A 2022 Systematic Review and Meta-Analysis of Enriched Therapeutic Diets and Nutraceuticals in Canine and Feline Osteoarthritis
Abstract
1. Introduction
2. Methods
2.1. Literature Search and Inclusion of Studies
2.2. Selection of Articles
2.3. Meta-Analysis: Construction and Validation of Analysis Scales for Data Extracted from Publications
2.4. Quality of Trials Assessment Scale (Table 1)
2.5. Efficacy Assessment Scale
2.6. Statistical Analyses
| Categories and Compounds Tested | References |
|---|---|
| Category 1. Omega-3-enriched therapeutic diets | |
| Green-lipped mussels | [21,22,23,24] |
| Fish oil | [25,26,27,28,29] |
| Category 2. Omega-3-based nutraceuticals | |
| Green-lipped mussels | [21,30,31,32,33,34] |
| Fish oil | [35,36,37] |
| Category 3. Collagen-based nutraceuticals | |
| Collagen | [38,39,40,41] |
| Collagen, glucosamine hydrochloride and chondroitin sulphate | [40,42] |
| Collagen-derived gelatine | [43] |
| NEM® | [44] |
| Ovopet® | [45] |
| MovoflexTM | [46] |
| Category 4. Nutraceuticals with chondroitin-glucosamine | |
| Chondroitin sulphate | [30] |
| Glucosamine hydrochloride, chondroitin sulphate and manganese | [47] |
| Glucosamine hydrochloride and chondroitin sulphate | [40,42,48] |
| Glucosamine hydrochloride, chondroitin sulphate, N-acetyl-D-glucosamine, ascorbic acid and zinc sulphate | [49,50] |
| Glucosamine hydrochloride, chondroitin sulphate and hyaluronic acid | [51] |
| Glucosamine hydrochloride, chondroitin sulphate and avocado and soya unsaponifiables | [52] |
| Category 5. Cannabinoid-based nutraceuticals | |
| Cannabidiol | [53,54,55,56,57] |
| Category 6. Nutraceuticals based on hydroxycitric acid | |
| Hydroxycitric acid | [39] |
| Hydroxycitric acid and chromemate | [39] |
| Hydroxycitric acid, chromemate and collagen | [39] |
| Category 7. Nutraceuticals based on calcium fructoborate | |
| Calcium fructoborate | [58] |
| Calcium fructoborate, glucosamine hydrochloride and chondroitin sulphate | [58] |
| Category 8. Composite Nutraceuticals | |
| Flexodol®/Flexxil® | [59] |
