Lipophilic Compounds and Antibacterial Activity of Opuntia ficus-indica Root Extracts from Algeria
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of O. ficus-indica Roots
2.2. Lipophilic Fraction of O. ficus-indica Roots
2.2.1. Extraction Yield
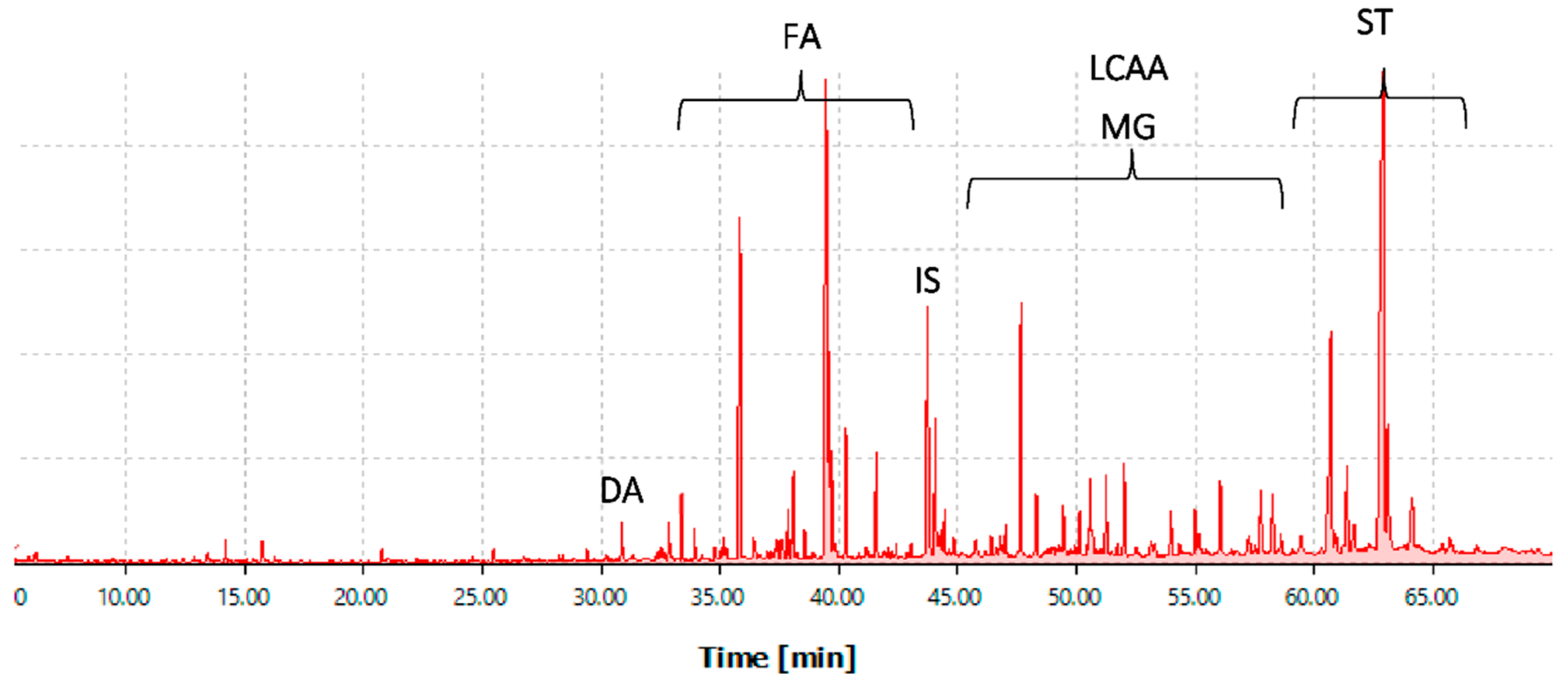
2.2.2. Lipophilic Composition
Fatty Acids
Long-Chain Aliphatic Alcohols
Sterols
Monoglycerides
Other Compounds Identified
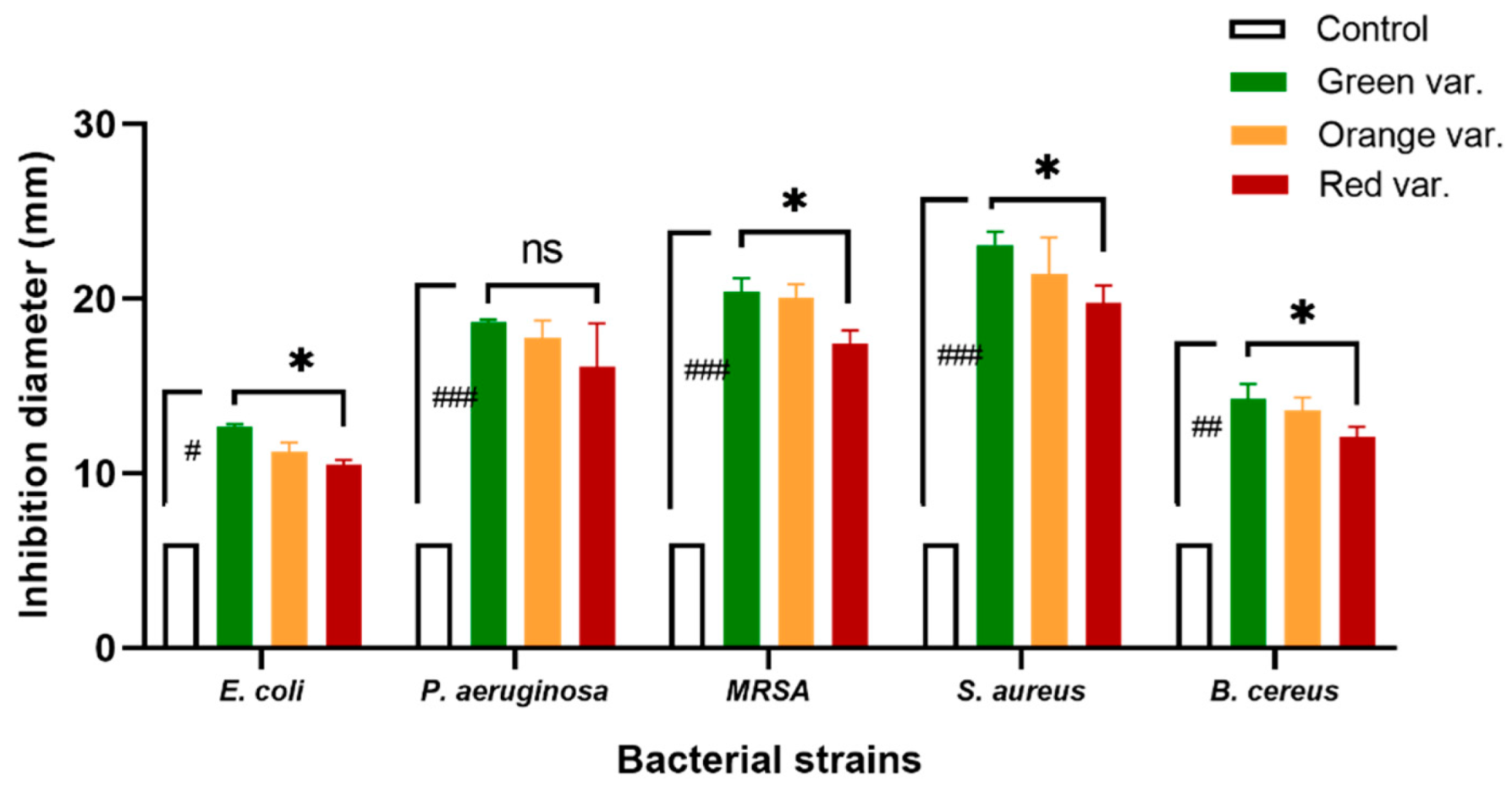
2.3. Antibacterial Activity
2.3.1. Bacteria Inhibition on Agar Medium
2.3.2. Minimal Inhibitory Concentration
3. Materials and Methods
3.1. Reagents
3.2. Harvest and Post-Harvest Processes
3.3. Physicochemical Proprieties Measurement
3.4. Extraction of Lipophilic Compounds
3.5. Gas Chromatography-Mass Spectrometry Analysis
3.6. Antibacterial Activity
3.6.1. Bacterial Strains and Culture Conditions
3.6.2. Agar Medium Diffusion Test
3.6.3. Determination of the Minimal Inhibition Concentration (MIC)
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Santos-Díaz, M.D.S.; Camarena-Rangel, N.G. Cacti for production of metabolites: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 8657–8667. [Google Scholar] [CrossRef] [PubMed]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Opuntia (Cactaceae) plant compounds, biological activities and prospects–A comprehensive review. Food Res. Int. 2018, 112, 328–344. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Komla, M.G. Wild Fruits: Composition, Nutritional Value and Products. Wild Fruits Compos. Nutr. Value Prod. 2019, 30, 333–358. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, A.; Sharma, R. Pharmacological actions of Opuntia ficus indica: A review. J. Appl. Pharm. Sci. 2012, 2, 15–18. [Google Scholar] [CrossRef]
- Nobel, P.S. Cactus roots: Depth and sensitivity to extreme temperatures. Acta Hortic. 2009, 811, 383–388. [Google Scholar] [CrossRef]
- Nobel, P.S.; de Cortázar, V.G. Growth and Predicted Productivity of Opuntia ficus-indica for Current and Elevated Carbon Dioxide. Agron. J. 1991, 83, 224–230. [Google Scholar] [CrossRef]
- Dubrovsky, J.G.; North, G.B.; Nobel, P.S. Root growth, developmental changes in the apex, and hydraulic conductivity for Opuntia ficus-indica during drought. New Phytol. 1998, 138, 75–82. [Google Scholar] [CrossRef]
- Mena, P.; Tassotti, M.; Andreu, L.; Nuncio-Jáuregui, N.; Legua, P.; Del Rio, D.; Hernández, F. Phytochemical characterization of different prickly pear (Opuntia ficus-indica (L.) Mill.) cultivars and botanical parts: UHPLC-ESI-MSn metabolomics profiles and their chemometric analysis. Food Res. Int. 2018, 108, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Cruz, D.M.; Pérez-Ramírez, I.F.; Delgado-García, J.; Mondragón-Jacobo, C.; Dector-Espinoza, A.; Reynoso-Camacho, R. An integral profile of bioactive compounds and functional properties of prickly pear (Opuntia ficus indica L.) peel with different tonalities Diana. Food Chem. 2019, 278, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, Betalain Pattern, and Antioxidant Properties of Cactus Pear (Opuntia spp.) Clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Chougui, N.; Louaileche, H.; Mohedeb, S.; Mouloudj, Y.; Hammoui, Y.; Tamendjari, A. Physico-chemical characterisation and antioxidant activity of some Opuntia ficus-indica varieties grown in North Algeria. Afr. J. Biotechnol. 2013, 12, 299–307. [Google Scholar] [CrossRef]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant Activities of Sicilian Prickly Pear (Opuntia ficus indica) Fruit Extracts and Reducing Properties of Its Betalains: Betanin and Indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef] [PubMed]
