Identification, Pathogenicity, and Genetic Diversity of Fusarium spp. Associated with Maize Sheath Rot in Heilongjiang Province, China
Abstract
1. Introduction
2. Results
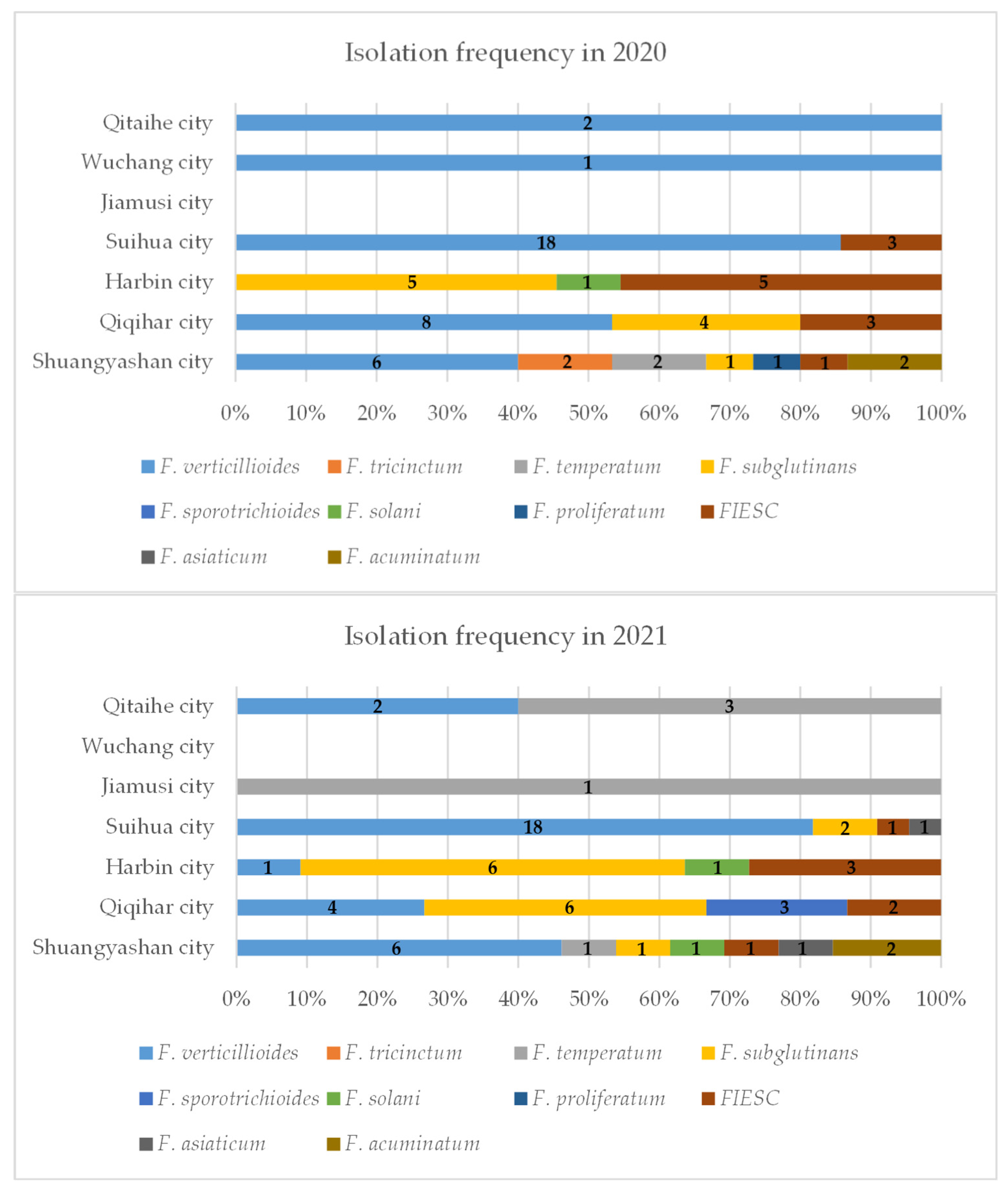
2.1. Isolation and Identification of Fusarium Isolates
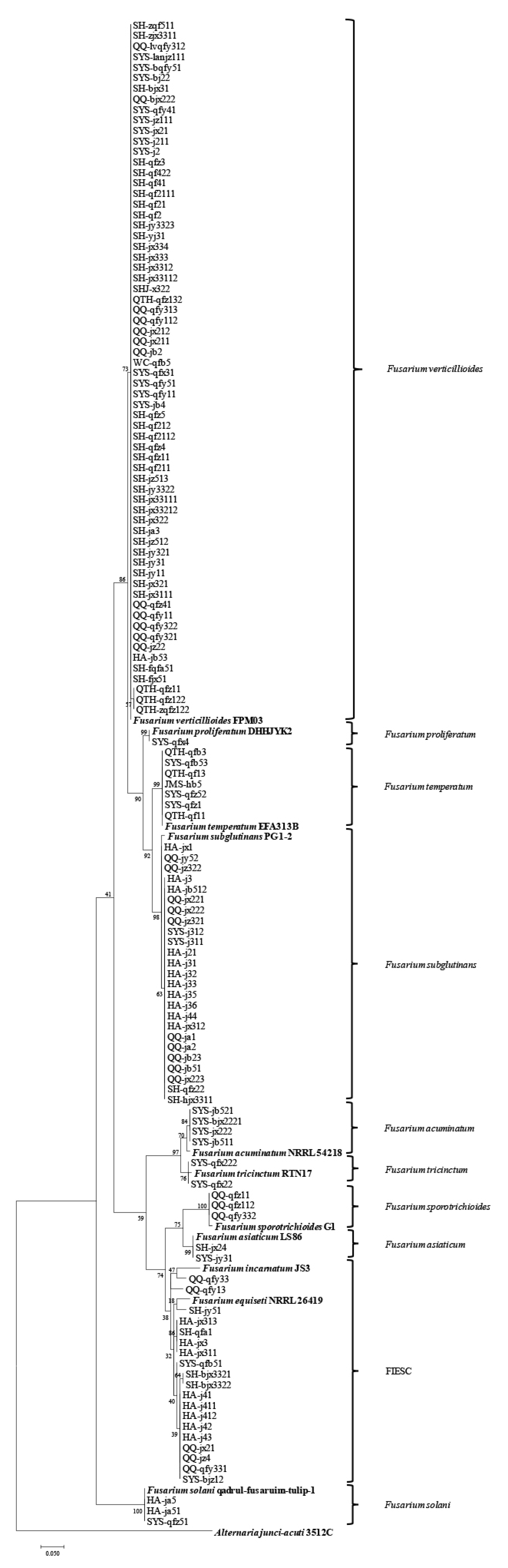
2.2. Phylogenetic Analysis of PCR-Generated DNA Sequences
2.3. Pathogenicity Tests
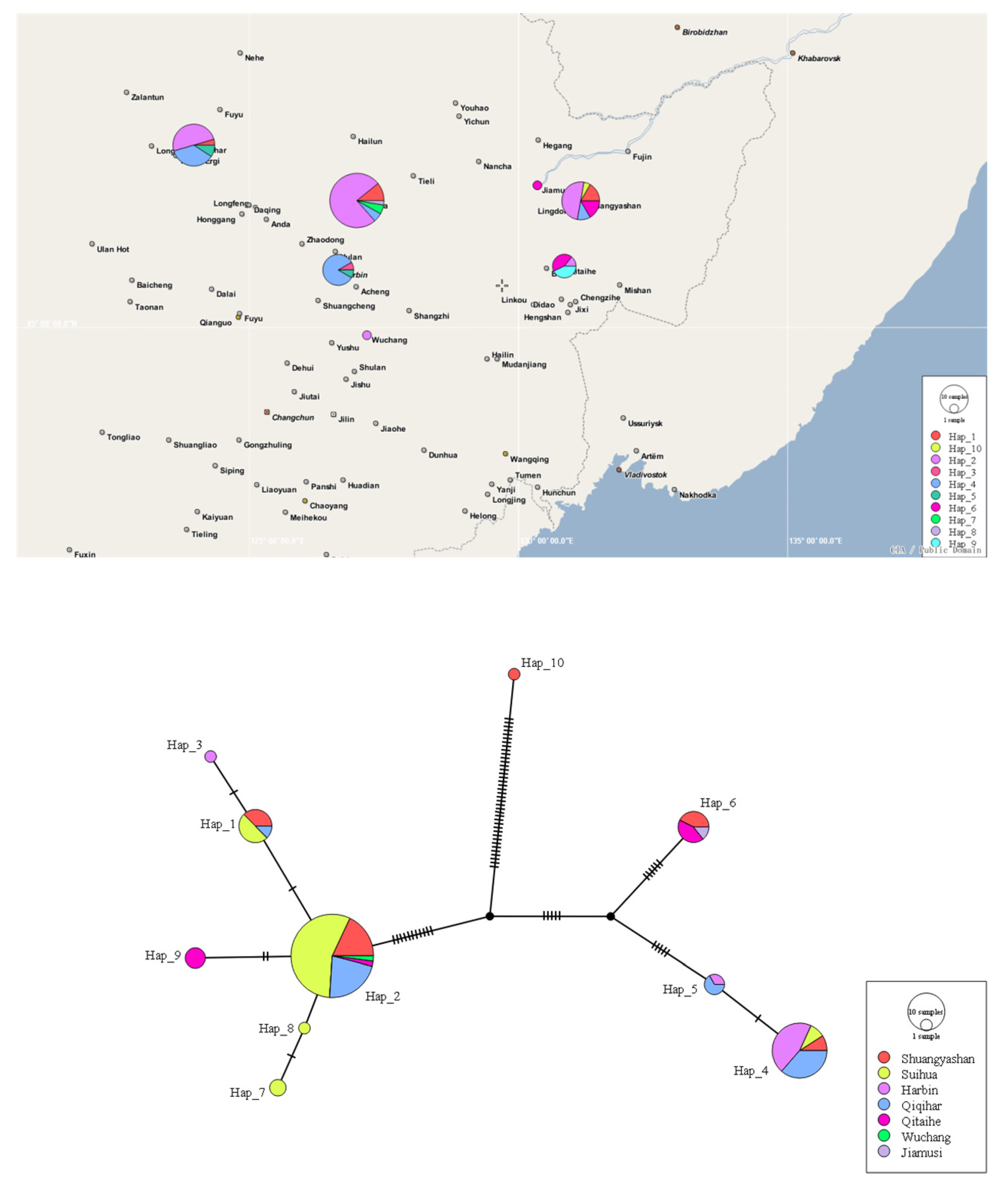
2.4. Haplotype Network
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Fusarium Isolation
4.2. Morphological Characterization
4.3. DNA Extraction and Sequencing
4.4. Phylogenetic Analysis of Fusarium Isolates
4.5. Pathogenicity Tests
4.6. Nucleotide Diversity and Haplotype Assignment within Species
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S. Review of maize market in 2020–2021 and prospect in 2021–2022. China Pig Ind. 2022, 17, 29–33. [Google Scholar]
- Wang, Q.L.; Zhang, N.N.; Liu, H. An empirical study on Maize supply response in China under the background of Market-oriented Reform-based on Provincial Panel data of three Northeast provinces from 2008 to 2019. Agric. Resour. Reg. China 2021, 42, 145–152. [Google Scholar]
- Xu, X.D.; Jiang, Y.; Wang, L.J.; Dong, H.Y.; Hu, L.; Lu, G.Z.; Liu, Z.H. Corn Sheath Rot—A New Disease Found in China. Sci. Agric. Sin. 2008, 41, 3083–3087. [Google Scholar]
- Hu, L.; Xu, X.D.; Jiang, Y.; Wang, L.J.; Xu, J.; Zhao, Y. Study on biological characteristics of corn sheath rot causal agent. Maize Sci. 2008, 16, 131–134. [Google Scholar]
- Li, P.P.; Cao, Z.Y.; Wang, K.; Zhai, H.; Jia, H.; Liu, N.; Li, S.H.; Hao, Z.M.; Gu, S.Q.; Dong, J.G. First report of Fusarium equiseti causing a sheath rot of corn in China. Plant Dis. 2014, 98, 998. [Google Scholar] [CrossRef]
- Wang, K.; Cao, Z.; Li, P.; Yin, H.; Liu, J.; Dong, J. Analysis of the correlation between diseased-degree of corn sheath rot and corn lodging and yield loss. Acta Phytophylacica Sin. 2015, 42, 949–956. [Google Scholar]
