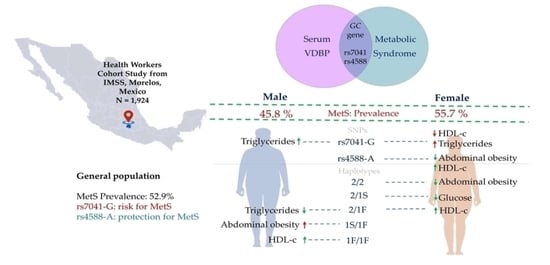
Unravelling the Contribution of the rs7041 and rs4588 Polymorphisms of the GC Gene and Serum VDBP Levels for Developing Metabolic Syndrome in the Mexican Population
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Sample
2.2. Genetic Association Analysis with MetS
2.3. Association between VDBP Serum Levels and Metabolic Syndrome
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Outcome Definition
4.3. Genotyping
4.4. Biochemical, Clinical, and Anthropometric Measures
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Eberly, L.E.; Prineas, R.; Cohen, J.D.; Vazquez, G.; Zhi, X.; Neaton, J.D.; Kuller, L.H. Metabolic Syndrome: Risk Factor Distribution and 18-Year Mortality in the Multiple Risk Factor Intervention Trial. Diabetes Care 2006, 29, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic Distribution of Metabolic Syndrome and Its Components in the General Adult Population: A Meta-Analysis of Global Data from 28 Million Individuals. Diabetes Res. Clin. Pract. 2022, 188. [Google Scholar] [CrossRef]
- Rojas-Martínez, R.; Aguilar-Salinas, C.A.; Romero-Martínez, M.; Castro-Porras, L.; Gómez-Velasco, D.; Mehta, R. Trends in the Prevalence of Metabolic Syndrome and Its Components in Mexican Adults, 2006-2018. Salud Publica Mex. 2021, 63, 713–724. [Google Scholar] [CrossRef]
- Bello-Chavolla, O.Y.; Vargas-Vázquez, A.; Antonio-Villa, N.E.; del Razo-Olvera, F.M.; Elías-López, D.; Aguilar-Salinas, C.A. A High Incidence of Metabolic Syndrome Traits in Mexicans Points at Obesity-Related Metabolic Dysfunction. Diabetes Metab. Syndr. Obes. 2021, 14, 1073–1082. [Google Scholar] [CrossRef]
- Prasad, G.; Bandesh, K.; Giri, A.K.; Kauser, Y.; Chanda, P.; Parekatt, V.; Mathur, S.; Madhu, S.V.; Venkatesh, P.; Bhansali, A.; et al. Genome-Wide Association Study of Metabolic Syndrome Reveals Primary Genetic Variants at CETP Locus in Indians. Biomolecules 2019, 9, 321. [Google Scholar] [CrossRef]
- Theik, N.W.Y.; Raji, O.E.; Shenwai, P.; Shah, R.; Kalluri, S.R.; Bhutta, T.H.; Hannoodee, H.; al Khalili, M.; Khan, S. Relationship and Effects of Vitamin D on Metabolic Syndrome: A Systematic Review. Cureus 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Kochhar, A. Interplay of Vitamin D and Metabolic Syndrome: A Review. Diabetes Metab. Syndr. 2016, 10, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemy, Z.; Shahdadian, F.; Moslemi, E.; Mirenayat, F.S.; Saneei, P. Serum Vitamin D Levels in Relation to Metabolic Syndrome: A Systematic Review and Dose-Response Meta-Analysis of Epidemiologic Studies. Obes. Rev. 2021, 22, e13223. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; García-Recio, E.; Luna-Bertos, E.; de Ruiz, C.; Illescas-Montes, R. Role of Vitamin D in the Metabolic Syndrome. Nutrients 2021, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Blum, C.B.; Ramakrishnan, R.; Dell, R.B.; Goodman, D.S. Turnover of the Plasma Binding Protein for Vitamin D and Its Metabolites in Normal Human Subjects. J. Clin. Endocrinol. Metab. 1981, 53, 1110–1116. [Google Scholar] [CrossRef]
- Kim, M.R.; Jeong, S.J. Relationship between Vitamin D Level and Lipid Profile in Non-Obese Children. Metabolites 2019, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Jassil, N.K.; Sharma, A.; Bikle, D.; Wang, X. Vitamin D binding protein and 25-hydroxyvitamin D levels: Emerging clinical applications. Endocr. Pract. 2017, 23, 605–613. [Google Scholar] [CrossRef]
- Gressner, O.A.; Gao, C.; Siluschek, M.; Kim, P.; Gressner, A.M. Inverse Association between Serum Concentrations of Actin-Free Vitamin D-Binding Protein and the Histopathological Extent of Fibrogenic Liver Disease or Hepatocellular Carcinoma. Eur. J. Gastroenterol. Hepatol. 2009, 21, 990–995. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10. [Google Scholar] [CrossRef]
- Rivera-Paredez, B.; Hidalgo-Bravo, A.; León-Reyes, G.; Antuna-Puente, B.; Flores, Y.N.; Salmerón, J.; Velázquez-Cruz, R. Association of GC Variants with Bone Mineral Density and Serum VDBP Concentrations in Mexican Population. Genes 2021, 12, 1176. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, D.; Liu, Y.; Feng, M.; Xu, Z.; Huang, H.; Zhang, L.; Li, W.; Li, X. The Association Between GC Gene Polymorphisms and Metabolic Syndrome in Chinese Rural Population: A Case-Control Study. Diabetes Metab. Syndr. Obes. 2022, 15, 165–174. [Google Scholar] [CrossRef]
- Karuwanarint, P.; Phonrat, B.; Tungtrongchitr, A.; Suriyaprom, K.; Chuengsamarn, S.; Schweigert, F.J.; Tungtrongchitr, R. Vitamin D-Binding Protein and Its Polymorphisms as a Predictor for Metabolic Syndrome. Biomark. Med. 2018, 12, 465–473. [Google Scholar] [CrossRef]
- Powe, C.E.; Evans, M.K.; Wenger, J.; Zonderman, A.B.; Berg, A.H.; Nalls, M.; Tamez, H.; Zhang, D.; Bhan, I.; Karumanchi, S.A.; et al. Vitamin D-Binding Protein and Vitamin D Status of Black Americans and White Americans. N. Engl. J. Med. 2013, 369, 1991–2000. [Google Scholar] [CrossRef]
