Comparative Study of M(Ⅱ)Al (M=Co, Ni) Layered Double Hydroxides for Silicone Foam: Characterization, Flame Retardancy, and Smoke Suppression
Abstract
1. Introduction
2. Results and Discussion
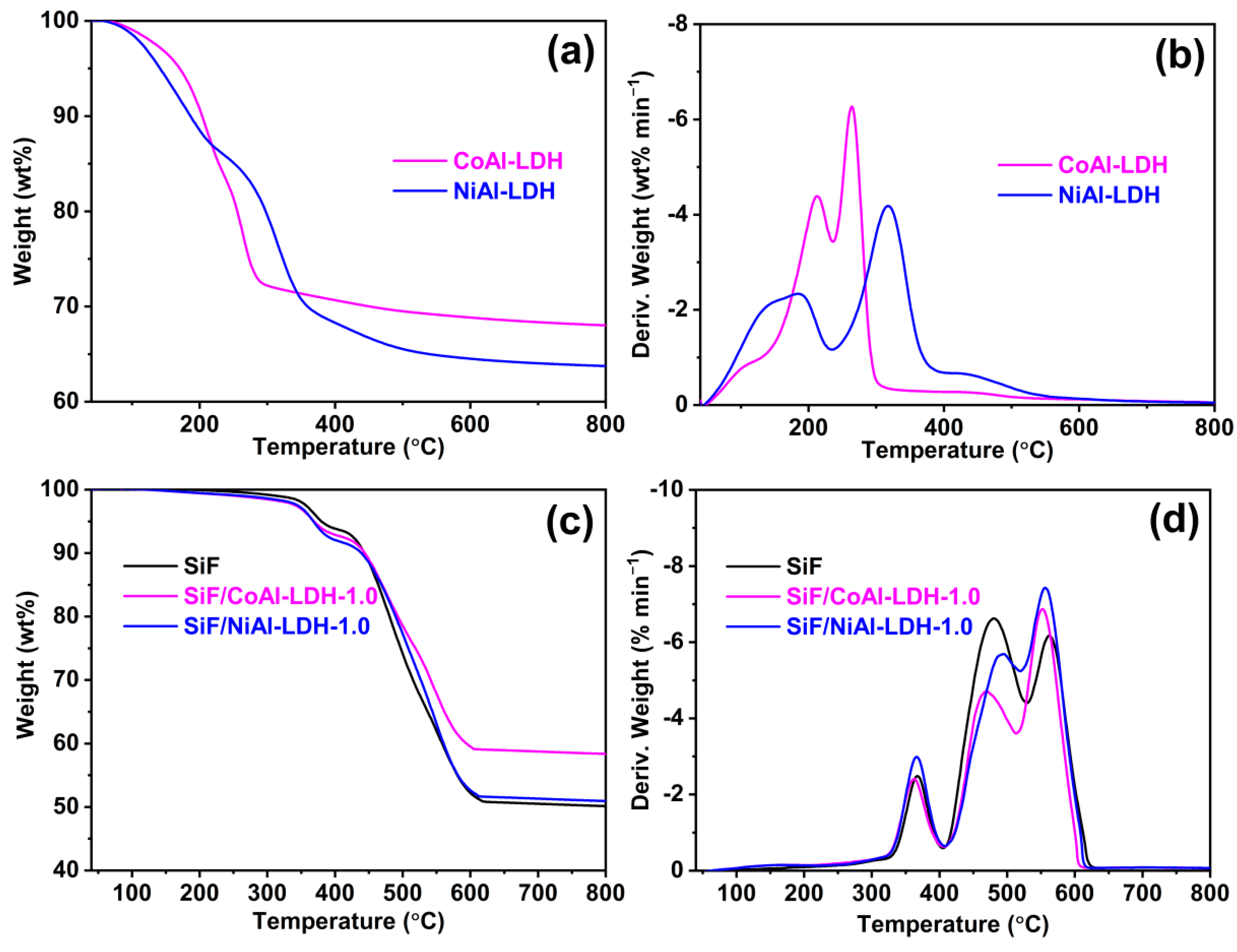
2.1. Characterization of CoAl-LDH and NiAl-LDH
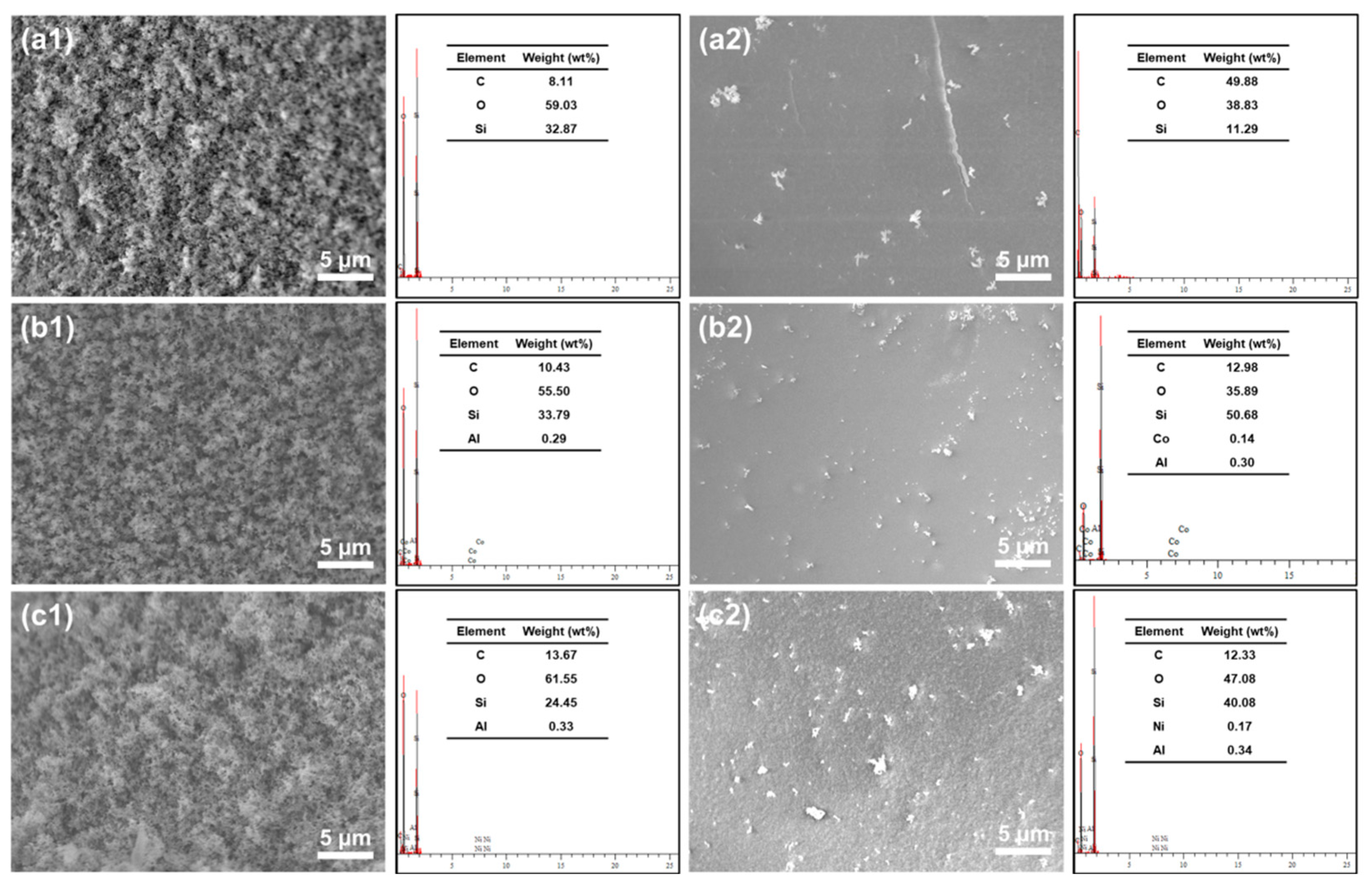
2.2. Morphological Analysis of the SiF/LDH Nanocomposites
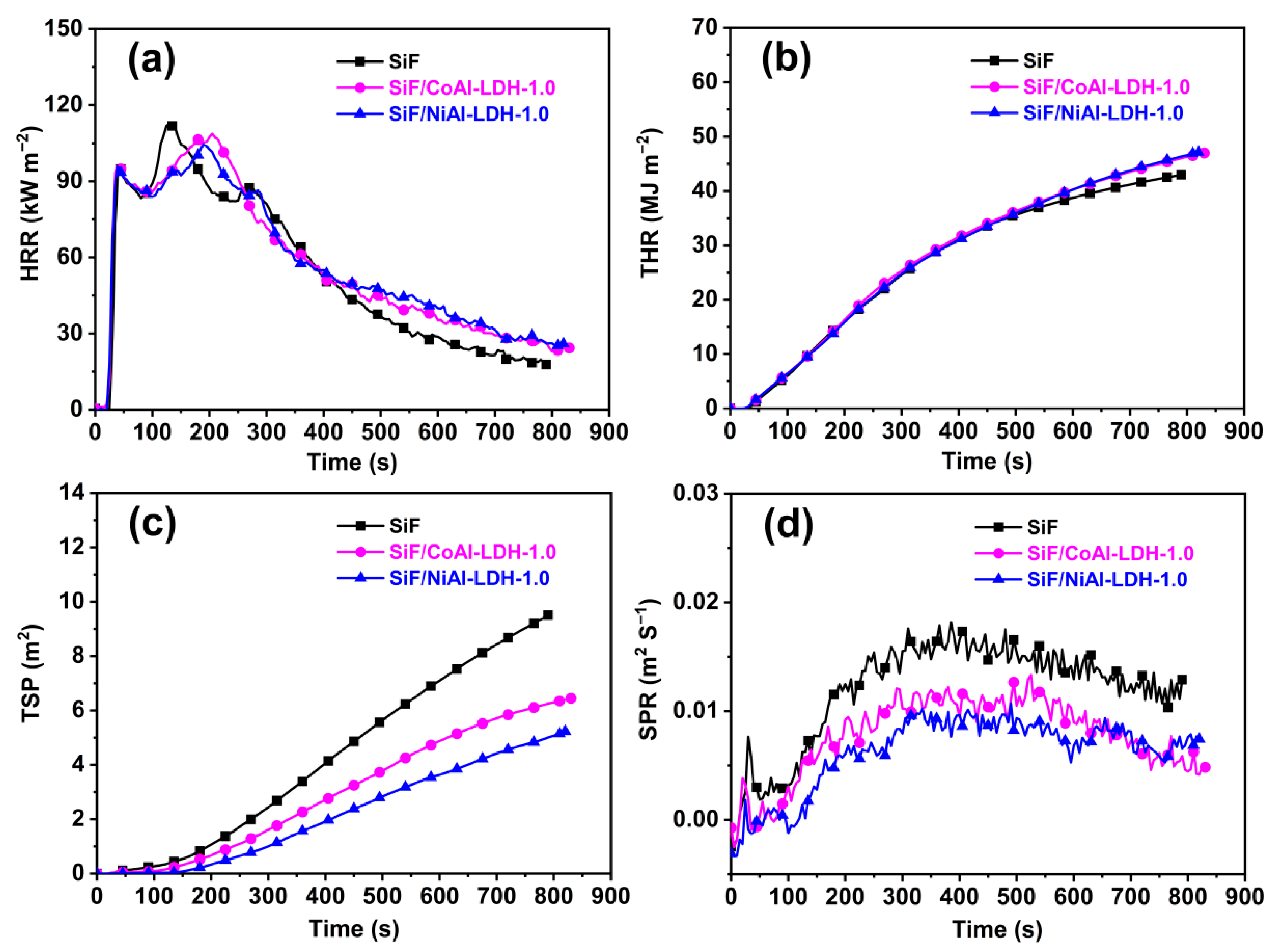
2.3. Flame Retardancy and Fire Behavior of the SiF/LDH Nanocomposites
2.4. Char Residue Analyses
2.5. Thermal Decomposition of CoAl-LDH, NiAl-LDH, and the SiF/LDH Nanocomposites
2.6. Mechanical Properties of the SiF/LDH Nanocomposites
3. Materials and Methods
3.1. Materials
3.2. Synthesis of CoAl-LDH and NiAl-LDH
3.3. Preparation of the SiF/LDH Nanocomposites
3.4. Characterizations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sherryna, A.; Tahir, M. Recent developments in layered double hydroxide structures with their role in promoting photocatalytic hydrogen production: A comprehensive review. Int. J. Energy Res. 2021, 46, 2093–2140. [Google Scholar] [CrossRef]
- Liu, B.W.; Zhao, H.B.; Wang, Y.Z. Advanced flame-retardant methods for polymeric materials. Adv. Mater. 2021, e2107905. [Google Scholar] [CrossRef]
- Gu, Z.; Atherton, J.J.; Xu, Z.P. Hierarchical layered double hydroxide nanocomposites: Structure, synthesis and applications. Chem. Commun. 2015, 51, 3024–3036. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Sadiku, E.R.; Mokhena, T.C. Morphology, thermal stability, and flammability properties of polymer-layered double hydroxide (LDH) nanocomposites: A Review. Crystals 2020, 10, 612. [Google Scholar] [CrossRef]
- Parida, K.M.; Mohapatra, L. Carbonate intercalated Zn/Fe layered double hydroxide: A novel photocatalyst for the enhanced photo degradation of azo dyes. Chem. Eng. J. 2012, 179, 131–139. [Google Scholar] [CrossRef]
- Han, X.; Zhang, S.; Lu, J.; Ma, Y.; Fu, N.; Wu, Y.; Liu, W.; Mei, D. Cu/Al layered double hydroxide by Co-precipitation with high anti-UV performance in waterborne varnish. Chem. Lett. 2019, 48, 866–869. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Chen, Z.; Wang, J.; Wang, S.; Hayat, T.; Wang, X. Layered double hydroxide intercalated with aromatic acid anions for the efficient capture of aniline from aqueous solution. J. Hazard. Mater. 2017, 321, 111–120. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Xiao, T.; Tang, Z.; Shen, J.; Li, H.; Zhou, Y.; Zou, Z. Urchin-like hierarchical CoZnAl-LDH/RGO/g-C3N4 hybrid as a Z-scheme photocatalyst for efficient and selective CO2 reduction. Appl. Catal. B Environ. 2019, 255, 117771–117784. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, J.; Zhang, W.; Pan, Y.T.; Wang, D.Y.; Yang, R. Synthesis of a novel dual layered double hydroxide hybrid nanomaterial and its application in epoxy nanocomposites. Chem. Eng. J. 2020, 381, 122777–122789. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Y.; Wang, J.; Zhou, C.; Tang, Q.; Rao, X. Calcined graphene/MgAl-layered double hydroxides for enhanced Cr(VI) removal. Chem. Eng. J. 2013, 221, 204–213. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. Preparation and characterization of microencapsulated LDHs with melamine-formaldehyde resin and its flame retardant application in epoxy resin. Polym. Adv. Technol. 2018, 29, 2147–2160. [Google Scholar] [CrossRef]
- Mascolo, G.; Mascolo, M.C. On the synthesis of layered double hydroxides (LDHs) by reconstruction method based on the “memory effect”. Microporous Mesoporous Mater. 2015, 214, 246–248. [Google Scholar] [CrossRef]
- Conterosito, E.; Palin, L.; Antonioli, D.; Riccardi, M.; Boccaleri, E.; Aceto, M.; Milanesio, M.; Gianotti, V. On the rehydration of organic layered double hydroxides to form low-ordered carbon/LDH nanocomposites. Inorganics 2018, 6, 79. [Google Scholar] [CrossRef]
- Pavel, O.D.; Stamate, A.-E.; Zăvoianu, R.; Bucur, I.C.; Bîrjega, R.; Angelescu, E.; Pârvulescu, V.I. Mechano-chemical versus co-precipitation for the preparation of Y-modified LDHs for cyclohexene oxidation and Claisen-Schmidt condensations. Appl. Catal. A Gen. 2020, 605, 117797–117807. [Google Scholar] [CrossRef]
- Szabados, M.; Ádám, A.A.; Traj, P.; Muráth, S.; Baán, K.; Bélteky, P.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Mechanochemical and wet chemical syntheses of CaIn-layered double hydroxide and its performance in a transesterification reaction compared to those of other Ca2M(III) hydrocalumites (M: Al, Sc, V, Cr, Fe, Ga) and Mg(II)-, Ni(II)-, Co(II)- or Zn(II)-based hydrotalcites. J. Catal. 2020, 391, 282–297. [Google Scholar]
