Androgen Receptor Gene CAG Repeat Length Varies and Affects Semen Quality in an Ethnic-Specific Fashion in Young Men from Russia
Abstract
1. Introduction
2. Results
2.1. Distribution of AR CAG Repeat Alleles and Association with Semen Parameters in the Entire Study Population
2.2. Distribution of AR CAG Repeat Alleles and Association with Semen Parameters in Different Ethnic Subgroups
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Semen Analysis
4.3. Hormone Assays
4.4. Genotyping
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aitken, R.J.; Baker, M.A. The role of genetics and oxidative stress in the etiology of male infertility-a unifying hypothesis? Front. Endocrinol. 2020, 11, 581838. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Borges, E., Jr.; Dutta, S.; Krajewska-Kulak, E. Decline in sperm count in European men during the past 50 years. Hum. Exp. Toxicol. 2018, 37, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Mínguez-Alarcón, L.; Williams, P.L.; Chiu, Y.H.; Gaskins, A.J.; Nassan, F.L.; Dadd, R.; Petrozza, J.; Hauser, R.; Chavarro, J.E.; Earth Study Team. Secular trends in semen parameters among men attending a fertility center between 2000 and 2017: Identifying potential predictors. Environ. Int. 2018, 121, 1297–1303. [Google Scholar] [CrossRef]
- Osadchuk, L.V.; Osadchuk, A.V. Role of CAG and GGC repeat polymorphisms in the androgen receptor gene in male fertility. Russ. J. Genet. 2022, 58, 247–264. [Google Scholar] [CrossRef]
- Pan, B.; Li, R.; Chen, Y.; Tang, Q.; Wu, W.; Chen, L.; Lu, C.; Pan, F.; Ding, H.; Xia, Y. Genetic association between androgen receptor gene CAG repeat length polymorphism and male infertility: A meta-analysis. Medicine 2016, 95, e2878. [Google Scholar] [CrossRef]
- Davey, R.A.; Grossmann, M. Androgen receptor structure, function and biology: From bench to bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar]
- Irvine, R.A.; Yu, M.C.; Ross, R.K.; Coetzee, G.A. The CAG and GGC microsatellites of the androgen receptor gene are in linkage disequilibrium in men with prostate cancer. Cancer Res. 1995, 55, 1937–1940. [Google Scholar]
- Sartor, O.; Zheng, Q.; Estham, J.A. Androgen receptor gene CAG repeat length varies in a race-specific fashion in men without prostate cancer. Urology 1999, 53, 378–380. [Google Scholar] [CrossRef]
- Nakabayashi, A.; Sueoka, K.; Matsuda, N.; Asada, H.; Tanigaki, R.; Sato, K.; Tajima, H.; Ogata, T.; Kuji, N.; Yoshimura, Y. Incidental deviation of short and long CAG repeats in the androgen receptor gene for Japanese male infertility. Reprod. Med. Biol. 2003, 2, 145–150. [Google Scholar] [CrossRef]
