Sustainable Production of Biosurfactant from Agro-Industrial Oil Wastes by Bacillus subtilis and Its Potential Application as Antioxidant and ACE Inhibitor
Abstract
1. Introduction
2. Results and Discussion
2.1. Sunflower and Rapeseed Cake Composition
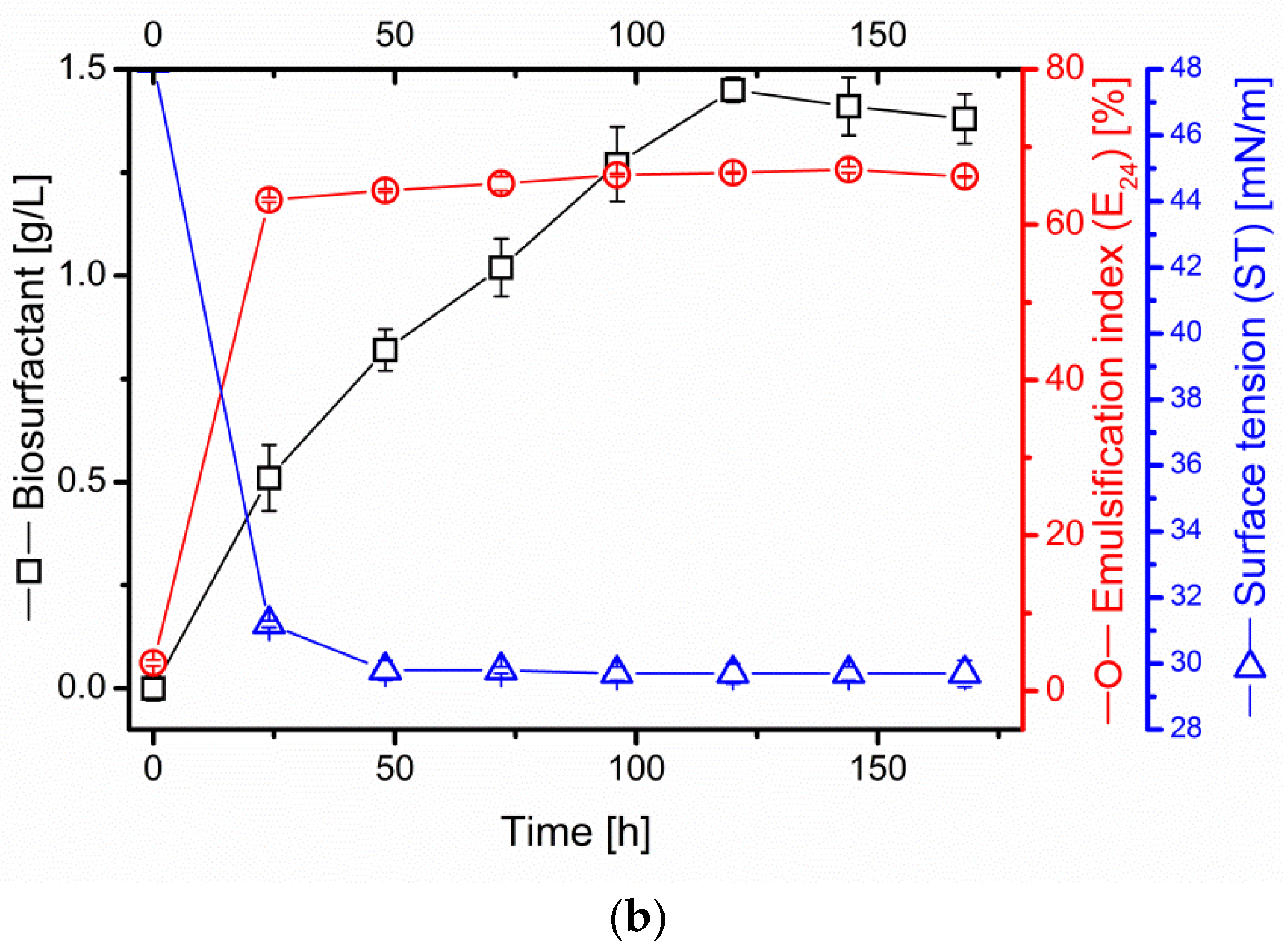
2.2. Kinetics of Surfactin Production in B. subtilis #309 Culture on Oil Cakes
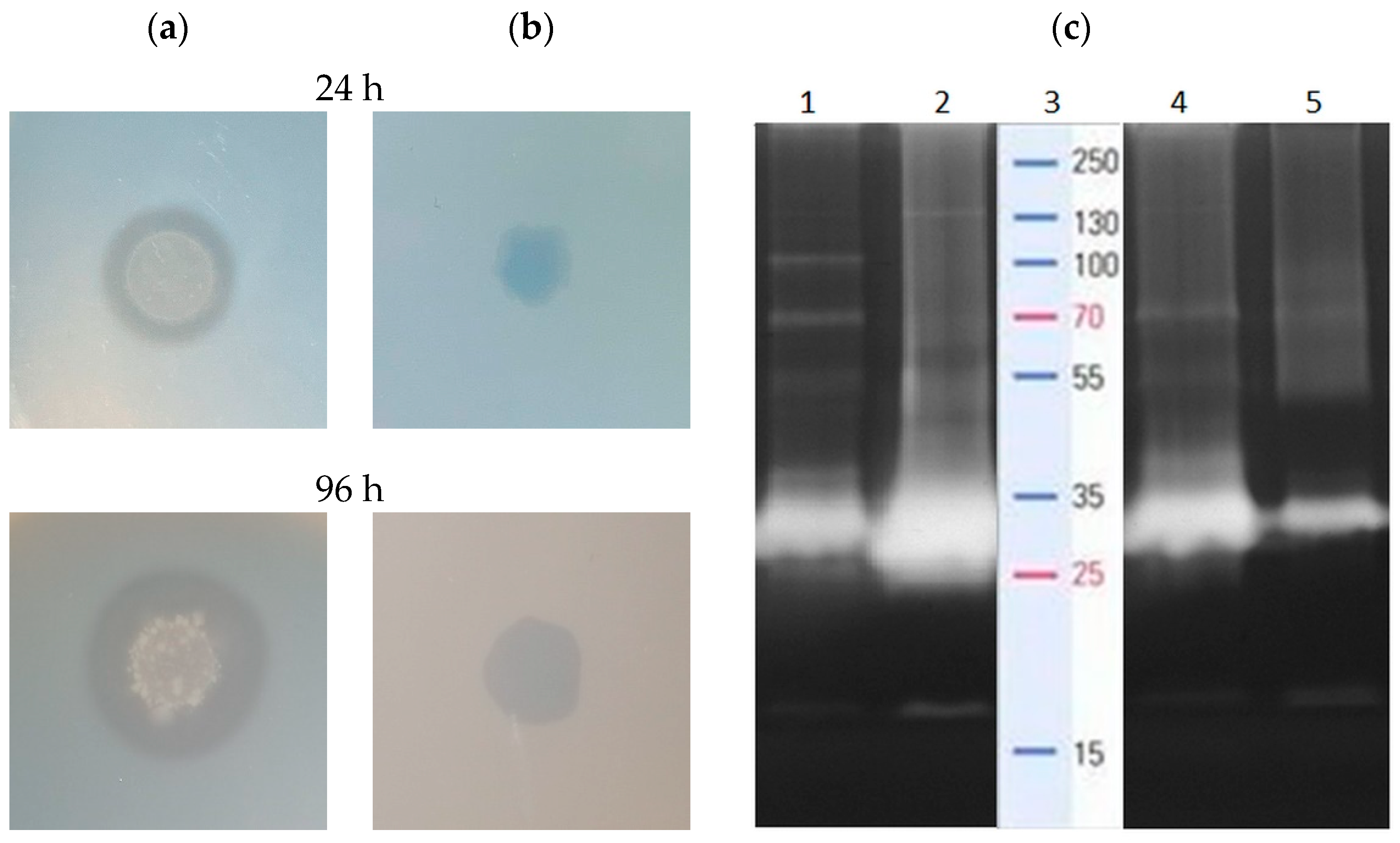
2.3. The Profile of Proteolytic and Lipolytic Enzymes Involved in Oil Cake Decomposition
2.4. Surfactin as an Oil Recovery Agent
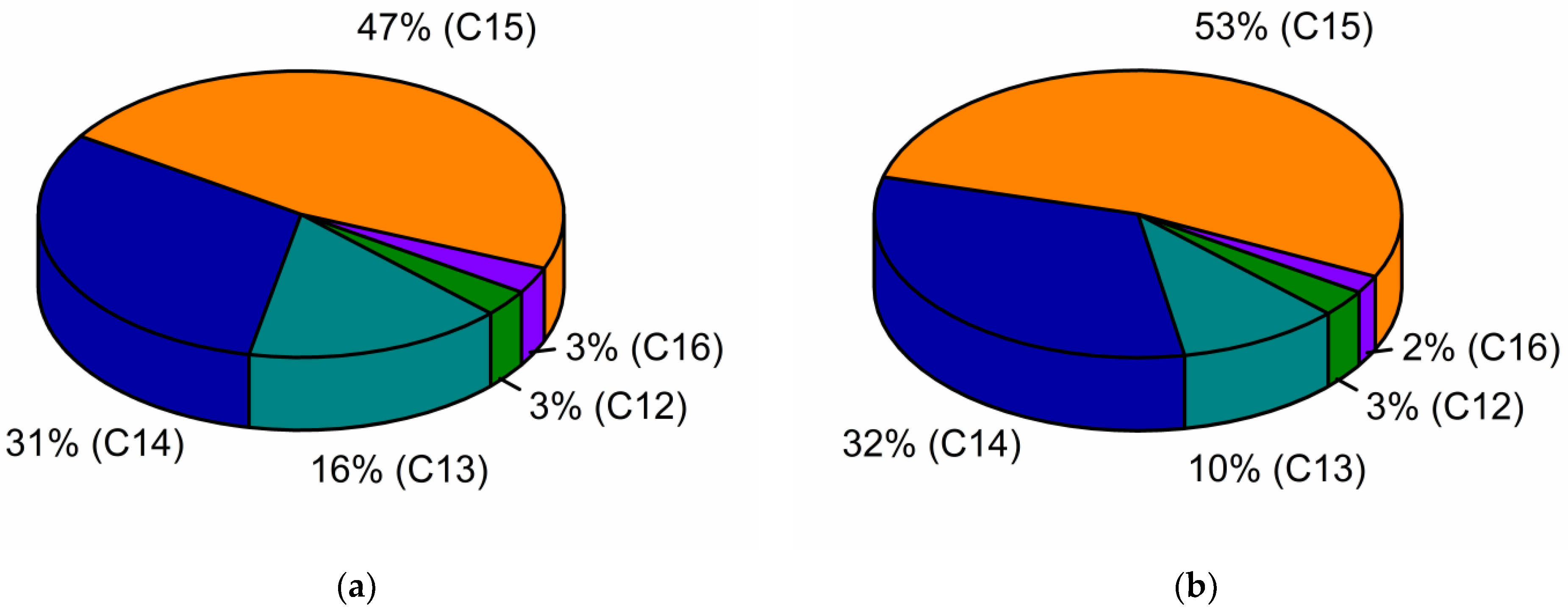
2.5. The Profile of Surfactin Homologues in the Sunflower and Rapeseed Cake Media
2.6. Antioxidant Activity of Surfactin
2.7. Surfactin as an Angiotensin-Converting Enzyme (ACE)-Inhibitory Agent
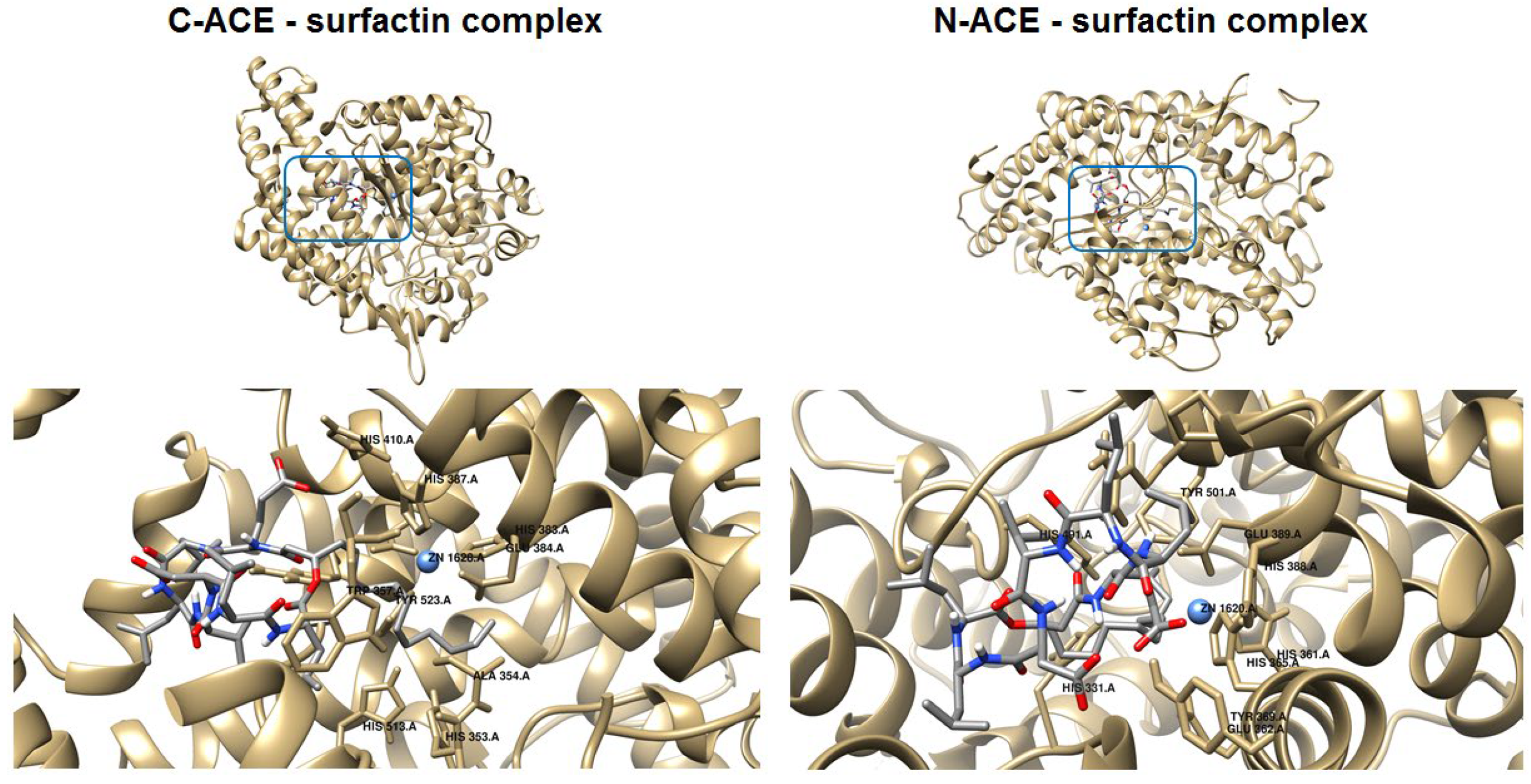
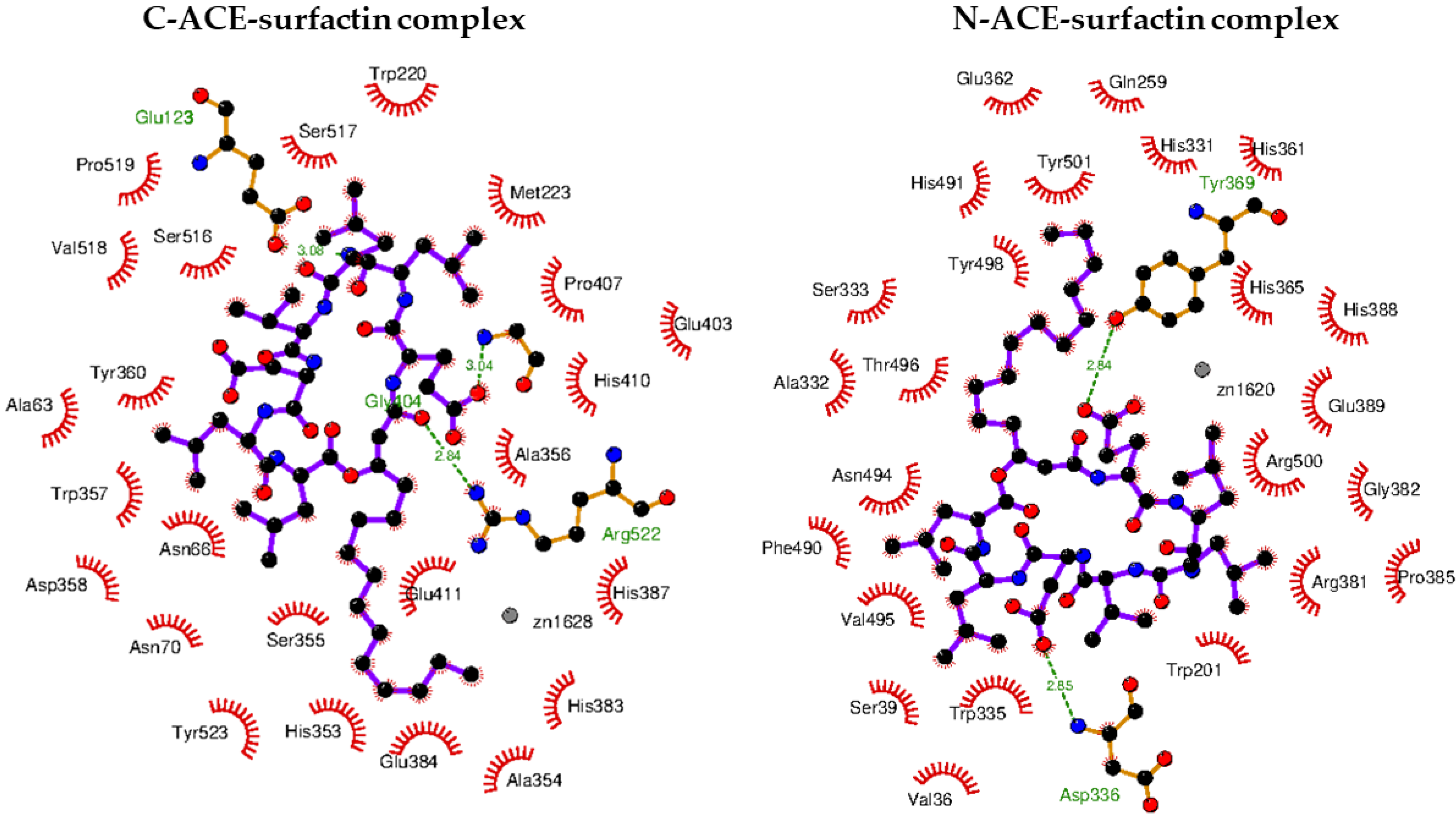
2.8. Molecular Docking of Surfactin C15 Homologue to the C- and N-Domains of ACE
3. Materials and Methods
3.1. Oil Seed Cakes and Composition Analysis
3.2. Surfactin Production Process Specificity in the Culture of B. subtilis #309 Performed on Oil Seed Cake Medium
3.3. Proteolytic and Lipolytic Properties of B. subtilis #309
3.3.1. Skim Milk and Tributyrin Agar Plate Assay
3.3.2. Proteolytic Enzyme Profile Determination
3.4. Oil Recovery Assay
3.5. Biosurfactant Recovery and Purification
3.6. Surfactin Homologue Profile Determination with Gas Chromatography–Mass Spectrometry (GC-MS)
3.7. Antioxidant Activity
3.8. Angiotensin-Converting Enzyme (ACE)-Inhibitory Activity
3.9. Molecular Docking of Surfactin to the C- and N-Domains of ACE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez, A.; Gea, T.; Sánchez, A.; Font, X. Agro-wastes and Inert Materials as Supports for the Production of Biosurfactants by Solid-state Fermentation. Waste Biomass Valoriz. 2020, 12, 1963–1976. [Google Scholar] [CrossRef]
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M.S. Surfactants: Toxicity, remediation and green surfactants. Environ. Chem. Lett. 2014, 12, 275–287. [Google Scholar] [CrossRef]
- Drakontis, C.E.; Amin, S. Biosurfactants: Formulations, properties, and applications. Curr. Opin. Colloid Interface Sci. 2020, 48, 77–90. [Google Scholar] [CrossRef]