- Chougui, N.; Tamendjari, A.; Hamidj, W.; Hallal, S.; Barras, A.; Richard, T.; Larbat, R. Oil composition and characterisation of phenolic compounds of Opuntia ficus-indica seeds. Food Chem. 2013, 139, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Taoufik, F.; Zine, S.; El Hadek, M.; Idrissi Hassani, L.M.; Gharby, S.; Harhar, H.; Matthäus, B. Oil content and main constituents of cactus seed oils Opuntia Ficus Indica of different origin in Morocco. Med. J. Nutr. Metab. 2015, 8, 85–92. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Kita, A.; Miedzianka, J.; Andreu-Coll, L.; Legua, P.; Hernandez, F. Characterization of Bioactive Compounds of Opuntia ficus-indica (L.) Mill. Seeds from Spanish Cultivars. Molecules 2020, 25, 5734. [Google Scholar] [CrossRef]
- Guevara-Figueroa, T.; Jiménez-Islas, H.; Reyes-Escogido, M.L.; Mortensen, A.G.; Laursen, B.B.; Lin, L.-W.W.; De León-Rodríguez, A.; Fomsgaard, I.S.; de la Rosa, A.P.; Barba de la Rosa, A.P.; et al. Proximate composition, phenolic acids, and flavonoids characterization of commercial and wild nopal ({Opuntia} spp.). J. Food Compos. Anal. 2010, 23, 525–532. [Google Scholar] [CrossRef]
- De Leo, M.; De Abreu, M.B.; Pawlowska, A.M.; Cioni, P.L.; Braca, A. Profiling the chemical content of Opuntia ficus-indica flowers by HPLC–PDA-ESI-MS and GC/EIMS analyses. Phytochem. Lett. 2010, 3, 48–52. [Google Scholar] [CrossRef]
- Ammar, I.; Ben Salem, M.; Harrabi, B.; Mzid, M.; Bardaa, S.; Sahnoun, Z.; Attia, H.; Ennouri, M. Anti-inflammatory activity and phenolic composition of prickly pear ({Opuntia} ficus-indica) flowers. Ind. Crops Prod. 2018, 112, 313–319. [Google Scholar] [CrossRef]
- Tesoriere, L.; Butera, D.; Pintaudi, A.M.; Allegra, M.; Livrea, M.A. Supplementation with cactus pear (Opuntia ficus-indica) fruit decreases oxidative stress in healthy humans: A comparative study with vitamin C. Am. J. Clin. Nutr. 2004, 80, 391–395. [Google Scholar] [CrossRef]
- Galati, E.M.; Monforte, M.T.; Miceli, N.; Mondello, M.R.; Taviano, M.F.; Galluzzo, M.; Tripodo, M.M. Opuntia ficus indica (L.) Mill, mucilages show cytoprotective effect on gastric mucosa in rat. Phyther. Res. 2007, 21, 344–346. [Google Scholar] [CrossRef]
- Benayad, Z.; Martinez-Villaluenga, C.; Frias, J.; Gomez-Cordoves, C.; Es-Safi, N.E. Phenolic composition, antioxidant and anti-inflammatory activities of extracts from Moroccan Opuntia ficus-indica flowers obtained by different extraction methods. Ind. Crops Prod. 2014, 62, 412–420. [Google Scholar] [CrossRef]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Gentile, C.; Livrea, M.A. Indicaxanthin inhibits NADPH oxidase (NOX)-1 activation and NF-κB-dependent release of inflammatory mediators and prevents the increase of epithelial permeability in IL-1β-exposed Caco-2 cells. Br. J. Nutr. 2014, 111, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Allegra, M.; De Cicco, P.; Ercolano, G.; Attanzio, A.; Busà, R.; Cirino, G.; Tesoriere, L.; Livrea, M.A.; Ianaro, A. Indicaxanthin from Opuntia Ficus Indica (L. Mill) impairs melanoma cell proliferation, invasiveness, and tumor progression. Phytomedicine 2018, 50, 19–24. [Google Scholar] [CrossRef]
- Serra, A.T.; Poejo, J.; Matias, A.A.; Bronze, M.R.; Duarte, C.M.M. Evaluation of Opuntia spp. derived products as antiproliferative agents in human colon cancer cell line (HT29). Food Res. Int. 2013, 54, 892–901. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Hernández-Reyes, A.; Uscanga-Palomeque, A.C.; Rodríguez-Padilla, C.; Martínez-Torres, A.C.; Gutiérrez-Uribe, J.A. Isorhamnetin glycoside isolated from Opuntia ficus-indica (L.) MilI induces apoptosis in human colon cancer cells through mitochondrial damage. Chem. Biol. Interact. 2019, 310, 108734. [Google Scholar] [CrossRef] [PubMed]
- Alimi, H.; Hfaiedh, N.; Bouoni, Z.; Sakly, M.; Ben Rhouma, K. Evaluation of antioxidant and antiulcerogenic activities of {Opuntia} ficus indica f. inermis flowers extract in rats. Environ. Toxicol. Pharmacol. 2011, 32, 406–416. [Google Scholar] [CrossRef]
