Relationship between Changes in Intestinal Microorganisms and Effect of High Temperature on the Growth and Development of Bombyx mori Larvae
Abstract
1. Introduction
2. Results
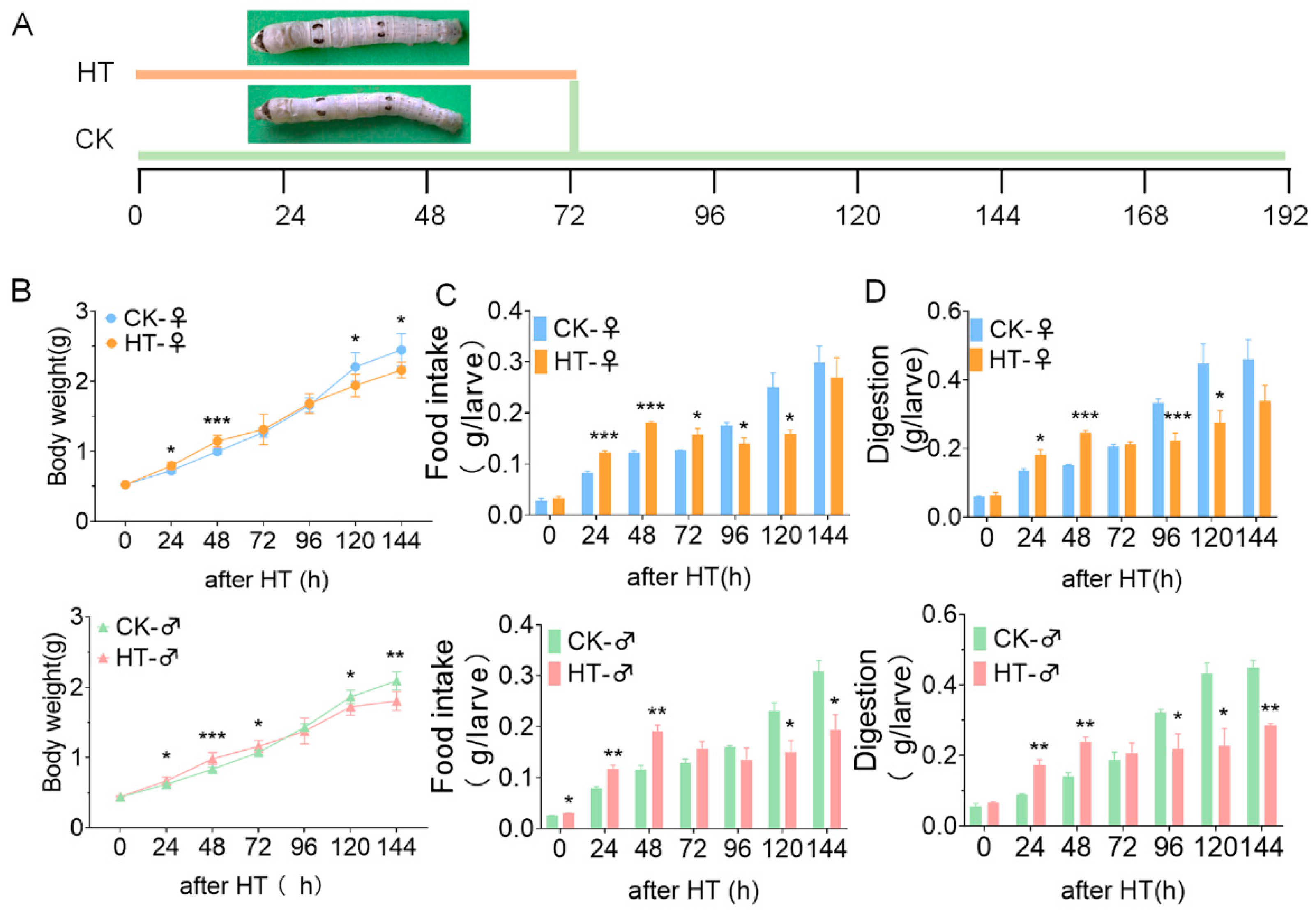
2.1. High Temperature Affects the Growth and Development of Silkworm Larvae
2.2. High Temperature Affects Growth and Development by Changing the Morphology and Structure of the Midgut of the Silkworm and the Activity of Related Digestive Enzymes
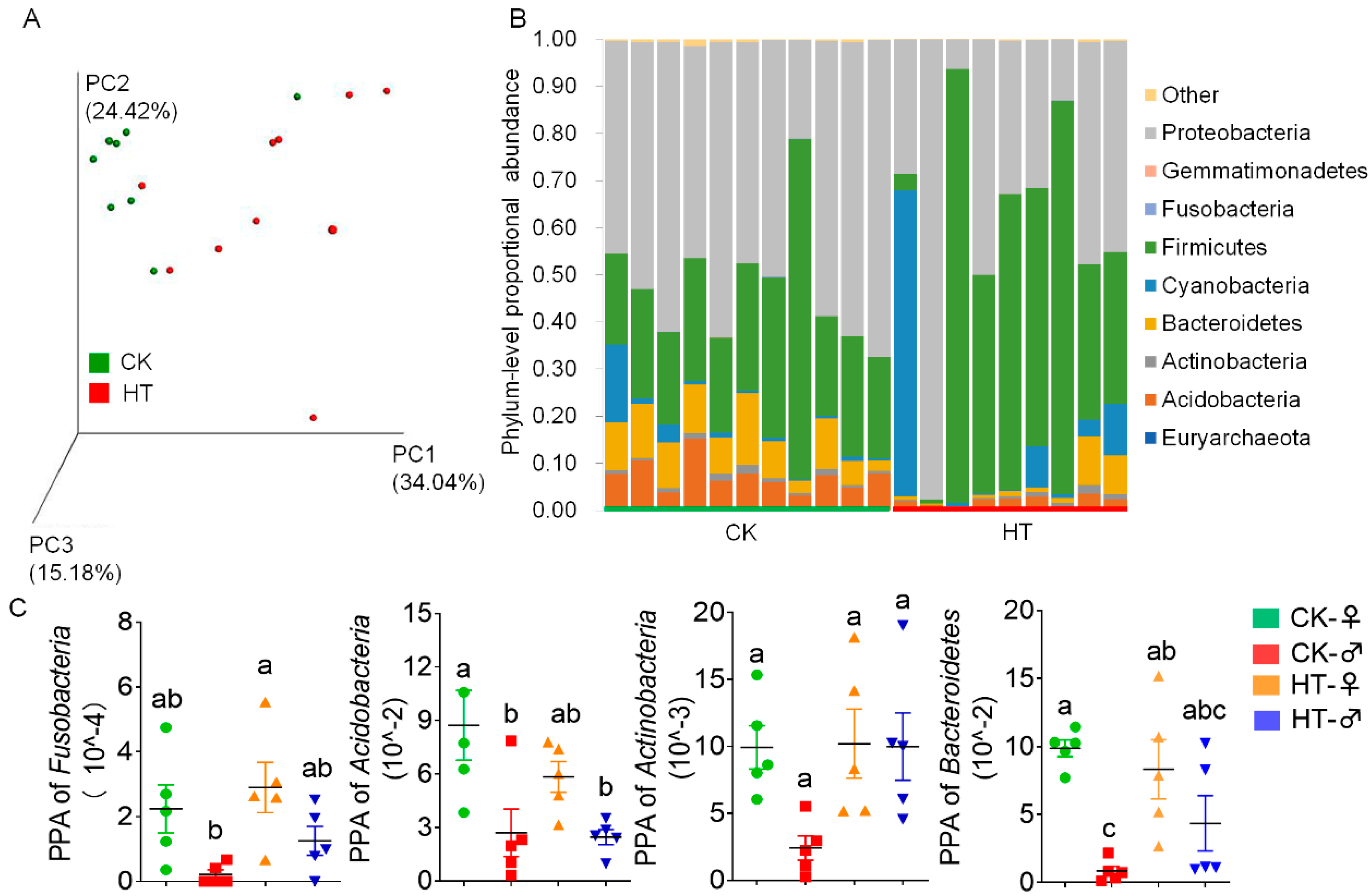
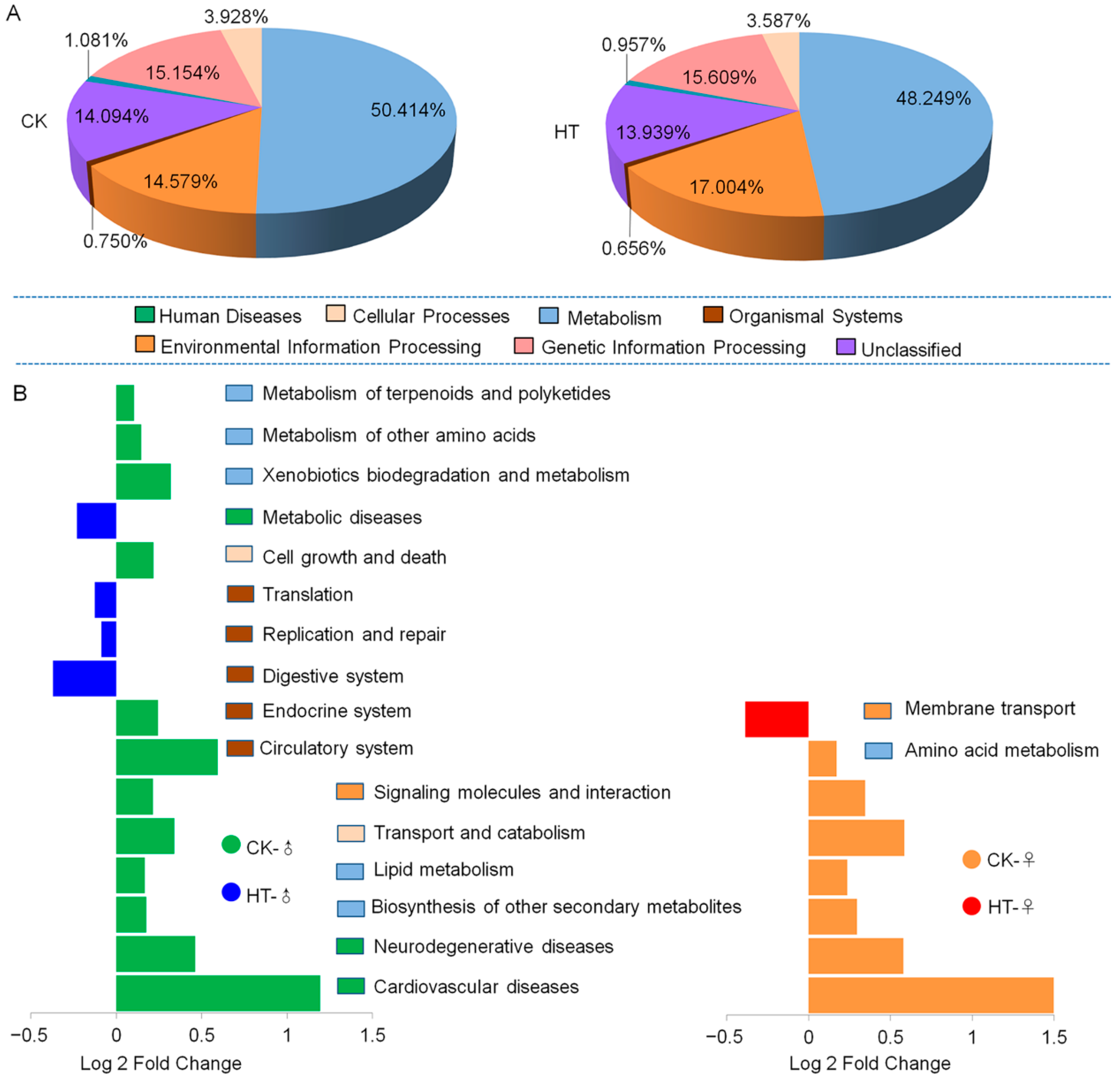
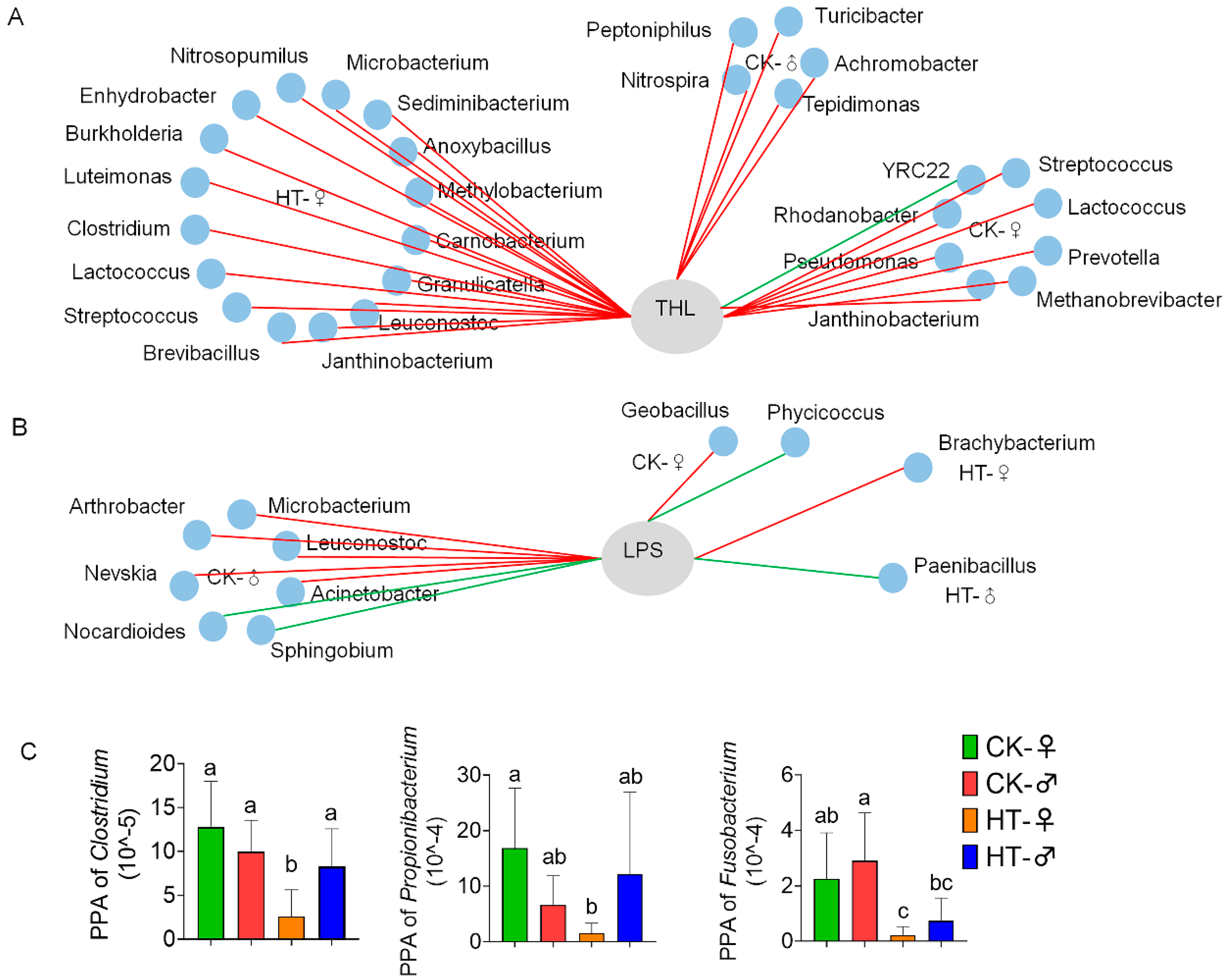
2.3. High-Temperature-Induced Changes in the Composition and Function of the Intestinal Microbiota of Silkworm Larvae Are One of the Main Causes of Abnormal Digestion, Absorption, Growth, and Development
3. Discussion
3.1. High Temperature Affects Silkworm Growth and Development by Changing the Intestinal Microbial Flora
3.2. The High-Temperature Treatment Did Not Cause a Significant Change in Protease Activity, but the Protein Content of Lipase Was Significantly Reduced
3.3. There Are Sex Differences in the Effects of High Temperature on the Intestinal Microbes of the Silkworm
4. Materials and Methods
4.1. Experimental Animal
4.2. DNA Extraction and High-Throughput Sequencing
4.3. Data Analysis
4.4. Quantitative Real-Time PCR
4.5. Hematoxylin-Eosin Staining (HE Staining)
4.6. Determination of Activity of Intestinal Digestive Juice Enzyme
4.7. Protein Content Determination
4.8. Growth and Development Index Survey