- Sun, L.; Wang, S.; Zhang, W.; Chi, F.; Hao, X.Y.; Bian, J.Y.; Li, Y. First report of sheath rot of corn caused by Fusarium verticillioides in northeast China. J. Plant Pathol. 2020, 102, 1301–1302. [Google Scholar] [CrossRef]
- Wang, W.; Wang, B.; Sun, X.; Qi, X.; Gong, G. Symptoms and pathogens diversity of maize Fusarium sheath rot in Sichuan province, China. Sci. Rep. 2021, 11, 2835. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.M.; Azeem, M.A.; Darwish, A.G.; Nafady, N.A.; Ibrahim, N.A. Fusarium: Biodiversity, Ecological Significances, and Industrial Applications. In Proceedings of the 3rd ISNPS, Avignon, France, 11–16 June 2016. [Google Scholar]
- Pfordt, A.; Romero, L.R.; Schiwek, S.; Karlovsky, P.; Tiedemann, A.V. Impact of environmental conditions and agronomic practices on the prevalence of Fusarium species associated with ear and stalk rot in maize. Pathogens 2020, 9, 236. [Google Scholar] [CrossRef]
- Schieber, R. A leaf blight of corn (Zea mays) incited by Fusarium moniliforme. Phytopathology 1968, 58, 554. [Google Scholar]
- Gai, X.T.; Yang, R.X.; Pan, X.J.; Yuan, Y.; Wang, S.N.; Liang, B.B.; Gao, Z.G. First Report of Fusarium incarnatum Causing Stalk Rot on Maize in China. Plant Dis. 2016, 100, 1010. [Google Scholar] [CrossRef]
- Wang, J.H.; Li, H.P.; Zhang, J.B.; Wang, B.T.; Liao, Y.C. First report of Fusarium maize ear rot caused by Fusarium meridionale in China. Plant Dis. 2014, 98, 279. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Qin, P.; Gao, Z.; Xu, J.; Xu, X. First report of seedling blight of maize caused by Fusarium asiaticum in Northeast China. Plant Dis. 2020, 105, 1206. [Google Scholar] [CrossRef] [PubMed]
- Mezzalama, M.; Guarnaccia, V.; Martino, I.; Tabome, G.; Gullino, M.L. First report of Fusarium commune causing root and crown rot on maize in Italy. Plant Dis. 2021, 105, 4156. [Google Scholar] [CrossRef]
- Kabeere, F.; Hampton, J.G.; Hill, M.J. Transmission of Fusarium graminearum (Schwabe) from maize seeds to seedlings. Seed Sci. Technol. 1997, 25, 245–252. [Google Scholar]