- Jeon, Y.E.; Yin, X.F.; Choi, D.B.; Lim, S.S.; Kang, I.J.; Shim, J.H. Inhibitory activity of aromadendrin from prickly pear (Opuntia ficus-indica) root on aldose reductase and the formation of advanced glycation end products. Food Sci. Biotechnol. 2011, 20, 1283–1288. [Google Scholar] [CrossRef]
- Argudín, M.Á.; Mendoza, M.C.; Rodicio, M.R. Food Poisoning and Staphylococcus aureus Enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef] [PubMed]
- Fluit, A.C.; Schmitz, F.J.; Verhoef, J.; Schmitz, F.-J. Frequency of Isolation of Pathogens from Bloodstream, Nosocomial Pneumonia, Skin and Soft Tissue, and Urinary Tract Infections Occurring in European Patients and the European SENTRY Participant Group. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Manabe Id, T.; Fujikura, Y.; Mizukami, K.; Akatsu, H.; Kudo, K. Pneumonia-associated death in patients with dementia: A systematic review and meta-analysis. PLoS ONE 2019, 14, 0213825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreu, L.; Nuncio-Jáuregui, N.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Antioxidant properties and chemical characterization of Spanish Opuntia ficus-indica Mill. cladodes and fruits. J. Sci. Food Agric. 2018, 98, 1566–1573. [Google Scholar] [CrossRef]
- Borland, A.M.; Hartwell, J.; Weston, D.J.; Schlauch, K.A.; Tschaplinski, T.J.; Tuskan, G.A.; Yang, X.; Cushman, J.C. Engineering crassulacean acid metabolism to improve water-use efficiency. Trends Plant Sci. 2014, 19, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Chougui, N.; Bachir-bey, M.; Tamendjari, A. Morphological and physicochemical diversity of prickly pears in Bejaia-Algeria. Int. J. Sci. Eng. Res. 2016, 7, 20. [Google Scholar]
- Nabil, B.; Ouaabou, R.; Ouhammou, M.; Saadouni, L.; Mahrouz, M. Impact of particle size on functional, physicochemical properties and antioxidant activity of cladode powder (Opuntia ficus-indica). J. Food Sci. Technol. 2020, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- El Kharrassi, Y.; Mazri, M.A.; Benyahia, H.; Benaouda, H.; Nasser, B.; El Mzouri, E.H. Fruit and juice characteristics of 30 accessions of two cactus pear species (Opuntia ficus indica and Opuntia megacantha) from different regions of Morocco. LWT-Food Sci. Technol. 2016, 65, 610–617. [Google Scholar] [CrossRef]
- Dubeux Junior, J.C.B.; Silva, N.G.M.; Santos, M.V.F.; Cunha, M.V.; Santos, D.C.; Lira, M.A.; Mello, A.C.L.; Pinto, M.S.C. Organic fertilization and plant population affect shoot and root biomass of forage cactus pear (Opuntia ficus-indica mill.). Acta Hortic. 2013, 995, 221–224. [Google Scholar] [CrossRef]
- Silva, R.P.; de Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Polar and lipophilic extracts characterization of roots, stalks, leaves and flowers of water hyacinth (Eichhornia crassipes), and insights for its future valorization. Ind. Crops Prod. 2015, 76, 1033–1038. [Google Scholar] [CrossRef]
- Rocchetti, G.; Pellizzoni, M.; Montesano, D.; Lucini, L. Italian opuntia ficus-indica cladodes as rich source of bioactive compounds with health-promoting properties. Foods 2018, 7, 24. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Mörsel, J.-T. Recovered lipids from prickly pear [Opuntia ficus-indica (L.) Mill] peel: A good source of polyunsaturated fatty acids, natural antioxidant vitamins and sterols. Food Chem. 2003, 83, 447–456. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Martínez-Cruz, O.; Paredes-Lopez, O. Phytochemical Content, Nutraceutical Potential and Biotechnological Applications of an Ancient Mexican Plant: Nopal (Opuntia ficus-indica). Curr. Nutr. Food Sci. 2014, 10, 196–217. [Google Scholar] [CrossRef]
- Bentley, G. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2007, 32, 82–84. [Google Scholar] [CrossRef]