- Tse, J.Y.; Liu, V.W.; Yeung, W.S.; Lau, E.Y.; Ng, E.H.; Ho, P.C. Molecular analysis of the androgen receptor gene in Hong Kong Chinese infertile men. J. Assist. Reprod. Genet. 2003, 20, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Esteban, E.; Rodon, N.; Via, M.; Gonzalez-Perez, E.; Santamaria, J.; Dugoujon, J.M.; Chennawi, F.E.; Melhaoui, M.; Cherkaoui, M.; Vona, G.; et al. Androgen receptor CAG and GGC polymorphisms in Mediterraneans: Repeat dynamics and population relationships. J. Hum. Genet. 2006, 51, 129–136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ackerman, C.M.; Lowe, L.P.; Lee, H.; Hayes, M.G.; Dyer, A.R.; Metzger, B.E.; Lowe, W.L.; Urbanek, M.; Hapo Study Cooperative Research Group. Ethnic variation in allele distribution of the androgen receptor (AR) (CAG) n repeat. J. Androl. 2012, 33, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Yong, E.L.; Loy, C.J.; Sim, K.S. Androgen receptor gene and male infertility. Hum. Reprod. Update 2003, 9, 1–7. [Google Scholar] [CrossRef]
- Xiao, F.; Lan, A.; Lin, Z.; Song, J.; Zhang, Y.; Li, J.; Gu, K.; Lv, B.; Zhao, D.; Zeng, S.; et al. Impact of CAG repeat length in the androgen receptor gene on male infertility-a meta-analysis. Reprod. Biomed. Online 2016, 33, 39–49. [Google Scholar] [CrossRef]
- Iwamoto, T.; Nozawa, S.; Yoshiike, M. Semen quality of Asian men. Reprod. Med. Biol. 2007, 6, 185–193. [Google Scholar] [CrossRef]
- Nordkap, L.; Joensen, U.N.; Blomberg Jensen, M.; Jørgensen, N. Regional differences and temporal trends in male reproductive health disorders: Semen quality may be a sensitive marker of environmental exposures. Mol. Cell Endocrinol. 2012, 355, 221–230. [Google Scholar] [CrossRef]
- Redmon, J.B.; Thomas, W.; Ma, W.; Drobnis, E.Z.; Sparks, A.; Wang, C.; Brazil, C.; Overstreet, J.W.; Liu, F.; Swan, S.H.; et al. Semen parameters in fertile US men: The Study for Future Families. Andrology 2013, 1, 806–814. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Buck Louis, G.M.; Toppari, J.; Andersson, A.M.; Eisenberg, M.L.; Jensen, T.K.; Jørgensen, N.; Swan, S.H.; Sapra, K.J.; et al. Male reproductive disorders and fertility trends: Influences of environment and genetic susceptibility. Physiol. Rev. 2016, 96, 55–97. [Google Scholar] [CrossRef]
- Osadchuk, L.; Tipisova, E.; Kleshchev, M.; Gorenko, I.; Osadchuk, A. Study of semen quality, reproductive hormone levels, and lipid levels in men from Arkhangelsk, a city in North of European Russia. Am. J. Men’s Health 2020, 14, 1557988320939714. [Google Scholar] [CrossRef]
- Osadchuk, L.V.; Shantanova, L.N.; Troev, I.V.; Kleshchev, M.A.; Osadchuk, A.V. Regional and ethnic differences in semen quality and reproductive hormones in Russia: A Siberian population-based cohort study of young men. Andrology 2021, 9, 1512–1525. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Davis-Dao, C.A.; Tuazon, E.D.; Sokol, R.Z.; Cortessis, V.K. Male infertility and variation in CAG repeat length in the androgen receptor gene: A meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 4319–4326. [Google Scholar] [CrossRef] [PubMed]