- Lauridsen, A.L.; Vestergaard, P.; Hermann, A.P.; Brot, C.; Heickendorff, L.; Mosekilde, L.; Nexo, E. Plasma Concentrations of 25-Hydroxy-Vitamin D and 1,25-Dihydroxy-Vitamin D Are Related to the Phenotype of Gc (Vitamin D-Binding Protein): A Cross-Sectional Study on 595 Early Postmenopausal Women. Calcif. Tissue Int. 2005, 77, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Paredez, B.; Hidalgo-Bravo, A.; León-Reyes, G.; León-Maldonado, L.S.; Aquino-Gálvez, A.; Castillejos-López, M.; Denova-Gutiérrez, E.; Flores, Y.N.; Salmerón, J.; Velázquez-Cruz, R. Total, Bioavailable, and Free 25-Hydroxyvitamin D Equally Associate with Adiposity Markers and Metabolic Traits in Mexican Adults. Nutrients 2021, 13, 3320. [Google Scholar] [CrossRef]
- Bland, R.; Markovic, D.; Hills, C.E.; Hughes, S.V.; Chan, S.L.F.; Squires, P.E.; Hewison, M. Expression of 25-Hydroxyvitamin D3-1alpha-Hydroxylase in Pancreatic Islets. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, M.M.; Speeckaert, R.; van Geel, N.; Delanghe, J.R. Vitamin D Binding Protein: A Multifunctional Protein of Clinical Importance. Adv. Clin. Chem. 2014, 63, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Michos, E.D.; Misialek, J.R.; Pankow, J.S.; Loehr, L.; Selvin, E.; Reis, J.P.; Gross, M.; Eckfeldt, J.H.; Folsom, A.R. Race and Vitamin D Binding Protein Gene Polymorphisms Modify the Association of 25-Hydroxyvitamin D and Incident Heart Failure: The ARIC (Atherosclerosis Risk in Communities) Study. JACC Heart Fail. 2015, 3, 347–356. [Google Scholar] [CrossRef]
- Tarighi, S.; Najafi, M.; Hossein-Nezhad, A.; Ghaedi, H.; Meshkani, R.; Moradi, N.; Fadaei, R.; Kazerouni, F.; Shanaki, M. Association Between Two Common Polymorphisms of Vitamin D Binding Protein and the Risk of Coronary Artery Disease: A Case-Control Study. J. Med. Biochem. 2017, 36, 349–357. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daffara, V.; Verdoia, M.; Rolla, R.; Nardin, M.; Marino, P.; Bellomo, G.; Carriero, A.; de Luca, G. Impact of Polymorphism Rs7041 and Rs4588 of Vitamin D Binding Protein on the Extent of Coronary Artery Disease. Nutr. Metab Cardiovasc. Dis. 2017, 27, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Rozmus, D.; Ciesielska, A.; Płomiński, J.; Grzybowski, R.; Fiedorowicz, E.; Kordulewska, N.; Savelkoul, H.; Kostyra, E.; Cieślińska, A. Vitamin D Binding Protein (VDBP) and Its Gene Polymorphisms-The Risk of Malignant Tumors and Other Diseases. Int J. Mol. Sci. 2020, 21, 7822. [Google Scholar] [CrossRef]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Bouillon, R.; Vanbaelen, H.; Demoor, P. 25-Hydroxyvitamin D and Its Binding Protein in Maternal and Cord Serum. J. Clin. Endocrinol. Metab. 1977, 45, 679–684. [Google Scholar] [CrossRef]
- Li, S.; Hu, L.; Zhang, C. Urinary Vitamin D-Binding Protein as a Marker of Ovarian Reserve. Reprod Biol. Endocrinol. 2021, 19, 80. [Google Scholar] [CrossRef]
