Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Agent for the Treatment of Liver Diseases
Abstract
1. Introduction
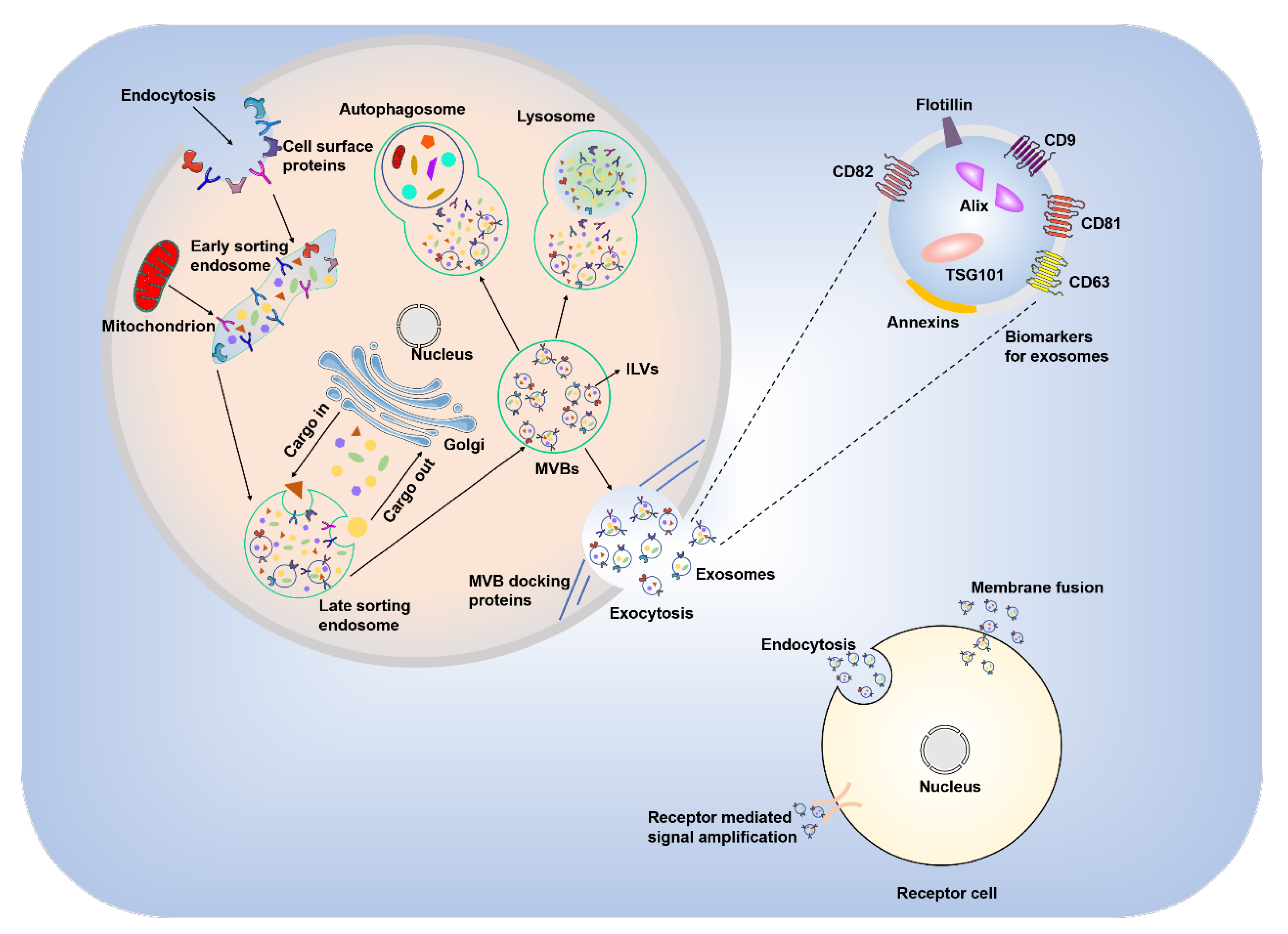
2. Biogenesis and General Physiological Functions of Exosomes
3. Contents and Physiological Functions of MSC-Derived Exosomes
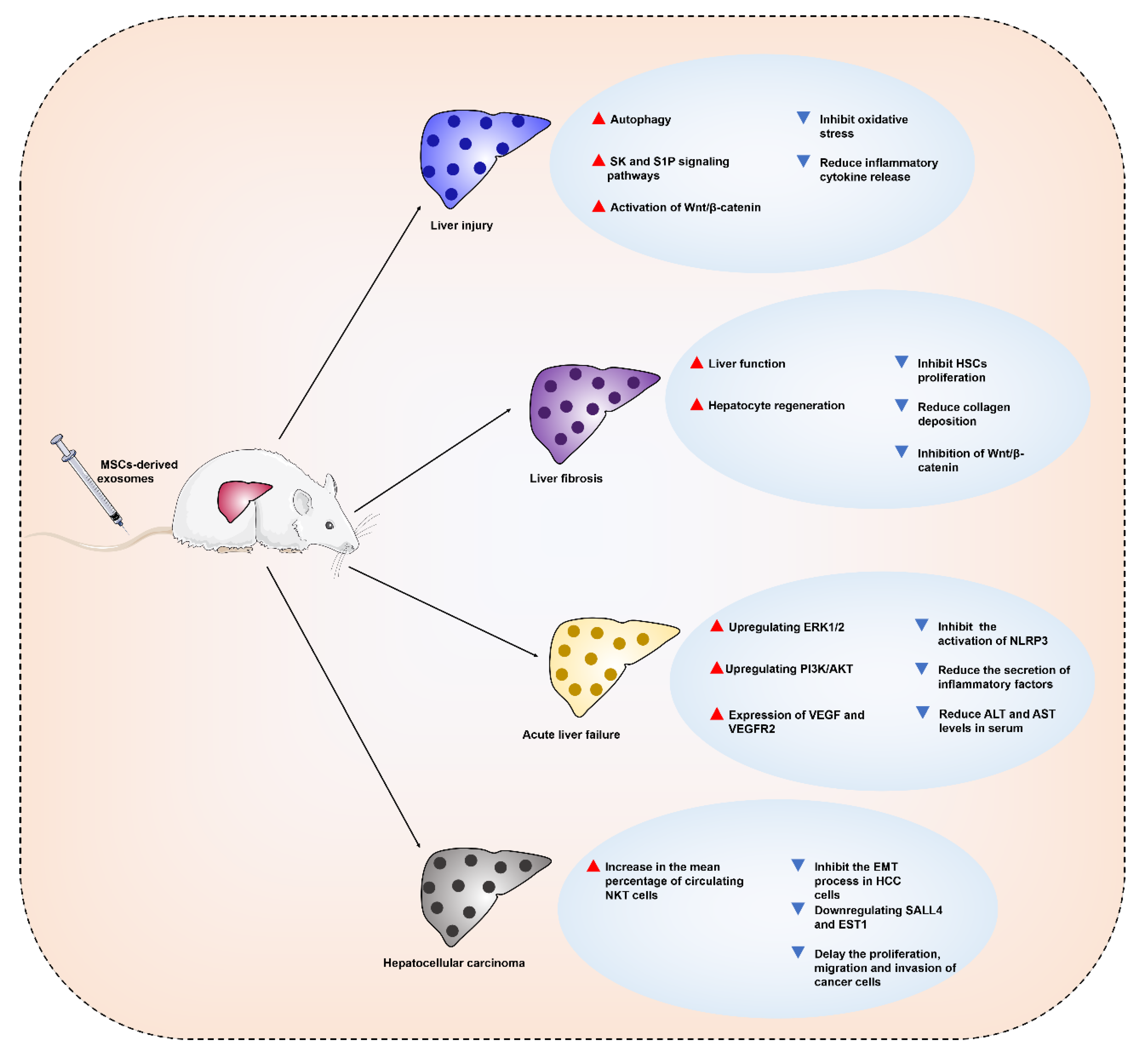
4. Therapeutic Applications and Mechanisms of MSC-Derived Exosomes in Liver Diseases
4.1. MSC-Derived Exosomes for the Treatment of Liver Injury
4.2. MSC-Derived Exosomes for the Treatment of Liver Fibrosis
4.3. MSC-Derived Exosomes for the Treatment of Acute Liver Failure
4.4. MSC-Derived Exosomes for the Treatment of Hepatocellular Carcinoma
5. Clinical Trials Using MSCs for the Treatment of Liver Diseases
6. MSC-Derived Exosomes: A Double-Edged Sword for Disease Treatment
7. Current Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfaifi, M.; Eom, Y.W.; Newsome, P.N.; Baik, S.K. Mesenchymal stromal cell therapy for liver diseases. J. Hepatol. 2018, 68, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Rocco, A.; Compare, D.; Angrisani, D.; Zamparelli, M.S.; Nardone, G. Alcoholic disease: Liver and beyond. World J. Gastroenterol. 2014, 20, 14652–14659. [Google Scholar] [CrossRef] [PubMed]
- Ringelhan, M.; McKeating, J.A.; Protzer, U. Viral hepatitis and liver cancer. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2017, 372, 20160274. [Google Scholar] [CrossRef]
- Lian, C.Y.; Zhai, Z.Z.; Li, Z.F.; Wang, L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem. Biol. Interact. 2020, 330, 109199. [Google Scholar] [CrossRef] [PubMed]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Brückner, S.; Winkler, S. The Therapeutic Promise of Mesenchymal Stem Cells for Liver Restoration. Trends Mol. Med. 2015, 21, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Song, F.Q.; Ren, L.N.; Guo, W.Q.; Wang, T.; Feng, Y.X.; Tang, L.J.; Li, K. The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases. J. Cell. Mol. Med. 2015, 19, 511–520. [Google Scholar] [CrossRef]
- Wu, M.C.; Meng, Q.H. Current understanding of mesenchymal stem cells in liver diseases. World J. Stem Cells 2021, 13, 1349–1359. [Google Scholar] [CrossRef]
- Jin, W.; Liang, X.; Brooks, A.; Futrega, K.; Liu, X.; Doran, M.R.; Simpson, M.J.; Roberts, M.S.; Wang, H. Modelling of the SDF-1/CXCR4 regulated in vivo homing of therapeutic mesenchymal stem/stromal cells in mice. PeerJ 2018, 6, e6072. [Google Scholar] [CrossRef]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol. Biol. 2016, 1416, 123–146. [Google Scholar]
- Chen, S.; Xu, L.; Lin, N.; Pan, W.; Hu, K.; Xu, R. Activation of Notch1 signaling by marrow-derived mesenchymal stem cells through cell-cell contact inhibits proliferation of hepatic stellate cells. Life Sci. 2011, 89, 975–981. [Google Scholar] [CrossRef]
- Gholamrezanezhad, A.; Mirpour, S.; Bagheri, M.; Mohamadnejad, M.; Alimoghaddam, K.; Abdolahzadeh, L.; Saghari, M.; Malekzadeh, R. in vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl. Med. Biol. 2011, 38, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Hardjo, M.; Masaka, T.; Tomiyama, K.; Mahmut, N.; Medina, R.J.; Niida, A.; Sonegawa, H.; Du, G.; Yong, R.; et al. Isolation of a bone marrow-derived stem cell line with high proliferation potential and its application for preventing acute fatal liver failure. Stem Cells 2007, 25, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Hu, X.J.; Li, Z.R.; Yan, R.H.; Li, D.; Wang, J.; Shan, H. In Vivo Bioluminescence Imaging of Transplanted Mesenchymal Stromal Cells and Their Rejection Mediated by Intrahepatic NK Cells. Mol. Imaging Biol. 2017, 19, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, W.J.; Ploemacher, R.E. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 2003, 17, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Ridge, S.M.; Sullivan, F.J.; Glynn, S.A. Mesenchymal stem cells: Key players in cancer progression. Mol. Cancer 2017, 16, 31. [Google Scholar] [CrossRef]