| DinamicTM | [60] |
| Curcuvet®-boswellic acid-glucosamine-chondroitin-omega-3-Vit. C, E-Saccharomyces cerevisiae | [61] |
| Category 9. Others | |
| Special protein milk concentrate | [62] |
| Curcumoids | [63] |
| Elk velvet antler | [64] |
| Boswellia serrata extracts | [65] |
| Avocado and soybean unsaponifiables | [66] |
| Yeast (Beta-1.3/1.6 glucans) | [67] |
| Brachystemma calycinum D don extracts | [68,69] |
| STA-LITE® polydextrose | [70] |
| S-adenosyl L-methionine (SAMe) | [71] |
| Crominex 3+ ® (chrome trivalent, Phyllanthus emblica, shilajit) | [72] |
| Shilajit (Asphaltum punjabianum) | [73] |
| Vitamin E | [74] |
| Terminalia chebula (Indian myrobolan) | [75] |
| Diets enriched with curcumoid extract, hydrolysed collagen and green tea extract | [76] |
| 4CYTETM Epiitalis® Forte (Biota orientalis) | [77] |
2.6.1. Quality of Trials
2.6.2. Analgesic Efficacy
2.6.3. Complementary Analyses
3. Results
3.1. Validation of the “Quality of Trial” Scale
3.2. Quality Assessment
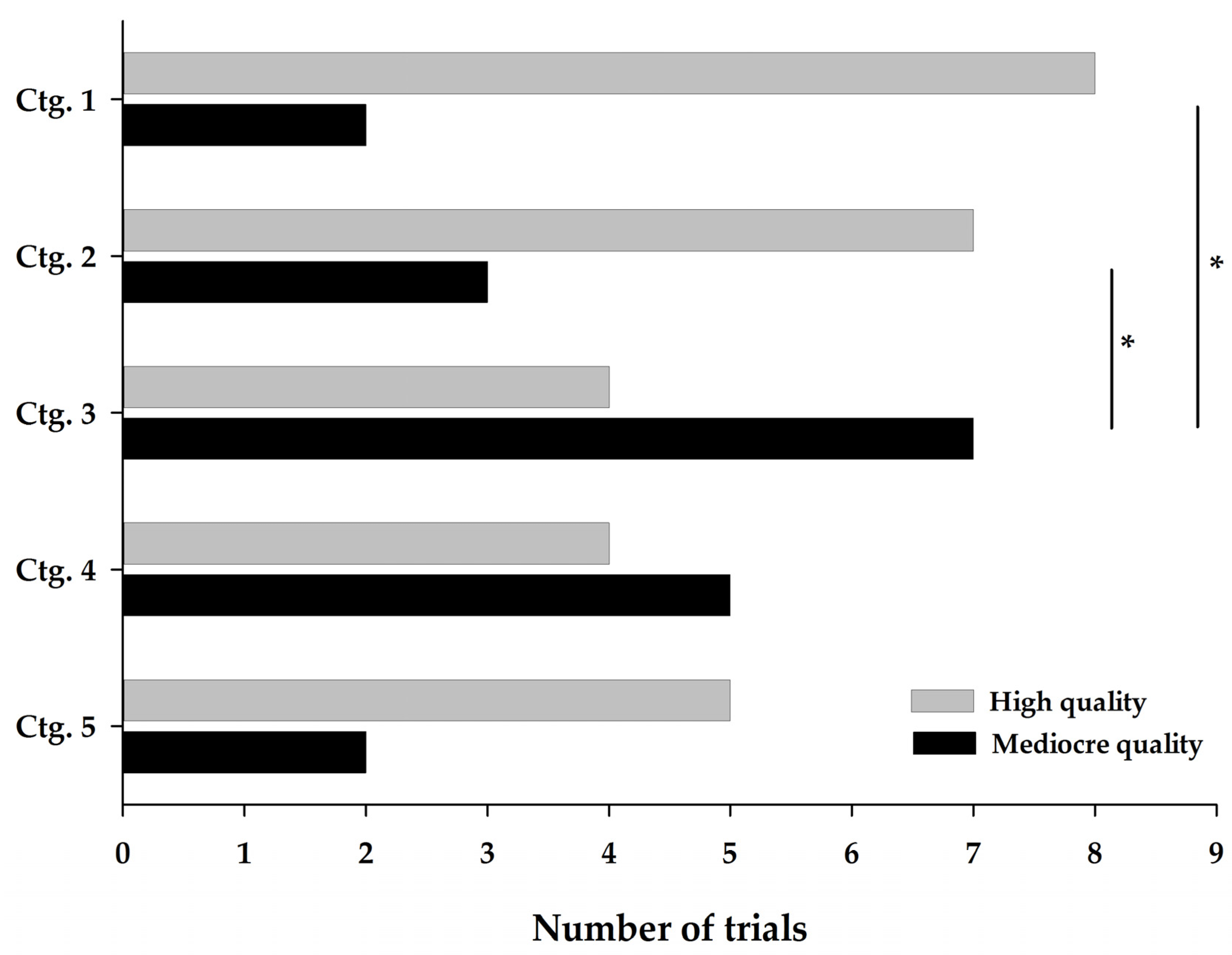
3.2.1. Descriptive Distribution of Quality
3.2.2. Effect of the Category on the Quality Total
3.3. Analgesic Efficacy Assessment
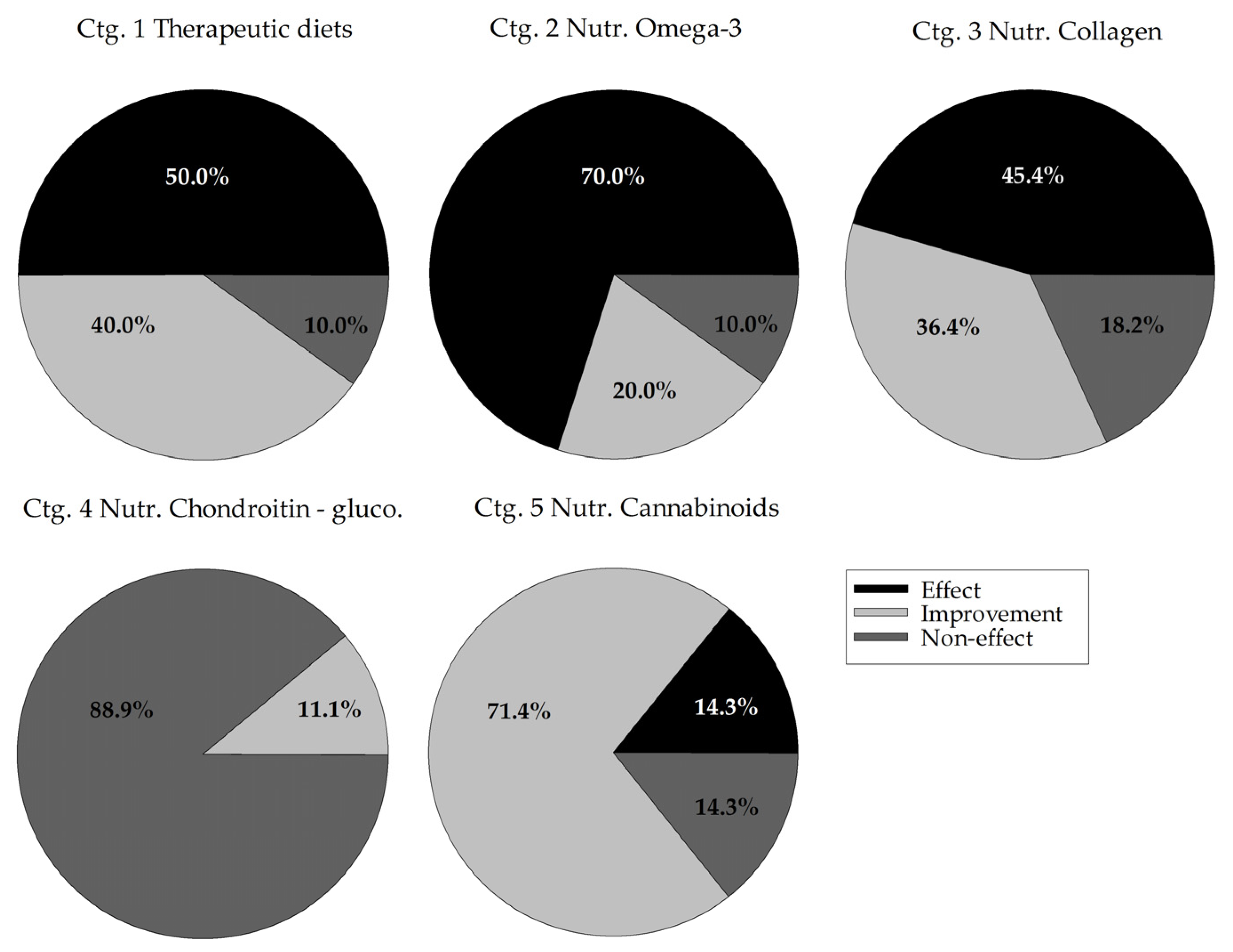
3.3.1. Descriptive Distribution of Efficacy
3.3.2. Effect of Category on Trial Efficacy
3.3.3. Complementary Analyses
4. Discussion
4.1. Review of the Work
4.2. Evaluation Scales: Trial Quality and Analgesic Efficacy
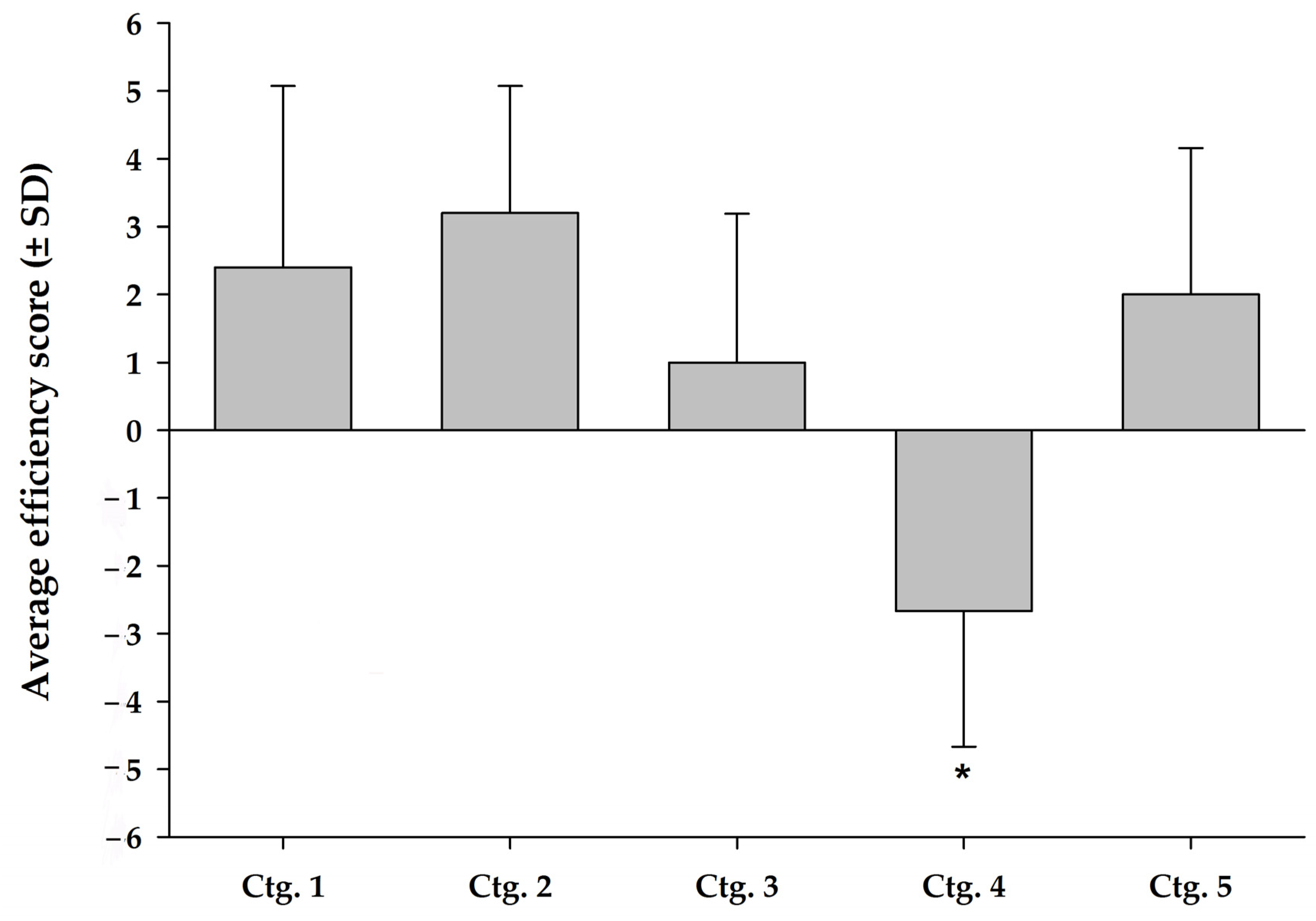
4.3. Combination of Quality of Trials and Analgesic Efficacy (Ctg. 1–5)
4.4. Enriched Therapeutic Diets (Ctg. 1) and Nutraceuticals (Ctg. 2) Based on Omega-3
4.5. Cannabinoid Nutraceuticals (Ctg. 5)
4.6. Collagen-Based Nutraceuticals (Ctg. 3)
4.7. Chondroitin-Glucosamine Nutraceuticals (Ctg. 4)
4.8. Nutraceuticals Based on Hydroxycitric Acid (Ctg. 6), Calcium Fructoborate (Ctg. 7) and Composite Nutraceuticals (Ctg. 8)
4.9. Other (Ctg. 9)
4.10. Effect Sizes
4.11. Potential Mechanism of Nutraceuticals Action
