functionalized with polyphenolic moieties. Pharmaceutics 2020, 12, 698. [Google Scholar] [CrossRef] [PubMed]
- Agawa, H.; Nakazono, M.; Nanbu, S.; Zaitsu, K. Chemiluminescence Change of Polyphenol Dendrimers with Different Core Molecules. Org. Lett. 2008, 10, 5171–5174. [Google Scholar] [CrossRef] [PubMed]
- Saberi, D.; Hashemi, H.; Ghanaatzadeh, N.; Moghadam, M.; Niknam, K. Ruthenium/dendrimer complex immobilized on silica-functionalized magnetite nanoparticles catalyzed oxidation of stilbenes to benzil derivatives at room temperature. Appl. Organomet. Chem. 2020, 34, e5563. [Google Scholar]
- Kurisawa, M.; Chung, J.E.; Kim, Y.J.; Uyama, H.; Kobayashi, S. Amplification of Antioxidant Activity and Xanthine Oxidase Inhibition of Catechin by Enzymatic Polymerization. Biomacromolecules 2003, 4, 469–471. [Google Scholar]
- Halkes, S.A.; Vrasidas, I.; Rooijer, G.R.; Berg, A.J.V.D.; Liskamp, R.M.; Pieters, R.J. Synthesis and biological activity of polygalloyl-dendrimers as stable tannic acid mimics. Bioorganic Med. Chem. Lett. 2002, 12, 1567–1570. [Google Scholar] [CrossRef]
- Nie, Z.; Liu, K.J.; Zhong, C.-J.; Wang, L.-F.; Yang, Y.; Tian, Q.; Liu, Y. Enhanced radical scavenging activity by antioxidant-functionalized gold nanoparticles: A novel inspiration for development of new artificial antioxidants. Free Radic. Biol. Med. 2007, 43, 1243–1254. [Google Scholar] [CrossRef]
- Gołąbek, A.; Kowalska, K.; Olejnik, A. Polyphenols as a Diet Therapy Concept for Endometriosis—Current Opinion and Future Perspectives. Nutrients 2021, 13, 1347. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2019, 57, 1205–1215. [Google Scholar]
- Guo, Y.; Sun, Q.; Wu, F.G.; Dai, Y.; Chen, X. Polyphenol-Containing Nanoparticles: Synthesis, Properties, and Therapeutic Delivery. Adv. Mater. 2021, 33, 2007356. [Google Scholar] [CrossRef]
- Oprea, O.B.; Popa, M.E.; Apostol, L.; Gaceu, L. Research on the Potential Use of Grape Seed Flour in the Bakery Industry. Foods 2022, 11, 1589. [Google Scholar] [PubMed]
- Bhaskara, V.K.; Mittal, B.; Mysorekar, V.V.; Amaresh, N.; Simal-Gandara, J. Resveratrol, cancer and cancer stem cells: A review on past to future. Curr. Res. Food Sci. 2020, 3, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.A.; Haytowitz, D.B.; Prior, R.L.; Gu, L.; Hammerstone, J.; Gebhardt, S.E.; Kelm, M.; Cunningham, D.; Beecher, G.R.; Holden, J.M. USDA Database for Proanthocyanidin Content of Selected Foods; U.S. Department of Agriculture: Washington, DC, UDA, 2004. Available online: http://www.nal.usda.gov/fnic/foodcomp (accessed on 24 July 2022).
