A Phosphorous-Based Bi-Functional Flame Retardant Based on Phosphaphenanthrene and Aluminum Hypophosphite for an Epoxy Thermoset
Abstract
1. Introduction
2. Results and Discussion
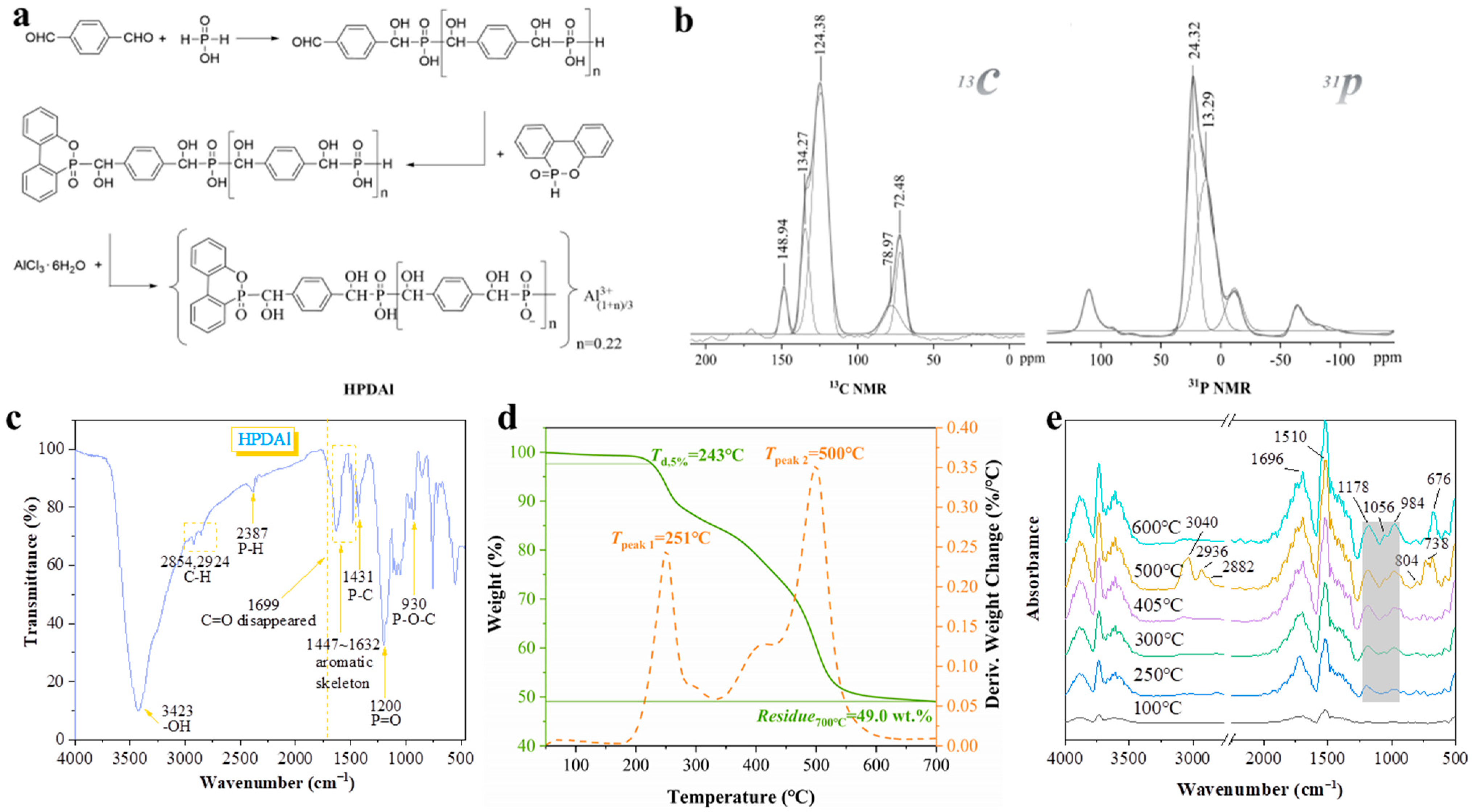
2.1. Characterization of HPDAl
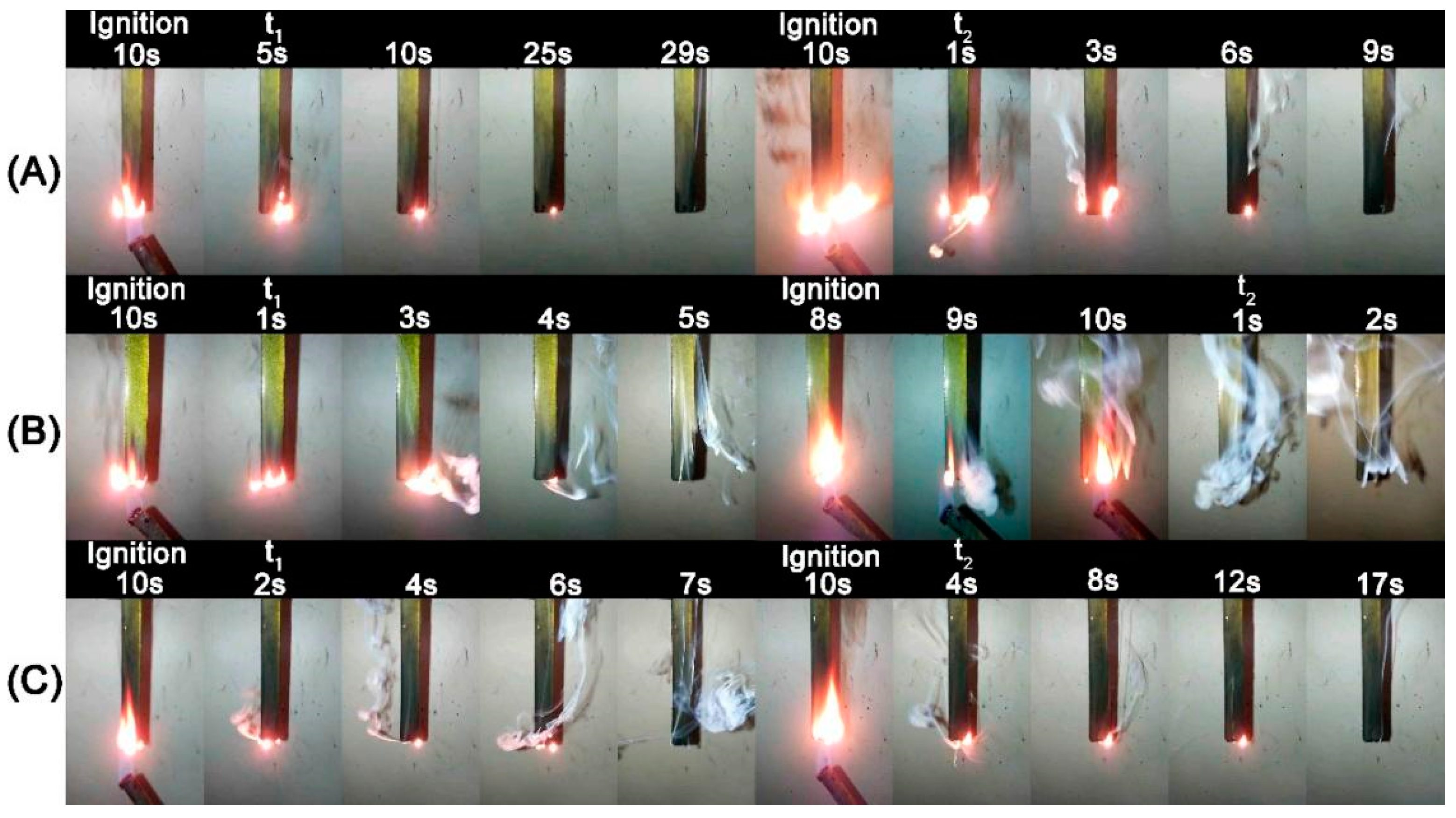
2.2. Flammability Analysis
| Samples | LOI (%) ± 1.0 | UL 94 | |||
|---|---|---|---|---|---|
| Dripping | Ranking | av-t1 (s) | av-t2 (s) | ||
| EP | 26.2 | Yes | NR | 145.6 | infinite |
| 1%HPDAl/EP | 30.4 | No | NR | 23.1 | 7.4 |
| 2%HPDAl/EP | 32.3 | No | V-0 | 3.1 | 2.9 |
| 3%HPDAl/EP | 32.3 | No | V-1 | 10.0 | 22.3 |
| AHP/EP | 28.8 | No | NR | 95.8 | 18.0 |
| DOPO/EP | 30.3 | No | NR | 22.6 | 35.7 |
| AHP/DOPO/EP | 29.8 | No | NR | 16.2 | 52.2 |
2.3. Thermal Decomposition Behavior Analysis
2.4. Fire Behaviour: Forced Combustion
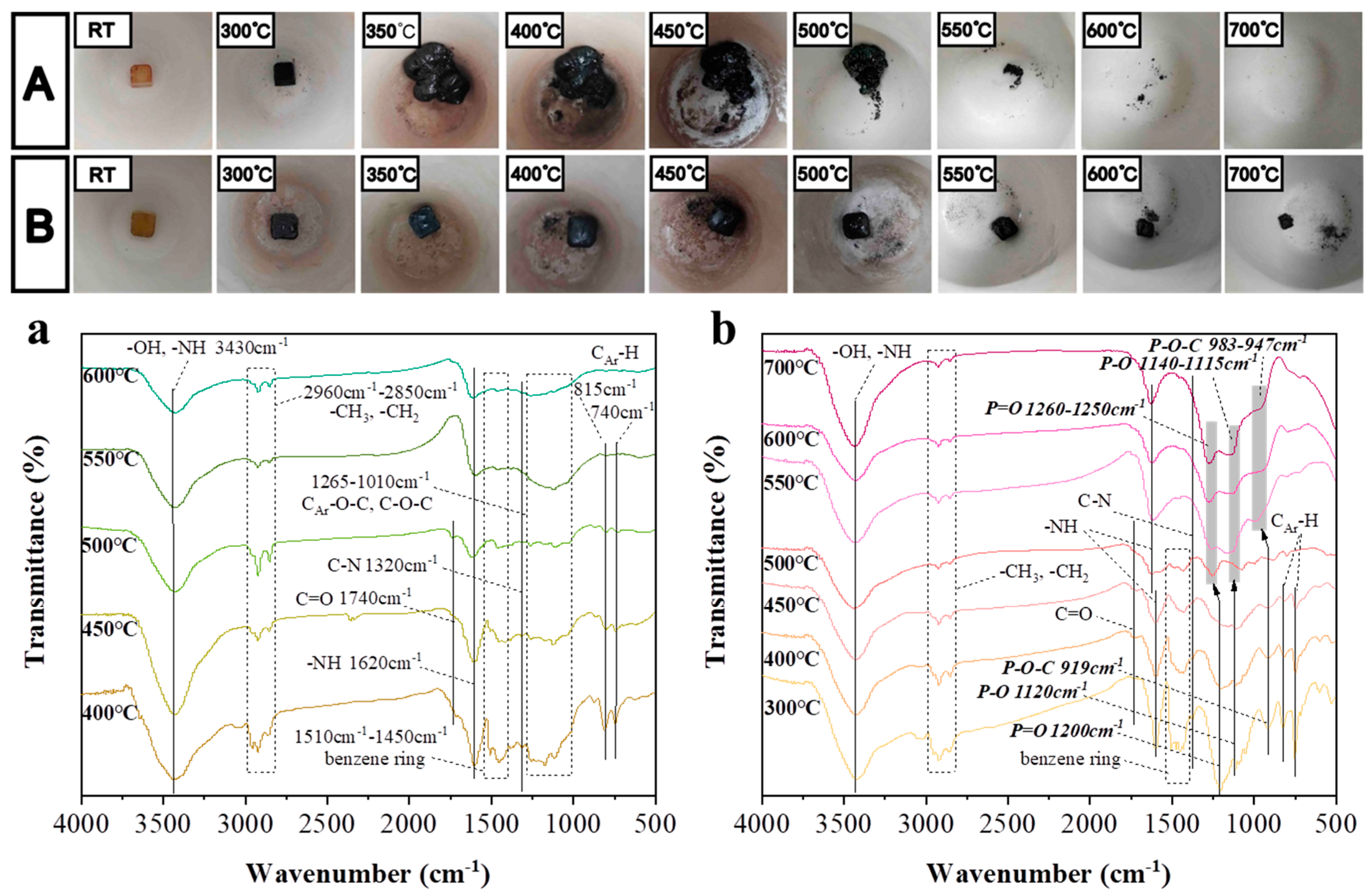
2.5. Morphologies of Final Char
2.6. Condensed Phase Flame Retardant Mechanism
2.7. Gas Phase Flame Retardant Mechanism
2.8. Mechanical Properties of Flame-Retardant EP
3. Methods and Materials
3.1. Materials
3.2. Synthesis of HPDAl
3.3. Preparation of Flame-Retardant EP and the Control Samples
3.4. Characterizations
3.4.1. Structure Characterization
3.4.2. Flame Retardancy Performance Characterization
3.4.3. Mechanism Research
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hergenrother, P.M.; Thompson, C.M.; Smith, J.G.; Connell, J.W.; Hinkley, J.A.; Lyon, R.E.; Moulton, R. Flame retardant aircraft epoxy resins containing phosphorus. Polymer 2005, 46, 5012–5024. [Google Scholar] [CrossRef]
- Dai, X.Y.; Li, P.H.; Sui, Y.L.; Zhang, C.L. Thermal and flame-retardant properties of intrinsic flame-retardant epoxy resin containing biphenyl structures and phosphorus. Eur. Polym. J. 2021, 147, 110319. [Google Scholar] [CrossRef]