- Wollenweber, H.W.; Sherbakoff, C.D.; Reinking, O.A.; Johann, H.; Bailey, A.A. Fundamentals for taxonomic studies of Fusarium. J. Agric. Res. 1925, 30, 833–843. [Google Scholar]
- Lima, C.S.; Pfenning, L.H.; Costa, S.S.; Campos, M.A.; Leslie, J.F. A new Fusarium lineage within the Gibberella fujikuroi species complex is the main causal agent of mango malformation disease in Brazil. Plant Pathol. 2010, 58, 33–42. [Google Scholar] [CrossRef]
- Vermeulen, M.; Rothmann, L.A.; Swart, W.J.; Gryzenhout, M. Fusarium casha sp. nov. and F. curculicola sp. nov. in the Fusarium fujikuroi Species Complex Isolated from Amaranthus cruentus and Three Weevil Species in South Africa. Diversity 2021, 10, 472. [Google Scholar] [CrossRef]
- Bashyal, B.M.; Aggarwal, R. Molecular identification of Fusarium species associated with bakanae disease of rice (Oryza sativa) in India. Indian J. Agric. Sci. 2013, 83, 71–76. [Google Scholar]
- Qiu, J.; Lu, Y.; He, D.; Lee, Y.W.; Ji, F.; Xu, J.; Shi, J. Fusarium fujikuroi Species Complex Associated with Rice, Maize, and Soybean from Jiangsu Province, China: Phylogenetic, Pathogenic, and Toxigenic Analysis. Plant Dis. 2020, 104, 2193–2201. [Google Scholar] [CrossRef]
- Prabhukarthikeyan, S.R.; Keerthana, U.; Nagendran, K.; Yadav, M.K.; Parameswaran, C.; Panneerselvam, P.; Rath, P.C. First Report of Fusarium proliferatum Causing Sheath Rot Disease of Rice in Eastern India. Plant Dis. 2021, 105, 704. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Chen, J.; Yi, C.; Li, Z.; Gao, C. First report of Fusarium fujikuroi causing bulb rot on Lilium lancifolium in China. Plant Dis. 2021, 105, 2254. [Google Scholar] [CrossRef] [PubMed]
- Tushar, B.; Rakholiya, K. Environment friendly way to management of Fusarium fruit rot disease of banana in vivo by essential oils. Int. J. Genet. 2020, 12, 798–800. [Google Scholar]
- Huang, S.P.; Wei, J.G.; Guo, T.X.; Li, Q.L.; Tang, L.H.; Mo, J.Y.; Wei, J.F.; Yang, X.B. First report of sheath rot caused by Fusarium proliferatum on Pisang Awak Banana (Musa ABB) in China. Plant Pathol. 2019, 101, 1271–1272. [Google Scholar] [CrossRef]