4.12. General Discussions and Conclusions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shearer, P. Epidemiology of orthopedic disease. In Orthopedic Conditions in Cats and Dogs; McNeill, E., Ed.; Royal Canin: Aimargues, France, 2011; Volume 21, pp. 24–25. [Google Scholar]
- Engelhardt, G.; Bögel, R.; Schnitzler, C.; Utzmann, R. Meloxicam: Influence on arachidonic acid metabolism: Part II. In vivo findings. Biochem. Pharmacol. 1996, 51, 29–38. [Google Scholar] [CrossRef]
- Serni, U.; Mannoni, A.; Benucci, M. Is there preliminary in-vivo evidence for an influence of nonsteroidal antiinflammatory drugs on progression in osteoarthritis? Part II-evidence from animal models. Osteoarthr. Cartil. 1999, 7, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Mabry, K.; Hill, T.; Tolbert, M.K. Prevalence of gastrointestinal lesions in dogs chronically treated with nonsteroidal anti-inflammatory drugs. J. Vet. Intern. Med. 2021, 35, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Astin, J.A. Why Patients Use Alternative Medicine Results of a National Study. JAMA 1998, 279, 1548–1553. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.S.; Lordan, R.; Horbańczuk, O.K.; Atanasov, A.G.; Chopra, I.; Horbańczuk, J.O.; Jóźwik, A.; Huang, L.; Pirgozliev, V.; Banach, M.; et al. The current use and evolving landscape of nutraceuticals. Pharmacol. Res. 2022, 175, 106001. [Google Scholar] [CrossRef]
- Grand View Research. Available online: https://www.grandviewresearch.com/industry-analysis/veterinary-dietary-supplements-market-report (accessed on 20 August 2022).
- Elrod, S.M.; Hofmeister, E.H. Veterinarians’ attitudes towards use of nutraceuticals. Can. J. Vet. Res. 2019, 83, 291–297. [Google Scholar]
- Finno, C.J. Veterinary Pet Supplements and Nutraceuticals. Nutr. Today 2020, 55, 97–101. [Google Scholar] [CrossRef]
- Taylor, C.L. Regulatory frameworks for functional foods and dietary supplements. Nutr. Rev. 2004, 62, 55–59. [Google Scholar] [CrossRef]
- Zeisel, S.H. Regulation of “nutraceuticals”. Science 1999, 285, 1853–1855. [Google Scholar] [CrossRef]
