Increasing the Grain Yield and Grain Protein Content of Common Wheat (Triticum aestivum) by Introducing Missense Mutations in the Q Gene
Abstract
:1. Introduction
2. Results
2.1. Phenotype of the Mutant ss1 Carrying the Qs1 Allele
2.2. Characterization of the Mutant NS8 Carrying the Qc1-N8 Allele
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Gene Cloning
4.3. Genotyping for Qc1-N8
4.4. Development of CAPS Marker for Qc1-N8
4.5. Processing Quality Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. FAO Database. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data (accessed on 26 July 2021).
- Kichey, T.; Heumez, E.; Pocholle, D.; Pageau, K.; Vanacker, H.; Dubois, F.; Le Gouis, J.; Hirel, B. Combined agronomic and physiological aspects of nitrogen management in wheat highlight a central role for glutamine synthetase. New Phytol. 2006, 169, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Liu, N.; Su, D.; Xue, Q.; Stewart, B.A.; Wang, Z. Effect of source-sink manipulation on accumulation of micronutrients and protein in wheat grains. J. Plant Nutr. Soil Sci. 2012, 175, 622–629. [Google Scholar] [CrossRef]
- Zheng, T.; Qi, P.F.; Cao, Y.L.; Han, Y.N.; Ma, H.L.; Guo, Z.R.; Wang, Y.; Qiao, Y.Y.; Hua, S.Y.; Yu, H.Y.; et al. Mechanisms of wheat (Triticum aestivum) grain storage proteins in response to nitrogen applications and its impacts on processing quality. Sci. Rep. 2018, 8, 11928. [Google Scholar] [CrossRef] [PubMed]
- Laidig, F.; Piepho, H.P.; Dirk Rentel, D.; Thomas Drobek, T.; Meyer, U.; Huesken, A. Breeding progress, environmental variation and correlation of winter wheat yield and quality traits in German official variety trials and on-farm during 1983–2014. Theor. Appl. Genet. 2017, 130, 223–245. [Google Scholar] [CrossRef]
- Mirosavljevic, M.; Momcilovic, V.; Zivancev, D.; Acin, V.; Jockovic, B.; Mikic, S.; Takac, V.; Dencic, S. Genetic improvement of grain yield and bread-making quality of winter wheat over the past 90 years under the Pannonian Plain conditions. Euphytica 2020, 216, 184. [Google Scholar] [CrossRef]
- Subira, J.; Peña, R.J.; Álvaro, F.; Ammar, K.; Ramdani, A.; Royo, C. Breeding progress in the pasta-making quality of durum wheat cultivars released in Italy and Spain during the 20th Century. Crop Pasture Sci. 2014, 65, 16–26. [Google Scholar] [CrossRef]
- Tabbita, F.; Pearce, S.; Barneix, A.J. Breeding for increased grain protein and micronutrient content in wheat: Ten years of the GPC-B1 gene. J. Cereal Sci. 2017, 73, 183–191. [Google Scholar] [CrossRef]
- Rasheed, A.; Xia, X.; Yan, Y.; Appels, R.; Mahmood, T.; He, Z. Wheat seed storage proteins: Advances in molecular genetics, diversity and breeding applications. J. Cereal Sci. 2014, 60, 11–24. [Google Scholar] [CrossRef]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef]
- Qi, P.F.; Wei, Y.; Yue, Y.W.; Yan, Z.H.; Zheng, Y.L. Biochemical and molecular characterization of gliadins. Mol. Biol. 2006, 40, 713–723. [Google Scholar] [CrossRef]
- Payne, P.I. Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Ann. Rev. Plant Physiol. 1987, 38, 141–153. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.F.; Chen, Q.; Wang, Y.; Guo, Z.R.; Xu, B.J.; Zhu, J.; Zhang, Y.Z.; Gong, X.; Luo, C.H.; Wu, W.; et al. Re-acquisition of the brittle rachis trait via a transposon insertion in domestication gene Q during wheat de-domestication. New Phytol. 2019, 224, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.J.; Fellers, J.P.; Trick, H.N.; Zhang, Z.; Tai, Y.; Gill, B.S.; Faris, J.D. Molecular characterization of the major wheat domestication gene Q. Genet. 2006, 172, 547–555. [Google Scholar] [CrossRef]
- Xie, Q.; Li, N.; Yang, Y.; Lv, L.; Yao, H.; Wei, R.; Sparkes, D.L.; Ma, Z.Q. Pleiotropic effects of the wheat domestication gene Q on yield and grain morphology. Planta 2018, 247, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.J.; Chen, Q.; Zheng, T.; Jiang, Y.F.; Qiao, Y.Y.; Guo, Z.R.; Cao, Y.L.; Wan, G.Y.; Zhang, Y.Z.; Zong, L.J.; et al. An overexpressed Q allele leads to increased spike density and improved processing quality. G3 Genes Genom. Genet. 2018, 8, 771–778. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Q.; Zhu, J.; Wang, Y.; Li, Y.; Li, Q.; Zhao, K.; Li, Y.; Tang, R.; Shi, X.; et al. The Qc5 allele increases wheat bread-making quality by regulating SPA and SPR. Int. J. Mol. Sci. 2022, 23, 7581. [Google Scholar] [CrossRef]