- Parodi, P.W. Has the association between saturated fatty acids, serum cholesterol and coronary heart disease been over emphasized? Int. Dairy J. 2009, 19, 345–361. [Google Scholar] [CrossRef]
- Briggs, M.A.; Petersen, K.S.; Kris-Etherton, P.M. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef]
- Kitahara, T.; Koyama, N.; Matsuda, J.; Aoyama, Y.; Hirakata, Y.; Kamihira, S.; Kohno, S.; Nakashima, M.; Sasaki, H. Antimicrobial Activity of Saturated Fatty Acids and Fatty Amines against Methicillin-Resistant Staphylococcus aureus. Biol. Pharm. Bull. 2004, 27, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Parfene, G.; Horincar, V.; Tyagi, A.K.; Malik, A.; Bahrim, G. Production of medium chain saturated fatty acids with enhanced antimicrobial activity from crude coconut fat by solid state cultivation of Yarrowia lipolytica. Food Chem. 2013, 136, 1345–1349. [Google Scholar] [CrossRef]
- Togashi, N.; Shiraishi, A.; Nishizaka, M.; Matsuoka, K.; Endo, K.; Hamashima, H.; Inoue, Y. Antibacterial activity of long-chain fatty alcohols against Staphylococcus aureus. Molecules 2007, 12, 139. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Kubo, A. Antibacterial Activity of Long-Chain Alcohols against Streptococcus mutans. J. Agric. Food Chem. 1993, 41, 2447–2450. [Google Scholar] [CrossRef]
- Mukherjee, K.; Tribedi, P.; Mukhopadhyay, B.; Sil, A.K. Antibacterial activity of long-chain fatty alcohols against mycobacteria. FEMS Microbiol. Lett. 2013, 338, 177–183. [Google Scholar] [CrossRef]
- Figueroa-Pérez, M.G.; Pérez-Ramírez, I.F.; Paredes-López, O.; Mondragón-Jacobo, C.; Reynoso-Camacho, R. Phytochemical composition and in vitro analysis of nopal (O. Ficus-Indica) cladodes at different stages of maturity. Int. J. Food Prop. 2018, 21, 1728–1742. [Google Scholar] [CrossRef]
- Salvo, F.; Galati, E.M.; Lo Curto, S.; Tripodo, M.M. Chemical Characterization of Opuntia ficus-indica Seed Oil. Acta Hortic. 2002, 581, 283–289. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Elmelegy, A.A.; Eldesoky, S.E.; Safwat, G. Phytochemical screening, antimicrobial, antioxidant, anticancer activities and nutritional values of cactus (Opuntia ficus-indica) pulp and peel. Fresenius Environ. Bull. 2019, 28, 18. [Google Scholar]
- Khémiri, I.; Bitri, L. Effectiveness of Opuntia ficus indica L. inermis Seed Oil in the Protection and the Healing of Experimentally Induced Gastric Mucosa Ulcer. Oxid. Med. Cell. Longev. 2019, 2019, 1568720. [Google Scholar] [CrossRef] [Green Version]
- Reiner, E.; Topliff, J.; Wood, J.D. Hypocholesterolemic agents derived from sterols of marine algae. Can. J. Biochem. Physiol. 1962, 40, 1401–1406. [Google Scholar] [CrossRef]
- Katan, M.B.; Grundy, S.M.; Jones, P.; Law, M.; Miettinen, T.; Paoletti, R. Efficacy and Safety of Plant Stanols and Sterols in the Management of Blood Cholesterol Levels. Mayo Clin. Proc. 2003, 78, 965–978. [Google Scholar] [CrossRef]
- Kim, J.A.; Son, J.H.; Song, S.B.; Yang, S.Y.; Kim, Y.H. Sterols isolated from seeds of Panax ginseng and their antiinflammatory activities. Pharmacogn. Mag. 2013, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Bok, J.W.; Lermer, L.; Chilton, J.; Klingeman, H.G.; Towers, G.H.N. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry 1999, 51, 891–898. [Google Scholar] [CrossRef]
- Pierre Luhata, L.; Usuki, T. Antibacterial activity of β-sitosterol isolated from the leaves of Odontonema strictum (Acanthaceae). Bioorg. Med. Chem. Lett. 2021, 48, 128248. [Google Scholar] [CrossRef] [PubMed]
- Téné, D.G.; Tih, A.E.; Kamdem, M.H.K.; Talla, R.M.; Diboue, P.H.B.; Melongo, Y.K.D.; Dzukoug, C.R.; Mmutlane, E.M.; Ndinteh, D.T.; Bodo, B.; et al. Antibacterial and antioxidant activities of compounds isolated from the leaves of Symphonia globulifera (Clusiaceae) and their chemophenetic significance. Biochem. Syst. Ecol. 2021, 99, 104345. [Google Scholar] [CrossRef]
