Ionizing Radiation from Radiopharmaceuticals and the Human Gut Microbiota: An Ex Vivo Approach
Abstract
1. Introduction
2. Results
2.1. Impact of Radiopharmaceuticals on Gut Microbiota
2.2. Phylotype-Level Dynamics of Microbiota after Irradiation
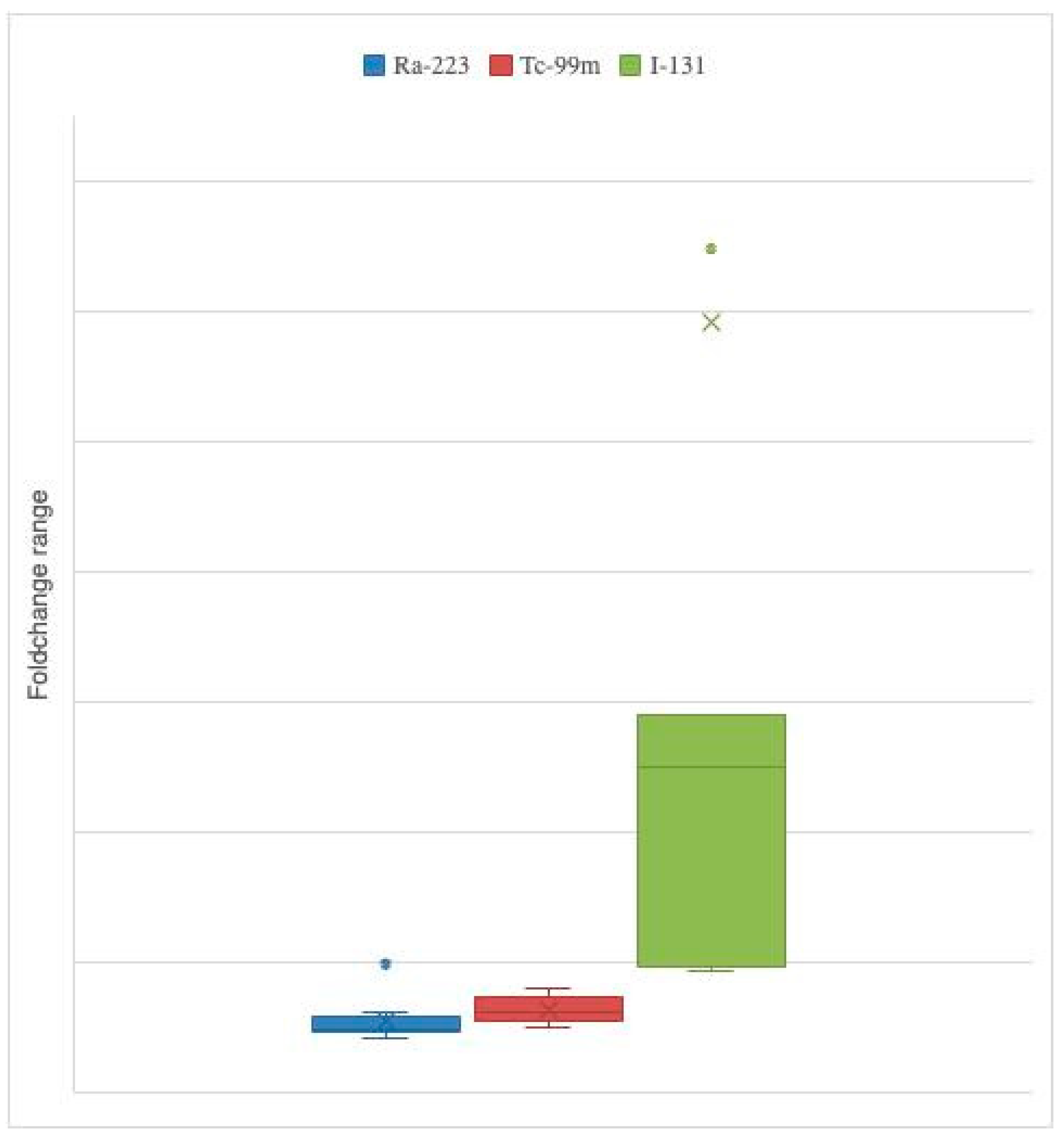
2.3. Variation Caused by Each Radiopharmaceutical
3. Discussion
3.1. Changes Regarding Each Radiopharmaceutical
3.2. Changes Regarding Each Taxa
4. Materials and Methods
4.1. Study Design
4.2. Stool Sample Processing and Ex Vivo Setup
4.3. Radiopharmaceuticals
4.4. DNA Extraction and Microbiota Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desouky, O.; Ding, N.; Zhou, G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef]
- Riley, P.A. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Röntgen, W.K. A new form of radiation. Science 1896, 3, 726–729. [Google Scholar] [CrossRef]
- Zhang, A.; Steen, T.Y. Gut Microbiomics-A Solution to Unloose the Gordian Knot of Biological Effects of Ionizing Radiation. J. Hered. 2018, 109, 212–221. [Google Scholar] [CrossRef]
- Andreyev, J. Gastrointestinal complications of pelvic radiotherapy: Are they of any importance? Gut 2005, 54, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, H.J.N.; Muls, A.C.; Norton, C.; Ralph, C.; Watson, L.; Shaw, C.; Lindsay, J.O. Guidance: The practical management of the gastrointestinal symptoms of pelvic radiation disease. Frontline Gastroenterol. 2015, 6, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Quastler, H. The nature of intestinal radiation death. Radiat. Res. 2012, 178, Av173–Av182. [Google Scholar] [CrossRef]
- Gerassy-Vainberg, S.; Blatt, A.; Danin-Poleg, Y.; Gershovich, K.; Sabo, E.; Nevelsky, A.; Daniel, S.; Dahan, A.; Ziv, O.; Dheer, R.; et al. Radiation induces proinflammatory dysbiosis: Transmission of inflammatory susceptibility by host cytokine induction. Gut 2018, 67, 97–107. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Kumagai, T.; Rahman, F.; Smith, A.M. The Microbiome and Radiation Induced-Bowel Injury: Evidence for Potential Mechanistic Role in Disease Pathogenesis. Nutrients 2018, 10, 1405. [Google Scholar] [CrossRef]
- Guarner, F.; Malagelada, J.R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E.; Wahl, R. Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Varela, E.; Martinez, C.; Antolin, M.; Llopis, M.; Dore, J.; Giralt, J.; Guarner, F.; Malagelada, J.R. The gut microbiota predispose to the pathophysiology of acute postradiotherapy diarrhea. Am. J. Gastroenterol. 2008, 103, 1754–1761. [Google Scholar] [CrossRef]