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B.; Laureano, O.; Ricardo Da Silva, J.M. Monomeric, oligomeric, and polymeric flavan-3-ol composition of wines and grapes from Vitis vinifera L. Cv. Graciano, Tempranillo, and Cabernet Sauvignon. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar] [PubMed]
- Milke, L.; Ferreira, P.; Kallscheuer, N.; Braga, A.; Vogt, M.; Kappelmann, J.; Oliveira, J.; Silva, A.R.; Rocha, I.; Bott, M.; et al. Modulation of the central carbon metabolism of Corynebacterium glutamicum improves malonyl-CoA availability and increases plant polyphenol synthesis. Biotechnol. Bioeng. 2019, 116, 1380–1391. [Google Scholar] [PubMed]
- Green, R.C. Physicochemical Properties and phenolic Composition of Selected Saskatchewan Fruits: Buffaloberry, Chokecherry and Sea Buckthorn. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2007. [Google Scholar]
- Price, K.R.; Bacon, J.R.; Rhodes, M.J. Effect of storage and domestic processing on the content and composition of flavonol glucosides in onion (Allium cepa). J. Agric. Food Chem. 1997, 45, 938–942. [Google Scholar]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar]
- Groenewoud, G.; Hundt, H.K.L. The microbial metabolism of condensed (+)-catechins by rat-caecal microflora. Xenobiotica 1986, 16, 99–107. [Google Scholar]
- Spencer, J.P.; Chaudry, F.; Pannala, A.S.; Srai, S.K.; Debnam, E.; Rice-Evans, C. Decomposition of cocoa procyanidins in the gastric milieu. Biochem. Biophys. Res. Commun. 2000, 272, 236–241. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Holt, R.R.; Lazarus, S.A.; Ensunsa, J.L.; Hammerstone, J.F.; Schmitz, H.H.; Keen, C.L. Stability of the Flavan-3-ols Epicatechin and Catechin and Related Dimeric Procyanidins Derived from Cocoa. J. Agric. Food Chem. 2002, 50, 1700–1705. [Google Scholar] [PubMed]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Brühl, L.; Matthäus, B. Extraction of oilseeds by SFE—A comparison with other methods for the determination of the oil content. Anal. Bioanal. Chem. 1999, 364, 631–634. [Google Scholar] [CrossRef]
- Dos Santos Freitas, L.; Jacques, R.A.; Richter, M.F.; Da Silva, A.L.; Caramão, E.B. Pressurized liquid extraction of vitamin E from Brazilian grape seed oil. J. Chromatogr. A 2008, 1200, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Fu, Y.-J.; Zu, Y.-G.; Tong, M.-H.; Wu, N.; Liu, X.-L.; Zhang, S. Supercritical carbon dioxide extraction of seed oil from Opuntia dillenii Haw. and its antioxidant activity. Food Chem. 2009, 114, 334–339. [Google Scholar] [CrossRef]
- Lilja, J.J.; Kivistö, K.T.; Backman, J.T.; Neuvonen, P.J. Effect of grapefruit juice dose on grapefruit juice-triazolam interaction: Repeated consumption prolongs triazolam half-life. Eur. J. Clin. Pharmacol. 2000, 56, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Rababah, T.M.; Ereifej, K.I.; Al-Mahasneh, M.A.; Ismaeal, K.; Hidar, A.-G.; Yang, W. Total Phenolics, Antioxidant Activities, and Anthocyanins of Different Grape Seed Cultivars Grown in Jordan. Int. J. Food Prop. 2008, 11, 472–479. [Google Scholar] [CrossRef]
- Martinello, M.; Hecker, G.; Pramparo, M.D.C. Grape seed oil deacidification by molecular distillation: Analysis of operative variables influence using the response surface methodology. J. Food Eng. 2007, 81, 60–64. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Passos, C.P.; Yilmaz, S.; Silva, C.M.; Coimbra, M.A. Enhancement of grape seed oil extraction using a cell wall degrading enzyme cocktail. Food Chem. 2009, 115, 48–53. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M.; Lester, P. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Schilling, S.; Alber, T.; Toepfl, S.; Neidhart, S.; Knorr, D.; Schieber, A.; Carle, R. Effects of pulsed electric field treatment of apple mash on juice yield and quality attributes of apple juices. Innov. Food Sci. Emerg. Technol. 2007, 8, 127–134. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A. Grape and wine phenolics: Observations and recent findings. Cienc. Investig. Agrar. 2008, 35, 107–120. [Google Scholar] [CrossRef]