- Schartel, B.; Braun, U.; Balabanovich, A.I.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.M.; Sandler, J.K.W.; Altstädt, V. Pyrolysis and fire behaviour of epoxy systems containing a novel 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-(DOPO)-based diamino hardener. Eur. Polym. J. 2008, 44, 704–715. [Google Scholar] [CrossRef]
- Müllera, P.; Bykovb, Y.; Döring, M. New star-shaped phosphorus-containing flame retardants based on acrylates for epoxy resins. Polym. Adv. Technol. 2013, 24, 834–840. [Google Scholar] [CrossRef]
- Wang, X.; Hua, Y.; Song, L.; Xing, W.Y.; Lu, H.D. Thermal degradation mechanism of flame retarded epoxy resins with a DOPO-substitued organophosphorus oligomer by TG-FTIR and DP-MS. J. Anal. Appl. Pyrol. 2011, 92, 164–170. [Google Scholar] [CrossRef]
- Peng, W.; Nie, S.B.; Xu, Y.X.; Yang, W. A tetra-DOPO derivative as highly efficient flame retardant for epoxy resins. Polym. Degrad. Stab. 2021, 193, 109715. [Google Scholar] [CrossRef]
- Braun, U.; Balabanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.; Sandler, J.K.W.; Altstädt, V.; et al. Influence of the oxidation state of phosphorus on the decomposition and fire behaviour of flame-retarded epoxy resin composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Modesti, M.; Besco, S.; Hrelja, D.; Donadi, S. Influence of phosphorus valency on thermal behaviour of flame retarded polyurethane foams. Polym. Degrad. Stab. 2011, 96, 1455–1461. [Google Scholar] [CrossRef]
- Schartel, B.; Balabanovich, A.I.; Braun, U.; Knoll, U.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.; Sandler, J.K.W.; Altstädt, V.; et al. Pyrolysis of epoxy resins and fire behavior of epoxy resin composites flame-retarded with 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide additives. J. Appl. Polym. Sci. 2010, 104, 2260–2269. [Google Scholar] [CrossRef]
- Qian, L.J.; Qiu, Y.; Liu, J.; Xin, F.; Chen, Y.J. The flame retardant group-synergistic-effect of a phosphaphenanthrene and triazine double-group compound in epoxy resin. J. Appl. Polym. Sci. 2014, 131, 39709. [Google Scholar] [CrossRef]