- Ferencz, Z.; Szabados, M.; Ádok-Sipiczki, M.; Kukovecz, Á.; Kónya, Z.; Sipos, P.; Pálinkó, I. Mechanochemically assisted synthesis of pristine Ca(II)Sn(IV)-layered double hydroxides and their amino acid intercalated nanocomposites. J. Mater. Sci. 2014, 49, 8478–8486. [Google Scholar] [CrossRef]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods—A review. Appl. Surf. Sci. 2016, 383, 200–213. [Google Scholar] [CrossRef]
- Xu, M.; Wei, M. Layered double hydroxide-based catalysts: Recent advances in preparation, structure, and applications. Adv. Funct. Mater. 2018, 28, 1802943–1802963. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Chen, F.; Yang, Q.; Luo, K.; Yao, F.; Wang, S.; Wang, X.; Wu, J.; Li, X.; Wang, D.; et al. Heterogeneous activation of peroxymonosulfate by Fe-Co layered doubled hydroxide for efficient catalytic degradation of Rhoadmine B. Chem. Eng. J. 2017, 321, 222–232. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Tran, H.N.; Nguyen, D.T.; Le, G.T.; Tomul, F.; Lima, E.C.; Woo, S.H.; Sarmah, A.K.; Nguyen, H.Q.; Nguyen, P.T.; Nguyen, D.D.; et al. Adsorption mechanism of hexavalent chromium onto layered double hydroxides-based adsorbents: A systematic in-depth review. J. Hazard. Mater. 2019, 373, 258–270. [Google Scholar] [CrossRef]
- Mittal, J. Recent progress in the synthesis of Layered Double Hydroxides and their application for the adsorptive removal of dyes: A review. J. Environ. Manag. 2021, 295, 113017. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, P.; Wei, Z.; Liu, T.; Yu, J.; Al-Ghamdi, A.A.; Wageh, S. Synthesis of reduced graphene oxide supported nickel-cobalt-layered double hydroxide nanosheets for supercapacitors. J. Colloid Interface Sci. 2021, 588, 637–645. [Google Scholar] [CrossRef]
- Lin, J.; Jia, H.; Liang, H.; Chen, S.; Cai, Y.; Qi, J.; Qu, C.; Cao, J.; Fei, W.; Feng, J. Hierarchical CuCo2S4@NiMn-layered double hydroxide core-shell hybrid arrays as electrodes for supercapacitors. Chem. Eng. J. 2018, 336, 562–569. [Google Scholar] [CrossRef]
- Li, S.; Yu, C.; Yang, J.; Zhao, C.; Zhang, M.; Huang, H.; Liu, Z.; Guo, W.; Qiu, J. A superhydrophilic “nanoglue” for stabilizing metal hydroxides onto carbon materials for high-energy and ultralong-life asymmetric supercapacitors. Energy Environ. Sci. 2017, 10, 1958–1965. [Google Scholar] [CrossRef]