- Mobasseri, N.; Babaei, F.; Karimian, M.; Nikzad, H. Androgen receptor (AR)-CAG trinucleotide repeat length and idiopathic male infertility: A case-control trial and a meta-analysis. EXCLI J. 2018, 17, 1167–1179. [Google Scholar] [PubMed]
- Al Zoubi, M.S.; Bataineh, H.; Rashed, M.; Al-Trad, B.; Aljabali, A.A.A.; Al-Zoubi, R.M.; Al Hamad, M.; Issam AbuAlArjah, M.; Batiha, O.; Al-Batayneh, K.M. CAG repeats in the androgen receptor gene is associated with oligozoospermia and teratozoospermia in infertile men in Jordan. Andrologia 2020, 52, e13728. [Google Scholar] [CrossRef]
- Katagiri, Y.; Neri, Q.V.; Takeuchi, T.; Moy, F.; Sills, E.S.; Palermo, G.D. Androgen receptor CAG polymorphism (Xq11-12) status and human spermatogenesis: A prospective analysis of infertile males and their offspring conceived by intracytoplasmic sperm injection. Int. J. Mol. Med. 2006, 18, 405–413. [Google Scholar] [CrossRef]
- Milatiner, D.; Halle, D.; Huerta, M.; Margalioth, E.J.; Cohen, Y.; Ben-Chetrit, A.; Gal, M.; Mimoni, T.; Eldar-Geva, T. Associations between androgen receptor CAG repeat length and sperm morphology. Hum. Reprod. 2004, 19, 1426–1430. [Google Scholar] [CrossRef][Green Version]
- Badran, W.A.; Fahmy, I.; Abdel-Megid, W.M.; Elder, K.; Mansour, R.; Kent-First, M. Length of androgen receptor-CAG repeats in fertile and infertile Egyptian men. J. Androl. 2009, 30, 416–425. [Google Scholar] [CrossRef]
- Khatami, S.R.; Galehdari, H.; Rasekh, A.; Mombeini, H.; Konar, E. Assessment of correlation between androgen receptor CAG repeat length and infertility in infertile men living in Khuzestan, Iran. Int. J. Fertil. Steril. 2015, 9, 189–196. [Google Scholar]
- Grigorova, M.; Punab, M.; Kahre, T.; Ivandi, M.; Tõnisson, N.; Poolamets, O.; Vihljajev, V.; Žilaitienė, B.; Erenpreiss, J.; Matulevičius, V.; et al. The number of CAG and GGN triplet repeats in the androgen receptor gene exert combinatorial effect on hormonal and sperm parameters in young men. Andrology 2017, 5, 495–504. [Google Scholar] [CrossRef]
- Thangaraj, K.; Joshi, M.B.; Reddy, A.G.; Gupta, N.J.; Chakravarty, B.; Singh, L. CAG repeat expansion in the androgen receptor gene is not associated with male infertility in Indian populations. J. Androl. 2002, 23, 815–818. [Google Scholar]
- Han, T.T.; Ran, J.; Ding, X.P.; Li, L.J.; Zhang, L.Y.; Zhang, Y.P.; Nie, S.S.; Chen, L. Cytogenetic and molecular analysis of infertile Chinese men: Karyotypic abnormalities, Y-chromosome microdeletions, and CAG and GGN repeat polymorphisms in the androgen receptor gene. Genet. Mol. Res. 2013, 12, 2215–2226. [Google Scholar] [CrossRef] [PubMed]