- Maier, T.; Schieber, A.; Kammerer, D.R.; Carle, R. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem. 2009, 112, 551–559. [Google Scholar] [CrossRef]

| Compound Group | General Structural Formula | Function | Representatives | |
|---|---|---|---|---|
| Flavonoids | Anthocyanidins | 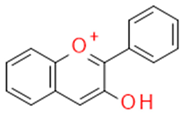 | Plant dyes | Cyanidine |
| Flavonols | 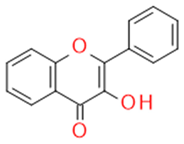 | Inhibitors of drug-metabolizing enzymes | Quercetin | |
| Flavanols | 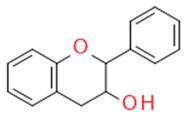 | The building blocks of proanthocyanides | Catechin, epicatechin | |
| Isoflavonoids | 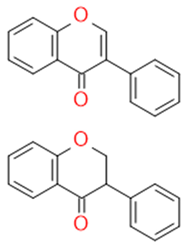 | Immune booster, estrogen stimulator | Isoflavone, genistein | |
| Flavons | 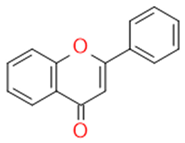 | Stimulates the function of cytochrome p450 | Apigenin | |
| Flavonones | 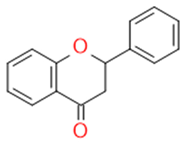 | Antidiabetics | Hesperetin, Naringenin, Eriodictyol | |
| Stilbenoid | Stilbene | 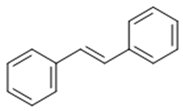 | Antioxidant | Resveratrol |
| Source | Compound Name | Classification | Structural Formula | Function |
|---|---|---|---|---|
| Grape seed and skin | Cyanidin | Anthocyanidin |  | Oxygen radical sequestration |
| Catechin/Epicatechin | Catechins flavan-3-ol | 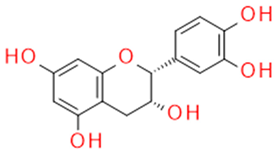 | Anticancer Antisclerotic Antidiabetic Free radical sequestration | |
| Quercetin | Flavonol | 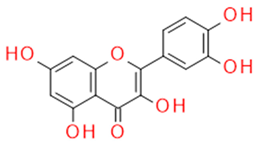 | Anti-inflammatory Antiallergic Anticancer Antioxidant | |
| Whole grapes | Resveratrol | Fitoalexin Stilbene | 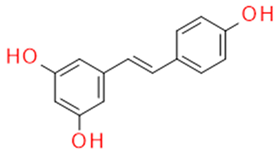 | Antioxidant Antimicrobial Anticancer Anti-inflammatory Blood glucose lowering |
| Rutin | Quercetin-3-rutinozide, flavonoid | 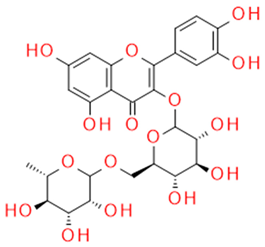 | Anti-inflammatory Vasoprotective Blood clotting inhibitor Antidiabetic |
| Physical Properties | Catechin | EC | EGC |
|---|---|---|---|
| Molecular weight (Mr) | 293 | 294 | 445 |
| Melting point, °C | 174 | 236 | 236 |
| Optical rotation, degree | 0° | 58.3° | 188° |
| Amax | 264–280 nm | ||
| Title | Method | Materials Needed | Literature |
|---|---|---|---|
| Antioxidant activity determination | FRAP method | FeCl3, triazine | [23] |
| András Boór total antioxidant content | 2,4,6-Tris(2-pyridyl)-s-triazine | [23,27] | |
| Determination of total polyphenol content | Folin Ciocalteu Reagent, Gallic acid, Na2CO3, Methanol | [23] | |
| Free radical scavenging activity (antiradical activity) | 1,1-Diphenyl-2-picrylhydrazine | [28] | |
| Determination of anthocyanin content | Dilution at 550 nm with 96% ethanol containing 2% HCL at 2% v/v, followed by spectrophotometry | [24] | |
| Determination of leucoanthocyanins | spectrophotometrically after heating with a 40:60 mixture of hydrochloric acid and butanol containing ferrous sulphate | [24] | |
| Determination of catechin content | reacted with sulphuric acid vanillin in an alcohol-diluted solution at 500 nm by spectrophotometry | Vanillin | [25] |
| Resveratrol content determination | directly to HPLC | [26] |
| Polyphenol Name | Molecular Mechanism of the Protective Effect | Cell Culture | Level | Ref. |
|---|---|---|---|---|
| Epigallocatechin, EGCG 1, ECG 2 | Lipoxygenase and cyclooxygenase inhibition | Human colon mucosa and tumor tissue | In vitro | [37] |
| EGCG, ECG | ARE 3-mediated gene expression through activation of MAPK 4 proteins (ERK, JNK, P38) | Hep G2 ARE in C8 cells | In vitro | [38] |
| Catechin, Proanthocyanidin B4 | Increases CAT 5, GST 6 and SOD 7 activity, increases intracellular GSH 8 levels | Heart H9C2 cells | In vitro | [39] |
| EGCG, Quercetin, ECG | Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase | Rat brain F0F1 ATPase | In vitro | [40] |