- Araújo, M.B.M.; Moreira, G.M.; Nascimento, L.V.; Nogueira, G.D.A.; Nascimento, S.R.D.; Pfenning, L.H.; Ambrósio, M.M.D. Fusarium rot of melon is caused by several Fusarium species. Plant Pathol. 2020, 70, 712–721. [Google Scholar] [CrossRef]
- Jacobs, A.; Van Wyk, P.S.; Marasas, W.F.O.; Wingfield, B.D.; Wingfield, M.J.; Coutinho, T.A. Fusarium ananatum sp. nov. in the Gibberella fujikuroi species complex from pineapples with fruit rot in south Africa. Fungal Biol. 2010, 114, 515–527. [Google Scholar] [CrossRef]
- Hsuan, H.M.; Salleh, B.; Zakaria, L. Molecular identification of Fusarium species in Gibberella fujikuroi species complex from rice, sugarcane and maize from Peninsular Malaysia. Int. J. Mol. Sci. 2011, 12, 6722–6732. [Google Scholar] [CrossRef]
- Donát, M.; Csaba, Z.; Zsuzsanna, K.; Árpád, S.; János, B. Identification of airborne propagules of the Gibberella fujikuroi species complex during maize production. Aerobiologia 2012, 28, 263–271. [Google Scholar] [CrossRef]
- Jarek, T.M.; dos Santos, Á.F.; Tessmann, D.J.; Vieira, E. Inoculation methods and aggressiveness of five Fusarium species against peach palm. Ciênc. Rural 2018, 48, e20170462. [Google Scholar] [CrossRef]
- Félix-Gastélum, R.; Mora-Carlón, B.A.; Leyva-Madrigal, K.Y.; Solano-Báez, A.R.; Pérez-Mora, J.L.; Guerra-Meza, O.; Mora-Romero, G.A. Sorghum Sheath Blight Caused by Fusarium spp. in Sinaloa, Mexico. Plant Dis. 2022, 106, 1454–1461. [Google Scholar] [CrossRef]
- Li, Y.L.; Yan, Z.B.; Wang, Y.H.; Zhou, Z. First report of Fusarium proliferatum causing leaf sheath rot on Clivia miniata in Henan province, China. Plant Dis. 2020, 104, 1552. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.; Forche, A. Haplotyping a Non-meiotic Diploid Fungal Pathogen Using Induced Aneuploidies and SNP/CGH Microarray Analysis. Methods Mol. Biol. 2017, 1551, 131–146. [Google Scholar] [PubMed]
- Liu, J.; Xu, M.M.; Wang, A.P.; Yin, H.F.; Wang, K.; Cao, Z.Y.; Dong, J.G. Resistant Evaluation and Yield Loss Common Corn Varieties to Corn Sheath Rot. Maize Sci. 2018, 26, 29–36. [Google Scholar]