- Tufan, A.C.; Satiroglu-Tufan, N.L.; Aydinuraz, B.; Satiroglu, M.H.; Aydos, K.; Bagci, H. No association of the CAG repeat length in exon 1 of the androgen receptor gene with idiopathic infertility in Turkish men: Implications and literature review. Tohoku, J. Exp. Med. 2005, 206, 105–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Batiha, O.; Haifawi, S.; Al-Smadi, M.; Burghel, G.J.; Naber, Z.; Elbetieha, A.M.; Bodoor, K.; Al Sumadi, A.; Swaidat, S.; Jarun, Y.; et al. Molecular analysis of CAG repeat length of the androgen receptor gene and Y chromosome microdeletions among Jordanian azoospermic infertile males. Andrologia 2018, 50, e12979. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, P.; Bogaert, V.; De Bacquer, D.; Goemaere, S.; Zmierczak, H.; Kaufman, J.M. Part of the interindividual variation in serum testosterone levels in healthy men reflects differences in androgen sensitivity and feedback set point: Contribution of the androgen receptor polyglutamine tract polymorphism. J. Clin. Endocrinol. Metab. 2007, 92, 3604–3610. [Google Scholar] [CrossRef]
- Huhtaniemi, I.T.; Pye, S.R.; Limer, K.L.; Thomson, W.; O’Neill, T.W.; Platt, H.; Payne, D.; John, S.L.; Jiang, M.; Boonen, S.; et al. Increased estrogen rather than decreased androgen action is associated with longer androgen receptor CAG repeats. J. Clin. Endocrinol. Metab. 2009, 94, 277–284. [Google Scholar] [CrossRef]
- De Naeyer, H.; Bogaert, V.; De Spaey, A.; Roef, G.; Vandewalle, S.; Derave, W.; Taes, Y.; Kaufman, J.M. Genetic variations in the androgen receptor are associated with steroid concentrations and anthropometrics but not with muscle mass in healthy young men. PLoS ONE 2014, 9, e86235. [Google Scholar]
- Asatiani, K.; von Eckardstein, S.; Simoni, M.; Gromoll, J.; Nieschlag, E. CAG repeat length in the androgen receptor gene affects the risk of male infertility. Int. J. Androl. 2003, 26, 255–261. [Google Scholar] [CrossRef]
- Kamieniczna, M.; Fraczek, M.; Malcher, A.; Rozwadowska, N.; Czernikiewicz, A.; Jedrzejczak, P.; Semczuk, M.; Kurpisz, M. Semen quality, hormonal levels, and androgen receptor gene polymorphisms in a population of young male volunteers from two different regions of Poland. Med. Sci. Monit. 2015, 21, 2494–2504. [Google Scholar] [CrossRef]
- Khan, H.L.; Bhatti, S.; Abbas, S.; Khan, Y.L.; Aslamkhan, M.; Gonzalez, R.M.M.; Gonzalez, G.R.; Aydin, H.H.; Trinidad, M.S. Tri-nucleotide consortium of androgen receptor is associated with low serum FSH and testosterone in asthenospermic men. Syst. Biol. Reprod. Med. 2018, 64, 112–121. [Google Scholar] [CrossRef]
- Ryan, C.P.; McDade, T.W.; Gettler, L.T.; Eisenberg, D.T.; Rzhetskaya, M.; Hayes, M.G.; Kuzawa, C.W. Androgen receptor CAG repeat polymorphism and hypothalamic-pituitary-gonadal function in Filipino young adult males. Am. J. Hum. Biol. 2017, 29, e22897. [Google Scholar] [CrossRef]