- Aragon, C.L.; Hofmeister, E.H.; Budsberg, S.C. Systematic review of clinical trials of treatments for osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 2007, 230, 514–521. [Google Scholar] [CrossRef]
- Sanderson, R.O.; Beata, C.; Flipo, R.M.; Genevois, J.P.; Macias, C.; Tacke, S.; Vezzoni, A.; Innes, J.F. Systematic review of the management of canine osteoarthritis. Vet. Rec. 2009, 164, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Vandeweerd, J.M.; Coisnon, C.; Clegg, P.; Cambier, C.; Pierson, A.; Hontoir, F.; Saegerman, C.; Gustin, P.; Buczinski, S. Systematic review of efficacy of nutraceuticals to alleviate clinical signs of osteoarthritis. J. Vet. Intern. Med. 2012, 26, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. Animals 2014, 4, 35–44. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Sena, E.; van der Worp, H.B.; Howells, D.; Macleod, M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 2007, 30, 433–439. [Google Scholar] [CrossRef]
- Suokas, A.K.; Sagar, D.R.; Mapp, P.I.; Chapman, V.; Walsh, D.A. Design, study quality and evidence of analgesic efficacy in studies of drugs in models of OA pain: A systematic review and a meta-analysis. Osteoarthr. Cartil. 2014, 22, 1207–1223. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bierer, T.L.; Bui, L.M. Improvement of arthritic signs in dogs fed green-lipped mussel (Perna canaliculus). J. Nutr. 2002, 132, 1634S–1636S. [Google Scholar] [CrossRef]
- Servet, E.; Biourge, V.; Marniquet, P. Dietary Intervention Can Improve Clinical Signs in Osteoarthritic Dogs. J. Nutr. 2006, 136, 1995S–1997S. [Google Scholar] [CrossRef]
- Lascelles, B.D.X.; DePuy, V.; Thomson, A.; Hansen, B.; Marcellin-Little, D.J.; Biourge, V.; Bauer, J.E. Evaluation of a therapeutic diet for feline degenerative joint disease. J. Vet. Intern. Med. 2010, 24, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Rialland, P.; Bichot, S.; Lussier, B.; Moreau, M.; Beaudry, F.; del Castillo, J.R.E.; Gauvin, D.; Troncy, E. Effect of a diet enriched with green-lipped mussel on pain behavior and functioning in dogs with clinical osteoarthritis. Can. J. Vet. Res. 2013, 77, 66–74. [Google Scholar] [PubMed]
