Modulators of Wnt Signaling Pathway Implied in Dentin Pulp Complex Engineering: A Literature Review
Abstract
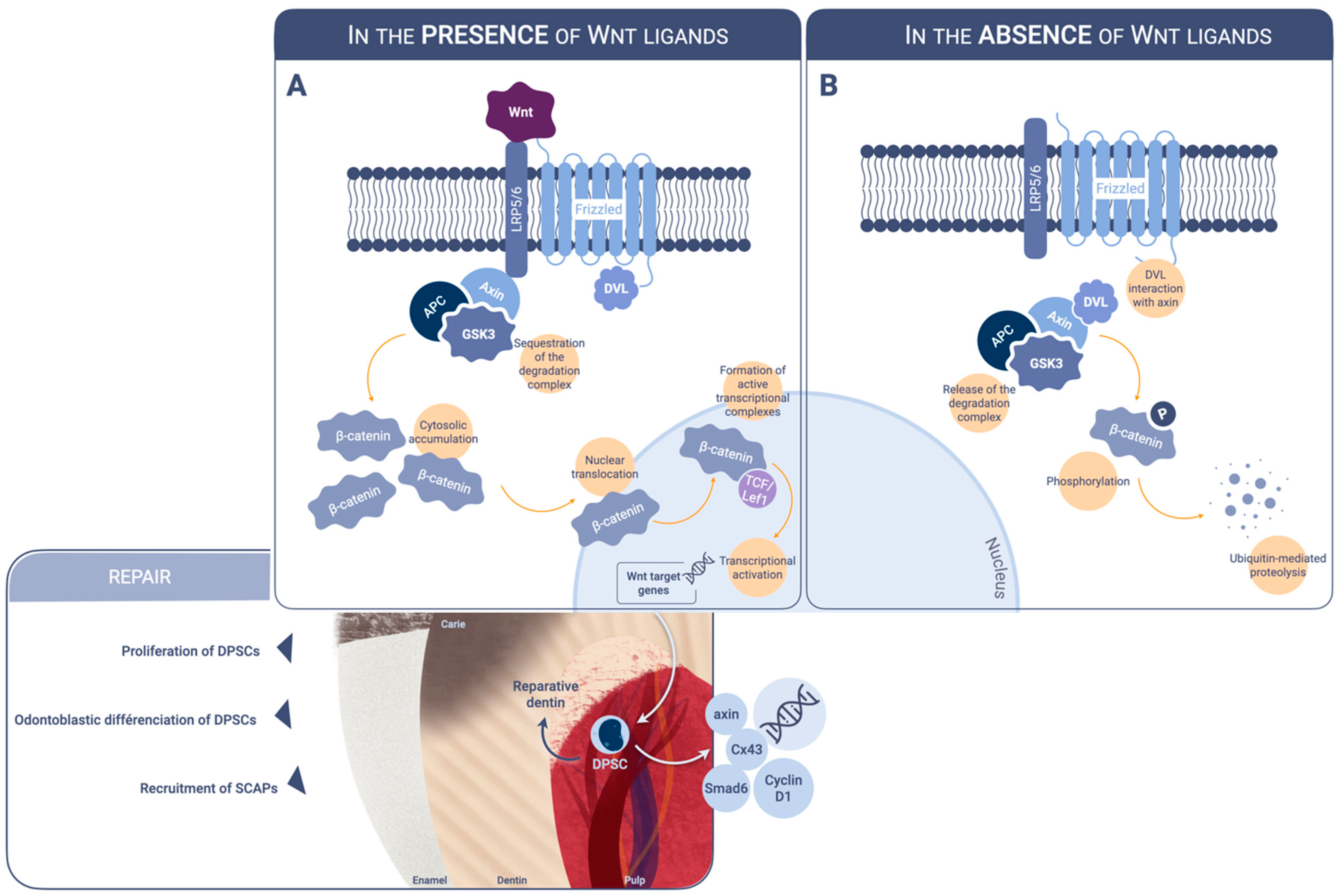
1. Introduction
2. Modulators of Wnt Beta-Catenin Signaling Acting on Dental Pulp Cells
2.1. Inorganic Calcium-Containing Materials
2.2. Small Molecule GSK3 Inhibitors
2.2.1. Tideglusib
2.2.2. NP928
2.2.3. Tivantinib
2.2.4. Lithium Chloride
2.3. R-Spondin 2
2.4. Wedelolactone
2.5. Semaphorin 3A and Its Receptor Neuropilin 1
2.6. Wnt3a Protein
2.7. Sclerostin
3. Cellular Metabolism Effect of Wnt
3.1. Epigenetic Remodeling in Human DPSCs under Wnt Ligant Exposure
3.2. Effect on Energetic Metabolism
3.2.1. Famotidine
3.2.2. Olanzapine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tziafas, D.; Economides, N. Formation of crystals on the surface of calcium hydroxide-containing materials in vitro. J Endod. 1999, 25, 539–542. [Google Scholar] [CrossRef]
- Schroder, U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J. Dent. Res. 1985, 64, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Njeh, A.; Uzunoglu, E. Is Pulp Inflammation a Prerequisite for Pulp Healing and Regeneration? Mediat. Inflamm. 2015, 2015, 347649. [Google Scholar] [CrossRef]
- Hilton, T.J. Keys to clinical success with pulp capping: A review of the literature. Oper. Dent. 2009, 34, 615–625. [Google Scholar] [CrossRef]
- Tran, X.V.; Gorin, C.; Willig, C.; Baroukh, B.; Pellat, B.; Decup, F.; Opsahl Vital, S.; Chaussain, C.; Boukpessi, T. Effect of a calcium-silicate-based restorative cement on pulp repair. J. Dent. Res. 2012, 91, 1166–1171. [Google Scholar] [CrossRef]
- Awawdeh, L.; Al-Qudah, A.; Hamouri, H.; Chakra, R.J. Outcomes of Vital Pulp Therapy Using Mineral Trioxide Aggregate or Biodentine: A Prospective Randomized Clinical Trial. J. Endod. 2018, 44, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Kuttler, Y. Classification of dentine into primary, secondary, and tertiary. Oral Surg. Oral Med. Oral Pathol. 1959, 12, 996–999. [Google Scholar] [CrossRef]
- Saito, K.; Nakatomi, M.; Ida-Yonemochi, H.; Ohshima, H. Osteopontin Is Essential for Type I Collagen Secretion in Reparative Dentin. J. Dent. Res. 2016, 95, 1034–1041. [Google Scholar] [CrossRef]
- Niehrs, C.; Acebron, S.P. Mitotic and mitogenic Wnt signalling. EMBO J. 2012, 31, 2705–2713. [Google Scholar] [CrossRef]