- Emokpae, M.A.; Brown, S.I. Effects of lifestyle factors on fertility: Practical recommendations for modification. Reprod. Fertil. 2021, 2, R13–R26. [Google Scholar] [CrossRef] [PubMed]
- Leisegang, K.; Dutta, S. Do lifestyle practices impede male fertility? Andrologia 2021, 53, e13595. [Google Scholar] [CrossRef] [PubMed]
- Kittles, R.A.; Young, D.; Weinrich, S.; Hudson, J.; Argyropoulos, G.; Ukoli, F.; Adams-Campbell, L.; Dunston, G.M. Extent of linkage disequilibrium between the androgen receptor gene CAG and GGC repeats in human populations: Implications for prostate cancer risk. Hum. Genet. 2001, 109, 253–261. [Google Scholar] [CrossRef]
- O’Hara, L.; Smith, L.B. Androgen receptor roles in spermatogenesis and infertility. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 595–605. [Google Scholar] [CrossRef] [PubMed]
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The role of the epididymis and the contribution of epididymosomes to mammalian reproduction. Int. J. Mol. Sci. 2020, 21, 5377. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H. Androgen actions in the testis and the regulation of spermatogenesis. Adv. Exp. Med. Biol. 2021, 1288, 175–203. [Google Scholar]
- Sharestani, S.; Kalantar, S.M.; Ghasemi, N.; Farashahi Yazd, E. CAG repeat polymorphism in androgen receptor and infertility: A case-control study. Int. J. Reprod. Biomed. 2021, 19, 845–851. [Google Scholar] [CrossRef]
- Von Eckardstein, S.; Syska, A.; Gromoll, J.; Kamischke, A.; Simoni, M.; Nieschlag, E. Inverse correlation between sperm concentration and number of androgen receptor CAG repeats in normal men. J. Clin. Endocrinol. Metab. 2001, 86, 2585–2590. [Google Scholar] [CrossRef]
- Heemers, H.V.; Tindall, D.J. Androgen receptor (AR) coregulators: A diversity of functions converging on and regulating the AR transcriptional complex. Endocr. Rev. 2007, 28, 778–808. [Google Scholar] [CrossRef]
- Nenonen, H.A.; Giwercman, A.; Hallengren, E.; Giwercman, Y.L. Non-linear association between androgen receptor CAG repeat length and risk of male subfertility-a meta-analysis. Int. J. Androl. 2011, 34, 327–332. [Google Scholar] [CrossRef]
- Mikhaylenko, D.S.; Sobol, I.Y.; Efremov, E.A.; Apolikhin, O.I.; Tanas, A.S.; Alekseev, B.Y.; Nemtsova, M.V. Genetic forms of male infertility: Main characteristics and practical aspects of laboratory diagnostics. Exp. Clin. Urol. 2020, 1, 96–104. (In Russian) [Google Scholar] [CrossRef]
- Chernykh, V.B.; Rudneva, S.A.; Sorokina, T.M.; Shileyko, L.V.; Ostroumova, T.V.; Ermolaeva, S.A.; Kurilo, L.F.; Ryzhkova, O.P.; Bliznets, E.A.; Chukhrova, A.L.; et al. An influence of androgen receptor (AR) gene CAG-polymorphism on spermatogenesis in infertile men. Androl. Genit. Surg. 2015, 16, 55–61. (In Russian) [Google Scholar]