- Fritsch, D.; Allen, T.A.; Dodd, C.E.; Jewell, D.E.; Sixby, K.A.; Leventhal, P.S.; Hahn, K.A. Dose-Titration effects of fish oil in Osteoarthritic dogs. J. Vet. Intern. Med. 2010, 24, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, D.A.; Allen, T.A.; Dodd, C.E.; Jewell, D.E.; Sixby, K.A.; Leventhal, P.S.; Brejda, J.; Hahn, K.A. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J. Am. Vet. Med. Assoc. 2010, 236, 535–539. [Google Scholar] [CrossRef]
- Roush, J.K.; Cross, A.R.; Renberg, W.C.; Dodd, C.E.; Sixby, K.A.; Fritsch, D.A.; Allen, T.A.; Jewell, D.E.; Richardson, D.C.; Leventhal, P.S.; et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J. Am. Vet. Med. Assoc. 2010, 236, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Roush, J.K.; Dodd, C.E.; Fritsch, D.A.; Allen, T.A.; Jewell, D.E.; Schoenherr, W.D.; Richardson, D.C.; Leventhal, P.S.; Hahn, K.A. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Troncy, E.; Del Castillo, J.R.E.; Bedard, C.; Gauvin, D.; Lussier, B. Effects of feeding a high omega-3 fatty acids diet in dogs with naturally occurring osteoarthritis. J. Anim. Physiol. Anim. Nutr. 2013, 97, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Dobenecker, B.; Beetz, Y.; Kienzle, E. A placebo-controlled double-blind study on the effect of nutraceuticals (chondroitin sulfate and mussel extract) in dogs with joint diseases as perceived by their owners. J. Nutr. 2002, 132, 1690S–1691S. [Google Scholar] [CrossRef]
- Pollard, B.; Guilford, W.G.; Ankenbauer-Perkins, K.L.; Hedderley, D. Clinical efficacy and tolerance of an extract of green-lipped mussel (Perna canaliculus) in dogs presumptively diagnosed with degenerative joint disease. N. Z. Vet. J. 2006, 54, 114–118. [Google Scholar] [CrossRef]
- Hielm-Björkman, A.; Tulamo, R.-M.; Salonen, H.; Raekallio, M. Evaluating Complementary Therapies for Canine Osteoarthritis Part I: Green-lipped Mussel (Perna canaliculus). Evid.-Based Complement. Altern. Med. eCAM 2009, 6, 365–373. [Google Scholar] [CrossRef]