- Li, L.; Clevers, H. Coexistence of quiescent and active adult stem cells in mammals. Science 2010, 327, 542–545. [Google Scholar] [CrossRef]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef] [PubMed]
- Schulte, G. Frizzleds and WNT/beta-catenin signaling--The black box of ligand-receptor selectivity, complex stoichiometry and activation kinetics. Eur. J. Pharmacol. 2015, 763, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Couve, E.; Osorio, R.; Schmachtenberg, O. Reactionary Dentinogenesis and Neuroimmune Response in Dental Caries. J. Dent. Res. 2014, 93, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Babb, R.; Chandrasekaran, D.; Neves, V.C.M.; Sharpe, P.T. Axin2-expressing cells differentiate into reparative odontoblasts via autocrine Wnt/beta-catenin signaling in response to tooth damage. Sci. Rep. 2017, 7, 3102. [Google Scholar] [CrossRef]
- Scheller, E.L.; Chang, J.; Wang, C.Y. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J. Dent. Res. 2008, 87, 126–130. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, Q.; Tian, H.; Lv, P.; Zhou, C.; Gao, X. Effects of WNT10A on proliferation and differentiation of human dental pulp cells. J. Endod. 2014, 40, 1593–1599. [Google Scholar] [CrossRef]
- Han, N.; Zheng, Y.; Li, R.; Li, X.; Zhou, M.; Niu, Y.; Zhang, Q. beta-catenin enhances odontoblastic differentiation of dental pulp cells through activation of Runx2. PLoS ONE 2014, 9, e88890. [Google Scholar] [CrossRef]
- Kim, T.H.; Bae, C.H.; Lee, J.C.; Ko, S.O.; Yang, X.; Jiang, R.; Cho, E.S. beta-catenin is required in odontoblasts for tooth root formation. J. Dent. Res. 2013, 92, 215–221. [Google Scholar] [CrossRef]
- Yoshida, S.; Wada, N.; Hasegawa, D.; Miyaji, H.; Mitarai, H.; Tomokiyo, A.; Hamano, S.; Maeda, H. Semaphorin 3A Induces Odontoblastic Phenotype in Dental Pulp Stem Cells. J. Dent. Res. 2016, 95, 1282–1290. [Google Scholar] [CrossRef]
- Rahman, S.U.; Oh, J.H.; Cho, Y.D.; Chung, S.H.; Lee, G.; Baek, J.H.; Ryoo, H.M.; Woo, K.M. Fibrous Topography-Potentiated Canonical Wnt Signaling Directs the Odontoblastic Differentiation of Dental Pulp-Derived Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 17526–17541. [Google Scholar] [CrossRef]