- Speeckaert, M.M.; Taes, Y.E.; de Buyzere, M.L.; Christophe, A.B.; Kaufman, J.M.; Delanghe, J.R. Investigation of the Potential Association of Vitamin D Binding Protein with Lipoproteins. Ann. Clin. Biochem. 2010, 47, 143–150. [Google Scholar] [CrossRef]
- Setayesh, L.; Amini, A.; Bagheri, R.; Moradi, N.; Yarizadeh, H.; Asbaghi, O.; Casazza, K.; Yekaninejad, M.S.; Wong, A.; Suzuki, K.; et al. Elevated Plasma Concentrations of Vitamin D-Binding Protein Are Associated with Lower High-Density Lipoprotein and Higher Fat Mass Index in Overweight and Obese Women. Nutrients 2021, 13, 3223. [Google Scholar] [CrossRef]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P.S. Macrophage-Mediated Inflammation in Metabolic Disease. Nat. Rev. Immunol 2011, 11, 738–749. [Google Scholar] [CrossRef]
- Yamamoto, N.; Homma, S. Vitamin D3 Binding Protein (Group-Specific Component) Is a Precursor for the Macrophage-Activating Signal Factor from Lysophosphatidylcholine-Treated Lymphocytes. Proc. Natl. Acad. Sci. USA 1991, 88, 8539–8543. [Google Scholar] [CrossRef]
- Borges, C.R.; Jarvis, J.W.; Oran, P.E.; Nelson, R.W. Population Studies of Vitamin D Binding Protein Microheterogeneity by Mass Spectrometry Lead to Characterization of Its Genotype-Dependent O-Glycosylation Patterns. J. Proteome Res. 2008, 7, 4143–4153. [Google Scholar] [CrossRef]
- Denova-Gutiérrez, E.; Flores, Y.N.; Gallegos-Carrillo, K.; Ramírez-Palacios, P.; Rivera-Paredez, B.; Muñoz-Aguirre, P.; Velázquez-Cruz, R.; Torres-Ibarra, L.; Meneses-León, J.; Méndez-Hernández, P.; et al. Health Workers Cohort Study: Methods and Study Design. Salud Publica Mex. 2016, 58, 708–716. [Google Scholar] [CrossRef]
- Freeman, J.; Wilson, K.; Spears, R.; Shalhoub, V.; Sibley, P. Performance Evaluation of Four 25-Hydroxyvitamin D Assays to Measure 25-Hydroxyvitamin D2. Clin. Biochem. 2015, 48, 1097–1104. [Google Scholar] [CrossRef]
- Martínez-Aguilar, M.M.; Aparicio-Bautista, D.I.; Ramírez-Salazar, E.G.; Reyes-Grajeda, J.P.; de la Cruz-Montoya, A.H.; Antuna-Puente, B.; Hidalgo-Bravo, A.; Rivera-Paredez, B.; Ramírez-Palacios, P.; Quiterio, M.; et al. Serum Proteomic Analysis Reveals Vitamin D-Binding Protein (VDBP) as a Potential Biomarker for Low Bone Mineral Density in Mexican Postmenopausal Women. Nutrients 2019, 11, 2853. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; López-Fontana, C.; Varo, J.J.; Sánchez-Villegas, A.; Martinez, J.A. Validation of the Spanish Version of the Physical Activity Questionnaire Used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef]
- Global Recommendations on Physical Activity for Health—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26180873/ (accessed on 2 August 2022).