- Endo, T.R.; Gill, B.S. The deletion stocks of common wheat. J. Hered. 1996, 87, 295–307. [Google Scholar] [CrossRef]
- Solomon, C.S.; Drea, S. Besides and beyond flowering: Other roles of EuAP2 genes in plant development. Genes 2019, 10, 994. [Google Scholar] [CrossRef]
- Greenwood, J.R.; Finnegan, E.J.; Watanabe, N.; Trevaskis, B.; Swain, S.M. New alleles of the wheat domestication gene Q reveal multiple roles in growth and reproductive development. Development 2017, 144, 1959–1965. [Google Scholar] [CrossRef] [Green Version]
- Weegels, P.L.; Hamer, R.J.; Schofield, J.D. Functional properties of wheat glutenin. J. Cereal Sci. 1996, 23, 1–18. [Google Scholar] [CrossRef]
- Masoni, A.; Ercoli, L.; Mariotti, M.; Arduini, I. Post-anthesis accumulation and remobilization of dry matter, nitrogen and phosphorus in durum wheat as affected by soil type. Eur. J. Agron. 2007, 26, 179–186. [Google Scholar] [CrossRef]
- Barnex, A.J. Physiology and biochemistry of source-regulated protein accumulation in the wheat grain. J. Plant Physiol. 2007, 164, 581–590. [Google Scholar] [CrossRef]
- He, G.; Zhang, Y.; Liu, P.; Jing, Y.; Zhang, L.; Zhu, Y.; Kong, X.; Zhao, H.; Zhou, Y.; Sun, J. The transcription factor TaLAX1 interacts with Q to antagonistically regulate grain threshability and spike morphogenesis in bread wheat. New Phytol. 2021, 230, 988–1002. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, M. Dosage effect of the spelta gene q of hexaploid wheat. Genetics 1963, 48, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Sissons, M. Role of durum wheat composition on the quality of pasta and bread. Food 2008, 2, 75–90. [Google Scholar]
- Porceddu, E.; Turchetta, T.; Masci, S.; D’Ovidio, R.; Lafiandra, D.; Kasarda, D.D.; Impiglia, A.; Nachit, M.M. Variation in endosperm protein composition and technological quality properties in durum wheat. Euphytica 1998, 100, 197–205. [Google Scholar] [CrossRef]
- Walsh, D.E.; Gilles, K.A. The influence of protein composition on spaghetti quality. Cereal Chem. 1971, 48, 544–553. [Google Scholar]
- Chuck, G.; Meeley, R.; Hake, S. Floral meristem initiation and meristem cell fate are regulated by the maize AP2 genes ids1 and sid1. Development 2008, 135, 3013–3019. [Google Scholar] [CrossRef]
- Dong, Z.; Alexander, M.; Chuck, G. Understanding grass domestication through maize mutants. Trends Genet. 2019, 35, 118–128. [Google Scholar] [CrossRef]
- Gil-Humanes, J.; Piston, F.; Martin, A.; Barro, F. Comparative genomic analysis and expression of the APETALA2-like genes from barley, wheat, and barley-wheat amphiploids. BMC Plant Biol. 2009, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; An, G. Two AP2 family genes, Supernumerary Bract (SNB) and Osindeterminate Spikelet 1 (OsIDS1), synergistically control inflorescence architecture and floral meristem establishment in rice. Plant J. 2012, 69, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, Z.; Zhang, X.; Liu, H.; Zhou, L.; Zhong, S.; Li, Y.; Zhu, C.; Lin, Z. krn1, a major quantitative trait locus for kernel row number in maize. New Phytol. 2019, 223, 1634–1646. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Li, Y.; Guo, Z.R.; Liu, C.H.; Wan, Y.F.; Hawkesford, M.; Zhu, J.; Wu, W.; Wei, M.Q.; et al. Post-translational cleavage of HMW-GS Dy10 allele improves the cookie-making quality in common wheat (Triticum aestivum). Mol. Breed. 2021, 41, 49. [Google Scholar] [CrossRef]
- AACC International. Basic Straight-Dough Bread-Baking Method—Long Fermentation (Method 10-09.01); Cereals & Grains Association: St. Paul, MN, USA, 2010; Available online: http://methods.aaccnet.org/ (accessed on 8 February 2014).