- Tang, S.; Qian, L.J.; Qiu, Y.; Dong, Y.P. High-performance flame retardant epoxy resin based on a bi-group molecule containing phosphaphenanthrene and borate groups. Polym. Degrad. Stab. 2018, 153, 210–219. [Google Scholar] [CrossRef]
- Qiu, Y.; Qian, L.J.; Feng, H.S.; Jin, S.L.; Hao, J.W. Toughening Effect and Flame-Retardant Behaviors of Phosphaphenanthrene/Phenylsiloxane Bigroup Macromolecules in Epoxy Thermoset. Macromolecules 2018, 51, 9992–10002. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Wang, B.B.; Liew, K.M.; Song, L.; Wang, C.M.; Hu, Y. Highly Effective P−P Synergy of a Novel DOPO-Based Flame Retardant for Epoxy Resin. Ind. Eng. Chem. Res. 2017, 56, 1245–1255. [Google Scholar] [CrossRef]
- Qian, L.J.; Ye, L.J.; Qiu, Y.; Qu, S.R. Thermal degradation behavior of the compound containing phosphaphenanthrene and phosphazene groups and its flame retardant mechanism on epoxy resin. Polymer 2011, 52, 5486–5493. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xu, B.; Wang, X.D.; Liu, Y.T. A phosphorous-based bi-functional flame retardant for rigid polyurethane foam. Polym. Degrad. Stab. 2021, 186, 109516. [Google Scholar] [CrossRef]
- Howell, B.A. Development of additive possessing both solid-phase and gas phase flame retardant activities. Polym. Degrad. Stabil. 2008, 93, 2052–2057. [Google Scholar] [CrossRef]
- Wang, J.Y.; Qian, L.J.; Huang, Z.G.; Fang, Y.Y.; Qiu, Y. Synergistic flame-retardant behavior and mechanisms of aluminum poly-hexamethylenephosphinate and phosphaphenanthrene in epoxy resin. Polym. Degrad. Stabil. 2016, 130, 173–181. [Google Scholar] [CrossRef]
- Xu, B.; Shao, L.S.; Wang, J.Y.; Liu, Y.T.; Qian, L.J. Enhancement of the intumescent flame retardant efficiency in polypropylene by synergistic charring effect of a hypophosphite/cyclotetrasiloxane bi-group compound. Polym. Degrad. Stabil. 2020, 181, 109281. [Google Scholar] [CrossRef]
- Wang, P.; Fu, X.; Kan, Y.; Wang, X.; Hu, Y. Two high-efficient DOPO-based phosphonamidate flame retardants for transparent epoxy resin. High. Perform. Polym. 2019, 31, 249–260. [Google Scholar] [CrossRef]