- Ahn, S.Y. The Role of MSCs in the Tumor Microenvironment and Tumor Progression. Anticancer Res. 2020, 40, 3039–3047. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Xunian, Z.; Kalluri, R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020, 111, 3100–3110. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef] [PubMed]
- Gang, D.; Yu, C.J.; Zhu, S.; Zhu, P.; Nasser, M.I. Application of mesenchymal stem cell-derived exosomes in kidney diseases. Cell. Immunol. 2021, 364, 104358. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, E.; Fujita, D.; Takahashi, M.; Oba, S.; Nishimatsu, H. Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes in Cardiovascular Disease. Adv. Exp. Med. Biol. 2017, 998, 179–185. [Google Scholar]
- Sun, Z.; Zhang, J.; Li, J.; Li, M.; Ge, J.; Wu, P.; You, B.; Qian, H. Roles of Mesenchymal Stem Cell-Derived Exosomes in Cancer Development and Targeted Therapy. Stem Cells Int. 2021, 2021, 9962194. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Huang, D.; Sang, C.; Zhong, T.; Zhang, Z.; Tang, Z. Advances in Mesenchymal Stem Cell-Derived Exosomes as Drug Delivery Vehicles. Front. Bioeng. Biotechnol. 2022, 9, 797359. [Google Scholar] [CrossRef]
- Juan, T.; Fürthauer, M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin. Cell Dev. Biol. 2018, 74, 66–77. [Google Scholar] [CrossRef]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef]
- Simpson, R.J.; Lim, J.W.; Moritz, R.L.; Mathivanan, S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev. Proteom. 2009, 6, 267–283. [Google Scholar] [CrossRef]
- Donoso-Quezada, J.; Ayala-Mar, S.; González-Valdez, J. The role of lipids in exosome biology and intercellular communication: Function, analytics and applications. Traffic 2021, 22, 204–220. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. Discovery of Double-Stranded Genomic DNA in Circulating Exosomes. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 275–280. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Hood, J.L.; San, R.S.; Wickline, S.A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011, 71, 3792–3801. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zeng, S.; Gong, Z.; Yan, Y. Exosome-based immunotherapy: A promising approach for cancer treatment. Mol. Cancer 2020, 19, 160. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. CCS 2021, 19, 47. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Shinagawa, K.; Castellino, F.J.; Schorey, J.S. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 2007, 110, 3234–3244. [Google Scholar] [CrossRef]
- Gastpar, R.; Gehrmann, M.; Bausero, M.A.; Asea, A.; Gross, C.; Schroeder, J.A.; Multhoff, G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005, 65, 5238–5247. [Google Scholar] [CrossRef]
- Anel, A.; Gallego-Lleyda, A.; de Miguel, D.; Naval, J.; Martínez-Lostao, L. Role of Exosomes in the Regulation of T-cell Mediated Immune Responses and in Autoimmune Disease. Cells 2019, 8, 154. [Google Scholar] [CrossRef]
- Nojima, H.; Freeman, C.M.; Schuster, R.M.; Japtok, L.; Kleuser, B.; Edwards, M.J.; Gulbins, E.; Lentsch, A.B. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J. Hepatol. 2016, 64, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Choi, D.Y.; Yun, S.J.; Choi, S.M.; Kang, J.W.; Jung, J.W.; Hwang, D.; Kim, K.P.; Kim, D.W. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J. Proteome Res. 2012, 11, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Yuan, O.; Lin, C.; Wagner, J.; Archard, J.A.; Deng, P.; Halmai, J.; Bauer, G.; Fink, K.D.; Fury, B.; Perotti, N.H.; et al. Exosomes Derived from Human Primed Mesenchymal Stem Cells Induce Mitosis and Potentiate Growth Factor Secretion. Stem Cells Dev. 2019, 28, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.B.; Fonsato, V.; Gatti, S.; Deregibus, M.C.; Sordi, A.; Cantarella, D.; Calogero, R.; Bussolati, B.; Tetta, C.; Camussi, G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J. Cell. Mol. Med. 2010, 14, 1605–1618. [Google Scholar] [CrossRef]