- Chalivendra, S.; Huang, F.N.; Busman, M.; Williams, W.P.; Ham, J.H. Low aflatoxin levels in aspergillus flavus-resistant maize are correlated with increased corn earworm damage and enhanced seed fumonisin. Front. Plant Sci. 2020, 11, 565323. [Google Scholar] [CrossRef]
- Kaur, H.; Mohan, C.; Vika, Y.; Singh, M. Pathogenic and molecular characterization of Fusarium moniliforme Sheld, the incitant of Fusarium maize stalk rot in the Punjab State of India. Maydica 2008, 59, 290–297. [Google Scholar]
- Li, L.; Qu, Q.; Cao, Z.; Guo, Z.; Dong, J. The relationship analysis on corn stalk rot and ear rot according to Fusarium species and fumonisin contamination in kernels. Toxins 2019, 11, 320. [Google Scholar] [CrossRef]
- Fumero, M.V.; Villani, A.; Susca, A.; Haidukowski, M.; Cimmarusti, M.T.; Toomajian, C.; Leslie, J.F.; Chulze, S.N.; Moretti, A. Fumonisin and beauvericin chemotypes and genotypes of the sister species Fusarium subglutinans and Fusarium temperatum. Appl. Environ. Microbiol. 2020, 86, e00133-20. [Google Scholar] [CrossRef]
- Munkvold, G.P. Fusarium Species and their Associated Mycotoxins. Methods Mol. Biol. 2017, 1542, 51–106. [Google Scholar]
- Sheng, W.; Wu, H.; Ji, W.; Li, Z.; Chu, F.; Wang, S. Visual Non-Instrumental On-Site Detection of Fumonisin B1, B2, and B3 in Cereal Samples Using a Clean-Up Combined with Gel-Based Immunoaffinity Test Column Assay. Toxins 2018, 10, 165. [Google Scholar] [CrossRef]
- Koné, D.; Badou, O.J.; Bomisso, E.L.; Camara, B.; Ake, S. In vitro activity of different fungicides on the growth in Mycosphaerella fijiensis var. difformis Stover and Dickson, Cladosporium musae Morelet and Deightoniella torulosa (Syd.) Ellis, isolated parasites of the banana phyllosphere in the Ivory Coast. Comptes Rendus Biol. 2009, 332, 448–455. [Google Scholar]
- Yi, R.H.; Zhu, X.R.; Zhou, E.X. On the evaluation of different methods by artificially inoculating with rice sheath blight fungus (Rhizoctonia solani). J. Guangzhou Univ. 2003, 3, 224–227. [Google Scholar]