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]

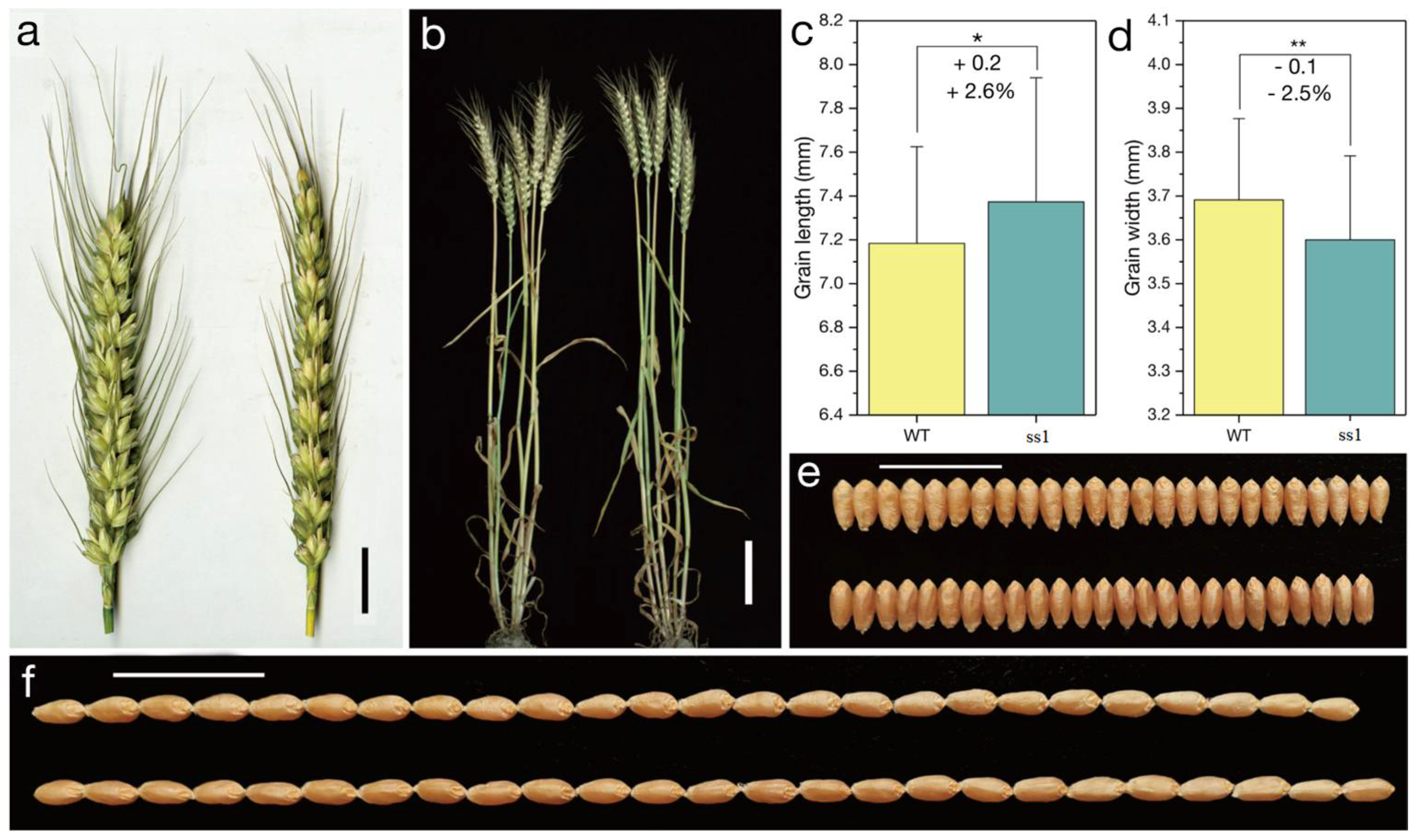
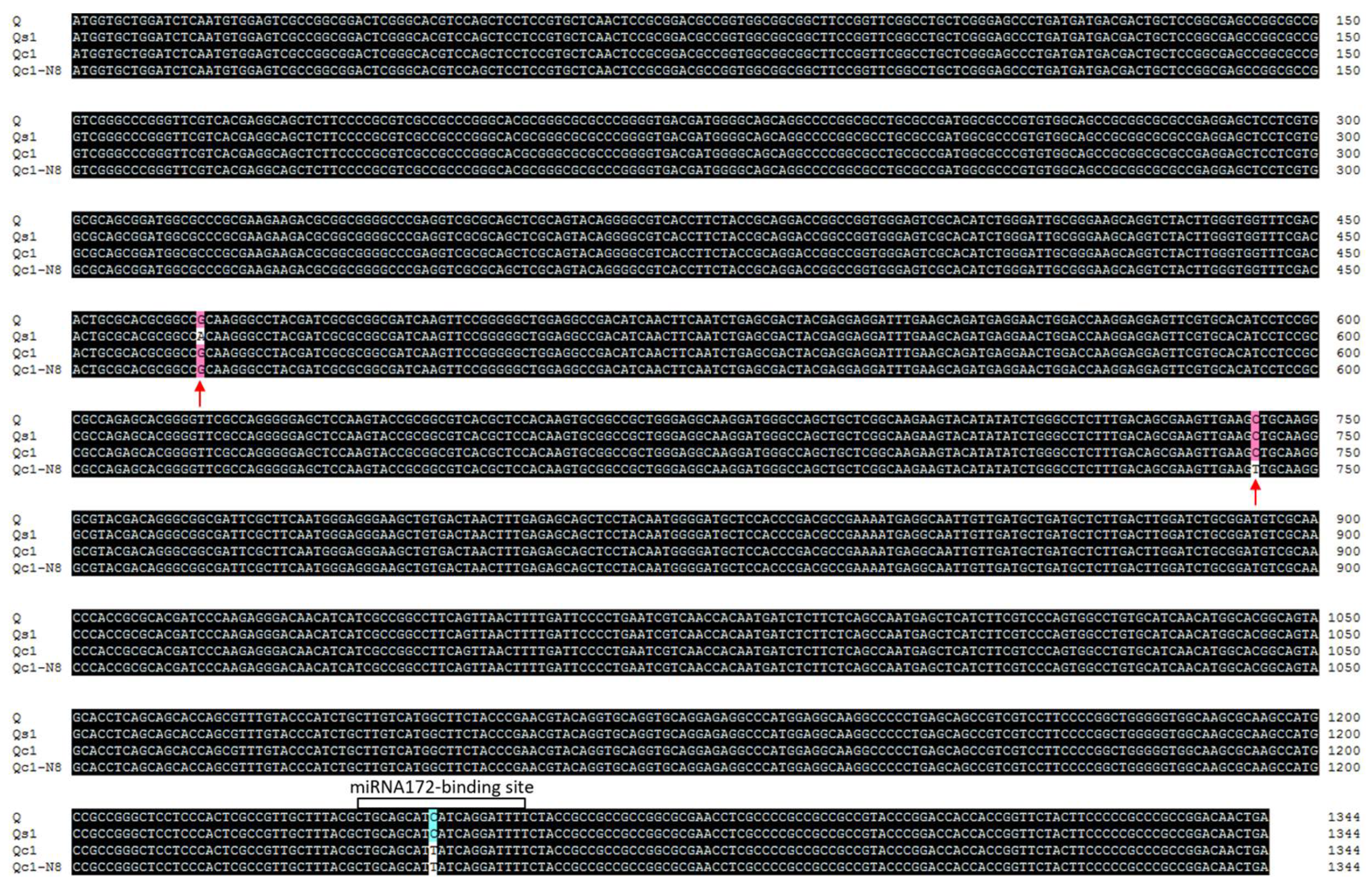

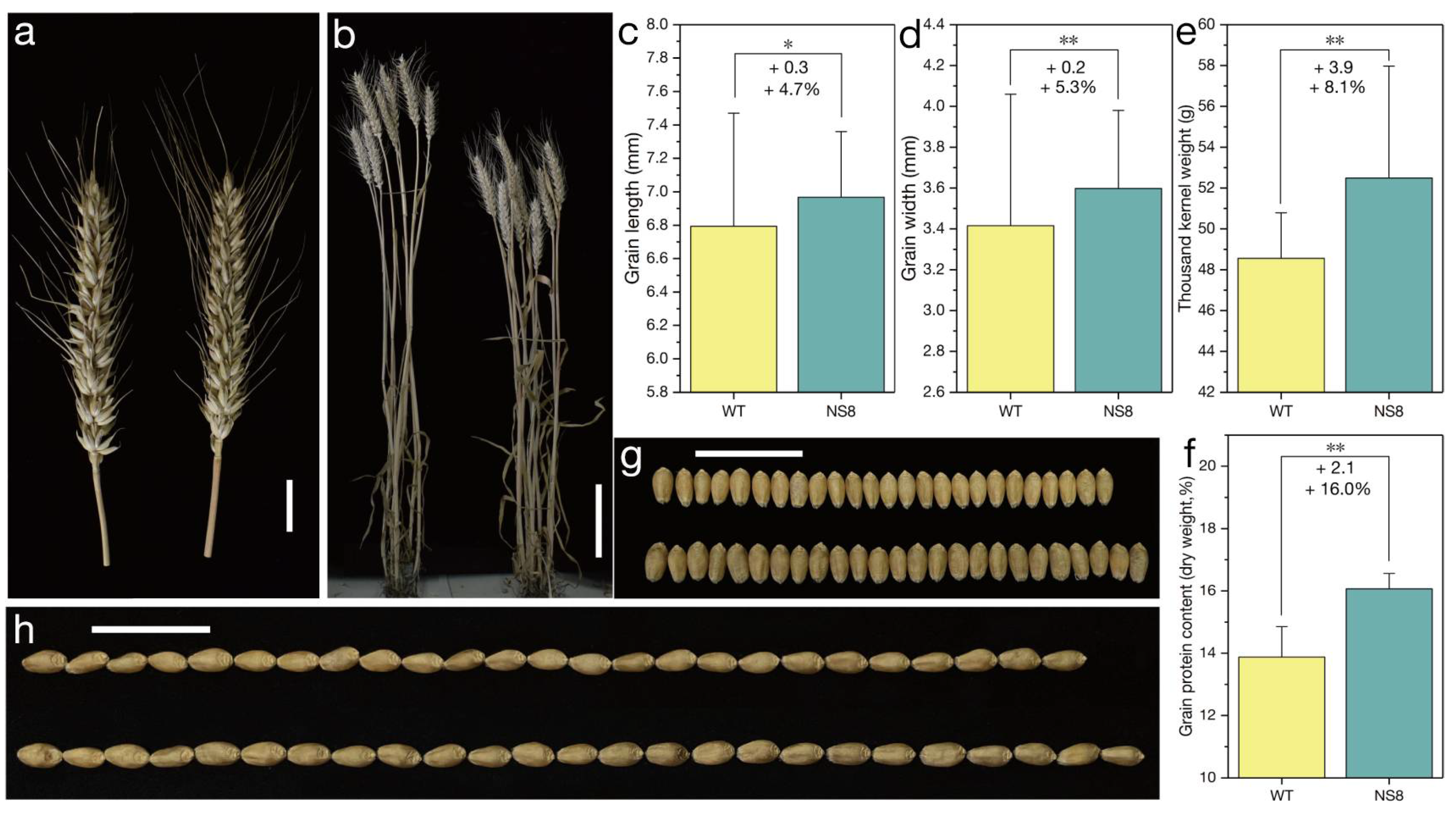

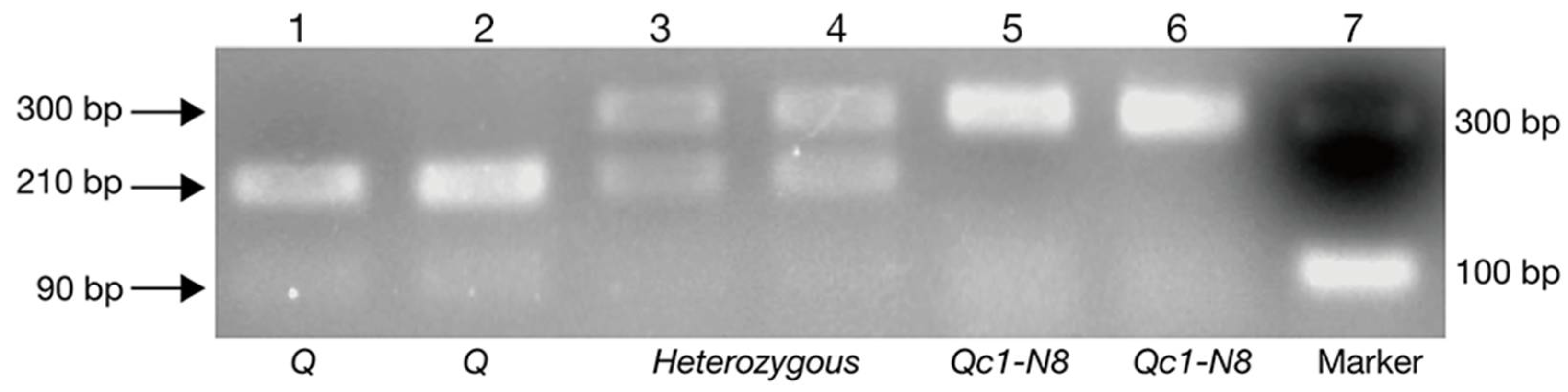
| Traits | Growing Season | ss1 | WT | E | G | E × G |
|---|---|---|---|---|---|---|
| Plant height (cm) | 2018–2019 2019–2020 | 83.41 ± 4.05 ** 71.82 ± 2.30 ** | 79.44 ± 3.09 67.11 ± 2.00 | 205.087 ** | 24.459 ** | 0.534 |
| Spike length (cm) | 2018–2019 2019–2020 | 14.65 ± 0.68 * 12.88 ± 0.80 ** | 14.06 ± 0.75 11.87 ± 0.46 | 123.331 ** | 15.408 ** | 2.680 |
| Spikelet number per main spike | 2018–2019 2019–2020 | 21.00 ± 1.15 ** 19.93 ± 0.70 ** | 22.94 ± 1.44 21.43 ± 1.45 | 15.038 ** | 24.858 ** | 0.110 |
| Spike density | 2018–2019 2019–2020 | 1.43 ± 0.07 ** 1.55 ± 0.10 ** | 1.63 ± 0.09 1.80 ± 0.08 | 45.434 ** | 84.944 ** | 13.78 |
| Grain number per main spike | 2018–2019 2019–2020 | 58.81 ± 5.83 * 50.43 ± 4.14 * | 65.13 ± 8.23 54.64 ± 4.25 | 43.376 ** | 8.860 ** | 0.044 |