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Hady, A.A.A.; Ramadan, M.M.; Lü, J.; Hashem, A.S. High-temperature shock consequences on the red flour beetle (Tribolium castaneum) and the rice weevil (Sitophilus oryzae). J. Therm. Biol. 2021, 100, 103062. [Google Scholar] [CrossRef] [PubMed]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. Camb. Philos. Soc. 2020, 95, 802–821. [Google Scholar] [CrossRef] [PubMed]
- Stazione, L.; Norry, F.M.; Sambucetti, P. Heat-hardening effects on mating success at high temperature in Drosophila melanogaster. J. Therm. Biol. 2019, 80, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.S.; Subbarao, S.V.; Misra, S.; Murty, U.S. Screening strains of the mulberry silkworm, Bombyx mori, for thermotolerance. J. Insect Sci. 2011, 11, 116. [Google Scholar] [CrossRef][Green Version]
- Jiang, L.; Xie, E.; Guo, H.; Sun, Q.; Liu, l.; Hao, Y.; Wang, Y.; Li, Q.; Xia, Q. Heat shock protein 19.9 (Hsp19.9) from Bombyx mori is involved in host protection against viral infection. Dev. Comp. Immunol. 2020, 114, 103790. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Kumar, D.; Cao, G.; Zhu, L.; Liu, B.; Zhu, M.; Liang, Z.; Kuang, S.; Chen, F.; Feng, Y.; et al. Effects of transient high temperature treatment on the intestinal flora of the silkworm Bombyx mori. Sci. Rep. 2017, 7, 3349. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Keesey, I.W.; Hansson, B.S.; Knaden, M. Gut microbiota affects development and olfactory behavior in Drosophila melanogaster. J. Exp. Biol. 2019, 222, jeb192500. [Google Scholar] [CrossRef]