- Endo, T.; Fujii, H.; Omura, M.; Shimada, T. Fast-track breeding system to introduce CTV resistance of trifoliate orange into citrus germplasm, by integrating early flowering transgenic plants with marker-assisted selection. Plant Biol. 2020, 20, 224. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Tabome, G.; Garibaldi, A.; Gullino, M.L. Alternaria leaf spot caused by Alternaria species: An emerging problem on ornamental plants in Italy. Plant Dis. 2020, 104, 2275–2287. [Google Scholar] [CrossRef]
- Hoffmann, A.; Funk, R.; Müller, M.E.H. Blowin’ in the Wind: Wind Dispersal Ability of Phytopathogenic Fusarium in a Wind Tunnel Experiment. Atmosphere 2021, 12, 1653. [Google Scholar] [CrossRef]
- Feldman, T.S.; O’Brien, H.E.; Arnold, A.E. Moths that Vector a Plant Pathogen also Transport Endophytic Fungi and Mycoparasitic Antagonists. Microb. Ecol. 2008, 56, 742–750. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Pfohl, K.; Karlovsky, P.; Dehne, H.W.; Altincicek, B. Dissemination of Fusarium proliferatum by mealworm beetle Tenebrio molitor. PLoS ONE 2018, 13, e0204602. [Google Scholar] [CrossRef]
- Yin, H.F.; Cao, Z.Y.; Wang, K.; Li, P.P.; Jia, H.; Dong, J.G. The influence of aphids occurrence on corn sheath rot. J. Agric. Univ. Hebei 2015, 38, 86–91. [Google Scholar]
- Arseniuk, E.; Scharen, A.L.; Czembor, H.J. Pathogenicity of seed transmitted Fusarium spp. to triticale seedlings. Mycotoxin Res. 1991, 7, 121–127. [Google Scholar] [CrossRef]
- Xu, X.; Yang, X.L.; Zhang, L.; Cao, H.S.; Cao, P.; Wang, X.J.; Xiang, W.S.; Zhao, J.W. First report of leaf blight caused by Limonomyces roseipellis on maize (Zea mays L.) in China. Plant Dis. 2021, 105, 2730. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Yang, X.L.; Cao, H.S.; Li, J.J.; Cao, P.; Guo, L.F.; Wang, X.J.; Zhao, J.W.; Xiang, W.S. Alternaria spp. associated with leaf blight of maize in Heilongjiang Province, China. Plant Dis. 2022, 106, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Professional: Ames, IA, USA, 2006; Volume 56, p. 1037. [Google Scholar]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.L.; Dai, H.; Wang, D.P.; Zhou, H.H.; He, W.Q.; Fu, Y.; Ibrahim, F.; Zhou, Y.; Gong, G.; Shang, J.; et al. Identification of Fusarium species associated with soybean root rot in Sichuan Province, China. Eur. J. Plant Pathol. 