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A review on natural surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Domínguez Rivera, Á.; Martínez Urbina, M.Á.; López y López, V.E. Advances on research in the use of agro-industrial waste in biosurfactant production. World J. Microbiol. Biotechnol. 2019, 35, 155. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; Guerra, J.M.C.; Sarubbo, L.A. Biosurfactants: Production and application prospects in the food industry. Biotechnol. Prog. 2020, 36, e3030. [Google Scholar] [CrossRef]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef]
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef]
- Zouari, R.; Hamden, K.; El Feki, A.; Chaabouni, K.; Makni-Ayadi, F.; Sallemi, F.; Ellouze-Chaabouni, S.; Ghribi-Aydi, D. Evaluation of Bacillus subtilis SPB1 biosurfactant effects on hyperglycemia, angiotensin I-converting enzyme (ACE) activity and kidney function in rats fed on high-fat–high-fructose diet. Arch. Physiol. Biochem. 2017, 123, 112–120. [Google Scholar] [CrossRef]
- Shoeb, E.; Akhlaq, F.; Badar, U.; Jameela Akhter, S.I. Classification and Industrial Applications of Biosurfactants. Acad. Res. Int. Part-I Nat. Appl. Sci. 2013, 4, 243–252. [Google Scholar]
- Peypoux, F.; Bonmatin, J.M.; Wallach, J. Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biotechnol. 1999, 51, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Seydlová, G.; Svobodová, J. Review of surfactin chemical properties and the potential biomedical applications. Cent. Eur. J. Med. 2008, 3, 123–133. [Google Scholar] [CrossRef]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5, 697. [Google Scholar] [CrossRef] [PubMed]
- Geneau-Sbartaï, Ć.; Leyris, J.; Silvestre, F.; Rigal, L. Sunflower cake as a natural composite: Composition and plastic properties. J. Agric. Food Chem. 2008, 56, 11198–11208. [Google Scholar] [CrossRef]
- Gültekin Subaşı, B.; Vahapoğlu, B.; Capanoglu, E.; Mohammadifar, M.A. A review on protein extracts from sunflower cake: Techno-functional properties and promising modification methods. Crit. Rev. Food Sci. Nutr. 2022, 62, 6682–6697. [Google Scholar] [CrossRef] [PubMed]