- Wang, A.; Ling, Z.; Yang, Z.; Kiela, P.R.; Wang, T.; Wang, C.; Cao, L.; Geng, F.; Shen, M.; Ran, X.; et al. Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: A pilot study. PLoS ONE 2015, 10, e0126312. [Google Scholar] [CrossRef]
- Johnson, L.B.; Riaz, A.A.; Adawi, D.; Wittgren, L.; Back, S.; Thornberg, C.; Osman, N.; Gadaleanu, V.; Thorlacius, H.; Jeppsson, B. Radiation enteropathy and leucocyte-endothelial cell reactions in a refined small bowel model. BMC Surg. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.; Park, S.J. High-throughput 16S rRNA gene sequencing reveals alterations of mouse intestinal microbiota after radiotherapy. Anaerobe 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Nam, Y.D.; Kim, H.J.; Seo, J.G.; Kang, S.W.; Bae, J.W. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLoS ONE 2013, 8, e82659. [Google Scholar] [CrossRef]
- Yi, Y.; Shen, L.; Shi, W.; Xia, F.; Zhang, H.; Wang, Y.; Zhang, J.; Wang, Y.; Sun, X.; Zhang, Z.; et al. Gut Microbiome Components Predict Response to Neoadjuvant Chemoradiotherapy in Patients with Locally Advanced Rectal Cancer: A Prospective, Longitudinal Study. Clin. Cancer Res. 2021, 27, 1329–1340. [Google Scholar] [CrossRef]
- Sahly, N.; Moustafa, A.; Zaghloul, M.; Salem, T.Z. Effect of radiotherapy on the gut microbiome in pediatric cancer patients: A pilot study. PeerJ 2019, 7, e7683. [Google Scholar] [CrossRef]
- Aerts, A.; Eberlein, U.; Holm, S.; Hustinx, R.; Konijnenberg, M.; Strigari, L.; van Leeuwen, F.W.B.; Glatting, G.; Lassmann, M. EANM position paper on the role of radiobiology in nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3365–3377. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.Y.A.; Nonnekens, J.; Aerts, A.; Baatout, S.; de Jong, M.; Cornelissen, B.; Pouget, J.P. Call to arms: Need for radiobiology in molecular radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1588–1590. [Google Scholar] [CrossRef] [PubMed]
- Morris, Z.S.; Wang, A.Z.; Knox, S.J. The Radiobiology of Radiopharmaceuticals. Semin. Radiat. Oncol. 2021, 31, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J.P.; Lozza, C.; Deshayes, E.; Boudousq, V.; Navarro-Teulon, I. Introduction to radiobiology of targeted radionuclide therapy. Front. Med. 2015, 2, 12. [Google Scholar] [CrossRef]