| Thousand kernel weight (g) | 2018–2019 2019–2020 | 44.08 ± 1.71 * 48.76 ± 1.25 * | 46.13 ± 0.43 51.90 ± 1.67 | 50.427 ** | 13.780 ** | 0.216 |
| Tiller number | 2018–2019 2019–2020 | 5.30 ± 1.03 3.85 ± 0.74 | 5.25 ± 1.33 4.25 ± 0.79 | 27.478 ** | 0.730 | 1.207 |
| Trails | Growing Season | ss1 | WT | E | G | E × G |
|---|---|---|---|---|---|---|
| Grain protein content (%; dry weight) | 2018–2019 2019–2020 | 13.12 ± 0.43 14.53 ± 0.60 | 13.45 ± 0.36 15.16 ± 0.76 | 30.313 ** | 2.562 | 0.323 |
| Zeleny sedimentation value (mL) | 2018–2019 2019–2020 | 32.65 ± 3.64 21.54 ± 2.51 | 27.73 ± 4.20 23.88 ± 1.98 | 29.114 ** | 0.301 | 4.315 |
| Wet gluten content (%) | 2018–2019 2019–2020 | 24.72 ± 1.60 27.21 ± 1.49 | 24.98 ± 3.06 29.66 ± 2.05 | 14.645 ** | 0.570 | 2.996 |
| Gluten index (%) | 2018–2019 2019–2020 | 92.87 ± 2.59 63.37 ± 9.02 | 87.32 ± 8.94 65.04 ± 6.69 | 65.599 ** | 0.004 | 0.315 |