- Hernández-Avila, M.; Romieu, I.; Parra, S.; Hernández-Avila, J.; Madrigal, H.; Willett, W. Validity and Reproducibility of a Food Frequency Questionnaire to Assess Dietary Intake of Women Living in Mexico City. Salud Publica Mex. 1998, 40, 133–140. [Google Scholar] [CrossRef]
- Hernández-Ávila, J.E.; González-Avilés, L.; Rosales-Mendoza, E. Manual de Usuario. SNUT Sistema de Evaluación de Hábitos Nutricionales y Consumo de Nutrimentos; Instituto Nacional de Salud Pública: Cuernavaca, Mexico, 2003. [Google Scholar]
| Total | Men | Women | ||||
|---|---|---|---|---|---|---|
| Without MetS | MetS | Without MetS | MetS | Without MetS | MetS | |
| n = 907 | n = 1017 | n = 317 | n = 268 | n = 590 | n = 749 | |
| Ages, (years) a | 45 (33–56) | 56 (48–65) * | 43 (33–54) | 51 (42–59) * | 47 (33–57) | 57 (50–66) * |
| Women, % | 65.1 | 73.7 * | - | - | - | - |
| Physical activity, % | ||||||
| Active | 38.8 | 31.4 * | 44.2 | 39.2 | 35.9 | 28.6 * |
| Smoking status, % | ||||||
| Past, % | 27.7 | 27.6 | 37.5 | 41.4 | 22.4 | 22.7 |
| Current, % | 12.7 | 12.1 | 21.1 | 20.5 | 8.1 | 9.1 |
| Vitamin D intake (UI/day) a | 152.7 (86.9–259.3) | 137.0 * (78.3–184.3) | 154.3 (83.2–264.5) | 129.1 (70.9–246.3) | 152.2 (89.5–255.7) | 139.6 (82.0–242.4) |
| Vitamin D levels (ng/mL) a | 22.3 (18–26.6) | 20.5 * (16.4–24.4) | 23 (19–28.3) | 21.3 * (17.1–25.7) | 21.9 (17.3–25.9) | 20.2 * (16.3–23.9) |
| VDBP (µmol/L) a | 270.2 (233.3–316) | 272.2 (229.2–316.1) | 267.2 (231.8–304.5) | 261.7 (223.6–303.6) | 272.2 (235.9–318.3) | 276.7 (232.1–318.8) |
| rs7041, % | ||||||
| TT | 29.3 | 26.0 | 28.7 | 22.4 | 29.5 | 27.2 |
| TG | 49.3 | 49.9 | 48.9 | 57.5 * | 49.6 | 47.1 |
| GG | 21.4 | 24.2 | 22.4 | 20.2 | 20.9 | 25.6 * |
| T | 53.9 | 50.9 | 53.2 | 51.1 | 54.3 | 50.8 |
| G | 46.1 | 49.1 | 46.9 | 48.9 | 45.7 | 49.2 |
| rs4588, % | ||||||
| CC | 60.0 | 63.2 | 62.5 | 63.4 | 58.7 | 63.2 |
| CA | 36.8 | 34.2 | 34.7 | 34.0 | 37.9 | 34.3 |
| AA | 3.2 | 2.6 | 2.8 | 2.6 | 3.4 | 2.5 |
| C | 78.4 | 80.0 | 79.8 | 80.4 | 77.7 | 80.3 |
| A | 21.6 | 19.7 | 20.2 | 19.6 | 22.3 | 19.7 |
| Diplotype, % | ||||||
| 1S/1S | 20.9 | 23.6 | 22.2 | 19.9 | 20.1 | 25.0 * |
| 1S/1F | 29.7 | 29.6 | 31.1 | 36.3 | 28.9 | 27.1 |
| 1F/1F | 10.0 | 10.6 | 9.5 | 7.5 | 10.3 | 11.7 |
| 2/2 | 3.0 | 2.5 | 2.5 | 2.6 | 3.3 | 2.4 |
| 2/1F | 16.5 | 13.1 * | 16.8 | 12.4 | 16.3 | 13.4 |
| 2/1S | 20.0 | 20.6 | 17.8 | 21.4 | 21.1 | 20.4 |
| Diet | ||||||
| Energy, (kcal/day) a | 1809 (1396–2392) | 1687 * (1237–2231) | 2026 (1554–2591) | 1852 * (1393–2504) | 1733 (1336–2319) | 1630 * (1198–2125) |
| Carbohydrate, (% energy) a | 65.3 (59.2–70.7) | 66.4 * (60.3–71.9) | 64.0 (58.5–70.1) | 65.1 (57.5–70.7) | 66.3 (59.7–71.0) | 66.9 * (61.3–72.6) |