- Cui, M.; Xiao, H.; Li, Y.; Zhou, L.; Zhao, S.; Luo, D.; Zheng, Q.; Dong, J.; Zhao, Y.; Zhang, X.; et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol. Med. 2017, 9, 448–461. [Google Scholar] [CrossRef]
- Lam, V.; Moulder, J.E.; Salzman, N.H.; Dubinsky, E.A.; Andersen, G.L.; Baker, J.E. Intestinal microbiota as novel biomarkers of prior radiation exposure. Radiat. Res. 2012, 177, 573–583. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Models Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sheikh Sajjadieh, M.R.; Kuznetsova, L.V.; Bojenko, V.B. Dysbiosis in ukrainian children with irritable bowel syndrome affected by natural radiation. Iran. J. Pediatr. 2012, 22, 364–368. [Google Scholar]
- Wang, Z.; Wang, Q.; Zhao, J.; Gong, L.; Zhang, Y.; Wang, X.; Yuan, Z. Altered diversity and composition of the gut microbiome in patients with cervical cancer. AMB Express 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- El Alam, M.B.; Sims, T.T.; Kouzy, R.; Biegert, G.W.G.; Jaoude, J.; Karpinets, T.V.; Yoshida-Court, K.; Wu, X.; Delgado-Medrano, A.Y.; Mezzari, M.P.; et al. A prospective study of the adaptive changes in the gut microbiome during standard-of-care chemoradiotherapy for gynecologic cancers. PLoS ONE 2021, 16, e0247905. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. Biochemistry of DNA lesions. Radiat. Res. Suppl. 1985, 8, S103–S111. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988, 35, 95–125. [Google Scholar] [CrossRef]
- Goudarzi, M.; Mak, T.D.; Jacobs, J.P.; Moon, B.H.; Strawn, S.J.; Braun, J.; Brenner, D.J.; Fornace, A.J., Jr.; Li, H.H. An Integrated Multi-Omic Approach to Assess Radiation Injury on the Host-Microbiome Axis. Radiat. Res. 2016, 186, 219–234. [Google Scholar] [CrossRef]
- Mavragani, I.V.; Laskaratou, D.A.; Frey, B.; Candéias, S.M.; Gaipl, U.S.; Lumniczky, K.; Georgakilas, A.G. Key mechanisms involved in ionizing radiation-induced systemic effects. A current review. Toxicol. Res. 2015, 5, 12–33. [Google Scholar] [CrossRef]
- Azzam, E.I.; Little, J.B. The radiation-induced bystander effect: Evidence and significance. Hum. Exp. Toxicol. 2004, 23, 61–65. [Google Scholar] [CrossRef]
- Radswiki, T.; Worsley, C. Tc-99m Pertechnetate. Reference Article. Available online: https://radiopaedia.org/articles/15559 (accessed on 24 January 2022).