- Kostik, V.; Memeti, S.; Bauer, B. Fatty acid composition of edible oils and fats. J. Hyg. Eng. Des. 2013, 4, 112–116. [Google Scholar]
- Chowdhury, K.; Banu, L.; Khan, S.; Latif, A. Studies on the Fatty Acid Composition of Edible Oil. Bangladesh J. Sci. Ind. Res. 2007, 42, 311–316. [Google Scholar] [CrossRef]
- Leming, R.; Lember, A. Chemical composition of expeller-extracted and cold-pressed rapeseed cake. Agraarteadus 2005, 16, 96–109. [Google Scholar]
- Jeong, H.S.; Kim, H.Y.; Ahn, S.H.; Oh, S.C.; Yang, I.; Choi, I.G. Optimization of enzymatic hydrolysis conditions for extraction of pectin from rapeseed cake (Brassica napus L.) using commercial enzymes. Food Chem. 2014, 157, 332–338. [Google Scholar] [CrossRef]
- Sakhno, L.O. Variability in the fatty acid composition of rapeseed oil: Classical breeding and biotechnology. Cytol. Genet. 2010, 44, 389–397. [Google Scholar] [CrossRef]
- Lewinska, A.; Zebrowski, J.; Duda, M.; Gorka, A.; Wnuk, M. Fatty acid profile and biological activities of linseed and rapeseed oils. Molecules 2015, 20, 22872–22880. [Google Scholar] [CrossRef] [PubMed]
- Paraszkiewicz, K.; Bernat, P.; Kuśmierska, A.; Chojniak, J.; Płaza, G. Structural identification of lipopeptide biosurfactants produced by Bacillus subtilis strains grown on the media obtained from renewable natural resources. J. Environ. Manag. 2018, 209, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Afzal, M.; Qamar, S.A.; Khalid, N. Optimization of biosurfactant production from chemically mutated strain of Bacillus subtilis using waste automobile oil as low-cost substrate. Environ. Sustain. 2020, 3, 405–413. [Google Scholar] [CrossRef]
- De Franҫa, Í.W.L.; Lima, A.P.; Lemos, J.A.M.; Lemos, C.G.F.; Melo, V.M.M.; De Sant’ana, H.B.; Gonҫalves, L.R.B. Production of a biosurfactant by Bacillus subtilis ICA56 aiming bioremediation of impacted soils. Catal. Today 2015, 255, 10–15. [Google Scholar] [CrossRef]
- Vedaraman, N.; Venkatesh, N. Production of surfactin by Bacillus subtilis MTCC 2423 from waste frying oils. Braz. J. Chem. Eng. 2011, 28, 175–180. [Google Scholar] [CrossRef]