- Ferguson, S.W.; Wang, J.; Lee, C.J.; Liu, M.; Neelamegham, S.; Canty, J.M.; Nguyen, J. The microRNA regulatory landscape of MSC-derived exosomes: A systems view. Sci. Rep. 2018, 8, 1419. [Google Scholar] [CrossRef]
- Deng, H.; Sun, C.; Sun, Y.; Li, H.; Yang, L.; Wu, D.; Gao, Q.; Jiang, X. Lipid, Protein, and MicroRNA Composition Within Mesenchymal Stem Cell-Derived Exosomes. Cell. Reprogram. 2018, 20, 178–186. [Google Scholar] [CrossRef]
- Lai, R.C.; Yeo, R.W.; Tan, K.H.; Lim, S.K. Mesenchymal stem cell exosome ameliorates reperfusion injury through proteomic complementation. Regen. Med. 2013, 8, 197–209. [Google Scholar] [CrossRef]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef]
- Wang, K. Molecular mechanisms of liver injury: Apoptosis or necrosis. Exp. Toxicol. Pathol. 2014, 66, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Feng, X.; Zhu, J.; Feng, B.; Yao, Q.; Pan, Q.; Yu, J.; Yang, J.; Li, L.; Cao, H. Mesenchymal stem cell treatment restores liver macrophages homeostasis to alleviate mouse acute liver injury revealed by single-cell analysis. Pharmacol. Res. 2022, 179, 106229. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, R.G. What is the most appropriate dose of N-acetylcysteine after massive acetaminophen overdose? Clin. Toxicol. 2019, 57, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Doyon, S.; Klein-Schwartz, W. Hepatotoxicity despite early administration of intravenous N-acetylcysteine for acute acetaminophen overdose. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2009, 16, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Y.; Pu, Z. Bone marrow mesenchymal stem cell-derived exosomes attenuate D-GaIN/LPS-induced hepatocyte apoptosis by activating autophagy in vitro. Drug Des. Dev. Ther. 2019, 13, 2887–2897. [Google Scholar] [CrossRef]
- Tan, C.Y.; Lai, R.C.; Wong, W.; Dan, Y.Y.; Lim, S.K.; Ho, H.K. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res. Ther. 2014, 5, 76. [Google Scholar] [CrossRef]
- Jiang, W.; Tan, Y.; Cai, M.; Zhao, T.; Mao, F.; Zhang, X.; Xu, W.; Yan, Z.; Qian, H.; Yan, Y. Human Umbilical Cord MSC-Derived Exosomes Suppress the Development of CCl4-Induced Liver Injury through Antioxidant Effect. Stem Cells Int. 2018, 2018, 6079642. [Google Scholar] [CrossRef]
- Tamura, R.; Uemoto, S.; Tabata, Y. Immunosuppressive effect of mesenchymal stem cell-derived exosomes on a concanavalin A-induced liver injury model. Inflamm. Regen. 2016, 36, 26. [Google Scholar] [CrossRef]
- Du, Y.; Li, D.; Han, C.; Wu, H.; Xu, L.; Zhang, M.; Zhang, J.; Chen, X. Exosomes from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells (hiPSC-MSCs) Protect Liver against Hepatic Ischemia/ Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 43, 611–625. [Google Scholar] [CrossRef]
- Xie, K.; Liu, L.; Chen, J.; Liu, F. Exosomes derived from human umbilical cord blood mesenchymal stem cells improve hepatic ischemia reperfusion injury via delivering miR-1246. Cell Cycle 2019, 18, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Liu, L.; Chen, J.; Liu, F. Exosomal miR-1246 derived from human umbilical cord blood mesenchymal stem cells attenuates hepatic ischemia reperfusion injury by modulating T helper 17/regulatory T balance. IUBMB Life 2019, 71, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Xu, Q.; Wu, Z.; Chen, Y.; Shu, Y.; Cao, X.; Chen, M.; Zhang, B.; Zhou, Y.; Yao, R.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res. Ther. 2020, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D. Liver fibrosis: Common mechanisms and antifibrotic therapies. Clin. Res. Hepatol. Gastroenterol. 2015, 39 (Suppl. 1), S51–S59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Yuan, W.G.; He, P.; Lei, J.H.; Wang, C.X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512–10522. [Google Scholar] [CrossRef]