- Altieri, C.; Bevilacqua, A.; Cardillo, D.; Sinigaglia, M. Effectiveness of fatty acids and their monoglycerides against gram-negative pathogens. Int. J. Food Sci. Technol. 2009, 44, 359–366. [Google Scholar] [CrossRef]
- Altieri, C.; Cardillo, D.; Bevilacqua, A.; Sinigaglia, M. Inhibition of Aspergillus spp. and Penicillium spp. by Fatty Acids and Their Monoglycerides. J. Food Prot. 2007, 70, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Altieri, C.; Bevilacqua, A.; Cardillo, D.; Sinigaglia, M. Antifungal activity of fatty acids and their monoglycerides against Fusarium spp. in a laboratory medium. Int. J. Food Sci. Technol. 2009, 44, 242–245. [Google Scholar] [CrossRef]
- Jialal, I.; Devaraj, S.; Venugopal, S.K. Oxidative Stress, Inflammation, and Diabetic Vasculopathies: The Role of Alpha Tocopherol Therapy. Free Radic. Res. 2002, 36, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Parafati, L.; Arena, E.; Grassenio, E.; Restuccia, C.; Fallico, B. Antioxidant and Antimicrobial Properties of Semi-Processed Frozen Prickly Pear Juice as Affected by Cultivar and Harvest Time. Foods 2020, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evid. Based Complement. Altern. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef] [PubMed]
- Welegerima, G.; Zemene, A. Antibacterial activity of Opuntia ficus-indica skin fruit extracts. Biotechnol. Int. 2017, 10, 74–83. [Google Scholar]
- Vaquero, M.J.R.; Alberto, M.R.; de Nadra, M.C.M. Antibacterial effect of phenolic compounds from different wines. Food Control 2007, 2, 93–101. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.; Kudanga, T. Phenolic compound profile and biological activities of Southern African Opuntia ficus-indica fruit pulp and peels. LWT 2019, 111, 337–344. [Google Scholar] [CrossRef]
- R’bia, O.; Chkioua, C.; Hellal, R.; Herchi, W.; Smiti, S.A. Opuntia ficus indica tohum yağ fraksiyonlarının antioksidan ve antibakteriyel aktiviteleri ve biyoaktif bileşikleri tanımlama. Turk. J. Biochem. 2017, 42, 481–491. [Google Scholar] [CrossRef]
- Ennouri, M.; Ammar, I.; Khemakhem, B.; Attia, H. Chemical composition and antibacterial activity of Opuntia ficus-indica f. inermis (cactus pear) flowers. J. Med. Food 2014, 17, 908–914. [Google Scholar] [CrossRef]
- Welegerima, G.; Zemene, A.; Tilahun, Y. Phytochemical composition and antibacterial activity of Opuntia ficus indica cladodes extracts. J. Med. Plants Stud. 2018, 6, 243–246. [Google Scholar]
- Bargougui, A.; Tag, H.M.; Bouaziz, M.; Triki, S. Antimicrobial, antioxidant, total phenols and flavonoids content of four cactus (opuntiaficus-indica) cultivars. Biomed. Pharmacol. J. 2019, 12, 1353–1368. [Google Scholar] [CrossRef]
- Ortega-Ortega, M.D.L.A.; Cruz-Cansino, N.D.S.; Alanís-García, E.; Delgado-Olivares, L.; Ariza-Ortega, J.A.; Ramírez-Moreno, E.; Manríquez-Torres, J.D.J. Optimization of ultrasound extraction of cactus pear (Opuntia ficus indica) seed oil based on antioxidant activity and evaluation of its antimicrobial activity. J. Food Qual. 2017, 2017, 9315360. [Google Scholar] [CrossRef]
- Mabotja, M.B.; Venter, S.L.; Du Plooy, C.P.; Kudanga, T.; Amoo, S.O. Phytochemical Content, Antioxidant, Alpha-Glucosidase Inhibitory and Antibacterial Activities of Spineless Cactus Pear Cultivars. Plants 2021, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Karadağ, A.E.; Demirci, B.; Polat, D.Ç.; Okur, M.E. Characterization of Opuntia ficus-indica (L.) Mill. fruit volatiles and antibacterial evaluation. Nat. Volatiles Essent. Oils 2018, 5, 35–38. [Google Scholar]