- Chen, W.H.; Liu, P.J.; Cheng, Y.B.; Liu, Y.; Wang, Q.; Duan, W.F. Flame retardancy mechanisms of melamine cyanurate in combination with aluminum diethylphosphinate in epoxy resin. J. Appl. Polym. Sci. 2019, 136, 47223. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, K.X.; Wang, X.; Chen, M.J.; Xin, F.; Liu, Z.G. Synergistic effects and flame-retardant mechanism of aluminum diethyl phosphinate in combination with melamine polyphosphate and aluminum oxide in epoxy resin. J. Therm. Anal. Calorim. 2018, 134, 1637–1646. [Google Scholar] [CrossRef]
- Ding, H.Y.; Huang, K.; Li, S.H.; Xu, L.N.; Xia, J.L.; Li, M. Flame retardancy and thermal degradation of halogen-free flame-retardant biobased polyurethane composites based on ammonium polyphosphate and aluminium hypophosphite. Polym. Test. 2017, 62, 325–334. [Google Scholar] [CrossRef]
- Wen, Y.; Cheng, Z.; Li, W.X.; Li, Z.; Liao, D.J.; Hu, X.P.; Pan, N.; Wang, D.Y.; Hull, T.R. A novel oligomer containing DOPO and ferrocene groups: Synthesis, characterization, and its application in fire retardant epoxy resin. Polym. Degrad. Stabil. 2018, 156, 111–124. [Google Scholar] [CrossRef]
- Zhang, W.C.; Li, X.M.; Yang, R.J. Blowing-out effect in epoxy composites flame retarded by DOPO-POSS and its correlation with amide curing agents. Polym. Degrad. Stabil. 2012, 97, 1314–1324. [Google Scholar] [CrossRef]
- Wang, P.; Cai, Z.S. Highly efficient flame-retardant epoxy resin with a novel DOPO-based triazole compound: Thermal stability, flame retardancy and mechanism. Polym. Degrad. Stabil. 2017, 137, 138–150. [Google Scholar] [CrossRef]
- Wu, H.Y.; Zeng, B.; Chen, J.M.; Wu, T.; Li, Y.T.; Liu, Y.Z.; Dai, L.Z. An intramolecular hybrid of metal polyhedral oligomeric silsesquioxanes with special titanium-embedded cage structure and flame retardant functionality. Chem. Eng. J. 2019, 374, 1304–1316. [Google Scholar] [CrossRef]