- Fiore, E.J.; Mazzolini, G.; Aquino, J.B. Mesenchymal Stem/Stromal Cells in Liver Fibrosis: Recent Findings, Old/New Caveats and Future Perspectives. Stem Cell Rev. Rep. 2015, 11, 586–597. [Google Scholar] [CrossRef]
- Ma, L.; Wei, J.; Zeng, Y.; Liu, J.; Xiao, E.; Kang, Y.; Kang, Y. Mesenchymal stem cell-originated exosomal circDIDO1 suppresses hepatic stellate cell activation by miR-141-3p/PTEN/AKT pathway in human liver fibrosis. Drug Deliv. 2022, 29, 440–453. [Google Scholar] [CrossRef]
- Sun, C.; Shi, C.; Duan, X.; Zhang, Y.; Wang, B. Exosomal microRNA-618 derived from mesenchymal stem cells attenuate the progression of hepatic fibrosis by targeting Smad4. Bioengineered 2022, 13, 5915–5927. [Google Scholar] [CrossRef]
- Rong, X.; Liu, J.; Yao, X.; Jiang, T.; Wang, Y.; Xie, F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res. Ther. 2019, 10, 98. [Google Scholar] [CrossRef]
- Chiabotto, G.; Ceccotti, E.; Tapparo, M.; Camussi, G.; Bruno, S. Human Liver Stem Cell-Derived Extracellular Vesicles Target Hepatic Stellate Cells and Attenuate Their Pro-fibrotic Phenotype. Front. Cell Dev. Biol. 2021, 9, 777462. [Google Scholar] [CrossRef]
- Wang, X.; Ning, Q. Immune mediated liver failure. EXCLI J. 2014, 13, 1131–1144. [Google Scholar] [PubMed]
- Blasco-Algora, S.; Masegosa-Ataz, J.; Gutiérrez-García, M.L.; Alonso-López, S.; Fernández-Rodríguez, C.M. Acute-on-chronic liver failure: Pathogenesis, prognostic factors and management. World J. Gastroenterol. 2015, 21, 12125–12140. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Han, M.; Chen, T.; Yan, W.; Ning, Q. Acute liver failure: Mechanisms of immune-mediated liver injury. Liver Int. Off. J. Int. Assoc. Study Liver 2010, 30, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.H.; Kim, S.Y.; Kim, Y.R.; Woo, S.Y.; Sung, S.H.; Kim, H.S.; Jung, S.C.; Jo, I.; Park, J.W. Tonsil-derived mesenchymal stem cells alleviate concanavalin A-induced acute liver injury. Exp. Cell Res. 2014, 326, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Rolando, N.; Wade, J.; Davalos, M.; Wendon, J.; Philpott-Howard, J.; Williams, R. The systemic inflammatory response syndrome in acute liver failure. Hepatology 2000, 32 Pt 1, 734–739. [Google Scholar] [CrossRef]
- Zhang, S.; Hou, Y.; Yang, J.; Xie, D.; Jiang, L.; Hu, H.; Hu, J.; Luo, C.; Zhang, Q. Application of mesenchymal stem cell exosomes and their drug-loading systems in acute liver failure. J. Cell. Mol. Med. 2020, 24, 7082–7093. [Google Scholar] [CrossRef]
- Liu, Y.; Lou, G.; Li, A.; Zhang, T.; Qi, J.; Ye, D.; Zheng, M.; Chen, Z. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine 2018, 36, 140–150. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, S.; Hu, H.; Yang, J.; Wang, X.; Ma, Y.; Jiang, J.; Wang, J.; Zhong, L.; Chen, M.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acute liver failure by reducing the activity of the NLRP3 inflammasome in macrophages. Biochem. Biophys. Res. Commun. 2019, 508, 735–741. [Google Scholar] [CrossRef]
- Wu, H.Y.; Zhang, X.C.; Jia, B.B.; Cao, Y.; Yan, K.; Li, J.Y.; Tao, L.; Jie, Z.G.; Liu, Q.W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acetaminophen-induced acute liver failure through activating ERK and IGF-1R/PI3K/AKT signaling pathway. J. Pharmacol. Sci. 2021, 147, 143–155. [Google Scholar] [CrossRef]