| (-)-epicatechin, procyanidin, EGCG, ECG | The recombinant human platelet Inhibition of 12-lipoxygenase and 15-lipoxygenase | J774A-1 cells | In vitro | [41] |
| Resveratrol | Inhibition of O-acyltransferase and sulfotransferase activity Prevention of oxidative DNA damage | Ovine ovarian tissue | In vitro | [42] |
| Inhibition of H2O2 production and PMO activity Increasing GSH levels and SOD activity Reducing PMO and oxidized GR levels | Mouse skin | Ex vivo | [43] | |
| Quercetin | Inhibits LDH cleavage Increases the activity of SOD, CAT, GSH, GPx 9 and GR 10 | HepG2 cells | In vitro | [44] |
| MDA and lipoperoxidation coupling Increase in Cu/Zn SOD and GPx mRNA levels | Rooster semen | In vitro | [45] | |
| Increasing the expression and activity of NQO1 11 | MCF 7 in human breast cancer cells | In vitro | [46] | |
| γ-GCS 12 level increase | Central neuron cells | In vitro | [47] | |
| Increasing ARE binding activity and transcriptional activity regulated by NRF2 13 Activation and stabilization of NRF2 Keap 1 14 reduces protein levels | Human B lymphoma cells | In vitro | [48] | |
| Reduction of PhIP-DNA adduct formation catalysed by O-acyl transferase and sulfotransferase | Primary culture of human mammary epithelial and adipose cells | In vitro | [49] | |
| Inhibits the expression and activity of CYP1A1/1A2 15 | In microsomes and intact Hep G2 cells | In vitro | [50] | |
| Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase | Caco-2 cell line | In vitro | [51] |
| Polyphenol Name | Molecular Mechanism of the Protective Effect | Cell Culture | Level | Ref. |
|---|---|---|---|---|
| Resveratrol | Inhibition of MMP-9 1 expression and activity | Cisplatin-resistant human OSCC cell line | In vitro | [52] |
| Promotion of myocardial vessel formation by induction of VEGF 2, Trx-1 3 and HO-1 4 | H9C2 cells | In vitro | [53] | |
| Inhibition of the expression and binding activity of MCP-1 5 and CCR2 6 receptors | Endometriotic stomal cells | In vitro | [54] | |
| Increase NO and NOS levels Increasing intracellular cGMP levels and reducing ANP 7 and BNP 8 levels | U2OS cells | In vitro | [55] | |
| Reduces monocyte cell adhesion to stimulated endothelium Reduces VCAM-1 9 mRNA and protein formation | Human vascular endothelial cells | In vitro | [56] | |
| EC | 7β-OH inhibition of cholesterol formation | Smooth muscle cells | In vitro | [57] |
| Quercetin | Increase serum LDL-bound PON-1 10 levels | HuH7 in human liver cell line | In vitro | [58] |
| Induction of IFN-γ 11 gene expression Inhibition of IL-4 12 gene expression | Peripheral blood in Human Peripheral-blood CD4+ T cells | In vitro | [59] | |
| Increase in intracellular GSH levels and activation of the γ-GCS 13 heavy subunit (GCS(h)) promoter | Central neuron cell line | In vitro | [47] | |
| Genistein Daidzein | They are incorporated into LDL, increasing its resistance to oxidation and its effectiveness in inhibiting cell proliferation | Human colon cancer cell line | Ex vivo, in vitro | [60] |
| EGCG, EGC | Inhibition of rat VSMC 14 precipitation on collagen and laminin Interference with VSMC integrin β1 receptor and ECM protein binding | Rat VSMC | In vitro | [61] |
| Procyanidins | Reducing the leukotriene-to-prostacyclin ratio in blood plasma | Human aortic endothelial cells | In vitro | [62] |
| Proanthocyanidin | Inhibition of CD36 mRNA expression | THP-1 cells | In vitro | [63] |
| Polyphenol Name | Molecular Mechanism of the Protective Effect | Cell Culture | Level | Ref |
|---|---|---|---|---|
| Resveratrol | Stimulates AMP kinase activity | Neuro2a in cells and primary neurons; MC3T3-E1 cells and primary osteoblasts | In vitro | [64] |
| Activation of phosphorylation of PKC Transcitrin selection to prevent Aβ1 aggregation 1 | Rat hippocampal cell culture; endothelial cell culture | In vitro | [65] | |
| Protection of dopaminergic neurons Activation of the sirtuin family of NAD-dependent histone deacetylases | Organotypic mid-brain slice culture; human umbilical vein endothelial cells | In vitro | [66] | |