- Liu, H.; Wu, W.H.; He, S.R.; Song, Q.Y.; Li, W.H.; Zhang, J.Y.; Qu, H.Q.; Xu, J.Z. Activated carbon spheres (ACS)@SnO2@NiO with a 3D nanospherical structure and its synergistic effect with AHP on improving the flame retardancy of epoxy resin. Polym. Adv. Technol. 2019, 30, 951–962. [Google Scholar] [CrossRef]
- Carja, I.D.; Serbezeanu, D.; Tachita, V.B.; Hamciuc, C.; Coroaba, A.; Lisa, G.; López, C.G.; Soriano, M.F.; Pérez, V.F.; Sánchez, M.D.R. A straightforward, eco-friendly and cost-effective approach towards flame retardant epoxy resins. J. Mater. Chem. A 2014, 2, 16230–16241. [Google Scholar] [CrossRef]
- Hua, X.; Yang, H.Y.; Jiang, Y.P.; He, H.L.; Liu, H.Y.; Huang, H.; Wan, C.J. Facile synthesis of a novel transparent hyperbranched phosphorous/nitrogen-containing flame retardant and its application in reducing the fire hazard of epoxy resin. J. Hazard. Mater. 2019, 379, 120793. [Google Scholar] [CrossRef]
- Zhang, W.C.; Fina, A.; Ferraro, G.; Yang, R.J. FTIR and GCMS analysis of epoxy resin decomposition products feeding the flame during UL 94 standard flammability test. Application to the understanding of the blowing-out effect in epoxy/polyhedral silsesquioxane formulations. J. Anal. Appl. Pyrol. 2018, 135, 271–280. [Google Scholar] [CrossRef]
- Qian, L.J.; Qiu, Y.; Wang, J.Y.; Xi, W. High-performance flame retardancy by char-cage hindering and free radical quenching effects in epoxy thermosets. Polymer 2015, 68, 262–269. [Google Scholar] [CrossRef]
- Brehme, S.; Schartel, B.; Goebbels, J.; Fischer, O.; Pospiech, D.; Bykov, Y.; Döring, M. Phosphorus polyester versus aluminium phosphinate in poly(butylene terephthalate) (PBT): Flame retardancy performance and mechanisms. Polym. Degrad. Stab. 2011, 96, 875–884. [Google Scholar] [CrossRef]
- Ma, W.; Xu, B.; Shao, L.S.; Liu, Y.T.; Chen, Y.J.; Qian, L.J. Synthesis of (1,4-Methylenephenylphosphinic acid) Piperazine and Its Application as a Flame Retardant in Epoxy Thermosets. Macromol. Mater. Eng. 2019, 304, 1900419. [Google Scholar] [CrossRef]