- Soontornvipart, K.; Mongkhon, N.; Nganvongpanit, K.; Kongtawelert, P. Effect of PCSO-524 on OA biomarkers and weight-bearing properties in canine shoulder and coxofemeral osteoarthritis. Thai J. Vet. Med. 2015, 45, 157–165. [Google Scholar]
- Vijarnsorn, M.; Kwananocha, I.; Kashemsant, N.; Jarudecha, T.; Lekcharoensuk, C.; Beale, B.; Peirone, B.; Lascelles, B.D.X. The effectiveness of marine based fatty acid compound (PCSO-524) and firocoxib in the treatment of canine osteoarthritis. BMC Vet. Res. 2019, 15, 349. [Google Scholar] [CrossRef] [PubMed]
- Hielm-Bjorkman, A.; Roine, J.; Elo, K.; Lappalainen, A.; Junnila, J.; Laitinen-Vapaavuori, O. An un-commissioned randomized, placebo-controlled double-blind study to test the effect of deep sea fish oil as a pain reliever for dogs suffering from canine OA. BMC Vet. Res. 2012, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Corbee, R.J.; Barnier, M.M.C.; Van de Lest, C.H.A.; Hazewinkel, H.A.W. The effect of dietary long-chain omega-3 fatty acid supplementation on owner’s perception of behaviour and locomotion in cats with naturally occurring osteoarthritis. J. Anim. Physiol. Anim. Nutr. 2013, 97, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Mehler, S.J.; May, L.R.; King, C.; Harris, W.S.; Shah, Z. A prospective, randomized, double blind, placebo-controlled evaluation of the effects of eicosapentaenoic acid and docosahexaenoic acid on the clinical signs and erythrocyte membrane polyunsaturated fatty acid concentrations in dogs with osteoarthritis. Prostaglandins Leukot. Essent. Fat. Acids 2016, 109, 1–7. [Google Scholar] [CrossRef]
- Deparle, L.A.; Gupta, R.C.; Canerdy, T.D.; Goad, J.T.; D’Altilio, M.; Bagchi, M.; Bagchi, D. Efficacy and safety of glycosylated undenatured type-II collagen (UC-II) in therapy of arthritic dogs. J. Vet. Pharmacol. Ther. 2005, 28, 385–390. [Google Scholar] [CrossRef]