| Water absorption (%) | 2018–2019 2019–2020 | 50.36 ± 0.71 58.74 ± 1.12 | 50.88 ± 1.67 58.28 ± 0.45 | 428.875 ** | 0.783 | 0.077 |
| Development time (s) | 2018–2019 2019–2020 | 78.00 ± 13.22 61.50 ± 10.71 | 77.67 ± 9.14 68.40 ± 10.46 | 7.440 * | 0.365 | 2.875 |
| Stability time (s) | 2018–2019 2019–2020 | 260.86 ± 121.40 193.20 ± 56.34 | 203.83 ± 66.50 230.40 ± 54.54 | 0.394 | 0.125 | 1.560 |
| Primers Name | Sequences (5′-3′) | Reference | Objective |
|---|---|---|---|
| AP2startF | ATGGTGCTGGATCTCAATGTGGAGTCGCCGGCGGA | [15] | Cloning of the genomic DNA sequence of Q gene |
| AP2.8R | CGCGGCCAAATCGGGGCAAAGGAATTCAAACGA | [15] | |
| AP2.2-1F | ATCTTAGCTGTATGGGCTCGTG | This study | |
| AP2.2-1R | TCAACGGAGATAGGGGTGTG | This study | |
| AP2.2-2F | AGGCTCCACATAAGTATATGATCGAGTC | This study | |
| AP2.2-2R | CTTAATTTCAGGAACGAACTTGTCG | This study | |
| AP2.16F | CTGCTTGGTGCGCTGCTCCACCAGCTTACTGAAA | [15] | |
| AP45.1R | CAGAAGGCCCAACGGTTAACGCAACAATGGC | [15] | |