- Fard-Esfahani, A.; Emami-Ardekani, A.; Fallahi, B.; Fard-Esfahani, P.; Beiki, D.; Hassanzadeh-Rad, A.; Eftekhari, M. Adverse effects of radioactive iodine-131 treatment for differentiated thyroid carcinoma. Nucl. Med. Commun. 2014, 35, 808–817. [Google Scholar] [CrossRef]
- Pashnehsaz, M.; Takavar, A.; Izadyar, S.; Zakariaee, S.S.; Mahmoudi, M.; Paydar, R.; Geramifar, P. Gastrointestinal Side Effects of the Radioiodine Therapy for the Patients with Differentiated Thyroid Carcinoma Two Days after Prescription. World J. Nucl. Med. 2016, 15, 173–178. [Google Scholar] [CrossRef]
- Oh, J.-R.; Ahn, B.-C. False-positive uptake on radioiodine whole-body scintigraphy: Physiologic and pathologic variants unrelated to thyroid cancer. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 362–385. [Google Scholar]
- Poeppel, T.D.; Handkiewicz-Junak, D.; Andreeff, M.; Becherer, A.; Bockisch, A.; Fricke, E.; Geworski, L.; Heinzel, A.; Krause, B.J.; Krause, T.; et al. EANM guideline for radionuclide therapy with radium-223 of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 824–845. [Google Scholar] [CrossRef] [PubMed]
- Flux, G.D. Imaging and dosimetry for radium-223: The potential for personalized treatment. Br. J. Radiol. 2017, 90, 20160748. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y. Internal radiation therapy: A neglected aspect of nuclear medicine in the molecular era. J. Biomed. Res. 2015, 29, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Sghir, A.; Gramet, G.; Suau, A.; Rochet, V.; Pochart, P.; Dore, J. Quantification of bacterial groups within human fecal flora by oligonucleotide probe hybridization. Appl. Env. Microbiol. 2000, 66, 2263–2266. [Google Scholar] [CrossRef] [PubMed]
- Lay, C.; Rigottier-Gois, L.; Holmstrøm, K.; Rajilic, M.; Vaughan, E.E.; de Vos, W.M.; Collins, M.D.; Thiel, R.; Namsolleck, P.; Blaut, M.; et al. Colonic microbiota signatures across five northern European countries. Appl. Environ. Microbiol. 2005, 71, 4153–4155. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Yamanouchi, K.; Tsujiguchi, T.; Sakamoto, Y.; Ito, K. Short-term follow-up of intestinal flora in radiation-exposed mice. J. Radiat. Res. 2019, 60, 328–332. [Google Scholar] [CrossRef]
- Garcia-Peris, P.; Velasco, C.; Lozano, M.A.; Moreno, Y.; Paron, L.; de la Cuerda, C.; Breton, I.; Camblor, M.; Garcia-Hernandez, J.; Guarner, F.; et al. Effect of a mixture of inulin and fructo-oligosaccharide on Lactobacillus and Bifidobacterium intestinal microbiota of patients receiving radiotherapy: A randomised, double-blind, placebo-controlled trial. Nutr. Hosp. 2012, 27, 1908–1915. [Google Scholar] [CrossRef]