- Peal, A.; D’Altilio, M.; Simms, C.; Alvey, M.; Gupta, R.C.; Goad, J.T.; Canerdy, T.D.; Bagchi, M.; Bagchi, D. Therapeutic efficacy and safety of undenatured type-II collagen (UC-II) alone or in combination with (-)-hydroxycitric acid and chromemate in arthritic dogs. J. Vet. Pharmacol. Ther. 2007, 30, 275–278. [Google Scholar] [CrossRef]
- Gupta, R.C.; Canerdy, T.D.; Lindley, J.; Konemann, M.; Minniear, J.; Carroll, B.A.; Hendrick, C.; Goad, J.T.; Rohde, K.; Doss, R.; et al. Comparative therapeutic efficacy and safety of type-II collagen (UC-II), glucosamine and chondroitin in arthritic dogs: Pain evaluation by ground force plate. J. Anim. Physiol. Anim. Nutr. 2012, 96, 770–777. [Google Scholar] [CrossRef]
- Stabile, M.; Samarelli, R.; Trerotoli, P.; Fracassi, L.; Lacitignola, L.; Crovace, A.; Staffieri, F. Evaluation of the Effects of Undenatured Type II Collagen (UC-II) as Compared to Robenacoxib on the Mobility Impairment Induced by Osteoarthritis in Dogs. Vet. Sci. 2019, 6, 72. [Google Scholar] [CrossRef]
- D’Altilio, M.; Peal, A.; Alvey, M.; Simms, C.; Curtsinger, A.; Gupta, R.C.; Canerdy, T.D.; Goad, J.T.; Bagchi, M.; Bagchi, D. Therapeutic Efficacy and Safety of Undenatured Type II Collagen Singly or in Combination with Glucosamine and Chondroitin in Arthritic Dogs. Toxicol. Mech. Methods 2007, 17, 189–196. [Google Scholar] [CrossRef]
- Beynen, A.C.; van Geene, H.W.; Grim, H.V.; Jacobs, P.; Van der Vlerk, T. Oral administration of gelatin hydrolysate reduces clinical signs of canine osteoarthritis in a double-blind, placebo-controlled trial. Am. J. Anim. Vet. Sci. 2010, 5, 102–106. [Google Scholar] [CrossRef]
- Ruff, K.J.; Kopp, K.J.; Von Behrens, P.; Lux, M.; Mahn, M.; Back, M. Effectiveness of NEM(®) brand eggshell membrane in the treatment of suboptimal joint function in dogs: A multicenter, randomized, double-blind, placebo-controlled study. Vet. Med. 2016, 7, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.; Gil-Quintana, E.; Fenaux, M.; Sanchez, N.; Torre, C. The efficacy of Ovopet® in the treatment of hip dysplasia in dogs. J. Vet. Med. Anim. Health 2018, 10, 198–207. [Google Scholar] [CrossRef]