- Jun, J.H.; Kim, J.Y.; Choi, J.H.; Lim, J.Y.; Kim, K.; Kim, G.J. Exosomes from Placenta-Derived Mesenchymal Stem Cells Are Involved in Liver Regeneration in Hepatic Failure Induced by Bile Duct Ligation. Stem Cells Int. 2020, 2020, 5485738. [Google Scholar] [CrossRef]
- Mu, X.M.; Wang, W.; Jiang, Y.Y.; Feng, J. Patterns of Comorbidity in Hepatocellular Carcinoma: A Network Perspective. Int. J. Environ. Res. Public Health 2020, 17, 3108. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, S.; Li, G.; Zhang, S.; Tang, X.; Ni, S.; Jian, X.; Xu, C.; Zhu, J.; Lu, M. The role of extracellular vesicles in mediating progression, metastasis and potential treatment of hepatocellular carcinoma. Oncotarget 2017, 8, 3683–3695. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, L.; Wu, M.; Cao, K.; Jiang, F.; Chen, D.; Li, N.; Li, W. The significance of exosomes in the development and treatment of hepatocellular carcinoma. Mol. Cancer 2020, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, J.; Zhang, Z.; Qian, Y.; Wang, G.; Duan, M.; Zhao, H.; Yang, Z.; Jiang, X. Mesenchymal stem cell-derived exosome: A tumor regulator and carrier for targeted tumor therapy. Cancer Lett. 2022, 526, 29–40. [Google Scholar] [CrossRef]
- Ko, S.F.; Yip, H.K.; Zhen, Y.Y.; Lee, C.C.; Lee, C.C.; Huang, C.C.; Ng, S.H.; Lin, J.W. Adipose-Derived Mesenchymal Stem Cell Exosomes Suppress Hepatocellular Carcinoma Growth in a Rat Model: Apparent Diffusion Coefficient, Natural Killer T-Cell Responses, and Histopathological Features. Stem Cells Int. 2015, 2015, 853506. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and inflammation: Inseparable actors of cancer progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef]
- Xu, Y.; Lai, Y.; Cao, L.; Li, Y.; Chen, G.; Chen, L.; Weng, H.; Chen, T.; Wang, L.; Ye, Y. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-451a represses epithelial-mesenchymal transition of hepatocellular carcinoma cells by inhibiting ADAM10. RNA Biol. 2021, 18, 1408–1423. [Google Scholar] [CrossRef]
- Ma, Y.S.; Liu, J.B.; Lin, L.; Zhang, H.; Wu, J.J.; Shi, Y.; Jia, C.Y.; Zhang, D.D.; Yu, F.; Wang, H.; et al. Exosomal microRNA-15a from mesenchymal stem cells impedes hepatocellular carcinoma progression via downregulation of SALL4. Cell Death Discov. 2021, 7, 224. [Google Scholar] [CrossRef]
- Li, Y.H.; Lv, M.F.; Lu, M.S.; Bi, J.P. Bone marrow mesenchymal stem cell-derived exosomal MiR-338-3p represses progression of hepatocellular carcinoma by targeting ETS1. J. Biol. Regul. Homeost. Agents 2021, 35, 617–627. [Google Scholar] [PubMed]
- Liu, J.; Ren, L.; Li, S.; Li, W.; Zheng, X.; Yang, Y.; Fu, W.; Yi, J.; Wang, J.; Du, G. The biology, function, and applications of exosomes in cancer. Acta Pharm. Sin. B 2021, 11, 2783–2797. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release Off. J. Control. Release Soc. 2015, 219, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Mehryab, F.; Rabbani, S.; Shahhosseini, S.; Shekari, F.; Fatahi, Y.; Baharvand, H.; Haeri, A. Exosomes as a next-generation drug delivery system: An update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020, 113, 42–62. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, G.; Zhang, K.; Cao, Q.; Liu, T.; Li, J. Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Res. Ther. 2021, 12, 561. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.; Shi, Y.; Zhao, L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J. Nanobiotechnol. 2018, 16, 103. [Google Scholar] [CrossRef]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef]
- Lou, G.; Chen, L.; Xia, C.; Wang, W.; Qi, J.; Li, A.; Zhao, L.; Chen, Z.; Zheng, M.; Liu, Y. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J. Exp. Clin. Cancer Res. CR 2020, 39, 4. [Google Scholar] [CrossRef]