- Yoshioka, S.; Takahashi, Y.; Abe, M.; Michikami, I.; Imazato, S.; Wakisaka, S.; Hayashi, M.; Ebisu, S. Activation of the Wnt/beta-catenin pathway and tissue inhibitor of metalloprotease 1 during tertiary dentinogenesis. J. Biochem. 2013, 153, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Deng, F.; Huang, E.; Yan, Z.; Wang, Z.; Deng, Y.; Zhang, Q.; Zhang, Z.; Ye, J.; et al. Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs). Biomaterials 2015, 39, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Semenov, M.; Han, C.; Baeg, G.H.; Tan, Y.; Zhang, Z.; Lin, X.; He, X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002, 108, 837–847. [Google Scholar] [CrossRef]
- Zeng, X.; Tamai, K.; Doble, B.; Li, S.; Huang, H.; Habas, R.; Okamura, H.; Woodgett, J.; He, X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 2005, 438, 873–877. [Google Scholar] [CrossRef]
- Coghlan, M.P.; Culbert, A.A.; Cross, D.A.; Corcoran, S.L.; Yates, J.W.; Pearce, N.J.; Rausch, O.L.; Murphy, G.J.; Carter, P.S.; Roxbee Cox, L.; et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 2000, 7, 793–803. [Google Scholar] [CrossRef]
- Sato, N.; Meijer, L.; Skaltsounis, L.; Greengard, P.; Brivanlou, A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2004, 10, 55–63. [Google Scholar] [CrossRef]
- Leone, A.; Volponi, A.A.; Renton, T.; Sharpe, P.T. In-Vitro regulation of odontogenic gene expression in human embryonic tooth cells and SHED cells. Cell Tissue Res. 2012, 348, 465–473. [Google Scholar] [CrossRef]
- Neves, V.C.; Babb, R.; Chandrasekaran, D.; Sharpe, P.T. Promotion of natural tooth repair by small molecule GSK3 antagonists. Sci. Rep. 2017, 7, 39654. [Google Scholar] [CrossRef]
- Zaugg, L.K.; Banu, A.; Walther, A.R.; Chandrasekaran, D.; Babb, R.C.; Salzlechner, C.; Hedegaard, M.A.B.; Gentleman, E.; Sharpe, P.T. Translation Approach for Dentine Regeneration Using GSK-3 Antagonists. J. Dent. Res. 2020, 99, 544–551. [Google Scholar] [CrossRef]
- Del Ser, T.; Steinwachs, K.C.; Gertz, H.J.; Andres, M.V.; Gomez-Carrillo, B.; Medina, M.; Vericat, J.A.; Redondo, P.; Fleet, D.; Leon, T. Treatment of Alzheimer’s disease with the GSK-3 inhibitor tideglusib: A pilot study. J. Alzheimers Dis. 2013, 33, 205–215. [Google Scholar] [CrossRef] [PubMed]
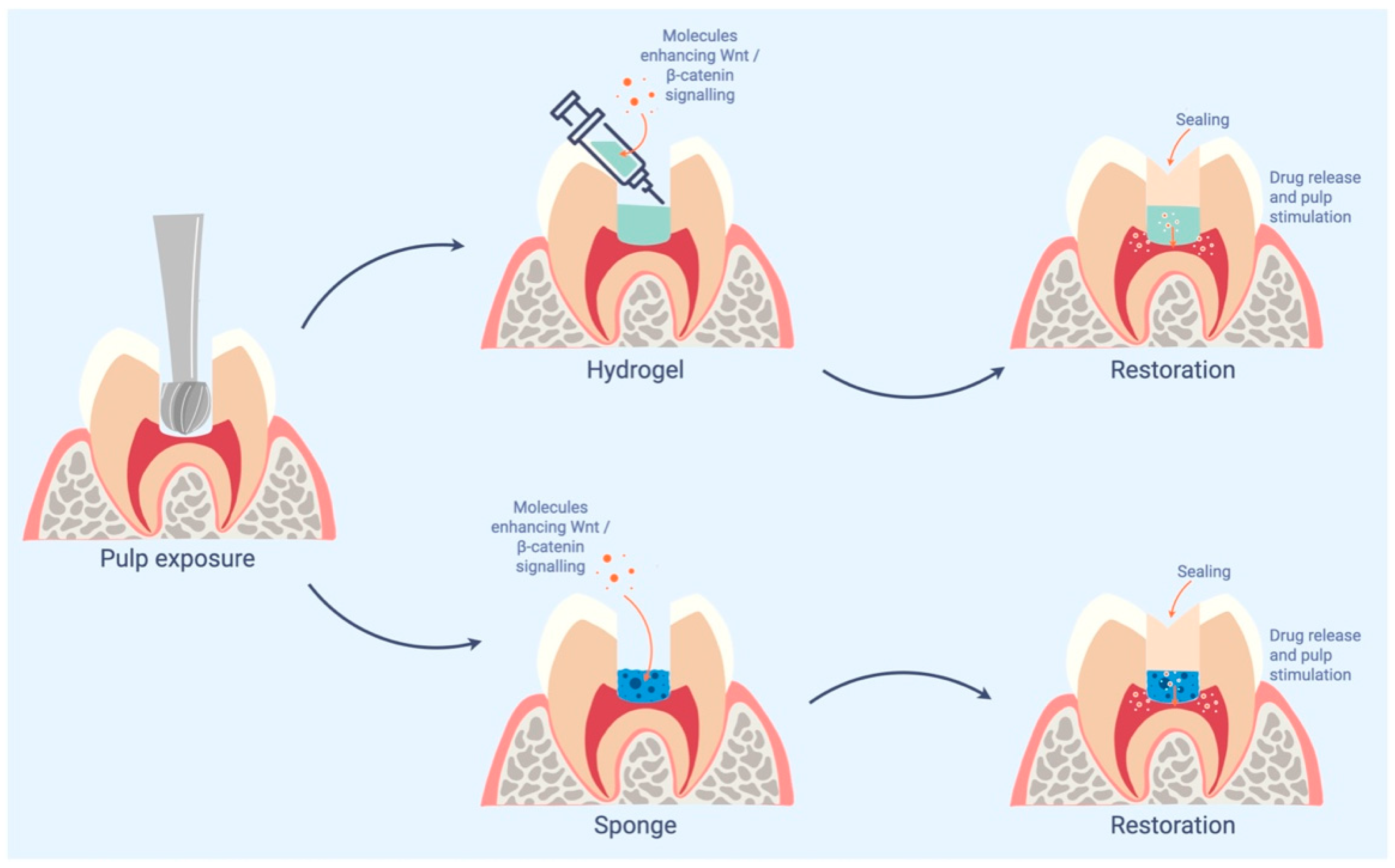