- Blando, F.; Russo, R.; Negro, C.; De Bellis, L.; Frassinetti, S. Antimicrobial and Antibiofilm Activity against Staphylococcus aureus of Opuntia ficus-indica (L.) Mill. Cladode Polyphenolic Extracts. Antioxidants 2019, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Correia, F.C.S.; Targanski, S.K.; Bomfim, T.R.D.; Da Silva, Y.S.A.D.; Violante, I.M.P.; De Carvalho, M.G.; De Sousa, P.T.; Silva, V.C.P.; Ribeiro, T.A.N. Chemical constituents and antimicrobial activity of branches and leaves of Cordia insignis (Boraginaceae). Rev. Virtual Quim. 2020, 12, 809–816. [Google Scholar] [CrossRef]
- Feng, Y.; Assani, I.; Wang, C.G.; Hou, P.L.; Zhao, S.F.; Ye, H.J.; Li, R.C.; Zhang, J.B.; Liao, Z.X. A New Aliphatic Ketone, Chemical Composition, Antibacterial, Antioxidant and In Vitro Cytotoxic Activities of Lepidium latifolium. ChemistrySelect 2020, 5, 8992–8997. [Google Scholar] [CrossRef]
- Doğan, A.; Otlu, S.; Çelebi, Ö.; Kilicle, P.A.; Sağlam, A.G.; Doğan, A.N.C.; Mutlu, N. An investigation of antibacterial effects of steroids. Turk. J. Vet. Anim. Sci. 2017, 41, 302–305. [Google Scholar] [CrossRef]
- Rabah, S.; Kouachi, K.; Ramos, P.A.B.; Gomes, A.P.; Almeida, A.; Haddadi-Guemghar, H.; Khodir, M.; Silvestre, A.J.D.; Santos, S.A.O. Unveiling the bioactivity potential of Allium triquetrum L. lipophilic fraction: Chemical characterization and in vitro antibacterial activity against methicillin-resistant staphylococcus aureus. Food Funct. 2020, 11, 5257–5265. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; Volume 1, ISBN 0935584145. [Google Scholar]
- Ramos, P.A.B.; Moreirinha, C.; Santos, S.A.O.; Almeida, A.; Freire, C.S.R.; Silva, A.M.S.; Silvestre, A.J.D. Valorisation of bark lipophilic fractions from three Portuguese Salix species: A systematic study of the chemical composition and inhibitory activity on Escherichia coli. Ind. Crops Prod. 2019, 132, 245–252. [Google Scholar] [CrossRef]
- Vilela, C.; Santos, S.A.O.; Oliveira, L.; Camacho, J.F.; Cordeiro, N.; Freire, C.S.R.; Silvestre, A.J.D. The ripe pulp of Mangifera indica L.: A rich source of phytosterols and other lipophilic phytochemicals. Food Res. Int. 2013, 54, 1535–1540. [Google Scholar] [CrossRef]
- Vilela, C.; Santos, S.A.O.; Villaverde, J.J.; Oliveira, L.; Nunes, A.; Cordeiro, N.; Freire, C.S.R.; Silvestre, A.J.D. Lipophilic phytochemicals from banana fruits of several Musa species. Food Chem. 2014, 162, 247–252. [Google Scholar] [CrossRef]
- Oliveira, C.S.D.; Moreira, P.; Resende, J.; Cruz, M.T.; Pereira, C.M.F.; Silva, A.M.S.; Santos, S.A.O.; Silvestre, A.J.D. Characterization and cytotoxicity assessment of the lipophilic fractions of different morphological parts of acacia dealbata. Int. J. Mol. Sci. 2020, 21, 1814. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Oliveira, C.S.D.; Trindade, S.S.; Abreu, M.H.; Rocha, S.S.M.; Silvestre, A.J.D. Bioprospecting for lipophilic-like components of five Phaeophyta macroalgae from the Portuguese coast. J. Appl. Phycol. 2016, 28, 3151–3158. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Trindade, S.S.; Oliveira, C.S.D.; Parreira, P.; Rosa, D.; Duarte, M.F.; Ferreira, I.; Cruz, M.T.; Rego, A.M.; Abreu, M.H.; et al. Lipophilic fraction of cultivated Bifurcaria bifurcata R. Ross: Detailed composition and in vitro prospection of current challenging bioactive properties. Mar. Drugs 2017, 15, 340. [Google Scholar] [CrossRef] [Green Version]
- Salvador, Â.C.; Simões, M.M.Q.; Silva, A.M.S.; Santos, S.A.O.; Rocha, S.M.; Silvestre, A.J.D. Vine waste valorisation: Integrated approach for the prospection of bioactive lipophilic phytochemicals. Int. J. Mol. Sci. 2019, 20, 4239. [Google Scholar] [CrossRef]
- SFM. Recommandations 2019 V.1.0 Janvier. Clin. Microbiol. Infect. 2019, 2, 144. [Google Scholar]
- Djenane, D.; Yangüela, J.; Montañés, L.; Djerbal, M.; Roncalés, P. Antimicrobial activity of Pistacia lentiscus and Satureja montana essential oils against Listeria monocytogenes CECT 935 using laboratory media: Efficacy and synergistic potential in minced beef. Food Control 2011, 22, 1046–1053. [Google Scholar] [CrossRef]