2018, 151, 563–577. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Y.X.; Wang, Z.Y.; Wen, J.; Hao, D.Y.; Li, S.J.; Zhang, Y. Study on Inoculation Methods of Ear Rot Caused by Fusarium verticilloides and Fusarium graminearum in Maize. J. Northeast. Agric. Sci. 2021, 46, 64–69. [Google Scholar]
- Leigh, J.W.; Bryant, D. Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef]
- Templeton, A.R.; Crandall, K.A.; Sing, C.F. A cladistics analysis of phenotypic associations with haplotypes inferred from restriction endo nuclease mapping and DNA sequence data III. Cladogram estimation. Genetics 1992, 132, 619–633. [Google Scholar] [CrossRef]
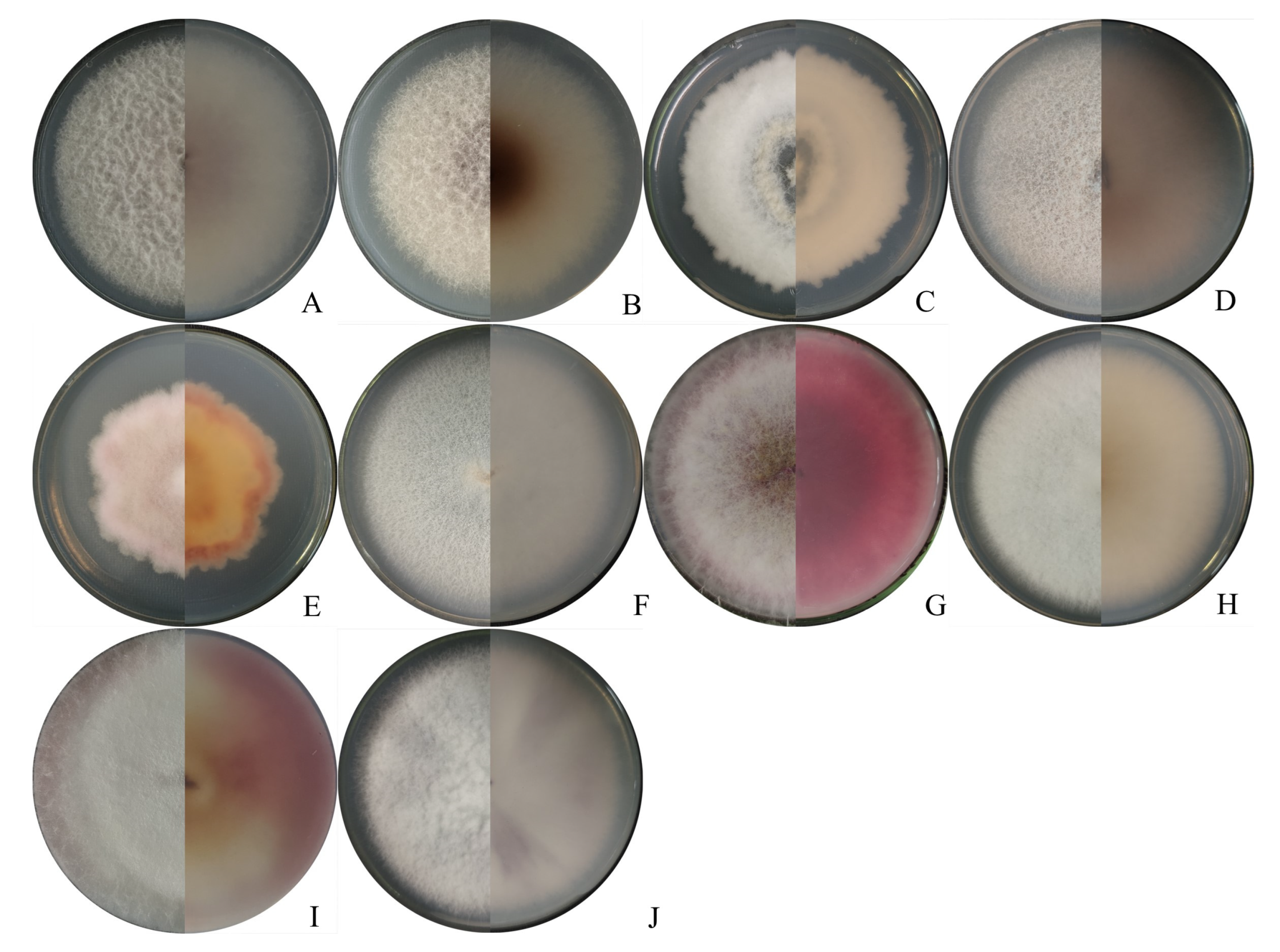
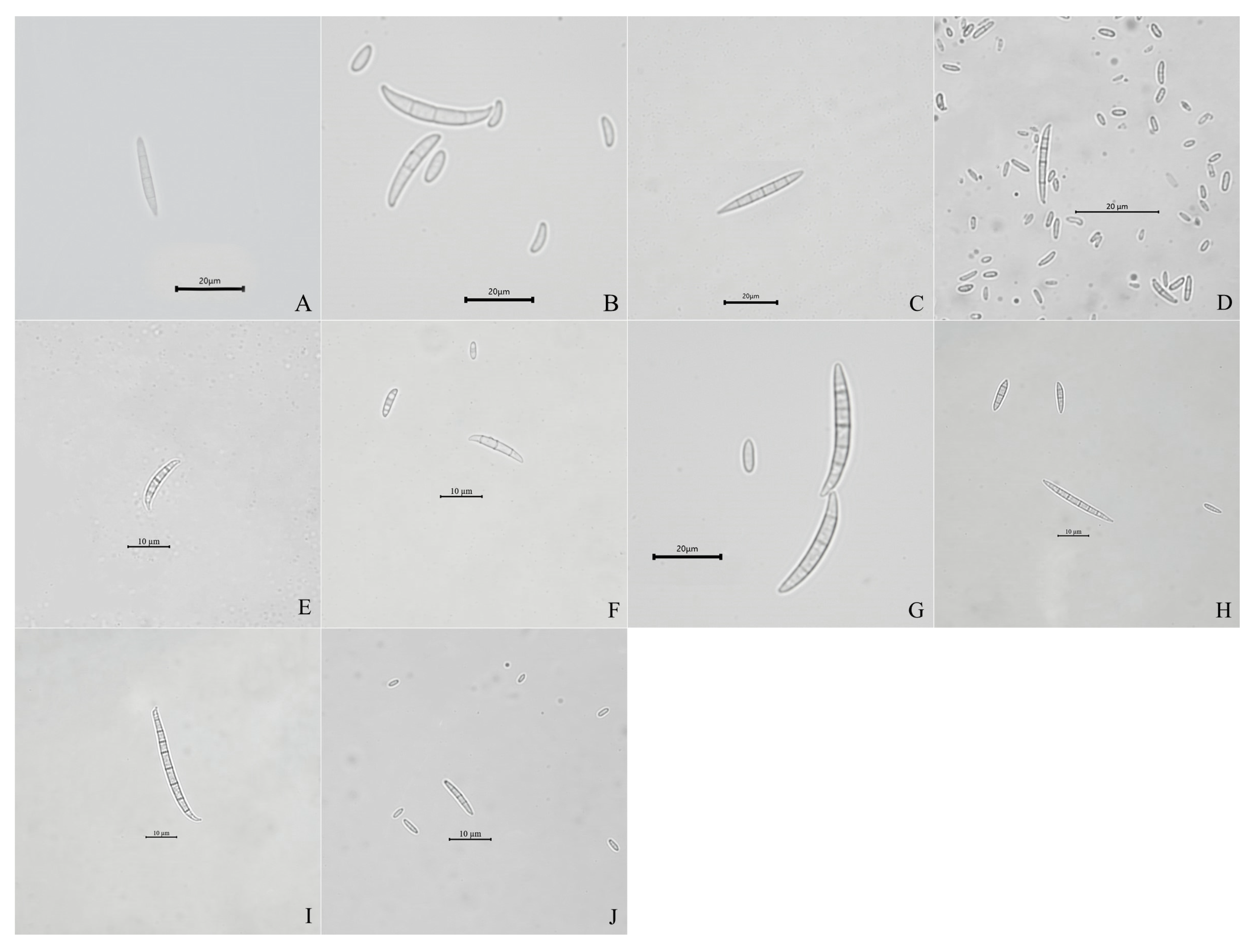

| Groups | Colonies Appearance | Conidia | |||
|---|---|---|---|---|---|
| Length (μm) | Width (μm) | Septum | Shape | ||
| F. verticillioides | White to greyish-purple mycelia | 16.6–23.4 | 2.3–3.4 | 3–4 | Slightly falcate to almost straight |
| F. subglutinans | Yellow mycelia, and then, turned lavender | 13.6–21.1 | 1.6–2.3 | 3–5 | Slightly falcate |
| FIESC | White to light beige with loosely floccose mycelia | 16.0–18.0 | 1.5–3 | 3-6 | Falcate |
| F. temperatum | White to peach-colored mycelia | 13.6–23.1 | 1.6–3.3 | 3-5 | Falcate |
| F. acuminatum | Abundant and partly carmine mycelia | 13.2–24.2 | 1.9–3.7 | 3-5 | Slender, equilaterally curved |
| F. solani | Dense and white mycelia | 16.0–26.0 | 1.5–2.8 | 2-4 | Sickle-shaped |
| F. sporotrichioides | White mycelia, and then, turned pink | 12.5–24.3 | 3.1–4.2 | 3-5 | Sickle-shaped |
| F. tricinctum | White to canary yellow, and dense mycelia | 12.5–26.0 | 1.5–3.0 | 3-5 | Falcate |
| F. asiaticum | White mycelia with pink pigmentation | 13.0–36.0 | 1.6–3.8 | 4-8 | Falcate |