- De Oliveira, J.G.; Garcia-Cruz, C.H. Properties of a biosurfactant produced by Bacillus pumilus using vinasse and waste frying oil as alternative carbon sources. Braz. Arch. Biol. Technol. 2013, 56, 155–160. [Google Scholar] [CrossRef]
- Moya Ramírez, I.; Tsaousi, K.; Rudden, M.; Marchant, R.; Jurado Alameda, E.; García Román, M.; Banat, I.M. Rhamnolipid and surfactin production from olive oil mill waste as sole carbon source. Bioresour. Technol. 2015, 198, 231–236. [Google Scholar] [CrossRef]
- Abas, M.R.; Kader, A.J.A.; Khalil, M.S.; Hamid, A.A.; Isa, M.H.M. Production of Surfactin from Bacillus subtilis ATCC 21332 by Using Treated Palm Oil Mill Effluent (POME) as fermentation media. Int. Conf. Food Agric. 2013, 55, 87–93. [Google Scholar]
- Bhange, K.; Chaturvedi, V.; Bhatt, R. Simultaneous production of detergent stable keratinolytic protease, amylase and biosurfactant by Bacillus subtilis PF1 using agro industrial waste. Biotechnol. Rep. 2016, 10, 94–104. [Google Scholar] [CrossRef]
- Long, X.; He, N.; He, Y.; Jiang, J.; Wu, T. Biosurfactant surfactin with pH-regulated emulsification activity for efficient oil separation when used as emulsifier. Bioresour. Technol. 2017, 241, 200–206. [Google Scholar] [CrossRef]
- Bezza, F.A.; Chirwa, E.M.N. Production and applications of lipopeptide biosurfactant for bioremediation and oil recovery by Bacillus subtilis CN2. Biochem. Eng. J. 2015, 101, 168–178. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, Q.; Gao, H.; Lai, H.; Wang, P. Production of lipopeptide biosurfactants by Bacillus atrophaeus 5-2a and their potential use in microbial enhanced oil recovery. Microb. Cell Fact. 2016, 15, 168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lin, J.; Wang, W.; Huang, H.; Li, S. Production of surfactin isoforms by Bacillus subtilis BS-37 and its applicability to enhanced oil recovery under laboratory conditions. Biochem. Eng. J. 2014, 95, 31–37. [Google Scholar] [CrossRef]
- Schmidt; de Oliveira, V.K. Hydrophobic Inducers to Enhanced Surfactin Production Using Cassava Wastewater as Low-Cost Culture Medium: A Prospection on New. Ph.D. Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2022. [Google Scholar]