- Jing, C.; Liu, X.; Yao, H.; Yan, P.; Zhao, G.; Bai, X.; Dong, B.; Dong, F.; Li, S.; Zhang, Y. Phase and morphology evolution of CoAl LDH nanosheets towards advanced supercapacitor applications. CrystEngComm 2019, 21, 4934–4942. [Google Scholar] [CrossRef]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-dimensional nanomaterials for biomedical applications: Emerging Trends and Future Prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef]
- Yan, L.; Gonca, S.; Zhu, G.; Zhang, W.; Chen, X. Layered double hydroxide nanostructures and nanocomposites for biomedical applications. J. Mater. Chem. B 2019, 7, 5583–5601. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Hong, W.; Chen, X. Nanoreinforcements of two-dimensional nanomaterials for flame retardant polymeric composites: An overview. Adv. Polym. Technol. 2019, 2019, 1–25. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; O’Hare, D.; Sun, L. Preparation of two dimensional layered double hydroxide nanosheets and their applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Di, Y.; Yang, Q.; Zhou, X. Composites of layered double hydroxide nanosheets, hydroxy-functionalized carbon nanotubes, and hydroxyapatite nanoparticles as flame retardants for epoxy resins. ACS Appl. Nano Mater. 2021, 4, 11753–11762. [Google Scholar] [CrossRef]
- Liu, C.; Li, P.; Xu, Y.J.; Liu, Y.; Zhu, P.; Wang, Y.Z. Epoxy/iron alginate composites with improved fire resistance, smoke suppression and mechanical properties. J. Mater. Sci. 2022, 57, 2567–2583. [Google Scholar] [CrossRef]
- Jian, R.K.; Pang, F.Q.; Lin, Y.C.; Bai, W.B. Facile construction of lamellar-like phosphorus-based triazole-zinc complex for high-performance epoxy resins. J. Colloid Interface Sci. 2022, 609, 513–522. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, P.; Liu, Y.; Zhu, P. Green flame-retardant flexible polyurethane foam based on polyphenol-iron-phytic acid network to improve the fire safety. Compos. B Eng. 2022, 239, 109958–109971. [Google Scholar] [CrossRef]
- Manzi-Nshuti, C.; Wang, D.; Hossenlopp, J.M.; Wilkie, C.A. Aluminum-containing layered double hydroxides: The thermal, mechanical, and fire properties of (nano)composites of poly(methyl methacrylate). J. Mater. Chem. 2008, 18, 3091–3102. [Google Scholar] [CrossRef]
- Shao, Z.B.; Cui, J.; Lin, X.B.; Li, X.L.; Jian, R.K.; Wang, D.Y. In-situ coprecipitation formed Fe/Zn-layered double hydroxide/ammonium polyphosphate hybrid material for flame retardant epoxy resin via synergistic catalytic charring. Compos. A Appl. Sci. Manuf. 2022, 155, 106841–106851. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, Y.; Zhang, X.; Kong, Q.; Zhang, J.; Liu, H.; Zhang, F. Improving the flame-retardant efficiency of layered double hydroxide with disodium phenylphosphate for epoxy resin. J. Therm. Anal. Calorim. 2019, 140, 149–156. [Google Scholar] [CrossRef]