| Green | Red | Orange | |
|---|---|---|---|
| Moisture (%) | 78.71 ± 0.16 b * | 80.87 ± 0.14 a | 76.99 ± 0.08 c |
| Acidity (% citric acid) | 0.24 ± 0.01 b | 0.16 ± 0.01 c | 0.26 ± 0.01 a |
| Brix (%) | 10.75 ± 0.00 | 8.75 ± 0.00 | 7.00 ± 0.00 |
| pH | 5.49 ± 0.26 b | 6.16 ± 0.09 a | 5.25 ± 0.19 b |
| Content mg g−1 Extract | Content mg kg−1 dw | ||||||
|---|---|---|---|---|---|---|---|
| RT (min) | Compound Name | Green var. | Orange var. | Red var. | Green var. | Orange var. | Red var. |
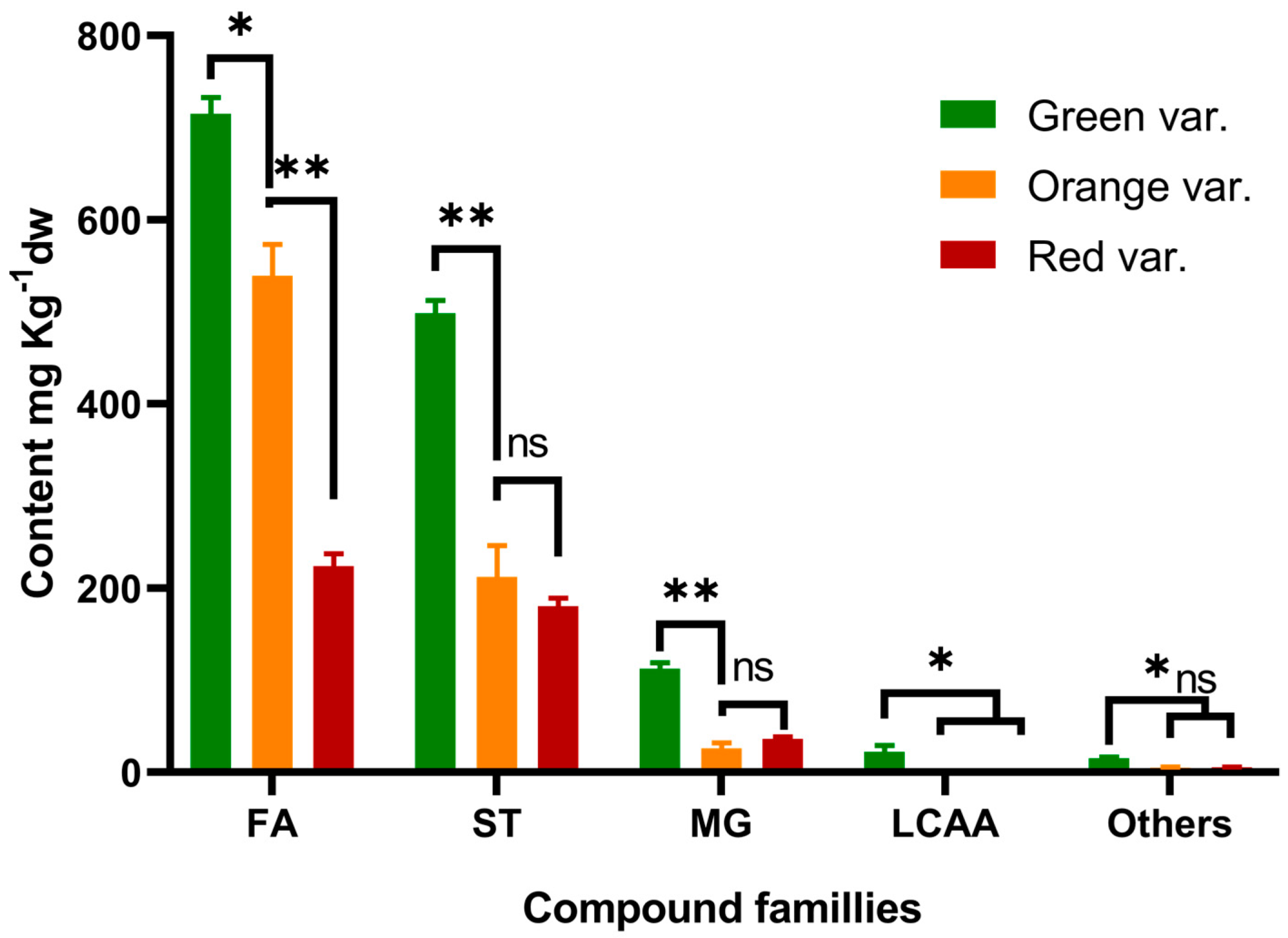
| Fatty acids | 123.75 a * | 115.74 a | 89.35 b | 715 a | 539 b | 224 c | |
| Saturated fatty acids | 61.32 b | 71.55 a | 51.85 c | 354 a | 349 a | 129 b | |
| 30.86 | Tetradecanoic acid | 1.17 b | 1.03 c | 2.04 a | 7 a | 5 b | 6 b |
| 33.37 | Pentadecanoic acid | 1.80 b | 2.23 a | 0.97 c | 10 b | 11 a | 3 c |
| 35.86 | Hexadecanoic acid | 29.51 b | 36.86 a | 24.46 c | 170 a | 183 a | 59 b |
| 38.08 | Heptadecanoic acid | 2.80 b | 3.90 a | 1.37 c | 16 b | 20 a | 3 c |
| 40.31 | Octadecanoic acid | 5.43 b | 7.16 a | 3.88 c | 31 b | 38 a | 8 c |
| 42.40 | Nonadecanoic acid | 0.40 b | 0.70 a | ND c | 2 b | 4 a | ND c |
| 44.44 | Eicosanoic acid | 1.37 b | 1.99 a | 1.04 c | 8 b | 9 a | 2 c |
| 46.41 | Heneicosanoic acid | 0.56 b | 0.79 a | 0.39 c | 3 b | 4 a | 1 c |
| 48.29 | Docosanoic acid | 1.93 a | 1.54 b | 1.26 c | 10 a | 8 b | 3 c |
| 50.12 | Tricosanoic acid | 1.55 b | 1.72 a | 1.36 c | 9 a | 8 a | 3 b |
| 52.00 | Tetracosanoic acid | 4.05 a | 2.61 c | 3.18 b | 23 a | 10 b | 8 c |
| 53.97 | Pentacosanoic acid | 2.09 a | 1.81 a | 1.89 a | 12 a | 6 b | 5 b |
| 56.04 | Hexacosanoic acid | 3.75 a | 4.29 a | 3.71 a | 22 a | 21 a | 10 b |
| 58.35 | Heptacosanoic acid | 3.07 a | 1.97 b | 2.98 a | 18 a | 9 b | 8 b |
| 60.51 | Octacosanoic acid | 1.83 b | 2.95 a | 3.32 a | 12 ab | 14 a | 9 b |
| Unsaturated fatty acids | 61.88 a | 32.20 b | 37.08 b | 358 a | 144 b | 94 c | |
| 32.84 | Pentadecenoic acid | 1.43 a | ND b | 1.44 a | 8 a | ND c | 4 b |
| 34.99 | (9Z)-Hexadec-9-enoic acid | 0.32 a | 0.20 b | 0.29 a | 2 a | 1 b | 1 b |
| 35.11 | (9E)-Hexadec-9-enoic acid | 0.63 a | 0.76 a | ND b | 3 a | 3 a | ND b |
| 37.37 | (10Z)-Heptadec-10-enoic acid | 0.57 a | 0.80 a | ND b | 3 a | 4 a | ND b |
| 37.53 | (10E)-Heptadec-10-enoic acid | 1.03 a | 1.26 a | ND b | 5 a | 3 b | ND c |
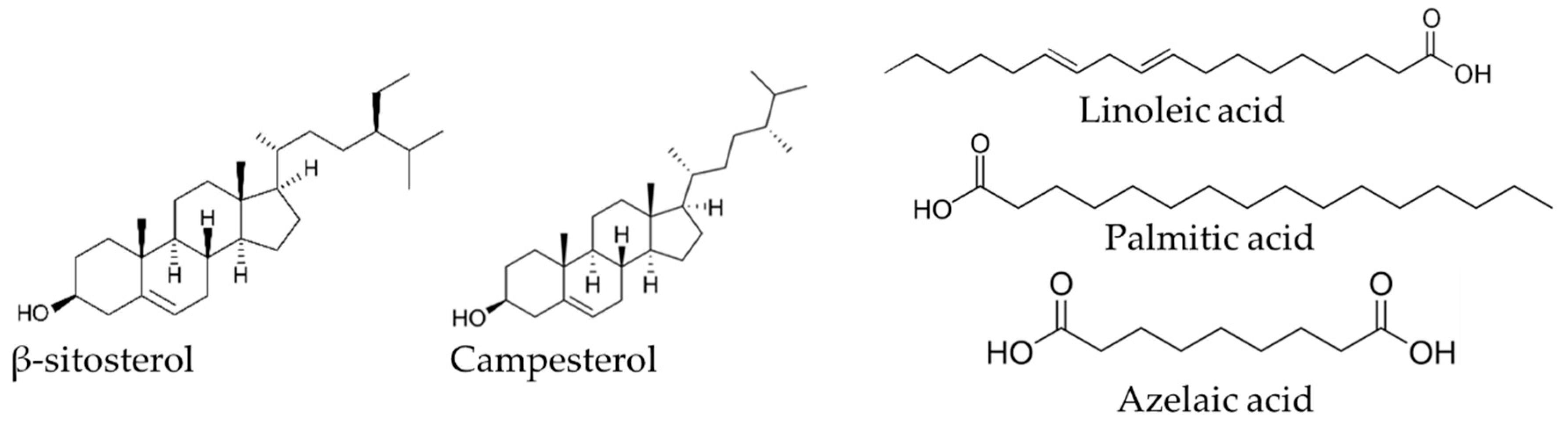
| 39.35 | (9Z, 12Z)-Octadeca-9,12-dienoic acid | 36.62 a | 4.76 c | 24.67 b | 213 a | 21 c | 60 b |
| 39.43 | (9Z, 12Z, 15Z)-Octadeca-9,12,15-trienoic acid | 6.49 a | ND c | 2.51 b | 38 a | ND c | 6 b |
| 39.53 | (9Z)-Octadec-9-enoic acid | 7.94 b | 20.40 a | 5.49 b | 45 b | 93 a | 14 c |
| 39.67 | (9E)-Octadec-9-enoic acid | 2.49 b | 4.02 a | 1.19 c | 16 a | 18 a | 4 b |
| 39.8 | Octadecenoic acid isomer | 0.42 a | ND b | ND b | 2 a | ND b | ND b |
| 41.51 | Nonadecadienoic acid isomer | 3.26 a | ND c | 1.49 b | 19 a | ND c | 4 b |
| 42.01 | Nonadecenoic acid isomer | 0.32 a | ND b | ND b | 2 a | ND b | ND b |
| 43.84 | Eicosenoic acid isomer | 0.35 a | ND b | ND b | 2 a | ND b | ND b |
| Diacids | 0.54 b | 11.99 a | 0.42 b | 3 b | 46 a | 1 b | |
| 29.36 | Azelaic acid (nonanedioic acid) | 0.54 b | 11.99 a | 0.42 b | 3 b | 46 a | 1 b |
| Long chain aliphatic alcohols | 2.82 a | ND b | ND b | 22 a | ND b | ND b | |
| 33.92 | Hexadecan-1-ol | 0.11 a | ND b | ND b | 4 a | ND b | ND b |
| 38.56 | Octadecan-1-ol | 0.06 a | ND b | ND b | 4 a | ND b | ND b |
| 46.78 | Docosan-1-ol | 0.52 a | ND b | ND b | 3 a | ND b | ND b |
| 50.46 | Tetracosan-1-ol | 0.32 a | ND b | ND b | 2 a | ND b | ND b |
| 54.31 | Hexacosan-1-ol | 0.37 a | ND b | ND b | 2 a | ND b | ND b |
| 58.58 | Octacosan-1-ol | 0.35 a | ND b | ND b | 2 a | ND b | ND b |
| 63.16 | Triacontan-1-ol | 1.09 a | ND b | ND b | 6 a | ND b | ND b |