- Hu, F.; Liu, Y.; Li, S. Rational strain improvement for surfactin production: Enhancing the yield and generating novel structures. Microb. Cell Fact. 2019, 18, 42. [Google Scholar] [CrossRef]
- González-Pérez, S.; Vereijken, J.M. Sunflower proteins: Overview of their physicochemical, structural and functional properties. J. Sci. Food Agric. 2007, 87, 2173–2191. [Google Scholar] [CrossRef]
- Chmielewska, A.; Kozłowska, M.; Rachwał, D.; Wnukowski, P.; Amarowicz, R.; Nebesny, E.; Rosicka-Kaczmarek, J. Canola/rapeseed protein–nutritional value, functionality and food application: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3836–3856. [Google Scholar] [CrossRef]
- Nitschke, M.; e Silva, S.S. Recent food applications of microbial surfactants. Crit. Rev. Food Sci. Nutr. 2018, 58, 631–638. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.K.; Kant, C.; Verma, H.; Kumar, D.; Singh, P.P.; Modi, A.; Droby, S.; Kesawat, M.S.; Alavilli, H.; et al. Microbial biosurfactant: A new frontier for sustainable agriculture and pharmaceutical industries. Antioxidants 2021, 10, 1472. [Google Scholar] [CrossRef]
- Abdollahi, S.; Tofighi, Z.; Babaee, T.; Shamsi, M.; Rahimzadeh, G.; Rezvanifar, H.; Saeidi, E.; Amiri, M.M.; Ashtiani, Y.S.; Samadi, N. Evaluation of anti-oxidant and anti-biofilm activities of biogenic surfactants derived from Bacillus amyloliquefaciens and Pseudomonas aeruginosa. Iran. J. Pharm. Res. 2020, 9, 115. [Google Scholar]
- Yalçin, E.; Çavuşoǧlu, K. Structural analysis and antioxidant activity of a biosurfactant obtained from Bacillus subtilis RW-I. Turk. J. Biochem. 2010, 35, 243–247. [Google Scholar]
- Shao, C.; Liu, L.; Gang, H.; Yang, S.; Mu, B. Structural diversity of the microbial surfactin derivatives from selective esterification approach. Int. J. Mol. Sci. 2015, 16, 1855–1872. [Google Scholar] [CrossRef] [PubMed]
- Tabbene, O.; Gharbi, D.; Slimene, I.B.; Elkahoui, S.; Alfeddy, M.N.; Cosette, P.; Mangoni, M.L.; Jouenne, T.; Limam, F. Antioxidative and DNA protective effects of bacillomycin D-like lipopeptides produced by B38 strain. Appl. Biochem. Biotechnol. 2012, 168, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Coates, D. The angiotensin converting enzyme (ACE). Int. J. Biochem. Cell Biol. 2003, 35, 769–773. [Google Scholar] [CrossRef]