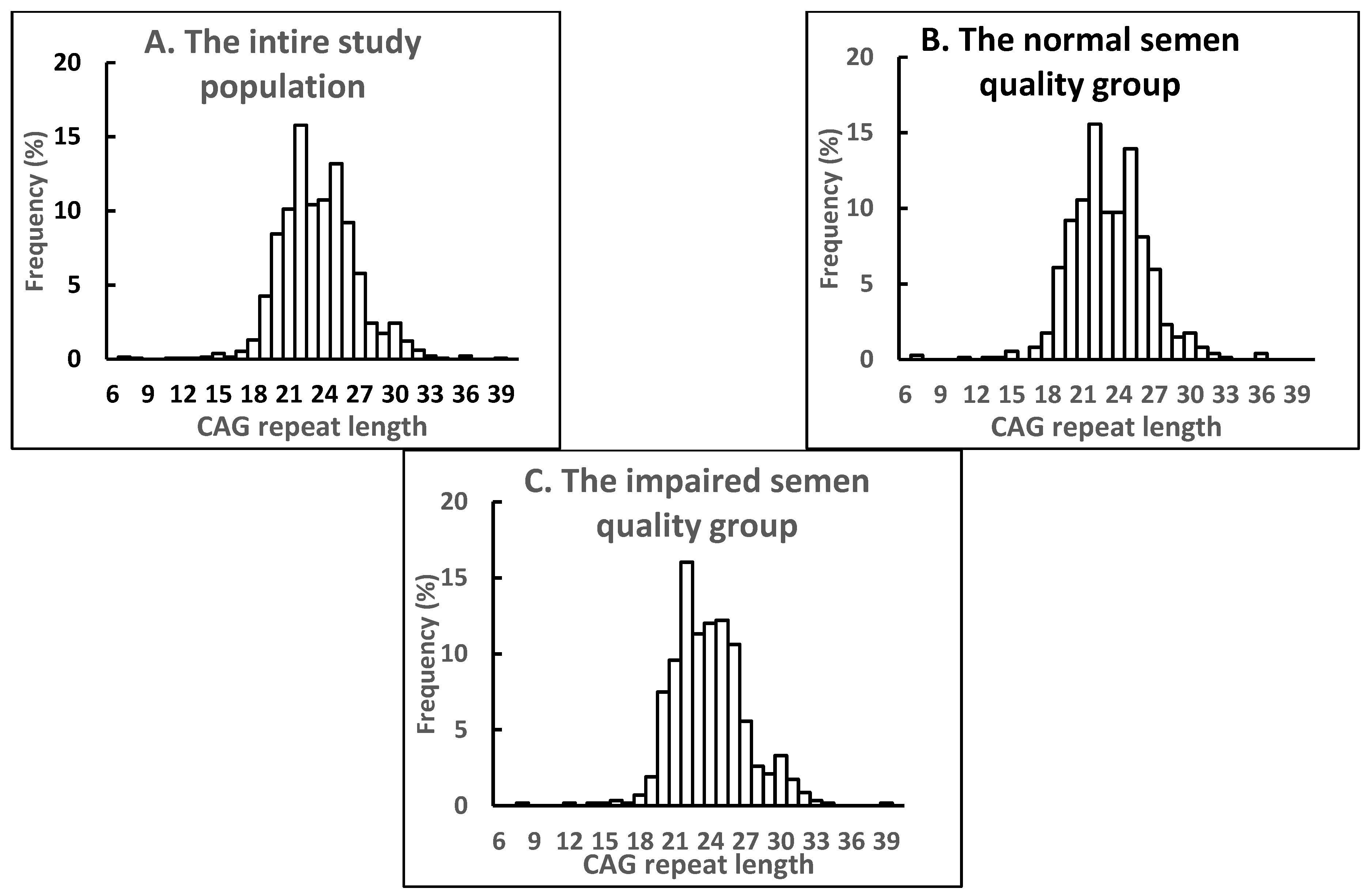
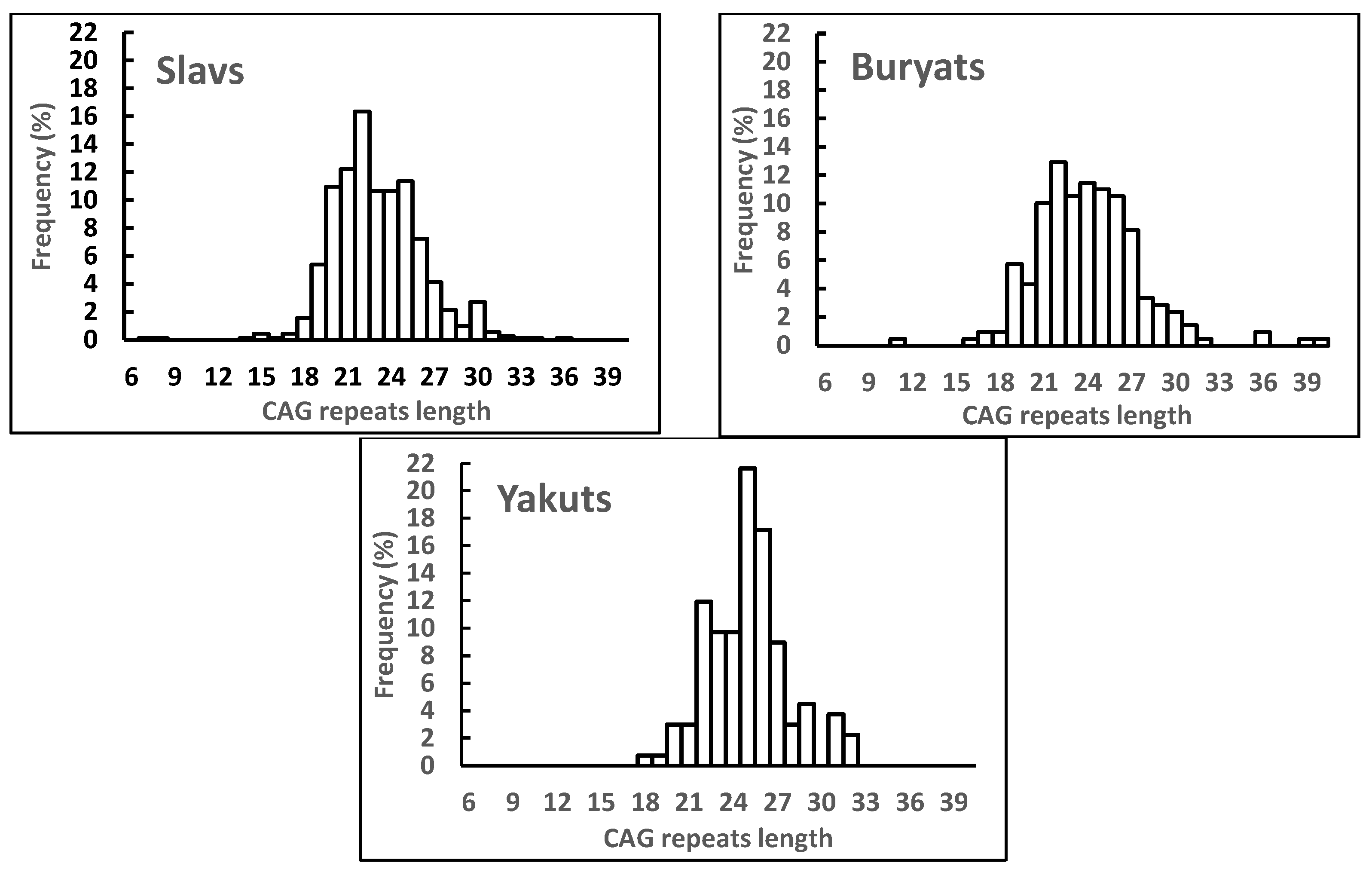
| Parameter | The Entire Russian Study Population (n = 1324) | Normal Semen Quality (n = 747) | Impaired Semen Quality (n = 577) | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | |
| Age (years) | 25.4 (7.1) | 23.0 (18–39) | 25.2 (7.0) | 23 (18–39) | 25.5 (7.4) | 23 (18–40) |
| Weight (kg) | 75.6 (14.0) | 73.6 (56.5–102.0) | 75.7 (13.9) | 74.0 (57.0–102.0) | 75.4 (14.3) | 74.0 (55.6–102.0) |
| Height (cm) | 177.1 (7.1) | 177 (165–189) | 177.5 (6.8) | 177.8 (166.7–188.0) | 176.6 (7.5) * | 176 (165–190) |
| BMI (kg/m2) | 24.1 (4.0) | 23.5 (18.7–31.4) | 24.0 (4.0) | 23.4 (18.5–31.3) | 24.1 (4.1) | 23.5 (18.8–31.4) |
| BTV (mL) | 39.0 (9.0) | 40 (25–54) | 40.5 (8.6) | 40 (28–56) | 37.1 (9.1) * | 36 (23–50) |
| Semen volume (mL) | 3.5 (1.6) | 3.3 (1.3–6.4) | 3.5 (1.6) | 3.3 (1.4–6.2) | 3.5 (1.6) | 3.3 (1.3–6.5) |
| Total sperm count (×106/ejaculate) | 169.7 (170.8) | 129.9 (7.8–441.6) | 237.4 (189.6) | 200.3 (61.4–488.6) | 82.5 (83.4) * | 57.3 (0.0–246.3) |
| Sperm concentration (×106/mL) | 49.3 (39.8) | 40.3 (3.4–129.5) | 69.5 (39.7) | 58.2 (25.7–147.4) | 23.1 (19.4) * | 18.8 (0–63.6) |
| Progressive motility (%) | 43.2 (26.7) | 40.7 (3.1–89.0) | 61.4 (18.1) | 60.5 (35.3–92.0) | 18.5 (13.0) * | 17.7 (1.3–41.8) |
| Normal morphology (%) | 6.6 (3.1) | 6.5 (2.0–11.8) | 8.2 (2.5) | 8.0 (4.8–13.0) | 4.4 (2.3) * | 4.0 (1.1–8.5) |
| LH (IU/L) | 3.6 (1.6) | 3.3 (1.5–6.4) | 3.4 (1.4) | 3.2 (1.5–6.0) | 3.9 (1.8) * | 3.6 (1.6–7.1) |
| FSH (IU/L) | 4.2 (3.1) | 3.6 (1.5–8.7) | 3.8 (2.0) | 3.5 (1.5–7.3) | 4.8 (4.0) * | 4.0 (1.5–10.3) |
| Testosterone (nmol/L) | 20.7 (7.6) | 19.7 (10.6–34.2) | 20.9 (7.4) | 20.1 (10.6–33.4) | 20.5 (7.9) | 19.0 (10.5–34.7) |
| Estradiol (nmol/L) | 0.21 (0.07) | 0.20 (0.12–0.32) | 0.20 (0.06) | 0.19 (0.11–0.32) | 0.21 (0.08) * | 0.20 (0.12–0.31) |
| Inhibin B (pg/mL) | 176.1 (66.5) | 169.9 (76.1–295.3) | 184.9 (64.8) | 173.3 (94.2–307.0) | 164.9 (67.1) * | 161.5 (55.3–277.6) |
| CAG repeat length | 23.5 (3.3) | 23 (19–29) | 23.2 (3.3) | 23 (19–29) | 23.9 (3.2) * | 24 (20–30) |
| Parameter | CAG Category (n = 1313) | ||
|---|---|---|---|
| CAG ≤ 19 (Short, n = 95) | 20 ≤ CAG ≤ 24 (Medium, n = 1023) | CAG ≥ 25 (Long, n = 195) | |
| Semen volume (mL) | 3.6 (1.6) | 3.5 (1.6) | 3.5 (1.6) |
| Total sperm count (×106/ejaculate) | 188.7 (131.5) a | 170.4 (167.7) ab | 158.6 (203.4) b |
| Sperm concentration (×106/mL) | 55.7 (40.1) a | 49.7 (39.6) ab | 44.8 (41.0) b |
| Progressive motility (%) | 51.3 (23.2) a | 43.1 (26.8) b | 39.9 (27.0) b |
| Normal morphology (%) | 7.9 (2.6) a | 6.5 (3.0) b | 6.4 (3.1) b |
| LH (IU/L) | 3.7 (1.6) | 3.6 (1.6) | 3.8 (1.7) |
| FSH (IU/L) | 4.7 (3.1) | 4.1 (3.9) | 4.4 (3.3) |