- Nayak, T.; Sengupta, I.; Dhal, P.K. A new era of radiation resistance bacteria in bioremediation and production of bioactive compounds with therapeutic potential and other aspects: An in-perspective review. J. Environ. Radioact. 2021, 237, 106696. [Google Scholar] [CrossRef]
- Hoyos-Hernandez, C.; Courbert, C.; Simonucci, C.; David, S.; Vogel, T.M.; Larose, C. Community structure and functional genes in radionuclide contaminated soils in Chernobyl and Fukushima. FEMS Microbiol. Lett. 2019, 366, fnz180. [Google Scholar] [CrossRef]
- Carter, S.R.; Zahs, A.; Palmer, J.L.; Wang, L.; Ramirez, L.; Gamelli, R.L.; Kovacs, E.J. Intestinal barrier disruption as a cause of mortality in combined radiation and burn injury. Shock 2013, 40, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Kuku, S.; Fragkos, C.; McCormack, M.; Forbes, A. Radiation-induced bowel injury: The impact of radiotherapy on survivorship after treatment for gynaecological cancers. Br. J. Cancer 2013, 109, 1504–1512. [Google Scholar] [CrossRef]
- Loos, M.; Quentmeier, P.; Schuster, T.; Nitsche, U.; Gertler, R.; Keerl, A.; Kocher, T.; Friess, H.; Rosenberg, R. Effect of preoperative radio(chemo)therapy on long-term functional outcome in rectal cancer patients: A systematic review and meta-analysis. Ann. Surg. Oncol. 2013, 20, 1816–1828. [Google Scholar] [CrossRef]
- Hwang, W.L.; Pike, L.R.G.; Royce, T.J.; Mahal, B.A.; Loeffler, J.S. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat. Rev. Clin. Oncol. 2018, 15, 477–494. [Google Scholar] [CrossRef]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hernandez, L.A.; Ruiz-Briseno, M.D.R.; Sanchez-Reyes, K.; Alvarez-Zavala, M.; Vega-Magana, N.; Lopez-Iniguez, A.; Diaz-Ramos, J.A.; Martinez-Ayala, P.; Soria-Rodriguez, R.A.; Ramos-Solano, M.; et al. Alterations in bacterial communities, SCFA and biomarkers in an elderly HIV-positive and HIV-negative population in western Mexico. BMC Infect. Dis. 2019, 19, 234. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.W.; Currie, V.; Richardson, A.J.; Duncan, G.; Holtrop, G.; Farquharson, F.; Louis, P.; Pinton, P.; Oswald, I.P. Porcine Small and Large Intestinal Microbiota Rapidly Hydrolyze the Masked Mycotoxin Deoxynivalenol-3-Glucoside and Release Deoxynivalenol in Spiked Batch Cultures In Vitro. Appl. Environ. Microbiol. 2018, 84, e02106–e02117. [Google Scholar] [CrossRef]
- Vanhoutte, T.; Huys, G.; Brandt, E.; Swings, J. Temporal stability analysis of the microbiota in human feces by denaturing gradient gel electrophoresis using universal and group-specific 16S rRNA gene primers. FEMS Microbiol. Ecol. 2004, 48, 437–446. [Google Scholar] [CrossRef]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Takada, T.; Tanaka, R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl. Environ. Microbiol. 2004, 70, 7220–7228. [Google Scholar] [CrossRef]