- Téfit, M.A.; Leulier, F. Lactobacillus plantarum favors the early emergence of fit and fertile adult Drosophila upon chronic undernutrition. J. Exp. Biol. 2017, 220, 900–907. [Google Scholar] [CrossRef]
- Leitão-Gonçalves, R.; Carvalho-Santos, Z.; Francisco, A.P.; Fioreze, G.T.; Anjos, M.; Baltazar, C.; Elias, A.P.; Itskov, P.M.; Piper, M.D.W.; Ribeiro, C. Commensal bacteria and essential amino acids control food choice behavior and reproduction. PLoS Biol. 2017, 15, e2000862. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Iatsenko, I.; Boquete, J.P.; Lemaitre, B. Microbiota-Derived Lactate Activates Production of Reactive Oxygen Species by the Intestinal NADPH Oxidase Nox and Shortens Drosophila Lifespan. Immunity 2018, 49, 929–942. [Google Scholar] [CrossRef]
- Rahmathulla, V.K.; Suresh, H.M. Seasonal variation in food consumption, assimilation, and conversion efficiency of Indian bivoltine hybrid silkworm, Bombyx mori. J. Insect Sci. 2012, 12, 82. [Google Scholar] [CrossRef]
- Kingsolver, J.; Moore, M.E.; Augustine, K.E.; Hill, C.A. Responses of Manduca sexta larvae to heat waves. J. Exp. Biol. 2021, 224, jeb236505. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ramanathan, V.; Victor, D.G. Global warming will happen faster than we think. Nature 2018, 564, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Huang, J.; Xia, J.; Lu, C. Biology of Sericulture; China Forestry Publishing House: Beijing, China, 2005; pp. 59–81. [Google Scholar]