- Liang, L.; Zhao, L.; Wang, Y.; Wang, Y. Treatment for Hepatocellular Carcinoma Is Enhanced When Norcantharidin is Encapsulated in Exosomes Derived from Bone Marrow Mesenchymal Stem Cells. Mol. Pharm. 2021, 18, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Liu, H.; Li, Y.; Fu, J.; Sun, Y.; Xu, R.; Lin, H.; Wang, S.; Lv, S.; et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 1), 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, H.; Shi, M.; Xu, R.; Fu, J.; Lv, J.; Chen, L.; Lv, S.; Li, Y.; Yu, S.; et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J. Gastroenterol. Hepatol. 2012, 27 (Suppl. 2), 112–120. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; Sabry, D.; Rashed, L.A.; Aref, W.M.; el-Ghobary, M.A.; Farhan, M.S.; Fouad, H.A.; Youssef, Y.A. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin. Transplant. 2013, 27, 607–612. [Google Scholar] [CrossRef]
- Lee, B.C.; Kang, I.; Yu, K.R. Therapeutic Features and Updated Clinical Trials of Mesenchymal Stem Cell (MSC)-Derived Exosomes. J. Clin. Med. 2021, 10, 711. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Rahman, H.S.; Markov, A.; Endjun, J.J.; Zekiy, A.O.; Chartrand, M.S.; Beheshtkhoo, N.; Kouhbanani, M.; Marofi, F.; Nikoo, M.; et al. Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res. Ther. 2021, 12, 297. [Google Scholar] [CrossRef]
- Lee, J.K.; Park, S.R.; Jung, B.K.; Jeon, Y.K.; Lee, Y.S.; Kim, M.K.; Kim, Y.G.; Jang, J.Y.; Kim, C.W. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE 2013, 8, e84256. [Google Scholar] [CrossRef]
- Pakravan, K.; Babashah, S.; Sadeghizadeh, M.; Mowla, S.J.; Mossahebi-Mohammadi, M.; Ataei, F.; Dana, N.; Javan, M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell. Oncol. 2017, 40, 457–470. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, G.; Zhang, Y.; Jiang, H.; Wang, W.; Zhao, D.; Hong, J.; Yu, H.; Qi, L. Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/β-catenin signaling pathway by targeting EZH2. Stem Cell Res. Ther. 2019, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Mo, C.; Guo, S.; Zhuang, J.; Huang, B.; Mao, X. Human bone marrow mesenchymal stem cells-derived microRNA-205-containing exosomes impede the progression of prostate cancer through suppression of RHPN2. J. Exp. Clin. Cancer Res. CR 2019, 38, 495. [Google Scholar] [CrossRef] [PubMed]
- Dostert, G.; Mesure, B.; Menu, P.; Velot, É. How Do Mesenchymal Stem Cells Influence or Are Influenced by Microenvironment through Extracellular Vesicles Communication? Front. Cell Dev. Biol. 2017, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, T.; Gai, C.; Deregibus, M.C.; Kholia, S.; Camussi, G. Cross Talk between Cancer and Mesenchymal Stem Cells through Extracellular Vesicles Carrying Nucleic Acids. Front. Oncol. 2016, 6, 125. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin. Immunol. 2018, 35, 69–79. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, Y.; Jiao, Z.; Wang, X.; Zhao, Y.; Li, Y.; Chen, H.; Yang, L.; Zhu, H.; Li, Y. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Tumor Growth Through Hedgehog Signaling Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 42, 2242–2254. [Google Scholar] [CrossRef]
- Qin, F.; Tang, H.; Zhang, Y.; Zhang, Z.; Huang, P.; Zhu, J. Bone marrow-derived mesenchymal stem cell-derived exosomal microRNA-208a promotes osteosarcoma cell proliferation, migration, and invasion. J. Cell. Physiol. 2020, 235, 4734–4745. [Google Scholar] [CrossRef]