| Protein, (% energy) a | 12.3 (10.6–14.0) | 12.6 * (11.0–14.4) | 12.3 (10.5–13.9) | 12.4 (10.8–14.3) | 12.3 (10.6–14.1) | 12.7 * (11.2–14.4) |
| Total fat, (% energy) a | 21.1 (17.3–21.5) | 20.5 * (17.0–24.3) | 20.8 (16.9–24.8) | 20.4 (16.7–25.2) | 21.3 (17.5–25.3) | 20.5 * (17.3–24.1) |
| Vitamin D intake (UI/day) a | 152.7 (86.9–259.3) | 137.0 * (78.3–243.0) | 154.3 (83.2–2645) | 129.1 (70.9–246.3) | 152.2 (89.5–255.7) | 139.6 (82.0–242.4) |
| Total | Men | Women | ||||
|---|---|---|---|---|---|---|
| OR (95%CI) | p Value | OR (95%CI) | p Value | OR (95%CI) | p Value | |
| rs7041 | ||||||
| TT | Ref. | Ref. | Ref. | |||
| TG | 1.24 (0.98–1.57) | 0.069 | 1.52 (0.99–2.30) | 0.051 | 1.15 (0.87–1.53) | 0.332 |
| GG | 1.37 (1.04–1.80) | 0.027 | 1.23 (0.73–2.05) | 0.435 | 1.45 (1.04–2.02) | 0.029 |
| TT | Ref. | Ref. | Ref. | |||
| TG + GG | 1.27 (1.03–1.59) | 0.027 | 1.43 (0.96–2.13) | 0.080 | 1.24 (0.95–1.62) | 0.106 |
| TT + TG | Ref. | Ref. | Ref. | |||
| GG | 1.18 (0.94–1.50) | 0.144 | 0.92 (0.60–1.41) | 0.707 | 1.33 (1.00–1.76) | 0.051 |
| rs4588 | ||||||
| CC | Ref. | Ref. | Ref. | |||
| CA | 0.81 (0.66–0.99) | 0.049 | 0.88 (0.61–1.27) | 0.480 | 0.79 (0.61–1.01) | 0.064 |
| AA | 0.57 (0.31–1.00) | 0.052 | 0.67 (0.23–1.96) | 0.465 | 0.50 (0.25–1.02) | 0.057 |
| CC | Ref. | Ref. | Ref. | |||
| CA + AA | 0.79 (0.65–0.97) | 0.023 | 0.86 (0.60–1.23) | 0.411 | 0.76 (0.60–0.97) | 0.030 |
| CC + CA | Ref. | Ref. | Ref. | |||
| AA | 0.60 (0.34–1.08) | 0.090 | 0.71 (0.24–2.05) | 0.522 | 0.55 (0.27–1.11) | 0.096 |
| Diplotype | ||||||
| 1S/1S | Ref. | Ref. | Ref. | |||
| 1S/1F | 0.90 (0.69–1.19) | 0.471 | 1.28 (0.79–2.07) | 0.319 | 0.77 (0.55–1.08) | 0.135 |
| 1F/1F | 0.98 (0.68–1.42) | 0.923 | 0.98 (0.48–2.00) | 0.952 | 0.95 (0.61–1.47) | 0.807 |
| 2/2 | 0.55 (0.29–1.03) | 0.062 | 0.95 (0.30–2.96) | 0.929 | 0.42 (0.20–0.90) | 0.025 |
| 2/1F | 0.62 (0.44–0.86) | 0.004 | 0.72 (0.40–1.31) | 0.285 | 0.57 (0.38–0.86) | 0.007 |
| 2/1S | 0.90 (0.69–1.22) | 0.514 | 1.24 (0.72–2.14) | 0.435 | 0.79 (0.55–1.14) | 0.215 |
| Total | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VDBP (µmol/L) | VDBP (µmol/L) | VDBP (µmol/L) | |||||||
| Low | Medium | High | Low | Medium | High | Low | Medium | High | |
| n = 642 | n = 641 | n = 641 | n = 195 | n = 195 | n = 195 | n = 447 | n = 446 | n = 446 | |
| <246.3 | 246.3–<299.5 | >299.5 | <241.2 | 241.2–<289.7 | >289.7 | <246.4 | 246.4–<304 | >304 | |
| Ages, (years) a | 53 (43–64) | 50 (39–61) | 52 * (40–61) | 48 (37–58) | 44 (36–55) | 46 (35–56) | 55 (46–66) | 53 (42–63) | 53 * (41–61) |