- Braun, L.; Keddie, B.A. A new tissue technique for evaluating effects of Bacillus thuringiensis toxins on insect midgut epithelium. J. Invertebr. Pathol. 1997, 69, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.A.; Coutu, C.; Theilmann, D.A.; Erlandson, M.A.; Hegedus, D.D. Proteomics analysis of Trichoplusia ni midgut epithelial cell brush border membrane vesicles. Insect Sci. 2019, 26, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.A.; Lemaitre, B. Gut-associated microbes of Drosophila melanogaster. Gut Microbes 2012, 3, 307–321. [Google Scholar] [CrossRef]
- Dayama, G.; Priya, S.; Niccum, D.E.; Khoruts, A.; Blekhman, R. Interactions between the gut microbiome and host gene regulation in cystic fibrosis. Genome Med. 2020, 12, 12. [Google Scholar] [CrossRef]
- EI Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bjursell, M.K.; Himrod, J.; Deng, S.; Carmichael, L.K.; Chiang, H.C.; Hooper, L.V.; Gordon, J.I. A genomic view of the human–Bacteroides thetaiotaomicron symbiosis. Science 2003, 299, 2074–2076. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef]
- Davila, A.M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.; Sanz, Y.; Tomé, D. Re-print of “intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host”. Pharmacol. Res. 2013, 68, 114–126. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Buchon, N.; Osman, D. All for one and one for all: Regionalization of the Drosophila intestine. Insect Biochem. Mol. Biol. 2015, 67, 2–8. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Martin-Nuñez, G.M.; Cornejo-Pareja, I.; Clemente-Postigo, M.; Tinahones, F.J. Gut Microbiota: The Missing Link Between Infection and Metabolic Disorders? Front. Endocrinol. 2021, 12, 639856. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Martinez, K.B.; Leone, V.; Chang, E.B. Western diets, gut dysbiosis, and metabolic diseases: Are they linked? Gut Microbes 2017, 8, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Brighenti, F.; Royall, D.; Jenkins, A.L.; Jenkins, D.J. Effect of rectal infusion of short chain fatty acids in human subjects. Am. J. Gastroenterol. 1989, 84, 1027–1033. [Google Scholar]
- Fast, P.G.; Angus, T.A. Effects of parasporal inclusions of Bacillus thuringiensis, var. sotto, Ishiwata on the permeability of the gut wall of Bombyx mori, (Linnaeus) larvae. J. Invertebr. Pathol. 1965, 20, 29–32. [Google Scholar] [CrossRef]
- Koch, F.; Thom, U.; Albrecht, E.; Weikard, R.; Nolte, W.; Kuhla, B.; Kuehn, C. Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc. Natl. Acad. Sci. USA 2019, 116, 10333–10338. [Google Scholar] [CrossRef] [PubMed]
- Vaishnavi, C. Translocation of gut flora and its role in sepsis. Indian J. Med. Microbiol. 2013, 31, 334–342. [Google Scholar] [CrossRef]