| EGCG, ECG, Myricetin | Inhibition of IL-6, IL-8, VEGF and PGE2 2 production Attenuation of COX-2 expression and NF-κB 3 activation Induction of MAPK phosphatase 1 expression Inhibition of phosphorylation of MAPK (p38 and JNK 4) | Human astrocytoma U373MG cell culture | In vitro | [67] |
| Attenuation of mitochondrial membrane potential rupture and release of CYT-C 5 Reducing caspase-9 and caspase-3 activity and increasing the BAX:BCL-2 ratio | Rat PC12 cells; HeLa cell line | In vitro | [68] | |
| Epicatechin | Protects neurons from programmed cell death induced by oxLDL 6 by inhibiting the activation of JNK, c-JUN and caspase-3 | Primer neuron cell culture | In vitro | [69] |
| Polyphenol Name | Molecular Mechanism of the Protective Effect | Cell Culture | Level | Ref. |
|---|---|---|---|---|
| Procyanidins | Inhibition of IL-1β transcription and secretion | ARPE-19 cells | In vitro | [72] |
| EGCG, ECG | Inducing programmed cell death by activating caspases 3, 8 and 9 | Caco-2 cells | In vitro | [73] |
| Inhibition of CD11b expression Inhibition of peripheral CD8+ T-cell migration and proliferation | HepG2 cells | In vitro | [74] | |
| Resveratrol | Inhibition of caspase-3 stimulation and IL-1β -induced cleavage of PARP | SH-SY5Y cells | In vitro | [75] |
| Inhibition of iNOS mRNA and protein expression by inhibiting NF-κB activation Inhibition of NO production | murine microglial cell line N9 | In vitro | [76] | |
| Activation of MAP kinase phosphatase | Prostate cells | In vitro | [77] | |
| Quercetin | Blocking the expression of ICAM-1 1, VCAM-1, and E-selectin Inhibition of PG synthesis and IL-6, 8 productions | HUVECs | In vitro | [78] |
| Inhibition of THP-1 adhesion and VCAM-1 expression activation | ARPE-19 cells | In vitro | [79] | |
| Inhibition of NO production and inhibition of iNOS 2 protein expression | hep g2 cells | In vitro | [80] | |
| Anthocyanins | Localization in endothelial cells Reduction of IL-8, MCP-1 and ICAM-1 activation | Caco-2 cells | In vitro | [81] |
| Polyphenol Name | Molecular Mechanism of the Protective Effect | Cell Culture | Level | Ref. |
|---|---|---|---|---|
| Resveratrol | Inhibition of cell proliferation and reduction of telomerase activity | Human cancer cell line HCT116 | In vitro | [86] |
| Stimulation of the P53-dependent pathway of programmed cell death | Human lung adenocarcinoma cells A549 | In vitro | [87] | |
| Inhibition of cell proliferation by interaction with the ERα 1-related PI3K pathway | Estrogen-sensitive MC3T3-E1 precursor cells | In vitro | [88] | |
| Inhibition of COX-2 expression through inhibition of MAPKs and AP-1 activation | RAW 264.7 macrophages | In vitro | [89] | |
| Reduction of expression of COX-1, COX-2, c-MYC, c-FOS, c-JUN, TGF-β 1 2 and TNF-α | Mucosal cell line | In vitro | [90] | |
| Inhibits oncogenic diseases through inhibition of protein kinase CKII activity | Human breast cancer mcf-7 cells | In vitro | [91] | |
| Inhibition of PKCα and PKCβI Ca2+-dependent activity | Smoth muscle cells | In vitro | [92] | |
| Prevents the formation of NB 3-DNS and NB-Hb 4 adducts | Hemoglobin of mice | In vivo | [93] | |
| Quercetin | Blocking EGFR tyrosine kinase activity | Xenografted NSCLC cells EGFR C797S mutation | In vitro | [94] |
| Quercetin, Myricetin | Inhibition of human CYP1A1 activity Inhibition of DE2 5 formation and B[a]P activation | O-deethylation of 7-ethoxyresorufin human lymphoblastoid TK6 cells | In vitro | [49] |
| Quercetin | Interaction with glycoprotein P and regulation of BCRP/ABCG2 6 activity | In two different cell lines expressing BCRP | In vitro | [95] |
| EGCG | Telomerase inhibition | In human cancer cells HeLa | In vitro | [96] |
| Polyphenol Name | Molecular Mechanism of the Protective Effect | Cell Culture | Level | Ref. |
|---|---|---|---|---|