- Jaerger, S.; Wypych, F. Thermal and flammability properties influenced by Zn/Al, Co/Al, and Ni/Al layered double hydroxide in low-density polyethylene nanocomposites. J. Appl. Polym. Sci. 2019, 137, 48737–48751. [Google Scholar] [CrossRef]
- Nagendra, B.; Rosely, C.V.S.; Leuteritz, A.; Reuter, U.; Gowd, E.B. Polypropylene/layered double hydroxide nanocomposites: Influence of LDH intralayer metal constituents on the properties of polypropylene. ACS Omega 2017, 2, 20–31. [Google Scholar] [CrossRef]
- Pang, Q.; Kang, F.; Deng, J.; Lei, L.; Lu, J.; Shao, S. Flame retardancy effects between expandable graphite and halloysite nanotubes in silicone rubber foam. RSC Adv. 2021, 11, 13821–13831. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-E.; Zhao, H.-B.; Cheng, J.-B.; Wang, T.; Fu, T.; Zhang, A.-N.; Wang, Y.-Z. An Effective Green Porous Structural Adhesive for Thermal Insulating, Flame-Retardant, and Smoke-Suppressant Expandable Polystyrene Foam. Engineering 2022. [Google Scholar] [CrossRef]
- Liu, B.-W.; Cao, M.; Zhang, Y.-Y.; Wang, Y.-Z.; Zhao, H.-B. Multifunctional protective aerogel with superelasticity over −196 to 500 °C. Nano Res. 2022, 15, 7797–7805. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, H.-B.; Cheng, J.-B.; Li, W.; Zhang, J.; Wang, Y.-Z. A green, durable and effective flame-retardant coating for expandable polystyrene foams. Chem. Eng. J. 2022, 440, 135807. [Google Scholar] [CrossRef]
- Liu, D.Y.; Zhao, B.; Wang, J.S.; Liu, P.W.; Liu, Y.Q. Flame retardation and thermal stability of novel phosphoramide/expandable graphite in rigid polyurethane foam. J. Appl. Polym. Sci. 2018, 135, 10. [Google Scholar] [CrossRef]
- Deng, S.B.; Liao, W.; Yang, J.C.; Cao, Z.J.; Wang, Y.Z. Flame-retardant and smoke-suppressed silicone foams with chitosan-based nanocoatings. Ind. Eng. Chem. Res. 2016, 55, 7239–7248. [Google Scholar] [CrossRef]
- Métivier, T.; Cassagnau, P. New trends in cellular silicone: Innovations and applications. J. Cell. Plast. 2018, 55, 151–200. [Google Scholar] [CrossRef]