- Chen, P.; Billiar, T. Gut Microbiota and Multiple Organ Dysfunction Syndrome (MODS). Adv. Exp. Med. Biol. 2020, 1238, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.R.; Xiao, Y.; Wu, F.Q.; Ye, M.Q.; Luo, G.Q.; Xing, D.X.; Li, L.; Yang, Q. Analysis of midgut gene expression profiles from different silkworm varieties after exposure to high temperature. Gene 2014, 549, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fang, Y.; Wang, L.P.; Zhu, W.J.; Ji, H.P.; Wang, H.Y.; Xu, S.Q.; Sima, Y.H. Transcriptome analysis of the Bombyx mori fat body after constant high temperature treatment shows differences between the sexes. Mol. Biol. Rep. 2014, 41, 6039–6049. [Google Scholar] [CrossRef] [PubMed]
- Belousova, I.; Pavlushin, S.; Subbotina, A.; Rudneva, N.; Martemyanov, V. Sex Specificity in Innate Immunity of Insect Larvae. J. Insect Sci. 2021, 21, 15. [Google Scholar] [CrossRef]
- Schwarzenbach, G.A.; Hosken, D.J.; Ward, P.I. Sex and immunity in the yellow dung fly Scathophaga stercoraria. J. Evol. Biol. 2005, 18, 455–463. [Google Scholar] [CrossRef]
- Bao, Y.; Qu, L.; Zhao, D.; Chen, L.; Jin, H.; Xu, L.; Cheng, J.; Zhang, C. The genome- and transcriptome-wide analysis of innate immunity in the brown planthopper, Nilaparvata lugens. BMC Genom. 2013, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhou, Z.; Lu, C.; Cheng, D.; Dai, F.; Li, B.; Zhao, P.; Zha, X.; Cheng, T.; Chai, C.; et al. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 2004, 306, 1937–1940. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysisof high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Kemp, P.F.; Aller, J.Y. Bacterial diversity in aquatic and other environments: What 16S rDNA libraries can tell us. FEMS Microbiol. Ecol. 2004, 47, 161–177. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Yuan, Q.; Du, B.; Jin, X.; Huang, X.; Li, Q.; Zhong, Y.; Pan, Z.; Xu, S.; Sima, Y. Relationship between Changes in Intestinal Microorganisms and Effect of High Temperature on the Growth and Development of Bombyx mori Larvae. Int. J. Mol. Sci. 2022, 23, 10289. https://doi.org/10.3390/ijms231810289
Sun X, Yuan Q, Du B, Jin X, Huang X, Li Q, Zhong Y, Pan Z, Xu S, Sima Y. Relationship between Changes in Intestinal Microorganisms and Effect of High Temperature on the Growth and Development of Bombyx mori Larvae. International Journal of Molecular Sciences. 2022; 23(18):10289. https://doi.org/10.3390/ijms231810289
Chicago/Turabian StyleSun, Xiaoning, Qian Yuan, Beibei Du, Xinye Jin, Xiyun Huang, Qiuying Li, Yueqiao Zhong, Zhonghua Pan, Shiqing Xu, and Yanghu Sima. 2022. "Relationship between Changes in Intestinal Microorganisms and Effect of High Temperature on the Growth and Development of Bombyx mori Larvae" International Journal of Molecular Sciences 23, no. 18: 10289. https://doi.org/10.3390/ijms231810289
APA StyleSun, X., Yuan, Q., Du, B., Jin, X., Huang, X., Li, Q., Zhong, Y., Pan, Z., Xu, S., & Sima, Y. (2022). Relationship between Changes in Intestinal Microorganisms and Effect of High Temperature on the Growth and Development of Bombyx mori Larvae. International Journal of Molecular Sciences, 23(18), 10289. https://doi.org/10.3390/ijms231810289