- Ghazala, I.; Bouassida, M.; Krichen, F.; Manuel Benito, J.; Ellouz-Chaabouni, S.; Haddar, A. Anionic lipopeptides from Bacillus mojavensis I4 as effective antihypertensive agents: Production, characterization, and identification. Eng. Life Sci. 2017, 17, 1244–1253. [Google Scholar] [CrossRef]
- Akif, M.; Schwager, S.L.; Anthony, C.S.; Czarny, B.; Beau, F.; Dive, V.; Sturrock, E.D.; Acharya, K.R. Novel mechanism of inhibition of human angiotensin-l-converting enzyme (ACE) by a highly specific phosphinic tripeptide. Biochem. J. 2011, 436, 53–59. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Pereira, J.F.B.; Rodrigues, L.R.; Coutinho, J.A.P.; Teixeira, J.A. Isolation and study of microorganisms from oil samples for application in Microbial Enhanced Oil Recovery. Int. Biodeterior. Biodegrad. 2012, 68, 56–64. [Google Scholar] [CrossRef]
- Janek, T.; Łukaszewicz, M.; Rezanka, T.; Krasowska, A. Isolation and characterization of two new lipopeptide biosurfactants produced by Pseudomonas fluorescens BD5 isolated from water from the Arctic Archipelago of Svalbard. Bioresour. Technol. 2010, 101, 6118–6123. [Google Scholar] [CrossRef]
- Janek, T.; Gudiña, E.J.; Połomska, X.; Biniarz, P.; Jama, D.; Rodrigues, L.R.; Rymowicz, W.; Lazar, Z. Sustainable Surfactin Production by Bacillus subtilis Using Crude Glycerol from Different Wastes. Molecules 2021, 26, 3488. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Xiao, D.; Xie, J.; Zeng, H.; Wang, Z.; Zhang, X.; Niu, Y.; Shen, Z.; Shen, J.; et al. Upregulation of neutrophil gelatinase-associated lipocalin in oesophageal squamous cell carcinoma: Significant correlation with cell differentiation and tumour invasion. J. Clin. Pathol. 2007, 60, 555–561. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Fernandes, E.C.; Rodrigues, A.I.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant production by Bacillus subtilis using corn steep liquor as culture medium. Front. Microbiol. 2015, 6, 59. [Google Scholar]