- Alaohali, A.; Salzlechner, C.; Zaugg, L.K.; Suzano, F.; Martinez, A.; Gentleman, E.; Sharpe, P.T. GSK3 Inhibitor-Induced Dentinogenesis Using a Hydrogel. J. Dent. Res. 2022, 101, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.; Jeay, S.; Li, Y.; Chen, C.R.; France, D.S.; Ashwell, M.A.; Hill, J.; Moussa, M.M.; Leggett, D.S.; Li, C.J. ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosine kinase with antitumor activity. Mol. Cancer Ther. 2010, 9, 1544–1553. [Google Scholar] [CrossRef]
- Kuenzi, B.M.; Remsing Rix, L.L.; Kinose, F.; Kroeger, J.L.; Lancet, J.E.; Padron, E.; Rix, U. Off-target based drug repurposing opportunities for tivantinib in acute myeloid leukemia. Sci. Rep. 2019, 9, 606. [Google Scholar] [CrossRef] [PubMed]
- Birjandi, A.A.; Suzano, F.R.; Sharpe, P.T. Drug Repurposing in Dentistry; towards Application of Small Molecules in Dentin Repair. Int. J. Mol. Sci. 2020, 21, 6394. [Google Scholar] [CrossRef] [PubMed]
- Shorter, E. The history of lithium therapy. Bipolar Disord. 2009, 11, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, X.; Qin, Y.; Wang, R.; Tang, J.; Cui, X.; Wang, T.; Liu, W.; Pan, H.; Li, B. Acceleration of bone regeneration by activating Wnt/beta-catenin signalling pathway via lithium released from lithium chloride/calcium phosphate cement in osteoporosis. Sci. Rep. 2017, 7, 45204. [Google Scholar] [CrossRef]
- Clement-Lacroix, P.; Ai, M.; Morvan, F.; Roman-Roman, S.; Vayssiere, B.; Belleville, C.; Estrera, K.; Warman, M.L.; Baron, R.; Rawadi, G. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 17406–17411. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, K.; Hayano, S.; Yanagita, T.; Kurosaka, H.; Kawanabe, N.; Itoh, S.; Ono, M.; Kuboki, T.; Kamioka, H.; Yamashiro, T. Topical application of lithium chloride on the pulp induces dentin regeneration. PLoS ONE 2015, 10, e0121938. [Google Scholar] [CrossRef]
- Ali, M.; Okamoto, M.; Komichi, S.; Watanabe, M.; Huang, H.; Takahashi, Y.; Hayashi, M. Lithium-containing surface pre-reacted glass fillers enhance hDPSC functions and induce reparative dentin formation in a rat pulp capping model through activation of Wnt/beta-catenin signaling. Acta Biomater. 2019, 96, 594–604. [Google Scholar] [CrossRef]