- Wu, N.J.; Li, X.T. Flame retardancy and synergistic flame retardant mechanisms of acrylonitrile-butadiene-styrene composites based on aluminum hypophosphite. Polym. Degrad. Stabil. 2014, 105, 265–276. [Google Scholar] [CrossRef]
- Chen, J.H.; Lu, J.H.; Pu, X.L.; Chen, L.; Wang, Y.Z. Recyclable, malleable and intrinsically flame-retardant epoxy resin with catalytic transesterification. Chemosphere 2022, 294, 133778. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.L.; Yang, R.J.; Zhang, W.C.; Li, D.H.; Jiao, Q.J. Synergistic barrier effect of aluminum phosphate on flame retardant polypropylene based on ammonium polyphosphate/dipentaerythritol system. Mater. Des. 2019, 181, 107913. [Google Scholar] [CrossRef]
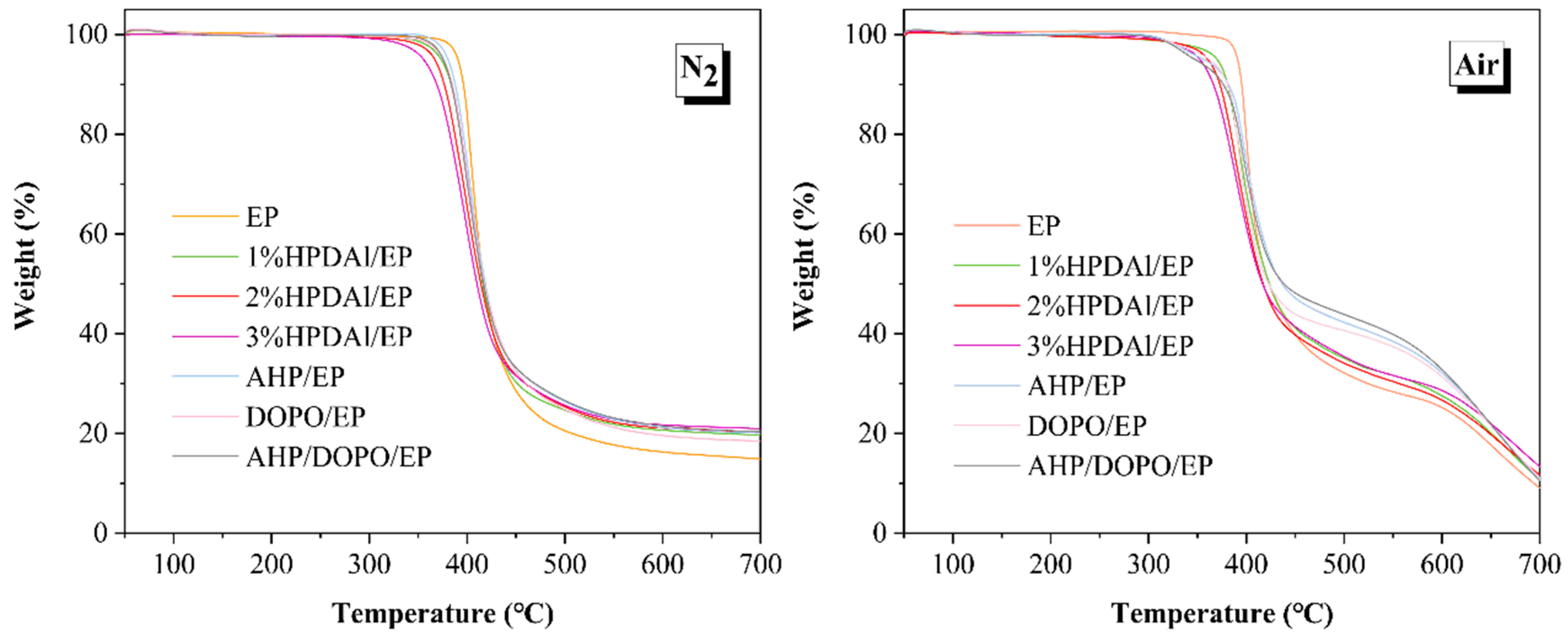

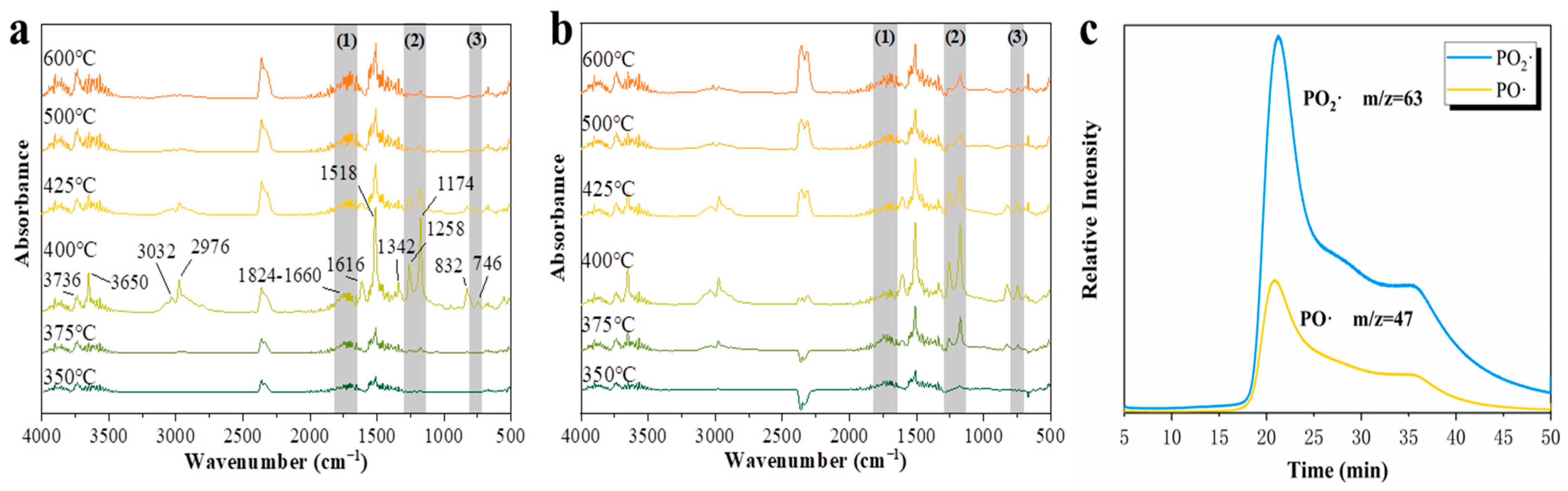
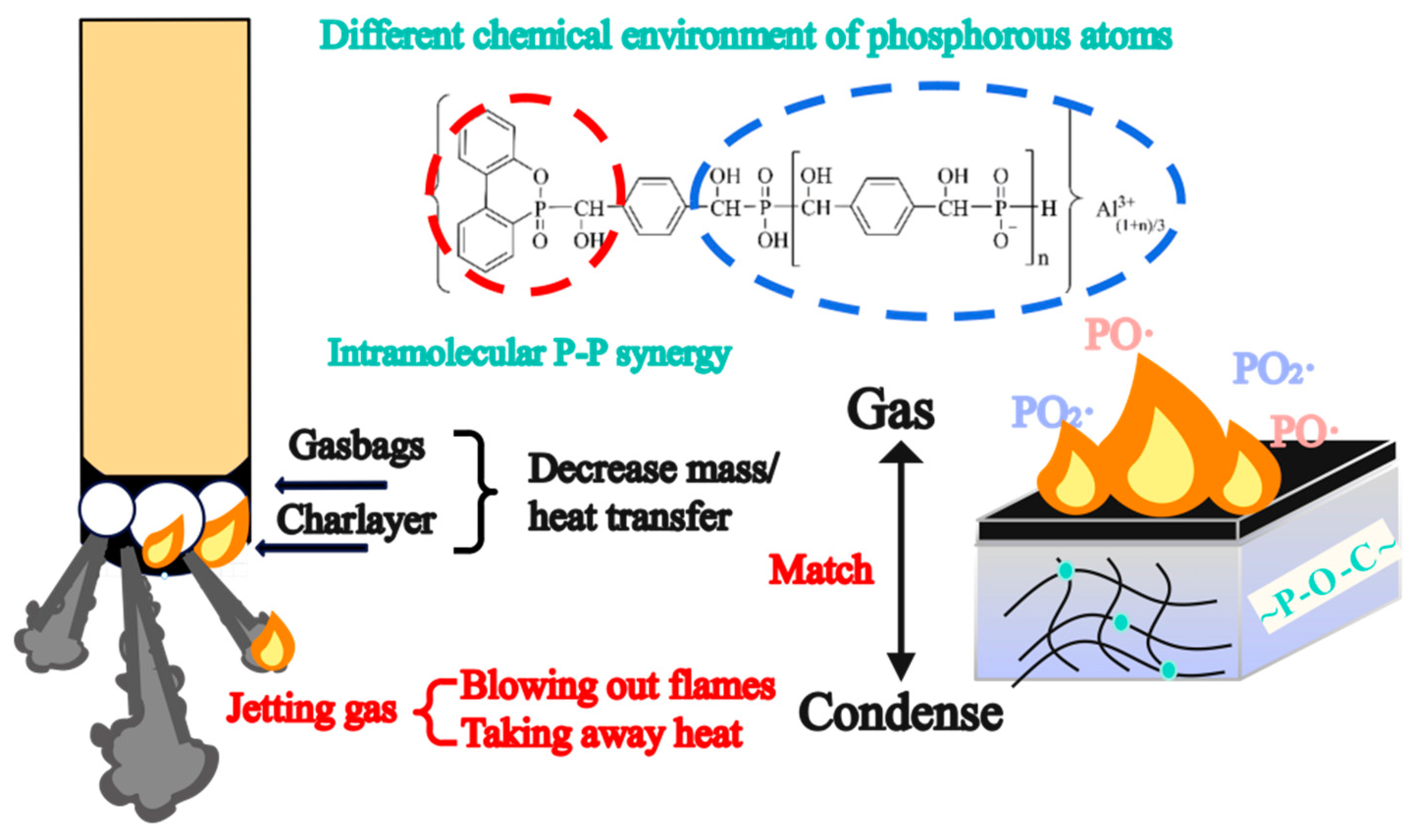
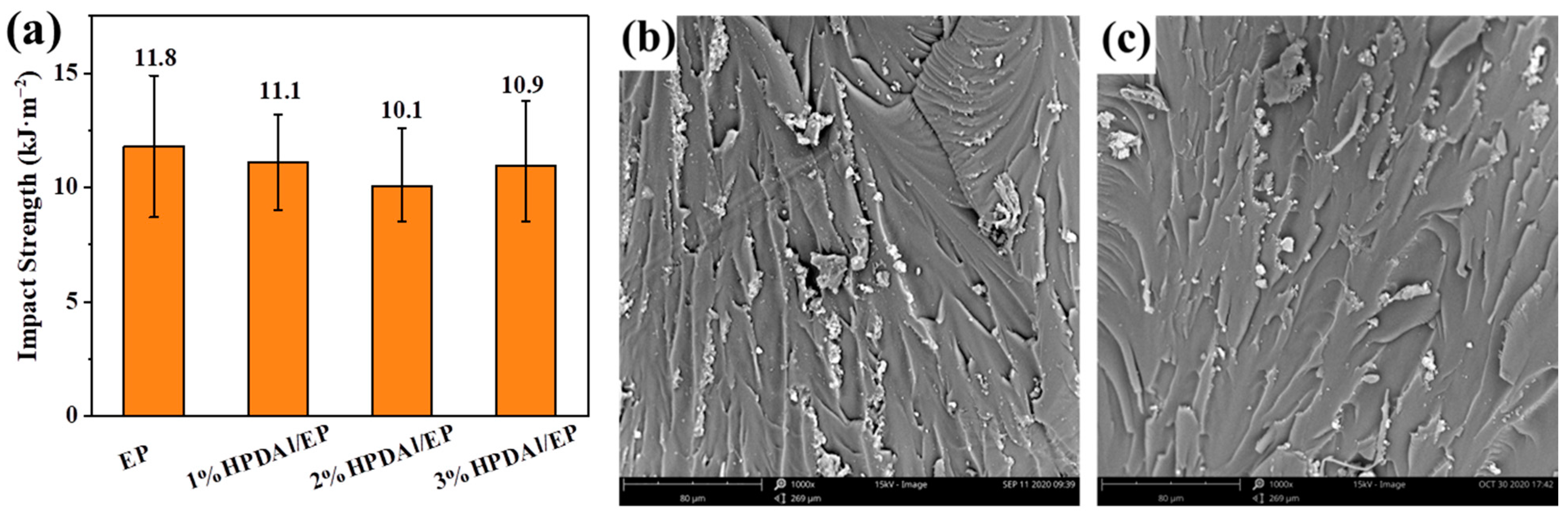
| Samples | N2 Atmosphere | Air Atmosphere | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Td,5% (°C) | Tpeak (°C) | Char Residue (wt.%) | Td,5% (°C) | Tpeak (°C) | Char Residue (wt.%) | |||||
| 500 °C | 600 °C | 700 °C | 500 °C | 600 °C | 700 °C | |||||
| EP | 391 | 405 | 20.5 | 16.3 | 14.9 | 388 | 398 | 31.7 | 24.6 | 9.3 |
| 1%HPDAl/EP | 373 | 402 | 24.7 | 20.7 | 19.7 | 370 | 395 | 34.3 | 27.1 | 10.7 |
| 2%HPDAl/EP | 367 | 399 | 25.4 | 21.1 | 20.3 | 361 | 389 | 34.5 | 27.3 | 11.8 |
| 3%HPDAl/EP | 355 | 398 | 25.7 | 21.8 | 21.0 | 353 | 391 | 35.2 | 28.8 | 13.1 |
| AHP/EP | 380 | 401 | 26.4 | 21.2 | 20.1 | 356 | 396 | 42.3 | 32.1 | 10.8 |
| DOPO/EP | 376 | 400 | 24.8 | 19.6 | 18.5 | 362 | 390 | 40.7 | 31.4 | 11.1 |
| AHP/DOPO/EP | 375 | 398 | 26.6 | 21.5 | 20.4 | 348 | 395 | 43.9 | 32.8 | 10.5 |
| Samples | TTI (s) | PHRR (kW m−2) | THR (MJ m−2) | TSP (m2) | TSR (m2 m−2) | av-EHC (MJ kg−1) | av-COY (kg kg−1) | av-CO2Y (kg kg−1) | Residue (wt.%) |
|---|---|---|---|---|---|---|---|---|---|
| EP | 56 ± 1 | 1420 ± 53 | 144 ± 5 | 52.2 ± 1.3 | 5770 ± 171 | 29.9 ± 0.3 | 0.13 ± 0.01 | 2.51 ± 0.08 | 7.70 ± 0.41 |