- Janek, T.; Rodrigues, L.R.; Gudiña, E.J.; Czyżnikowska, Ż. Metal-Biosurfactant Complexes Characterization: Binding, Self-Assembly and Interaction with Bovine Serum Albumin. Int. J. Mol. Sci. 2019, 20, 2864. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. Total phenolic content and antioxidant properties of eclipse black beans (Phaseolus vulgaris L.) as affected by processing methods. J. Food Sci. 2008, 73, H19–H27. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Aluko, R.E. Multifunctional Cationic Peptide Fractions from Flaxseed Protein Hydrolysates. Plant Foods Hum. Nutr. 2012, 67, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Application of the PM6 method to modeling proteins. J. Mol. Model. 2009, 15, 765–805. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Morris, G.; Huey, R.; Linkstrom, W.; Sanner, M.; Belew, R.; Goodsell, D. Olson AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2010, 30, 2785–2791. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. Ligplot: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. Des. Sel. 1995, 8, 127–134. [Google Scholar] [CrossRef] [PubMed]

| Component | Sunflower Cake | Rapeseed Cake |
|---|---|---|
| Dry Matter (DM), (%) | 93.8 ± 0.5 | 93.1 ± 0.5 |
| Crude Protein, (% of DM) | 21.4 ± 0.8 | 30.2 ± 1.2 |
| Fiber | ||
| Neutral Detergent Fiber (NDF), (% of DM) | 34.4 ± 1.7 | 22.6 ± 1.1 |
| Acid Detergent Fiber (ADF), (% of DM) | 29.4 ± 1.5 | 21.0 ± 1.0 |
| Acid Detergent Lignin (ADL), (% of DM) | 9.49 ± 0.9 | 8.79 ± 0.9 |
| FA, (% of DM) | 13.5 ± 0.3 | 12.9 ± 0.5 |
| FA Composition, (%) | ||
| Palmitic C16:0 | 2.82 ± 0.1 | 2.44 ± 0.1 |
| Oleic C18:1 | 34.96 ± 0.2 | 77.84 ± 0.4 |
| Linoleic C18:2 | 55.67 ± 0.6 | 8.05 ± 0.2 |
| Others | 6.55 ± 0.2 | 11.67 ± 0.1 |
| Ash, (% of DM) | 5.85 ± 0.4 | 5.53 ± 0.3 |
| Raw Material | Time (h) | Oil Recovered (%) |
|---|---|---|
| Sunflower cake | 0 | 0.8 ± 0.2 |
| 24 | 14.5 ± 0.6 | |
| 48 | 23.1 ± 1.1 | |
| 72 | 26.8 ± 0.4 | |
| 96 | 30.4 ± 0.1 | |
| 120 | 31.6 ± 0.8 | |
| 144 | 31.0 ± 1.1 | |
| 168 | 30.4 ± 1.0 | |
| Rapeseed cake | 0 | 1.2 ± 0.3 |
| 24 | 22.6 ± 1.2 | |
| 48 | 25.2 ± 0.8 | |
| 72 | 27.1 ± 0.2 | |
| 96 | 31.3 ± 0.2 | |
| 120 | 37.1 ± 0.9 | |
| 144 | 36.2 ± 0.4 | |
| 168 | 33.1 ± 1.2 |
| Surfactin | Antioxidant Activity | ||
| ABTS | DPPH | FRAP | |
| TEAC [µM/g] | 3.77 ± 0.22 | 11.67 ± 0.8 | 0.76 ± 0.096 |