- Alaohali, A.; Brauer, D.S.; Gentleman, E.; Sharpe, P.T. A modified glass ionomer cement to mediate dentine repair. Dent. Mater. 2021, 37, 1307–1315. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Papachristou, E.; Koidis, P.; Geurtsen, W. Wnt/beta-catenin signaling regulates Dental Pulp Stem Cells’ responses to pulp injury by resinous monomers. Dent. Mater. 2015, 31, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Kazanskaya, O.; Glinka, A.; del Barco Barrantes, I.; Stannek, P.; Niehrs, C.; Wu, W. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev. Cell 2004, 7, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kim, K.A.; De Vera, J.; Palencia, S.; Wagle, M.; Abo, A. R-Spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical Wnt/beta-catenin pathway. Proc. Natl. Acad. Sci. USA 2009, 106, 2331–2336. [Google Scholar] [CrossRef]
- Sato, T.; van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; van den Born, M.; Barker, N.; Shroyer, N.F.; van de Wetering, M.; Clevers, H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011, 469, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Takata, N.; Abbey, D.; Fiore, L.; Acosta, S.; Feng, R.; Gil, H.J.; Lavado, A.; Geng, X.; Interiano, A.; Neale, G.; et al. An Eye Organoid Approach Identifies Six3 Suppression of R-spondin 2 as a Critical Step in Mouse Neuroretina Differentiation. Cell Rep. 2017, 21, 1534–1549. [Google Scholar] [CrossRef] [PubMed]
- Takegami, Y.; Ohkawara, B.; Ito, M.; Masuda, A.; Nakashima, H.; Ishiguro, N.; Ohno, K. R-spondin 2 facilitates differentiation of proliferating chondrocytes into hypertrophic chondrocytes by enhancing Wnt/beta-catenin signaling in endochondral ossification. Biochem. Biophys. Res. Commun. 2016, 473, 255–264. [Google Scholar] [CrossRef]
- Arima, M.; Hasegawa, D.; Yoshida, S.; Mitarai, H.; Tomokiyo, A.; Hamano, S.; Sugii, H.; Wada, N.; Maeda, H. R-spondin 2 promotes osteoblastic differentiation of immature human periodontal ligament cells through the Wnt/beta-catenin signaling pathway. J. Periodontal. Res. 2019, 54, 143–153. [Google Scholar] [CrossRef]
- Gong, Y.; Yuan, S.; Sun, J.; Wang, Y.; Liu, S.; Guo, R.; Dong, W.; Li, R. R-Spondin 2 Induces Odontogenic Differentiation of Dental Pulp Stem/Progenitor Cells via Regulation of Wnt/beta-Catenin Signaling. Front. Physiol. 2020, 11, 918. [Google Scholar] [CrossRef]
- Wang, C.; Song, Y.; Gu, Z.; Lian, M.; Huang, D.; Lu, X.; Feng, X.; Lu, Q. Wedelolactone Enhances Odontoblast Differentiation by Promoting Wnt/beta-Catenin Signaling Pathway and Suppressing NF-kappaB Signaling Pathway. Cell. Reprogram. 2018, 20, 236–244. [Google Scholar] [CrossRef]