- Kang, F.R.; Wang, C.P.; Deng, J.; Yang, K.; Ma, L.; Pang, Q.T. Flame retardancy and smoke suppression of silicone foams with microcapsulated aluminum hypophosphite and zinc borate. Polym. Adv. Technol. 2020, 31, 654–664. [Google Scholar] [CrossRef]
- Kang, F.R.; Deng, J.; Jiao, D.S.; He, L.Q.; Wang, W.F.; Liu, Z.C. Microfluidic fabrication of polysiloxane/dimethyl methylphosphonate flame-retardant microcapsule and its application in silicone foams. Polym. Adv. Technol. 2019, 30, 1269–1278. [Google Scholar] [CrossRef]
- Kang, F.; Wang, C.; Deng, J.; Wang, W.; Li, X. Effects of talc/hollow glass beads on the flame retardancy of silicone foams. Mater. Res. Express 2019, 6, 95318–95326. [Google Scholar] [CrossRef]
- Zhou, L.L.; Li, W.X.; Zhao, H.B.; Wang, J.S.; Zhao, B. NiTi-layered double hydroxide nanosheets toward high-efficiency flame retardancy and smoke suppression for silicone foam. Polym. Degrad. Stab. 2022, 204, 110104. [Google Scholar] [CrossRef]
- Long, Y.W.; Zeng, H.Y.; Li, H.B.; Xu, S.; Lv, S.B.; Li, Z.; Tian, Z.F. Hierarchical Co3O4@CoAl hydrotalcite grown on Ni foam for high-performance asymmetric supercapacitors. Appl. Clay Sci. 2021, 210, 106136. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Q.; Hou, J.; Pei, L.; Li, Y.; Ai, S. Platinum nanocrystals supported on CoAl mixed metal oxide nanosheets derived from layered double hydroxides as catalysts for selective hydrogenation of cinnamaldehyde. J. Catal. 2015, 331, 193–202. [Google Scholar] [CrossRef]
- Chen, L.; Sun, B.; Wang, X.; Qiao, F.; Ai, S. 2D ultrathin nanosheets of Co-Al layered double hydroxides prepared in l-asparagine solution: Enhanced peroxidase-like activity and colorimetric detection of glucose. J. Mater. Chem. B 2013, 1, 2268–2274. [Google Scholar] [CrossRef]
- Liu, L.; Hu, X.; Zeng, H.Y.; Yi, M.Y.; Shen, S.G.; Xu, S.; Cao, X.; Du, J.Z. Preparation of NiCoFe-hydroxide/polyaniline composite for enhanced-performance supercapacitors. J. Mater. Sci. Technol. 2019, 35, 1691–1699. [Google Scholar] [CrossRef]
- Tao, J.; Yu, X.; Liu, Q.; Liu, G.; Tang, H. Internal electric field induced S-scheme heterojunction MoS2/CoAl LDH for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 585, 470–479. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, X.; Wang, G.; Jin, Z. Rational design of a core–shell-shaped flowerlike Mn0.05Cd0.95S@NiAl-LDH structure for efficient hydrogen evolution. Catal. Lett. 2020, 151, 634–647. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Bai, X.; Li, Y.; Zhang, J.; Zhao, M.; Huang, X.; Feng, C.; Zhao, Y. Exfoliation of layered double hydroxides by use of zwitterionic surfactants in aqueous solution. J. Dispers. Sci. Technol. 2019, 40, 811–818. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, N.; Shi, Z.; Ye, Z.; Gao, Q.; Zhi, M.; Hong, Z. Preparation of Ni-Al layered double hydroxide hollow microspheres for supercapacitor electrode. Chem. Eng. J. 2018, 338, 55–61. [Google Scholar] [CrossRef]
- Yu, C.; Cao, Z.; Chen, S.; Wang, S.; Zhong, H.; Ma, X. In situ selenylation of molybdate ion intercalated Co-Al layered double hydrotalcite for high-performance electrocatalytic oxygen evolution reaction. J. Taiwan Inst. Chem. Eng. 2021, 119, 166–176. [Google Scholar] [CrossRef]
- Rao, W.; Shi, J.; Yu, C.; Zhao, H.-B.; Wang, Y.-Z. Highly efficient, transparent, and environment-friendly flame-retardant coating for cotton fabric. Chem. Eng. J. 2021, 424, 130556. [Google Scholar] [CrossRef]
- Ning, K.; Zhou, L.-L.; Zhao, B. A novel aminothiazole-based cyclotriphosphazene derivate towards epoxy resins for high flame retardancy and smoke suppression. Polym. Degrad. Stab. 2021, 190, 109651–109661. [Google Scholar] [CrossRef]
- Liu, Z.P.; Ma, R.Z.; Osada, M.; Iyi, N.; Ebina, Y.; Takada, K.; Sasaki, T. Synthesis, anion exchange, and delamination of Co-Al layered double hydroxide: Assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J. Am. Chem. Soc. 2006, 128, 4872–4880. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.M.; Wang, Z.Y.; Zhang, T.G.; Fu, F.; Yang, N. Synthesis and characterization of Co–Al–CO3 layered double-metal hydroxides and assessment of their friction performances. Appl. Clay Sci. 2012, 59–60, 36–41. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.-M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Qiu, J.; Lai, X.; Fang, W.; Li, H.; Zeng, X. An efficient strategy for simultaneously improving tracking resistance and flame retardancy of addition-cure liquid silicone rubber. Polym. Degrad. Stab. 2017, 144, 176–186. [Google Scholar] [CrossRef]
- Kuma, K.; Paplawsky, W.; Gedulin, B.; Arrhenius, G. Mixed-valence hydroxides as bioorganic host minerals. Orig. Life Evol. Biosph. 1989, 19, 573–602. [Google Scholar] [CrossRef]
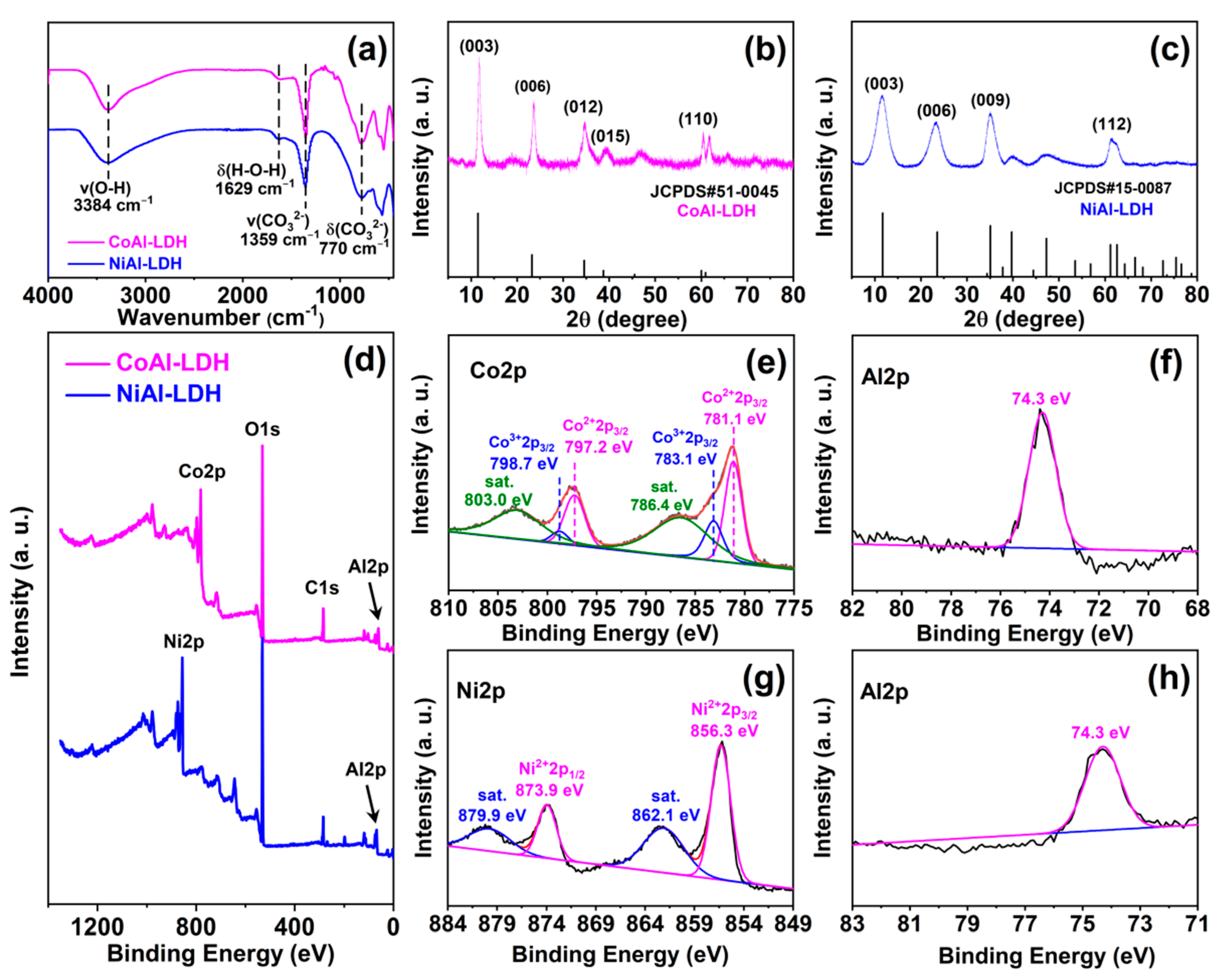

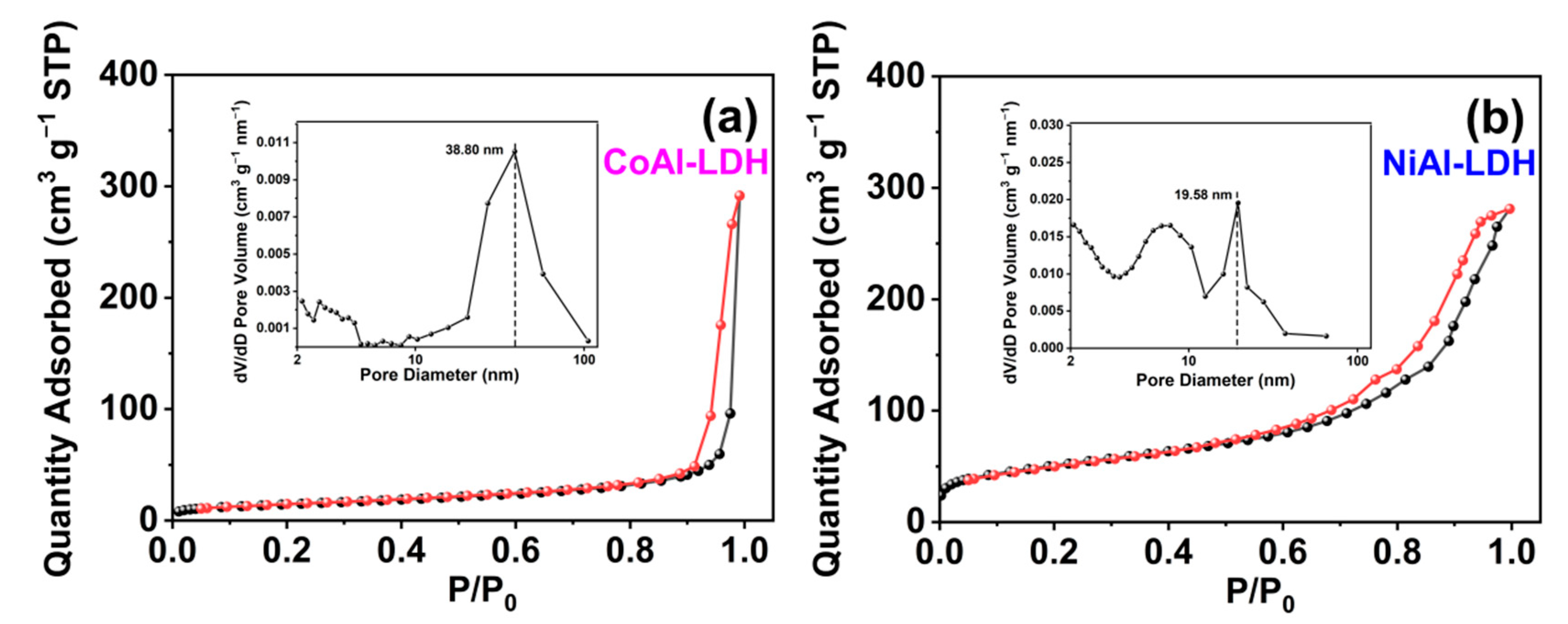



| Samples | Metal Element | Element Content (%) | Metal Elements’ Molar Ratios |