| ACE-Inhibitory Activity | |||
| IC50 [mg/mL] | 0.62 ± 0.04 | ||
| Complex | ΔGbinding [kcal/mol] | ΔGintermolecular [kcal/mol] | ΔGvdw ΔGhbond ΔGdesolv [kcal/mol] | Electrostatic Energy [kcal/mol] | Hydrogen Bonds | Hydrophobic Interactions |
|---|---|---|---|---|---|---|
| C-ACE–surfactin C15 | −9.5 | −17.0 | −17.1 | 0.1 | Gly104 Glu123 Agr522 | Ala63 Asn66 Asn70 Gly104 Glu123 Trp220 Met233 His353 Ala354 Ser355 Ala356 Trp357 Asp358 Tyr360 His383 Glu384 His387 Glu403 Pro407 His410 Glu411 Ser516 Ser517 Val518 Pro519 Arg522 Tyr523 |
| N-ACE–surfactin C15 | −9.2 | −16.7 | −16.8 | 0.1 | Asp336 Tyr369 | Val36 Ser39 Trp201 Gln256 His331 Ala332 Ser333 Trp335 Asp336 His361 Glu362 His365 Tyr369 Arg381 Gly382 Pro385 His388 Glu389 Tyr489 Phe490 His491 Val492 Asn494 Thr496 Arg500 Tyr501 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciurko, D.; Czyżnikowska, Ż.; Kancelista, A.; Łaba, W.; Janek, T. Sustainable Production of Biosurfactant from Agro-Industrial Oil Wastes by Bacillus subtilis and Its Potential Application as Antioxidant and ACE Inhibitor. Int. J. Mol. Sci. 2022, 23, 10824. https://doi.org/10.3390/ijms231810824
Ciurko D, Czyżnikowska Ż, Kancelista A, Łaba W, Janek T. Sustainable Production of Biosurfactant from Agro-Industrial Oil Wastes by Bacillus subtilis and Its Potential Application as Antioxidant and ACE Inhibitor. International Journal of Molecular Sciences. 2022; 23(18):10824. https://doi.org/10.3390/ijms231810824
Chicago/Turabian StyleCiurko, Dominika, Żaneta Czyżnikowska, Anna Kancelista, Wojciech Łaba, and Tomasz Janek. 2022. "Sustainable Production of Biosurfactant from Agro-Industrial Oil Wastes by Bacillus subtilis and Its Potential Application as Antioxidant and ACE Inhibitor" International Journal of Molecular Sciences 23, no. 18: 10824. https://doi.org/10.3390/ijms231810824
APA StyleCiurko, D., Czyżnikowska, Ż., Kancelista, A., Łaba, W., & Janek, T. (2022). Sustainable Production of Biosurfactant from Agro-Industrial Oil Wastes by Bacillus subtilis and Its Potential Application as Antioxidant and ACE Inhibitor. International Journal of Molecular Sciences, 23(18), 10824. https://doi.org/10.3390/ijms231810824