- Hayashi, M.; Nakashima, T.; Taniguchi, M.; Kodama, T.; Kumanogoh, A.; Takayanagi, H. Osteoprotection by semaphorin 3A. Nature 2012, 485, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, X.; Feng, X.; Gu, Z.; Gu, Y.; Lian, M.; Xiao, J.; Cao, P.; Zheng, K.; Gu, X.; et al. NRP1 Accelerates Odontoblast Differentiation of Dental Pulp Stem Cells Through Classical Wnt/beta-Catenin Signaling. Cell. Reprogram. 2017, 19, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Vijaykumar, A.; Root, S.H.; Mina, M. Wnt/beta-Catenin Signaling Promotes the Formation of Preodontoblasts In Vitro. J. Dent. Res. 2021, 100, 387–396. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bardet, C.; Mouraret, S.; Liu, B.; Singh, G.; Sadoine, J.; Dhamdhere, G.; Smith, A.; Tran, X.V.; Joy, A.; et al. Wnt Acts as a Prosurvival Signal to Enhance Dentin Regeneration. J. Bone Miner Res. 2015, 30, 1150–1159. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef]
- Li, X.; Ominsky, M.S.; Niu, Q.T.; Sun, N.; Daugherty, B.; D’Agostin, D.; Kurahara, C.; Gao, Y.; Cao, J.; Gong, J.; et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J. Bone Miner Res. 2008, 23, 860–869. [Google Scholar] [CrossRef]
- Amri, N.; Djole, S.X.; Petit, S.; Babajko, S.; Coudert, A.E.; Castaneda, B.; Simon, S.; Berdal, A. Distorted Patterns of Dentinogenesis and Eruption in Msx2 Null Mutants: Involvement of Sost/Sclerostin. Am. J. Pathol. 2016, 186, 2577–2587. [Google Scholar] [CrossRef][Green Version]
- Naka, T.; Yokose, S. Spatiotemporal expression of sclerostin in odontoblasts during embryonic mouse tooth morphogenesis. J. Endod. 2011, 37, 340–345. [Google Scholar] [CrossRef]
- Collignon, A.M.; Amri, N.; Lesieur, J.; Sadoine, J.; Ribes, S.; Menashi, S.; Simon, S.; Berdal, A.; Rochefort, G.Y.; Chaussain, C.; et al. Sclerostin Deficiency Promotes Reparative Dentinogenesis. J. Dent. Res. 2017, 96, 815–821. [Google Scholar] [CrossRef]
- Satoh, A.; Brace, C.S.; Rensing, N.; Cliften, P.; Wozniak, D.F.; Herzog, E.D.; Yamada, K.A.; Imai, S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430. [Google Scholar] [CrossRef]
- Ou, Y.; Zhou, Y.; Liang, S.; Wang, Y. Sclerostin promotes human dental pulp cells senescence. PeerJ 2018, 6, e5808. [Google Scholar] [CrossRef]