| Testosterone (nmol/L) | 19.1 (6.24) | 20.8 (7.7) | 21.3 (7.9) |
| Estradiol (nmol/L) | 0.19 (0.05) a | 0.21 (0.08) ab | 0.21 (0.06) b |
| Inhibin B (pg/mL) | 165.9 (64.2) | 178.5 (66.5) | 169.1 (68.0) |
| CAG Repeat Length | |||
|---|---|---|---|
| Ethnicity | n | Mean (SD) | Median (5–95) |
| Slavs | 697 | 23.0 ± 3.1 a | 23 (19–29) |
| Buryats | 208 | 24.0 ± 3.5 b | 24 (19–30) |
| Yakuts | 134 | 25.0 ± 2.7 c | 25 (21–31) |
| Parameter | Slavs (n = 697) | Buryats (n = 208) | Jakuts (n = 134) | |||
|---|---|---|---|---|---|---|
| Normal Semen Quality (n = 399) | Impaired Semen Quality (n = 298) | Normal Semen Quality (n = 130) | Impaired Semen Quality (n = 78) | Normal Semen Quality (n = 59) | Impaired Semen Quality (n = 75) | |
| Semen volume (mL) | 3.6 (1.7) | 3.7 (1.8) | 3.3 (1.4) | 3.1 (1.2) | 3.1 (1.3) | 3.1 (1.2) |
| Total sperm count (×106/ejaculate) | 262.7 (199.8) | 84.2 (85.8) * | 186.2 (135.3) | 63.2 (54.8) * | 152.4 (82.7) | 51.2 (50.6) * |
| Sperm concentration (×106/mL) | 76.4 (42.1) | 22.1 (18.8) * | 60.0 (37.4) | 20.2 (14.2) * | 52.2 (24.5) | 17.1 (13.9) * |
| Progressive motility (%) | 61.2 (18.3) | 15.6 (10.3) * | 63.1 (19.7) | 17.6 (10.8) * | 54.7 (15.4) | 18.1 (11.0) * |
| Normal morphology (%) | 8.4 (2.8) | 4.6 (2.4) * | 7.9 (2.4) | 5.0 (2.3) * | 6.2 (1.9) | 3.4 (1.9) * |
| LH (IU/L) | 3.3 (1.4) | 3.8 (1.7) * | 3.9 (1.5) | 4.1 (1.8) | 3.3 (1.4) | 3.8 (1.7) * |
| FSH (IU/L) | 3.5 (2.0) | 4.1 (3.6) + | 4.3 (2.0) | 5.4 (3.9) | 4.6 (1.7) | 5.8 (4.0) |
| Testosterone (nmol/L) | 21.2 (7.5) | 21.0 (8.1) | 19.0 (5.9) | 18.8 (6.8) | 20.9 (6.8) | 20.5 (7.9) |
| Estradiol (nmol/L) | 0.19 (0.07) | 0.20 (0.09) | 0.22 (0.06) | 0.24 (0.10) | 0.22 (0.05) | 0.23 (0.05) |
| Inhibin B (pg/mL) | 195.8 (65.4) | 173.4 (68.3) * | 152.5 (61.3) | 137.7 (65.8) | 167.1 (47.6) | 154.0 (53.9) |
| CAG repeats length | 22.8 (2.9) | 23.3 (3.3) * | 23.6 (3.4) | 24.6 (3.7) | 25.2 (2.8) | 24.7 (2.6) |
| Slavs (n = 697) | |||
|---|---|---|---|
| Parameter | CAG Category | ||
| CAG ≤ 19 (Short, n = 59) | 20 ≤ CAG ≤ 24 (Medium, n = 428) | CAG ≥ 25 (Long, n = 210) | |
| Semen volume (mL) | 3.7 (1.8) | 3.7 (1.7) | 3.7 (1.6) |
| Total sperm count (×106/ejaculate) | 200.4 (136.0) | 184.6 (149.9) | 196.7 (251.8) |
| Sperm concentration (×106/mL) | 58.9 (44.1) | 53.6 (42.2) | 54.0 (47.0) |
| Progressive motility (%) | 50.4 (23.4) a | 43.5 (27.6) ab | 40.8 (27.3) b |
| Normal morphology (%) | 7.7 (2.8) a | 7.0 (3.2) ab | 6.6 (3.3) b |
| LH (IU/L) | 3.4 (1.4) | 3.5 (1.6) | 3.7 (1.5) |
| FSH (IU/L) | 4.4 (3.1) | 3.7 (2.8) | 3.6 (1.9) |
| Testosterone (nmol/L) | 19.4 (6.4) | 21.3 (7.8) | 21.7 (7.8) |
| Estradiol (nmol/L) | 0.19 (0.06) | 0.20 (0.08) | 0.19 (0.06) |
| Inhibin B (pg/mL) | 169.2 (65.2) | 188.9 (67.0) | 183.2 (72.7) |
| Buryats (n = 208) | |||
| Parameter | CAG Category | ||