|---|---|---|---|
| CoAl-LDH | Co | 42.71 | Co: Al = 2.32 |
| Al | 8.42 | ||
| NiAl-LDH | Ni | 30.78 | Ni: Al = 2.28 |
| Al | 6.21 |
| Samples | Density (g cm−3) | LOI (vol%) | UL-94 (3.2 mm) | |||
|---|---|---|---|---|---|---|
| t1 (s) | t2 (s) | Rating | Dripping | |||
| SiF | 0.28 ± 0.01 | 28.7 | >30 | - | NR | No |
| SiF/CoAl-LDH-1.0 | 0.27 ± 0.01 | 29.6 | 25 ± 2 | 1 ± 1 | V-1 | No |
| SiF/NiAl-LDH-1.0 | 0.26 ± 0.02 | 29.6 | 14 ± 3 | 8 ± 1 | V-1 | No |
| Samples | pHRR (kW m−2) | tp (s) | THR (MJ m−2) | TSP (m2) | FIGRA (kW m−2·s−1) | Residue (%) |
|---|---|---|---|---|---|---|
| SiF | 112.8 | 130 | 43.0 | 9.5 | 0.87 | 76.0 |
| SiF/CoAl-LDH-1.0 | 108.7 | 205 | 47.0 | 6.4 | 0.53 | 79.5 |
| SiF/NiAl-LDH-1.0 | 104.3 | 190 | 47.1 | 5.2 | 0.55 | 79.9 |
| Samples | T5% (°C) | Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) | Char Residue at 800 °C (wt%) |
|---|---|---|---|---|---|
| CoAl-LDH | 171 | 213 | 264 | - | 68.0 |
| NiAl-LDH | 141 | 184 | 317 | - | 63.7 |
| SiF | 380 | 367 | 480 | 562 | 50.1 |
| SiF/CoAl-LDH-1.0 | 369 | 363 | 470 | 552 | 58.4 |
| SiF/NiAl-LDH-1.0 | 368 | 366 | 495 | 557 | 50.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.-L.; Li, W.-X.; Zhao, H.-B.; Zhao, B. Comparative Study of M(Ⅱ)Al (M=Co, Ni) Layered Double Hydroxides for Silicone Foam: Characterization, Flame Retardancy, and Smoke Suppression. Int. J. Mol. Sci. 2022, 23, 11049. https://doi.org/10.3390/ijms231911049
Zhou L-L, Li W-X, Zhao H-B, Zhao B. Comparative Study of M(Ⅱ)Al (M=Co, Ni) Layered Double Hydroxides for Silicone Foam: Characterization, Flame Retardancy, and Smoke Suppression. International Journal of Molecular Sciences. 2022; 23(19):11049. https://doi.org/10.3390/ijms231911049
Chicago/Turabian StyleZhou, Lin-Lin, Wen-Xiong Li, Hai-Bo Zhao, and Bin Zhao. 2022. "Comparative Study of M(Ⅱ)Al (M=Co, Ni) Layered Double Hydroxides for Silicone Foam: Characterization, Flame Retardancy, and Smoke Suppression" International Journal of Molecular Sciences 23, no. 19: 11049. https://doi.org/10.3390/ijms231911049
APA StyleZhou, L.-L., Li, W.-X., Zhao, H.-B., & Zhao, B. (2022). Comparative Study of M(Ⅱ)Al (M=Co, Ni) Layered Double Hydroxides for Silicone Foam: Characterization, Flame Retardancy, and Smoke Suppression. International Journal of Molecular Sciences, 23(19), 11049. https://doi.org/10.3390/ijms231911049