- Liao, C.; Wang, Y.; Ou, Y.; Wu, Y.; Zhou, Y.; Liang, S. Effects of sclerostin on lipopolysaccharide-induced inflammatory phenotype in human odontoblasts and dental pulp cells. Int. J. Biochem. Cell Biol. 2019, 117, 105628. [Google Scholar] [CrossRef]
- Uribe-Etxebarria, V.; Luzuriaga, J.; Garcia-Gallastegui, P.; Agliano, A.; Unda, F.; Ibarretxe, G. Notch/Wnt cross-signalling regulates stemness of dental pulp stem cells through expression of neural crest and core pluripotency factors. Eur. Cell. Mater. 2017, 34, 249–270. [Google Scholar] [CrossRef]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Uribe-Etxebarria, V.; Garcia-Gallastegui, P.; Perez-Garrastachu, M.; Casado-Andres, M.; Irastorza, I.; Unda, F.; Ibarretxe, G.; Subiran, N. Wnt-3a Induces Epigenetic Remodeling in Human Dental Pulp Stem Cells. Cells 2020, 9, 652. [Google Scholar] [CrossRef]
- Asghari, M.; Nasoohi, N.; Hodjat, M. High glucose promotes the aging of human dental pulp cells through Wnt/beta-catenin signaling. Dent. Med. Probl. 2021, 58, 39–46. [Google Scholar] [CrossRef]
- Medak, K.D.; Townsend, L.K.; Hahn, M.K.; Wright, D.C. Female mice are protected against acute olanzapine-induced hyperglycemia. Psychoneuroendocrinology 2019, 110, 104413. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florimond, M.; Minic, S.; Sharpe, P.; Chaussain, C.; Renard, E.; Boukpessi, T. Modulators of Wnt Signaling Pathway Implied in Dentin Pulp Complex Engineering: A Literature Review. Int. J. Mol. Sci. 2022, 23, 10582. https://doi.org/10.3390/ijms231810582
Florimond M, Minic S, Sharpe P, Chaussain C, Renard E, Boukpessi T. Modulators of Wnt Signaling Pathway Implied in Dentin Pulp Complex Engineering: A Literature Review. International Journal of Molecular Sciences. 2022; 23(18):10582. https://doi.org/10.3390/ijms231810582
Chicago/Turabian StyleFlorimond, Marion, Sandra Minic, Paul Sharpe, Catherine Chaussain, Emmanuelle Renard, and Tchilalo Boukpessi. 2022. "Modulators of Wnt Signaling Pathway Implied in Dentin Pulp Complex Engineering: A Literature Review" International Journal of Molecular Sciences 23, no. 18: 10582. https://doi.org/10.3390/ijms231810582
APA StyleFlorimond, M., Minic, S., Sharpe, P., Chaussain, C., Renard, E., & Boukpessi, T. (2022). Modulators of Wnt Signaling Pathway Implied in Dentin Pulp Complex Engineering: A Literature Review. International Journal of Molecular Sciences, 23(18), 10582. https://doi.org/10.3390/ijms231810582