| Proanthocyanidins | Accelerate programmed cell death by altering the cdki-cdk-cyclin cascade and reducing mitochondrial membrane potential through activation of cascade 3 | Human epidermoid carcinoma A431 cells | In vitro | [97] |
| Quercetin | Inhibition of phosphorylation of JNK and P38 MRK by ROS 1-mediated signaling | Murine macrophage cell line RAW 264.7 | In vitro | [98] |
| Actin/PKB and ERK1/2 signaling cascade to affect neuronal functionality | P19 neuronal cells | In vitro | [99] | |
| Resveratrol | Inhibits monocyte NO, MAPK and PI3K-dependent CCR2 binding | Rat fibroblast-like synoviocyte RSC-364 cell line | In vitro | [100] |
| Inhibit cardiac fibroblast division via NO-cGMP signaling | Rat heart in fibroblast culture | In vitro | [101] | |
| Activates phase II genes through regulation of ARE/EpRE activation Modifies the performance of KeapI by binding NRF2 | Lung cancer cells | In vitro | [102] |
| Polyphenol Name | Molecular Mechanism of the Protective Effect | Cell Culture | Level | Ref. |
|---|---|---|---|---|
| EGCG, Quercetin | Inhibition of programmed cell death through regulation of BCL-2 and BAX Inducing nuclear transactivation of P53 Reducing the activity of caspase 3 Blockade of JNK and P38 MARK-related singletons | 3T3-L1 preadipocytes | In vitro | [103] |
| Cy3G 1 | Increases eNOS expression and activity NO production triggering Regulation of phosphorylation of eNOS and AKT increase cGMP production | Endothelial cell line | In vitro | [104] |
| EGCG | Endothelium-dependent vasodilator effect Activates phosphatidylinositol 3-kinase, AKT, and eNOS. | HUVEC | In vitro | [105] |
| Increases the activity of eNOS Induces continuous activation of AKT, ERK1/2, and eNOS Phosphorylation of Ser1179 | Calf aortic endothelial cells | In vitro | [106] | |
| Catechins | Chicken CAM 2 angiogenin-like protein reduces angiogen-induced vascularization | In chicken cells | In vitro | [107] |
| Proanthocya-nidin | Reducing VCAM-1 expression Reduces TNFα-induced T cell binding to HUVEC | Primary HUVEC | In vitro | [108] |
| Procyanidine, flavan-3-ols | They inhibit the activity of ACE 3 | Two substrates | In vitro | [13] |
| Polyphenol Name | Molecular Mechanism of the Protective Effect | Cell Culture | Level | Ref. |
|---|---|---|---|---|
| EGCG, ECG | Inhibits SGLT1 and sodium-free GLUT | Polarized Caco-2 intestinal cells | In vitro | [109] |
| Quercetin | Reduces blood sugar levels Inhibits SVCT1 1 and GLUT2 | Intestinal cell model | In vitro | [110] |
| Tannin, anthocyanin | Inhibition of α-amylase and α-glucosidase | On 2-chloro-4-nitrophenyl-4-O-β-D-galactopyranosyl maltosyl substrate | In vitro | [111] |
| Polyphenol Name | Molecular Mechanism of the Protective Effect | Cell Culture | Level | Ref. |
|---|---|---|---|---|
| Resveratrol | Stimulates P21 expression and arrests the cell cycle in G1 phase | A375SM malignant melanoma | In vitro | [112] |
| Inhibition of cyclin D1/D2-cdk6 cyclin D1/D2-cdk4 cyclin E-cdk2 complexes | MCF7 cells | In vitro | [113] | |
| Decreases cyclin D1/Cdk4 complex and stimulates expression of cyclin E and A | Melanoma cells | In vitro | [114] | |
| Decrease the hyperphosphorylated form of pRb and increase the hypophosphorylated form of pRb Decrease expression of E2F (1–5) transcription factors and their heterodimer partners DP1, DP2 Leads to cell cycle arrest in the G0/G1 phase | Embryonic rat heart cell line | In vitro | [115] | |
| Proanthocyani dines | Inhibit expression of cyclin B1, D1, A1 and 𝛃-catenin | Human cancer cell lines | In vitro | [116] |
| They stop the cell cycle in the G1-S phase | VMSC at human hepatocellular carcinoma cells | In vitro | [117] |
| Type of Activity | Polyphenol Name | Molecular Mechanism of the Protective Effect | Cell Culture | Level | Ref. |
|---|---|---|---|---|---|
| Anti-HIV effect | Proanthocyanidins | Inhibits expression of the HIV-preventing chaperones CCR2b, CCR3, and CCR5. | Normal peripheral mononuclear cells | In vitro | [125] |