| 1%HPDAl/EP | 52 ± 2 | 1188 ± 36 | 117 ± 4 | 41.7 ± 1.4 | 4722 ± 142 | 27.2 ± 0.1 | 0.12 ± 0.01 | 2.20 ± 0.07 | 12.50 ± 0.35 |
| 2%HPDAl/EP | 50 ± 2 | 1072 ± 32 | 107 ± 3 | 40.1 ± 1.2 | 4536 ± 136 | 27.0 ± 0.1 | 0.13 ± 0.00 | 2.20 ± 0.06 | 14.50 ± 0.39 |
| 3%HPDAl/EP | 49 ± 1 | 1128 ± 34 | 115 ± 3 | 41.9 ± 1.2 | 4740 ± 139 | 27.2 ± 0.1 | 0.15 ± 0.01 | 2.19 ± 0.07 | 14.00 ± 0.32 |
| AHP/EP | 58 ± 2 | 1329 ± 40 | 139 ± 4 | 46.2 ± 1.5 | 5097 ± 155 | 29.8 ± 0.2 | 0.17 ± 0.02 | 2.50 ± 0.08 | 15.37 ± 0.45 |
| DOPO/EP | 52 ± 2 | 1351 ± 41 | 129 ± 4 | 54.8 ± 2.1 | 6060 ± 181 | 27.7 ± 0.2 | 0.20 ± 0.02 | 2.29 ± 0.09 | 12.01 ± 0.37 |
| AHP/DOPO/EP | 56 ± 1 | 1223 ± 37 | 133 ± 4 | 49.1 ± 1.5 | 5021 ± 152 | 28.9 ± 0.3 | 0.16 ± 0.01 | 2.40 ± 0.06 | 13.89 ± 0.42 |
| Samples | Flame-Inhibition Effect (%) | Charring Effect (%) | Barrier and Protective Effect (%) |
|---|---|---|---|
| 1%HPDAl/EP | 9.0 | 5.2 | −2.9 |
| 2%HPDAl/EP | 9.7 | 7.4 | −1.8 |
| 3%HPDAl/EP | 9.0 | 6.8 | 1.3 |
| AHP/EP | 0.3 | 8.3 | 3.4 |
| DOPO/EP | 7.4 | 4.7 | −6.1 |
| AHP/DOPO/EP | 3.0 | 6.7 | −0.6 |
| Element Contents | C (%) | N (%) | O (%) | P (%) | Al (%) |
|---|---|---|---|---|---|
| 1%HPDAl/EP | 88.25 | 1.21 | 9.42 | 0.52 | 0.60 |
| 2%HPDAl/EP | 85.98 | 1.67 | 10.65 | 1.07 | 0.47 |
| 3%HPDAl/EP | 83.27 | 2.71 | 11.67 | 1.98 | 0.44 |
| AHP/EP | 70.24 | 5.88 | 19.39 | 1.47 | 3.03 |
| DOPO/EP | 84.65 | 4.81 | 9.87 | 0.67 | 0.00 |
| AHP/DOPO/EP | 79.86 | 3.80 | 13.39 | 1.54 | 1.41 |
| Samples | P Ratio in Residues (%) | Char Yield (%) | Initial P Ratio in Samples (%) | Reserved P Ratio in Total P (%) | Released P Ratio in Total P (%) |
|---|---|---|---|---|---|
| 1%HPDAl/EP | 0.52 | 12.50 | 0.14 | 46.4 | 53.6 |
| 2%HPDAl/EP | 1.07 | 14.50 | 0.29 | 53.5 | 46.5 |
| 3%HPDAl/EP | 1.98 | 14.00 | 0.43 | 64.5 | 35.5 |
| AHP/EP | 1.47 | 15.37 | 0.29 | 77.9 | 22.1 |
| DOPO/EP | 0.67 | 12.01 | 0.29 | 27.7 | 72.3 |
| AHP/DOPO/EP | 1.54 | 13.89 | 0.29 | 73.8 | 26.2 |