| CAG ≤ 20 (short, n = 27) | 21 ≤ CAG ≤ 27 (medium, n = 156) | CAG ≥ 28 (long, n = 25) | |
| Semen volume (mL) | 3.0 (1.1) | 3.2 (1.4) | 3.2 (1.3) |
| Total sperm count (×106/ejaculate) | 133.9 (97.3) | 146.0 (133.0) | 95.3 (91.0) |
| Sperm concentration (×106/mL) | 46.2 (28.7) a | 47.1 (38.1) ab | 30.1 (27.3) b |
| Progressive motility (%) | 52.4 (21.6) + | 46.6 (28.1) | 36.1 (28.7) + |
| Normal morphology (%) | 7.9 (2.6) a | 6.8 (2.7) ab | 6.3 (3.1) b |
| LH (IU/L) | 4.4 (1.8) | 3.8 (1.5) | 4.2 (2.0) |
| FSH (IU/L) | 5.6 (3.4) | 4.4 (2.2) | 5.5 (5.1) |
| Testosterone (nmol/L) | 17.7 (4.9) | 19.0 (6.2) | 19.1 (5.7) |
| Estradiol (nmol/L) | 0.21 (0.04) | 0.23 (0.08) | 0.25 (0.10) |
| Inhibin B (pg/mL) | 144.0 (65.6) | 149.2 (59.9) | 134.5 (79.4) |
| Yakuts (n = 134) | |||
| Parameter | CAG Category | ||
| CAG ≤ 21 (short, n = 10) | 22 ≤ CAG ≤ 27 (medium, n = 94) | CAG ≥ 28 (long, n = 30) | |
| Semen volume (mL) | 2.5 (0.7) | 3.1 (1.3) | 3.2 (1.4) |
| Total sperm count (×106/ejaculate) | 65.5 (62.1) | 102.0 (74.4) | 110.2 (117.4) |
| Sperm concentration (×106/mL) | 25.7 (24.0) | 35.7 (27.1) | 33.2 (25.3) |
| Progressive motility (%) | 29.0 (18.7) | 36.8 (23.6) | 38.3 (21.4) |
| Normal morphology (%) | 5.4 (2.0) | 4.7 (2.4) | 4.8 (2.3) |
| LH (IU/L) | 3.1 (1.2) | 3.6 (1.6) | 3.8 (1.6) |
| FSH (IU/L) | 4.6 (1.6) | 5.2 (3.2) | 5.5 (3.5) |
| Testosterone (nmol/L) | 20.6 (6.2) | 20.0 (6.7) | 23.0 (9.2) |
| Estradiol (nmol/L) | 0.22 (0.06) | 0.22 (0.05) | 0.23 (0.06) |
| Inhibin B (pg/mL) | 162.1 (53.7) | 156.7 (48.3) | 168.7 (59.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osadchuk, L.; Vasiliev, G.; Kleshchev, M.; Osadchuk, A. Androgen Receptor Gene CAG Repeat Length Varies and Affects Semen Quality in an Ethnic-Specific Fashion in Young Men from Russia. Int. J. Mol. Sci. 2022, 23, 10594. https://doi.org/10.3390/ijms231810594
Osadchuk L, Vasiliev G, Kleshchev M, Osadchuk A. Androgen Receptor Gene CAG Repeat Length Varies and Affects Semen Quality in an Ethnic-Specific Fashion in Young Men from Russia. International Journal of Molecular Sciences. 2022; 23(18):10594. https://doi.org/10.3390/ijms231810594
Chicago/Turabian StyleOsadchuk, Ludmila, Gennady Vasiliev, Maxim Kleshchev, and Alexander Osadchuk. 2022. "Androgen Receptor Gene CAG Repeat Length Varies and Affects Semen Quality in an Ethnic-Specific Fashion in Young Men from Russia" International Journal of Molecular Sciences 23, no. 18: 10594. https://doi.org/10.3390/ijms231810594
APA StyleOsadchuk, L., Vasiliev, G., Kleshchev, M., & Osadchuk, A. (2022). Androgen Receptor Gene CAG Repeat Length Varies and Affects Semen Quality in an Ethnic-Specific Fashion in Young Men from Russia. International Journal of Molecular Sciences, 23(18), 10594. https://doi.org/10.3390/ijms231810594