- Deng, Y.; Julaiti, A.; Ran, W.; He, Y. Bone marrow mesenchymal stem cells-derived exosomal microRNA-19b-3p targets SOCS1 to facilitate progression of esophageal cancer. Life Sci. 2021, 278, 119491. [Google Scholar] [CrossRef]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, C.; Shi, H.; Zhang, B.; Zhang, L.; Zhang, X.; Wang, S.; Wu, X.; Yang, T.; Huang, F.; et al. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: Novel biomarkers and a mechanism for gastric cancer. Br. J. Cancer 2014, 110, 1199–1210. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, L.; Li, Y.; Zhang, X.; Gu, J.; Yan, Y.; Xu, X.; Wang, M.; Qian, H.; Xu, W. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012, 315, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, Z.C.; Piorońska, W.; Battello, N.; Zimmer, A.D.; Dewidar, B.; Han, M.; Pereira, S.; Blagojevic, B.; Castven, D.; Charlestin, V.; et al. Severe metabolic alterations in liver cancer lead to ERK pathway activation and drug resistance. EBioMedicine 2020, 54, 102699. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shi, L.; Xiao, T.; Xue, J.; Li, J.; Wang, P.; Wu, L.; Dai, X.; Ni, X.; Liu, Q. microRNA-21, via the HIF-1α/VEGF signaling pathway, is involved in arsenite-induced hepatic fibrosis through aberrant cross-talk of hepatocytes and hepatic stellate cells. Chemosphere 2021, 266, 129177. [Google Scholar] [CrossRef] [PubMed]
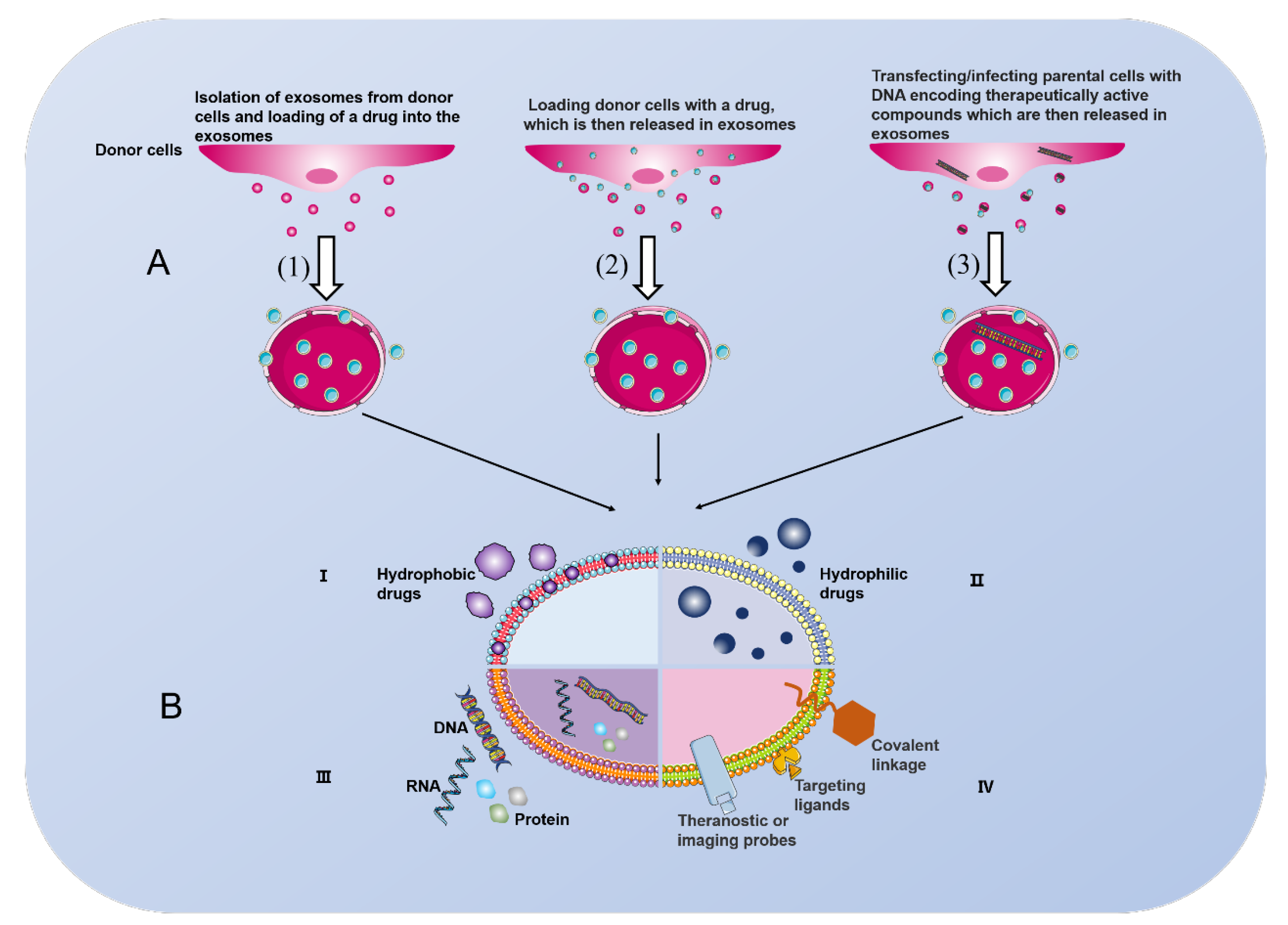
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.E.; Manangon, E.; Hood, J.L.; Wickline, S.A.; Fernandez, D.P.; Johnson, W.P.; Gale, B.K. A review of exosome separation techniques and characterization of B16-F10 mouse melanoma exosomes with AF4-UV-MALS-DLS-TEM. Anal. Bioanal. Chem. 2014, 406, 7855–7866. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef]
- Bec, N.; Bonhoure, A.; Henry, L.; Berry, L.; Larroque, C.; Coux, O.; Stoebner, P.E.; Vidal, M. Proteasome 19S RP and translation preinitiation complexes are secreted within exosomes upon serum starvation. Traffic 2019, 20, 516–536. [Google Scholar] [CrossRef]
- Bister, N.; Pistono, C.; Huremagic, B.; Jolkkonen, J.; Giugno, R.; Malm, T. Hypoxia and extracellular vesicles: A review on methods, vesicular cargo and functions. J. Extracell. Vesicles 2020, 10, e12002. [Google Scholar] [CrossRef]
- Pachler, K.; Lener, T.; Streif, D.; Dunai, Z.A.; Desgeorges, A.; Feichtner, M.; Öller, M.; Schallmoser, K.; Rohde, E.; Gimona, M. A Good Manufacturing Practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy 2017, 19, 458–472. [Google Scholar] [CrossRef]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko, E.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, e99263. [Google Scholar] [CrossRef]
- Laggner, M.; Gugerell, A.; Bachmann, C.; Hofbauer, H.; Vorstandlechner, V.; Seibold, M.; Lechner, G.G.; Peterbauer, A.; Madlener, S.; Demyanets, S.; et al. Reproducibility of GMP-compliant production of therapeutic stressed peripheral blood mononuclear cell-derived secretomes, a novel class of biological medicinal products. Stem Cell Res. Ther. 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
| Diseases | Source of MSCs | Injection Route | Test Evaluation Subjects | Phase | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| HBV-related acute-on-chronic liver failure | UCB-MSCs | Peripheral veins | Safety and efficacy | Phase II | NCT02812121 |
| Liver failure caused by hepatitis B | Autologous MMSCs | The proper hepatic artery | The short-term efficacy and long-term prognosis | Unknown | NCT00956891 |
| Acute-on-chronic liver failure caused by HBV | HUC-MSCs | Peripheral vein | Safety and efficacy | Phase I/II | NCT01724398 |
| HBV-related liver failure | Allogeneic HBM-MSCs and HUC-MSCs | Peripheral vein | Safety and efficacy | Phase I/II | NCT01844063 |
| The early and middle stage of liver cirrhosis on the basis of HBV infection | Autologous BMSCs | Portal vein | The therapeutic efficacy | Phase II | NCT00993941 |