| Q-mRNA-F2-123 | TCGGAGATGGTGCTGGAT | This study | Cloning the full open reading frame of Q gene |
| Q-mRNA-R1-1479 | GCCAGCTTCAGTTGTCCG | This study | |
| QF7 | GACCAGCCAGTAGTGTCACC | This study | Genotyping of Qc1-N8 allele |
| QR7 | TCTTGCAGTTCCATCCGTCC | This study | |
| N8-CAPS-F1 | ACTAGAGTGAGTGAGCAAAGATT | This study | CAPS marker for Qc1-N8 allele |
| N8-CAPS-R1 | AGCAACTGTTAGGCTCCACATAA | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Guo, Z.; Shi, X.; Wei, M.; Fan, Y.; Zhu, J.; Zheng, T.; Wang, Y.; Kong, L.; Deng, M.; et al. Increasing the Grain Yield and Grain Protein Content of Common Wheat (Triticum aestivum) by Introducing Missense Mutations in the Q Gene. Int. J. Mol. Sci. 2022, 23, 10772. https://doi.org/10.3390/ijms231810772
Chen Q, Guo Z, Shi X, Wei M, Fan Y, Zhu J, Zheng T, Wang Y, Kong L, Deng M, et al. Increasing the Grain Yield and Grain Protein Content of Common Wheat (Triticum aestivum) by Introducing Missense Mutations in the Q Gene. International Journal of Molecular Sciences. 2022; 23(18):10772. https://doi.org/10.3390/ijms231810772
Chicago/Turabian StyleChen, Qing, Zhenru Guo, Xiaoli Shi, Meiqiao Wei, Yazhen Fan, Jing Zhu, Ting Zheng, Yan Wang, Li Kong, Mei Deng, and et al. 2022. "Increasing the Grain Yield and Grain Protein Content of Common Wheat (Triticum aestivum) by Introducing Missense Mutations in the Q Gene" International Journal of Molecular Sciences 23, no. 18: 10772. https://doi.org/10.3390/ijms231810772
APA StyleChen, Q., Guo, Z., Shi, X., Wei, M., Fan, Y., Zhu, J., Zheng, T., Wang, Y., Kong, L., Deng, M., Cao, X., Wang, J., Wei, Y., Jiang, Q., Jiang, Y., Chen, G., Zheng, Y., & Qi, P. (2022). Increasing the Grain Yield and Grain Protein Content of Common Wheat (Triticum aestivum) by Introducing Missense Mutations in the Q Gene. International Journal of Molecular Sciences, 23(18), 10772. https://doi.org/10.3390/ijms231810772