| Sterols | 86.26 a | 49.61 b | 66.78 ab | 499 a | 212 b | 180 b | |
| 60.73 | Campesterol | 14.53 a | 6.29 c | 9.95 b | 84 a | 28 b | 26 b |
| 61.35 | Stigmasterol | 4.33 a | 0.39 b | 4.35 a | 25 a | 2 c | 12 b |
| 62.65 | β-Sitosterol | 60.88 a | 34.91 b | 47.23 ab | 352 a | 158 b | 130 b |
| 62.81 | Stigmastanol | 6.53 ab | 8.02 a | 5.25 b | 38 a | 24 b | 12 c |
| Monoglycerides | 19.04 a | 4.27 c | 14.00 b | 112 a | 26 c | 36 b | |
| 45.77 | 2,3-Dihydroxypropyl pentadecanoate | 0.63 a | ND b | ND b | 3 a | ND b | ND b |
| 47.00 | 1,3-Dihydroxypropan-2-yl hexadecanoate | 0.84 a | 0.10 c | 0.42 b | 5 a | 1 b | 1 b |
| 47.68 | 2,3-Dihydroxypropyl hexadecanoate | 8.56 a | 1.99 c | 4.86 b | 49 a | 10 c | 12 b |
| 49.44 | 2,3-Dihydroxypropyl heptadecanoate | 1.30 a | 0.58 b | 0.62 b | 9 a | 3 b | 2 b |
| 49.92 | 1,3-Dihydroxypropan-2-yl (9Z,12Z)-octadeca-9,12-dienoate | 0.28 b | ND c | 0.43 a | 2 a | ND c | 1 b |
| 50.56 | 2,3-Dihydroxypropyl (9Z,12Z)-octadeca-9,12-dienoate | 2.24 b | 0.10 c | 4.99 a | 12 a | 5 b | 12 a |
| 50.59 | 2,3-Dihydroxypropyl (9Z)-octadec-9-enoate | 0.62 a | 0.26 b | ND c | 5 a | 1 b | ND c |
| 50.67 | 2,3-Dihydroxypropyl (9Z)-octadec-9-enoate | ND b | ND b | 0.97 a | ND b | ND b | 3 a |
| 51.23 | 2,3-Dihydroxypropyl octadecanoate | 2.44 a | 0.71 c | 1.29 b | 14 a | 3 b | 3 b |
| 53.11 | 2,3-Dihydroxypropyl nonadecanoate | 0.42 a | ND b | ND b | 4 a | ND b | ND b |
| 55.14 | 2,3-Dihydroxypropyl icosanoate | 0.88 a | 0.33 c | 0.43 b | 5 a | 2 b | 1 c |
| 59.33 | 2,3-Dihydroxypropyl docosanoate | 0.80 a | 0.20 b | ND c | 4 a | 1 b | ND c |
| Others | 2.32 a | 0.90 c | 1.89 b | 15 a | 5 b | 6 b | |
| 14.20 | Glycerol | 0.77 a | 0.81 a | 0.45 b | 6 a | 4 b | 1 c |
| 38.21 | (9E)-Octadec-9-enoic acid ethyl ester | ND b | 0.09 a | ND b | ND b | 1 a | ND b |
| 57.25 | α-Tocopherol | 1.55 a | traces b | 1.44 a | 9 a | traces c | 4 b |
| Total | 234.19 a | 170.52 b | 172.02 b | 1363 a | 783 b | 446 c | |
| Green var. | Orange var. | Red var. | |
|---|---|---|---|
| Gram-negative strains | |||
| E. coli ATCC 25922 | 550.00 ± 0.00 a | 566.67 ± 5.77 b | 606.00 ± 5.48 c |
| P. aeruginosa ATCC 27853 | 777.50 ± 5.00 a | 826.00 ± 5.48 b | 947.50 ± 5.00 c |
| Gram positive strains | |||
| MRSA MU45 (Mec C) | 263.33 ± 5.77 a | 316.67 ± 5.77 b | 343.33 ± 5.77 c |
| S. aureus ATCC 29213 | 253.33 ± 5.77 a | 296.67 ± 5.77 b | 303.33 ± 5.77 b |
| B. cereus ATCC 10876 | 76.67 ± 5.77 a | 83.33 ± 5.77 a | 86.67 ± 5.77 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benramdane, E.; Chougui, N.; Ramos, P.A.B.; Makhloufi, N.; Tamendjari, A.; Silvestre, A.J.D.; Santos, S.A.O. Lipophilic Compounds and Antibacterial Activity of Opuntia ficus-indica Root Extracts from Algeria. Int. J. Mol. Sci. 2022, 23, 11161. https://doi.org/10.3390/ijms231911161
Benramdane E, Chougui N, Ramos PAB, Makhloufi N, Tamendjari A, Silvestre AJD, Santos SAO. Lipophilic Compounds and Antibacterial Activity of Opuntia ficus-indica Root Extracts from Algeria. International Journal of Molecular Sciences. 2022; 23(19):11161. https://doi.org/10.3390/ijms231911161
Chicago/Turabian StyleBenramdane, Elias, Nadia Chougui, Patrícia A. B. Ramos, Nawal Makhloufi, Abderezak Tamendjari, Armando J. D. Silvestre, and Sónia A. O. Santos. 2022. "Lipophilic Compounds and Antibacterial Activity of Opuntia ficus-indica Root Extracts from Algeria" International Journal of Molecular Sciences 23, no. 19: 11161. https://doi.org/10.3390/ijms231911161
APA StyleBenramdane, E., Chougui, N., Ramos, P. A. B., Makhloufi, N., Tamendjari, A., Silvestre, A. J. D., & Santos, S. A. O. (2022). Lipophilic Compounds and Antibacterial Activity of Opuntia ficus-indica Root Extracts from Algeria. International Journal of Molecular Sciences, 23(19), 11161. https://doi.org/10.3390/ijms231911161