| Radiopharmaceutical | Aliquots ID | Reference Gene | Firmicutes | Bacteroidetes | Proteobacteria | Actinobacteria | Prevotella | Lactobacillus | Bifidobacterium | Atopobacter | Clostridium leptum | Clostridium coccoides | Bacteroides fragilis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tc-99m Samples | Non irradiated #1 | 11.72 | 13.25 | 13.64 | 18.89 | 25.68 | 17.28 | 24.22 | 17.38 | 18.38 | 15.27 | 14.38 | 14.61 |
| Tc-99m #1 | 11.70 | 12.63 | 13.41 | 18.55 | 24.08 | 17.04 | 23.56 | 16.62 | 17.38 | 15.08 | 14.07 | 14.57 | |
| Tc-99m #2 | 12.11 | 13.23 | 13.55 | 19.20 | 26.32 | 17.36 | 24.20 | 18.36 | 18.29 | 15.18 | 14.72 | 14.73 | |
| Tc-99m #3 | 11.16 | 12.65 | 12.72 | 18.09 | 24.46 | 16.44 | 23.10 | 17.05 | 17.46 | 14.98 | 13.88 | 13.58 | |
| Ra-223 Samples | Non irradiated #2 | 10.25 | 11.77 | 11.73 | 19.44 | 25.50 | 12.52 | 21.45 | 19.67 | 17.09 | 12.90 | 14.11 | 13.95 |
| Ra-223 #1 | 9.82 | 11.07 | 11.42 | 19.00 | 25.19 | 12.17 | 21.14 | 19.09 | 16.72 | 12.65 | 12.85 | 13.22 | |
| Ra-223 #2 | 10.40 | 11.77 | 12.16 | 19.77 | 25.91 | 12.93 | 21.53 | 19.27 | 17.31 | 12.97 | 13.25 | 14.23 | |
| Ra-223 #3 | 10.11 | 11.58 | 11.53 | 19.02 | 25.78 | 12.41 | 21.41 | 19.65 | 17.14 | 12.88 | 12.88 | 13.10 | |
| I-131 Samples | Non irradiated#3 | 13.78 | 16.60 | 15.93 | 22.21 | 33.56 | 20.71 | 26.74 | 23.23 | 24.01 | 19.74 | 20.03 | 17.39 |
| I-131 #1 | 10.95 | 12.96 | 12.20 | 17.10 | 24.93 | 16.91 | 22.17 | 17.61 | 17.70 | 14.88 | 15.02 | 13.11 | |
| I-131 #2 | 11.08 | 12.91 | 12.26 | 16.78 | 23.89 | 17.12 | 22.20 | 18.26 | 17.40 | 14.34 | 14.88 | 13.29 | |
| I-131 #3 | 11.02 | 12.94 | 12.23 | 16.94 | 24.41 | 17.02 | 22.19 | 17.94 | 17.55 | 14.61 | 14.95 | 13.20 |
| Taxa | FC Ra-223 | FC Tc-99m | FC I-131 |
|---|---|---|---|
| Firmicutes | 1.11 | 1.27 | 1.87 |
| Bacteroidetes | 0.92 | 1.27 | 1.91 |
| Proteobacteria | 1.03 | 1.16 | 5.68 |
| Actinobacteria | 0.83 | 1.58 | 83.58 |
| Prevotella spp. | 0.92 | 1.21 | 1.91 |
| Lactobacillus spp. | 0.97 | 1.45 | 3.46 |
| Bifidobacterium spp. | 1.14 | 0.98 | 5.78 |
| Atopobacter spp. | 0.93 | 1.52 | 12.95 |
| Clostridium leptum | 0.95 | 1.09 | 5.15 |
| Clostridium coccoides | 1.97 | 1.07 | 4.98 |
| Bacteroides fragilis | 1.23 | 1.19 | 2.69 |
| Sample Identification | Sample Weight (g) | Administered Activity (MBq) | |
|---|---|---|---|
| Tc-99m | Tc-99m #1 | 8 | 0.056 |
| Tc-99m #2 | 7 | 0.049 | |
| Tc-99m #3 | 9 | 0.046 | |
| Non irradiated #1 | 10 | ||
| Ra-223 | Ra-223 #1 | 4 | 0.134 |
| Ra-223 #2 | 13 | 0.511 | |
| Ra-223 #3 | 6 | 0.246 | |
| Non irradiated #2 | 8 | ||
| I-131 | I-131 #1 | 9 | 0.082 |