| Sensory effect | Proanthocyanidins, Resveratrol | Enhancing VEGF expression | Pigment cell culture; retinal ARPE-19 cells | In vitro | [124] |
| Liver protection | Genistein | Reduces experimental liver damage by preventing lipid peroxidation and enhancing the antioxidant system | Rat and Human hepatocyte-derived cell lines (ie HepG2 and Hep3B) | In vitro | [127] |
| Polyphenol Name | Molecular Mechanism of the Protective Effect | Target Organ/Disease | Type of Investigation | Biomarker | Animals | Ref. |
|---|---|---|---|---|---|---|
| Lipophilic Grape Seed Proanthocyanidin (LGSP) | Apoptosis via decreasing the expression of cyclin D1 and CDK 4 and increasing the expression of the tumor suppressors p21 and p27; activation of cleaved fragments of caspases 3, caspases 9, and PARP | PC3 Human Prostate Cancer Cell xenograft | xenograft model via oral gavage LGSP | Ki67 and cleaved caspase 3 immunostaining | PC3-derived mouse | [150] |
| Grape Seed Proanthocyanidin (GSP) | GSP induces autophagy, and inhibition of autophagy increased apoptosis in HepG2 cells; inducing the phosphorylation of mitogen-activated protein kinase (MAPK) pathway-associated proteins (p-JNK, p-ERK and p-p38 MAPK); reduces the expression of survivin | HepG2 (human liver cancer cells)-derived xenografts | xenograft model via oral gavage GSP | Ki67 immunostaining | nude mouse | [151] |
| Grape Seed Procyanidin | decrease the inflammation by PPAR-γ/COX-2 pathway | Pulmonary arterial hypertension model | treated with normoxia/cigarette smoke | mPAP, PVR, RVHI, WT%, and WA% was detected in the rats | Sprague Dawley rats | [152] |
| Grape Seed Proanthocyanidin (GSP) | endothelial nitric oxide synthase expression in lung tissue and plasma NO level were increased; Ca2+ level in pulmonary arterial smooth muscle cell (PASMC) was decreased; transcription of inflammatory factors such as myeloperoxidase, interleukin (IL)-1β, IL-6 and tumor necrosis factor alpha (TNF-α) was down-regulated in lung tissue; nuclear factor-κB pathway was inhibited as IκBα was less phosphorylated; TNFα-induced PASMC overproliferation could be inhibited | Pulmonary arterial hypertension model | treated with monocrotaline | Haemodynamic index, mean pulmonary arterial pressure (mPAP), cardiac output (CO), pulmonary vessel resistance (PVR), right ventricular hypertrophy index (RVHI), WT%, WA%, pulmonary blood pressure NO assay, cytosolic Ca2+ detection | Sprague Dawley rats | [153] |
| Grape Seed Proanthocyanidin (GSP) | promoted locomotor recovery, reduced neuronal apoptosis, increased neuronal preservation, and regulated microglial polarization; microglial polarization and prevents neuronal apoptosis, possibly by the TLR4-mediated NF-κB and PI3K/AKT signaling pathways | Spinal cord injury | T9 vertebral laminectomy | Locomotor Recovery Assessment; Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL) Assay; Annexin V-FITC/PI Assays; NO assay, Immunofluorescence staining: NeuN, GFAP, CD86, CD206, p-NF-κB-p65, p-AKT | Sprague Dawley rats | [154] |
| Red grape seed and skin extract | GSSE was effective in protecting dopamine neurons from 6-OHDA toxicity by reducing apoptosis, the level of reactive oxygen species (ROS) and inflammation; reducing the cleaved caspase-3 activity that helps inhibit 6-OHDA-induced mDA neuron death in a cellular model of PD; decreases ROS production induced by 6- OHDA in ESC-derived DA neurons; decreases phospho-NF-kB p65 activation induced by 6-OHDA in dopaminergic neurons; rescues motor deficits induced by 6- OHDA; prevents the loss of midbrain dopaminergic neurons (mDA) in a 6-OHDA mouse model of PD; prevents the loss of SOD1 level induced by 6-OHDA lesion | Parkinson’s disease | neurotoxin 6-hydroxydopamine (6-OHDA), which induces oxidative damage and mimics the degeneration of dopaminergic neurons observed in Parkinson’s disease | Immunostaining: MAP2, AB5622, r tyrosine hydroxylase, caspase-3, phosphorylated NF-kB p65; ROS assay, | mice | [155] |