| Samples | DGEBA/DDM g/g | DDM (g) | HPDAl | AHP a | DOPO (g) | P-Content (wt.%) | |||
|---|---|---|---|---|---|---|---|---|---|
| (g) | (wt.%) | (g) | (wt.%) | (g) | (wt.%) | ||||
| EP | 100.0/25.3 | 25.3 | - | - | - | - | - | - | 0.00 |
| 1%HPDAl/EP | 100.0/25.3 | 25.3 | 1.3 | 1.0 | - | - | - | - | 0.14 |
| 2%HPDAl/EP | 100.0/25.3 | 25.3 | 2.5 | 2.0 | - | - | - | - | 0.29 |
| 3%HPDAl/EP | 100.0/25.3 | 25.3 | 3.9 | 3.0 | - | - | - | - | 0.43 |
| AHP/EP | 100.0/25.3 | 25.3 | - | - | 0.893 | 0.7 | - | - | 0.29 |
| DOPO/EP | 100.0/25.3 | 25.3 | - | - | - | - | 2.610 | 2.0 | 0.29 |
| AHP/DOPO/EP | 100.0/25.3 | 25.3 | - | - | 0.702 | 0.6 | 0.560 | 0.4 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Liu, Y.; Wei, S.; Zhao, S.; Qian, L.; Chen, Y.; Shan, H.; Zhang, Q. A Phosphorous-Based Bi-Functional Flame Retardant Based on Phosphaphenanthrene and Aluminum Hypophosphite for an Epoxy Thermoset. Int. J. Mol. Sci. 2022, 23, 11256. https://doi.org/10.3390/ijms231911256
Xu B, Liu Y, Wei S, Zhao S, Qian L, Chen Y, Shan H, Zhang Q. A Phosphorous-Based Bi-Functional Flame Retardant Based on Phosphaphenanthrene and Aluminum Hypophosphite for an Epoxy Thermoset. International Journal of Molecular Sciences. 2022; 23(19):11256. https://doi.org/10.3390/ijms231911256
Chicago/Turabian StyleXu, Bo, Yanting Liu, Simiao Wei, Siheng Zhao, Lijun Qian, Yajun Chen, Hao Shan, and Qinglei Zhang. 2022. "A Phosphorous-Based Bi-Functional Flame Retardant Based on Phosphaphenanthrene and Aluminum Hypophosphite for an Epoxy Thermoset" International Journal of Molecular Sciences 23, no. 19: 11256. https://doi.org/10.3390/ijms231911256
APA StyleXu, B., Liu, Y., Wei, S., Zhao, S., Qian, L., Chen, Y., Shan, H., & Zhang, Q. (2022). A Phosphorous-Based Bi-Functional Flame Retardant Based on Phosphaphenanthrene and Aluminum Hypophosphite for an Epoxy Thermoset. International Journal of Molecular Sciences, 23(19), 11256. https://doi.org/10.3390/ijms231911256