| HBV-related liver cirrhosis | Autologous BMSCs | Hepatic artery | Safety and efficacy | Phase I/II | NCT01724697 |
| Liver cirrhosis resulting from chronic HBV | Allogenic BMSCs | Portal vein or hepatic artery | The therapeutic efficacy | Phase II | NCT01223664 |
| Liver cirrhosis with refractory ascites | Autologous BMSCs | Liver artery | The effect of MSCs in the patients | Phase III | NCT01854125 |
| Alcoholic liver cirrhosis | BMSCs | Hepatic artery catheterizations | Safety and efficacy | Phase II | NCT01741090 |
| Liver cirrhosis | UC-MSCs | Intravenously | Safety and efficacy | Phase I/II | NCT01220492 |
| Decompensated cirrhosis | HUC-MSCs | Intravenously | Safety and efficacy | Phase II | NCT05224960 |
| Liver cirrhosis due to biliary atresia | UC-MSCs | Hepatic artery | Safety and efficacy | Phase I/II | NCT04522869 |
| Liver cirrhosis | UC-MSCs | Hepatic artery | Safety and efficacy | Phase I/II | NCT01224327 |
| Liver cirrhosis | UC-MSCs | Hepatic artery and intravenously | The efficacy of different interventional therapies | Phase I/II | NCT01233102 |
| Source of Exosomes | Study Purposes | Study Results | References |
|---|---|---|---|
| Human BMSC-derived exosomes | To study the molecular mechanism of MSCs on the growth of human osteosarcoma and human gastric cancer cells | Promoting MG63 and SGC7901 cell growth through the activation of Hedgehog signaling pathway | [116] |
| BMSC-derived exosomes | To explore the impact of the miR-208a-enriched exosomes derived from BMSCs on osteosarcoma cells. | Promoting proliferation, migration and invasion of osteosarcoma cells partly through downregulation of PDCD4 and activation of ERK1/2 pathway | [117] |
| Adult BMSC-derived exosomes | To explore the role of BMSC-derived exosomes mediating miR-19b-3p in EC cell growth | BMSC-derived exosomal microRNA-19b-3p targets SOCS1 to facilitate progression of esophageal cancer | [118] |
| Hypoxic BMSC-derived exosomes | To further elucidate the communication between BMSC-derived exosomes and cancer cells in the hypoxic niche | Exosome-mediated transfer of miR-193a-3p, miR-210-3p and miR-5100, could promote invasion of lung cancer cells by activating STAT3 signaling-induced EMT | [119] |
| Exosomes derived from gastric cancer tissue-derived MSCs | To explore the expression and role of miRNAs in gastric cancer stromal cells | Favouring gastric cancer progression by transferring exosomal miRNAs to gastric cancer cells | [120] |
| Human BMSC-derived exosomes | To explore the mechanism of MSCs xenograft promoting tumor growth | Enhancing VEGF expression in tumor cells by activating ERK1/2 pathway to promote tumor growth in vivo | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Luo, Q.; Que, H.; Wang, N.; Gong, P.; Gu, J. Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Agent for the Treatment of Liver Diseases. Int. J. Mol. Sci. 2022, 23, 10972. https://doi.org/10.3390/ijms231810972
Ding Y, Luo Q, Que H, Wang N, Gong P, Gu J. Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Agent for the Treatment of Liver Diseases. International Journal of Molecular Sciences. 2022; 23(18):10972. https://doi.org/10.3390/ijms231810972
Chicago/Turabian StyleDing, Yi, Qiulin Luo, Hanyun Que, Nan Wang, Puyang Gong, and Jian Gu. 2022. "Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Agent for the Treatment of Liver Diseases" International Journal of Molecular Sciences 23, no. 18: 10972. https://doi.org/10.3390/ijms231810972
APA StyleDing, Y., Luo, Q., Que, H., Wang, N., Gong, P., & Gu, J. (2022). Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Agent for the Treatment of Liver Diseases. International Journal of Molecular Sciences, 23(18), 10972. https://doi.org/10.3390/ijms231810972