| Polyphenol Name | Molecular Mechanism of Therapeutic Effect | Target Organ/Disease | Type of Investigation | Biomarker | Patients | Ref. |
|---|---|---|---|---|---|---|
| Resveratrol | STAT3/HIF-1/VEGF pathway | Rheumatoid arthritis | Randomized controlled clinical trial | CRP, DAS28-ESR, ESR, IL-6, MMP-3, RF, TNF-α, ucOC | 100 | [156] |
| Grape seed extract | Reduces FPG, TC, LDL cholesterol, and triglycerides levels; | Glycemic control | Randomized controlled clinical trial | serum TC, LDL, VLDL, HDL colesterol, triglycerides level | 50 | [157] |
| Grape seed extract | Suppress lipoxygenase pathways; increase pro-inflammatory leukotrienes | Inflammation | Randomized controlled clinical trial | CRP, pro-inflammatory leukotrienes, cytokine pattern | 50 | [157] |
| Grape seed extract | VEGF, anti-inflammatory activity through cytokines (TNF, IL-1, IL-6, IL-14), antibacterial activity, antioxidant activity | Wound healing after Cesarean section | Randomized controlled clinical trial | REEDA scale (redness, edema, ecchymosis, discharge, and approximation) | 129 | [158] |
| Grape seed procyanidin extract | inhibit the proinflammatory and procarcinogenic COX-2/PGE2 pathways; 15-lipoxygenase (15-LOX) and 15-Hydroxyeicosatetraenoic acid (15-HETE) pathways | Lung cancer | Randomized controlled clinical trial | Ki67 proliferative labeling index; serum miR-19a, -19b, and -106b | 287 (146/control 141) | [159] |
| Grape seed procyanidin extract | COX-2/PGE2 pathways | Lung cancer | Randomized controlled clinical trial | Serum PGE3 and leukotriene B5 (LTB5) | 287 | [160] |
| Grape seed extract | Reduces TNF and IL-6 level, and TG and VLDL level decreases and HDL-C level increases. It protects against atherosclerosis | Cardiovascular prevention in obesity | Randomized, double-blinded, placebo-controlled clinical trial | visceral adiposity index (VAI), and atherogenic index of plasma (AIP); plasma LDL-C level | 50 (25/25) | [161] |
| Grape seed extract | Increases glucose transport | insulin resistance in metabolic syndrome | Randomized controlled clinical trial | Plasma FBG, TG, HDL-C and insulin level | 48 (24/24) | [162] |
| Red grape seed extract | Reduces TNF and IL-6 level, TG and VLDL level decreases, and HDL-C level increases. | hyperlipidaemia | Randomized controlled clinical trial | apolipoprotein AI and paraoxonase activity | 70 | [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegedüs, I.; Andreidesz, K.; Szentpéteri, J.L.; Kaleta, Z.; Szabó, L.; Szigeti, K.; Gulyás, B.; Padmanabhan, P.; Budan, F.; Máthé, D. The Utilization of Physiologically Active Molecular Components of Grape Seeds and Grape Marc. Int. J. Mol. Sci. 2022, 23, 11165. https://doi.org/10.3390/ijms231911165
Hegedüs I, Andreidesz K, Szentpéteri JL, Kaleta Z, Szabó L, Szigeti K, Gulyás B, Padmanabhan P, Budan F, Máthé D. The Utilization of Physiologically Active Molecular Components of Grape Seeds and Grape Marc. International Journal of Molecular Sciences. 2022; 23(19):11165. https://doi.org/10.3390/ijms231911165
Chicago/Turabian StyleHegedüs, Imre, Kitti Andreidesz, József L. Szentpéteri, Zoltán Kaleta, László Szabó, Krisztián Szigeti, Balázs Gulyás, Parasuraman Padmanabhan, Ferenc Budan, and Domokos Máthé. 2022. "The Utilization of Physiologically Active Molecular Components of Grape Seeds and Grape Marc" International Journal of Molecular Sciences 23, no. 19: 11165. https://doi.org/10.3390/ijms231911165
APA StyleHegedüs, I., Andreidesz, K., Szentpéteri, J. L., Kaleta, Z., Szabó, L., Szigeti, K., Gulyás, B., Padmanabhan, P., Budan, F., & Máthé, D. (2022). The Utilization of Physiologically Active Molecular Components of Grape Seeds and Grape Marc. International Journal of Molecular Sciences, 23(19), 11165. https://doi.org/10.3390/ijms231911165