| BMI (Kg/m2) a | 26.6 (24.1–29.7) | 26.9 (24.2–30) | 26.7 (23.7–29.6) | 26.5 (24.2–29.1) | 27 (24.7–29.4) | 26.3 (23.6–28.8) | 26.8 (24.1–29.9) | 26.9 (24–30.3) | 26.9 (23.9–30.1) |
| Women, % | 65.7 | 67.9 | 75.2 * | - | - | - | - | - | - |
| Physical activity, % | |||||||||
| Active | 35.1 | 35.7 | 33.9 | 39.5 | 44.6 | 41.5 | 32.2 | 31.2 | 32.1 |
| Smoking status, % | |||||||||
| Past, % | 27.6 | 27.9 | 27.5 | 38 | 38 | 42 | 21.5 | 22.2 | 24 |
| Current, % | 14.0 | 11.5 | 11.5 | 21 | 21 | 20.5 | 10.3 | 7.2 | 8.5 |
| VD intake (UI/day) a | 144.6 (84–252.7) | 147.3 (85.3–260.4) | 137.2 (80.7–239.4) | 141.6 (70.1–271) | 147.3 (75.8–261.3) | 145.2 (78.8–243.7) | 149.6 (89.9–246) | 146.9 (87.1–255.9) | 133.8 (81–240) |
| VD levels (ng/mL) a | 20.9 (17.1–24.7) | 21.7 (17.7–25.8) | 21 (16.8–25.6) | 22.3 (18.5–26.9) | 22.2 (18.5–27.2) | 21.8 (17.6–27.4) | 20.4 (16.3–23.8) | 21.3 (17.2–25.4) | 20.8 (16.6–24.9) |
| MetS, % | 53.6 | 51.0 | 54.0 | 49.2 | 42.6 | 45.6 | 55.0 | 56.1 | 56.7 |
| WC (cm) a | 94 (87–102) | 94 (87–102) | 93 (85–99) * | 97 (90–104) | 96 (91–102) | 95 (88–101) | 93 (85–101) | 92 (85–101) | 92 (85–99) |
| Systolic BP, (mmHg) a | 119 (198–131) | 118 (108–129) | 116 (106–128) * | 122 (113–132) | 121 (114–130) | 120 (111–131) | 117 (107–131) | 117 (106–128) | 114 (105–127) * |
| Diastolic BP, (mmHg) a | 74 (67–81) | 74 (68–81) | 74 (68–80) | 76 (70–83) | 78 (71–84) | 77 (69–85) | 72 (65–79) | 73 (66–80) | 73 (67–79) |
| FPG (mg/dL) a | 97 (91–106) | 96 (90–105) | 97 (90–106) | 98 (91–107) | 98 (92–106) | 99 (92–109) | 97 (91–105) | 95 (89–104) | 97 (90–105) |
| Triglycerides (mg/dL) a | 147 (108–203) | 154 (109–210) | 163 (119–212) * | 167 (116–241) | 169 (113–244) | 169 (126–256) | 141 (105–189) | 153 (109–204) | 163 (117–207) * |
| HDL-c (mg/dL) a | 43 (36–51) | 44 (37.51) | 45 (39–53) * | 39 (34–45) | 40 (34–45) | 40 (35–48) * | 45 (39–54) | 46 (39–54) | 46 (40–54) * |
| rs7041 | |||||||||
| TT | 32.7 | 27.6 | 22.2 * | 33.3 | 23.1 | 21 * | 32.9 | 29 | 22.9 * |
| TG | 46.7 | 50.3 | 51.8 | 47.2 | 55.9 | 55.4 | 46.1 | 49.4 | 49.1 |
| GG | 20.6 | 22 | 26.0 * | 19.5 | 21 | 23.6 | 21.0 | 21.6 | 28.0 * |
| T | 56.1 | 52.8 | 48.1* | 56.9 | 51 | 48.7 * | 55.9 | 53.7 | 47.4 * |
| G | 43.9 | 47.2 | 51.9 * | 43.1 | 49 | 51.3 * | 44.1 | 46.3 | 52.6 * |
| rs4588 | |||||||||
| CC | 52.3 | 64.7 | 68.1 * | 55.4 | 65.1 | 68.2 * | 51.2 | 63.2 | 69.2 * |
| CA | 43.3 | 33.2 | 29.7 * | 40.5 | 32.8 | 29.7 * | 44.5 | 34.1 | 23 * |
| AA | 4.4 | 2.0 | 2.2 * | 4.1 | 2.1 | 2.1 | 4.3 | 2.7 | 1.8 * |
| C | 74 | 81 | 83 * | 75.6 | 82 | 83 * | 73.4 | 80.3 | 83.7 * |
| A | 26 | 18 | 17 * | 24.4 | 18 | 17 * | 26.5 | 19.7 | 16.3 * |
| Diplotype, % | |||||||||