| F. proliferatum | White mycelia with dark violet pigmentation | 13.6–22.3 | 3.1–4.2 | 3-4 | Slender |
| Fusarium Species | Disease Index a |
|---|---|
| F. tricinctum | 88.9–100 (94.4 ± 6.4) a |
| F. proliferatum | 88.9–100 (94.4 ± 5.6) a |
| F. asiaticum | 33.3–100 (73.6 ± 20.5) ab |
| F. acuminatum | 33.3–88.9 (68.1 ± 17.3) ab |
| F. solani | 22.2–100 (61.1 ± 39.0) ab |
| F. sporotrichioides | 33.3–100 (58.3 ± 29.2) b |
| F. temperatum | 44.4–77.8 (58.3 ± 16.7) b |
| F. verticillioides | 33.3–100 (57.4 ± 26.7) b |
| F. subglutinans | 22.2–100 (53.7 ± 32.5) b |
| FIESC | 11.1–77.8 (42.3 ± 22.6) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Xu, X.; Wang, S.; Zhang, L.; Shen, G.; Teng, H.; Yang, C.; Song, C.; Xiang, W.; Wang, X.; et al. Identification, Pathogenicity, and Genetic Diversity of Fusarium spp. Associated with Maize Sheath Rot in Heilongjiang Province, China. Int. J. Mol. Sci. 2022, 23, 10821. https://doi.org/10.3390/ijms231810821
Yang X, Xu X, Wang S, Zhang L, Shen G, Teng H, Yang C, Song C, Xiang W, Wang X, et al. Identification, Pathogenicity, and Genetic Diversity of Fusarium spp. Associated with Maize Sheath Rot in Heilongjiang Province, China. International Journal of Molecular Sciences. 2022; 23(18):10821. https://doi.org/10.3390/ijms231810821
Chicago/Turabian StyleYang, Xilang, Xi Xu, Shuo Wang, Li Zhang, Guijin Shen, Haolin Teng, Chunbo Yang, Chunru Song, Wensheng Xiang, Xiangjing Wang, and et al. 2022. "Identification, Pathogenicity, and Genetic Diversity of Fusarium spp. Associated with Maize Sheath Rot in Heilongjiang Province, China" International Journal of Molecular Sciences 23, no. 18: 10821. https://doi.org/10.3390/ijms231810821
APA StyleYang, X., Xu, X., Wang, S., Zhang, L., Shen, G., Teng, H., Yang, C., Song, C., Xiang, W., Wang, X., & Zhao, J. (2022). Identification, Pathogenicity, and Genetic Diversity of Fusarium spp. Associated with Maize Sheath Rot in Heilongjiang Province, China. International Journal of Molecular Sciences, 23(18), 10821. https://doi.org/10.3390/ijms231810821