| I-131 #2 | 12 | 0.100 | |
| I-131 #3 | 8 | 0.098 | |
| Non irradiated #3 | 7 |
| Study Ref. | Primers | Target | Sequence | Gram | Phylum | Order |
|---|---|---|---|---|---|---|
| [56] | 534/358 | 16S | ATTACCGCGGCTGCTGG | Bacterial (universal) | ||
| CCTACGGGAGGCAGCAG | ||||||
| [57] | Firm | Firmicutes | GGAGYATGTGGTTTAATTCGAAGCA | Gram+ | Firmicutes | |
| AGCTGACGACAACCATGCAC | ||||||
| [59] | Lac | Lactobacillus spp. | AGCAGTAGGGAATCTTCCA | Lactobacilliales | ||
| CATTYCACCGCTACACATG | ||||||
| [60] | Atopo | Atopobacter spp. | GGGTTGAGAGACCGACC | |||
| CGGRGCTTCTTCTGCAGG | ||||||
| [60] | Ccoc | Clostridium coccoides | AAATGACGGTACCTGACTAA | Clostridialles | ||
| CTTTGAGTTTCATTCTTGCGAA | ||||||
| [60] | Clept | Clostridium leptum | GCACAAGCAGTGGAGT | |||
| CTTCCTCCGTTTTGTCAA | ||||||
| [57] | Act | Actinobacteria | TACGGCCGCAAGGCTA | Actinobacteria | ||
| TCRTCCCCACCTTCCTCCG | ||||||
| [59] | Bifid | Bifidobacterium spp. | CTCCTGGAAACGGGTGG | |||
| GGTGTTCTTCCCGATATCTACA | ||||||
| [57] | Bact | Bacteroidetes | GGARCATGTGGTTTAATTCGATGAT | Gram− | Bacteroidetes | |
| AGCTGACGACAACCATGCAG | ||||||
| [60] | Bfra | Bacteroides fragilis | ATAGCCTTTCGAAAGRAAGAT | |||
| CCAGTATCAACTGCAATTTTA | ||||||
| [58] | Prevo-F BacPre-R | Prevotella spp. | CRCRCRGTAAACGATGGATG | |||
| TTGAGTTTCACCGTTGCCGG | ||||||
| [57] | Prot | Proteobacteria | TCGTCAGCTCGTGTYGTGA | Proteobacteria | ||
| CGTAAGGGCCATGATG | ||||||
| [58] | Ent | Enterobacteria | GACCTCGCGAGAGCA | |||
| CCTACTTCTTTTGCAACCCA | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, A.; Oliveira, A.; Guedes, C.; Fernandes, R.; Soares, R.; Barata, P. Ionizing Radiation from Radiopharmaceuticals and the Human Gut Microbiota: An Ex Vivo Approach. Int. J. Mol. Sci. 2022, 23, 10809. https://doi.org/10.3390/ijms231810809
Fernandes A, Oliveira A, Guedes C, Fernandes R, Soares R, Barata P. Ionizing Radiation from Radiopharmaceuticals and the Human Gut Microbiota: An Ex Vivo Approach. International Journal of Molecular Sciences. 2022; 23(18):10809. https://doi.org/10.3390/ijms231810809
Chicago/Turabian StyleFernandes, Ana, Ana Oliveira, Carla Guedes, Rúben Fernandes, Raquel Soares, and Pedro Barata. 2022. "Ionizing Radiation from Radiopharmaceuticals and the Human Gut Microbiota: An Ex Vivo Approach" International Journal of Molecular Sciences 23, no. 18: 10809. https://doi.org/10.3390/ijms231810809
APA StyleFernandes, A., Oliveira, A., Guedes, C., Fernandes, R., Soares, R., & Barata, P. (2022). Ionizing Radiation from Radiopharmaceuticals and the Human Gut Microbiota: An Ex Vivo Approach. International Journal of Molecular Sciences, 23(18), 10809. https://doi.org/10.3390/ijms231810809