| 1S/1S | 20 | 21.3 | 25.6 * | 19 | 20.7 | 23.6 | 20.4 | 20.7 | 27.4 * |
| 1S/1F | 23.7 | 31.6 | 33.5 * | 27.8 | 36.3 | 36.4 | 22 | 29.3 | 32.4 * |
| 1F/1F | 9.1 | 12.5 | 9.4 | 8.7 | 8.8 | 8.2 | 9.5 | 13.9 | 10 |
| 2/2 | 4.1 | 1.9 | 2.2 | 4.1 | 1.6 | 2.1 | 3.9 | 2.7 | 1.8 |
| 2/1F | 19.8 | 13.6 | 10.7 * | 20.6 | 13 | 10.8 | 19.9 | 12.7 | 11.3 * |
| 2/1S | 23.3 | 19.1 | 18.6 * | 19.6 | 19.7 | 19 | 24.4 | 20.7 | 17 * |
| VDBP Levels | ||||||
|---|---|---|---|---|---|---|
| Total | Men | Women | ||||
| Variable | Rho | p Value | Rho | p Value | Rho | p Value |
| Age | −0.088 | 0.0001 | −0.043 | 0.3036 | −0.129 | <0.001 |
| Glucose | −0.012 | 0.6068 | 0.019 | 0.6401 | −0.015 | 0.5948 |
| Waist circumference | −0.065 | 0.0044 | −0.074 | 0.0744 | −0.049 | 0.0706 |
| HDL-c | 0.075 | 0.0011 | 0.074 | 0.0737 | 0.047 | 0.0875 |
| Triglycerides | 0.065 | 0.0044 | 0.044 | 0.2891 | 0.087 | 0.0015 |
| Systolic BP | −0.082 | 0.0003 | −0.090 | 0.0288 | −0.065 | 0.0167 |
| Diastolic BP | −0.021 | 0.3585 | −0.018 | 0.6718 | 0.005 | 0.8533 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo-Bravo, A.; Rivera-Paredez, B.; León-Reyes, G.; Patiño, N.; Castillejos-López, M.; Salmerón, J.; Velázquez-Cruz, R. Unravelling the Contribution of the rs7041 and rs4588 Polymorphisms of the GC Gene and Serum VDBP Levels for Developing Metabolic Syndrome in the Mexican Population. Int. J. Mol. Sci. 2022, 23, 10581. https://doi.org/10.3390/ijms231810581
Hidalgo-Bravo A, Rivera-Paredez B, León-Reyes G, Patiño N, Castillejos-López M, Salmerón J, Velázquez-Cruz R. Unravelling the Contribution of the rs7041 and rs4588 Polymorphisms of the GC Gene and Serum VDBP Levels for Developing Metabolic Syndrome in the Mexican Population. International Journal of Molecular Sciences. 2022; 23(18):10581. https://doi.org/10.3390/ijms231810581
Chicago/Turabian StyleHidalgo-Bravo, Alberto, Berenice Rivera-Paredez, Guadalupe León-Reyes, Nelly Patiño, Manuel Castillejos-López, Jorge Salmerón, and Rafael Velázquez-Cruz. 2022. "Unravelling the Contribution of the rs7041 and rs4588 Polymorphisms of the GC Gene and Serum VDBP Levels for Developing Metabolic Syndrome in the Mexican Population" International Journal of Molecular Sciences 23, no. 18: 10581. https://doi.org/10.3390/ijms231810581
APA StyleHidalgo-Bravo, A., Rivera-Paredez, B., León-Reyes, G., Patiño, N., Castillejos-López, M., Salmerón, J., & Velázquez-Cruz, R. (2022). Unravelling the Contribution of the rs7041 and rs4588 Polymorphisms of the GC Gene and Serum VDBP Levels for Developing Metabolic Syndrome in the Mexican Population. International Journal of Molecular Sciences, 23(18), 10581. https://doi.org/10.3390/ijms231810581