- Muller, C.; Enomoto, M.; Buono, A.; Steiner, J.M.; Lascelles, B.D.X. Placebo-controlled pilot study of the effects of an eggshell membrane-based supplement on mobility and serum biomarkers in dogs with osteoarthritis. Vet. J. 2019, 253, 105379. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Dupuis, J.; Bonneau, N.H.; Desnoyers, M. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet. Rec. 2003, 152, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Maihasap, P.; Soontornwipart, K.; Techaarpornkul, N. Clinical effect of glucosamine and chondroitin contained nutraceutical on osteoarthritis in dogs after anterior cruciate ligament rupture surgical repair. Thai J. Vet. Med. 2014, 44, 67–73. [Google Scholar]
- McCarthy, G.; O’Donovan, J.; Jones, B.; McAllister, H.; Seed, M.; Mooney, C. Randomised double-blind, positive-controlled trial to assess the efficacy of glucosamine/chondroitin sulfate for the treatment of dogs with osteoarthritis. Vet. J. 2007, 174, 54–61. [Google Scholar] [CrossRef]
- Sul, R.M.; Chase, D.; Parkin, T.; Bennett, D. Comparison of meloxicam and a glucosamine-chondroitin supplement in management of feline osteoarthritis: A double-blind randomised, placebo-controlled, prospective trial. Vet. Comp. Orthop. Traumatol. 2014, 27, 20–26. [Google Scholar] [CrossRef]
- Alves, J.C.; Santos, A.M.; Jorge, P.I. Effect of an Oral Joint Supplement When Compared to Carprofen in the Management of Hip Osteoarthritis in Working Dogs. Top. Companion Anim. Med. 2017, 32, 126–129. [Google Scholar] [CrossRef]
- Scott, R.M.; Evans, R.; Conzemius, M.G. Efficacy of an oral nutraceutical for the treatment of canine osteoarthritis. A double-blind, randomized, placebo-controlled prospective clinical trial. Vet. Comp. Orthop. Traumatol. 2017, 30, 318–323. [Google Scholar] [CrossRef]
- Gamble, L.J.; Boesch, J.M.; Frye, C.W.; Schwark, W.S.; Mann, S.; Wolfe, L.; Brown, H.; Berthelsen, E.S.; Wakshlag, J.J. Pharmacokinetics, safety, and clinical efficacy of cannabidiol treatment in osteoarthritic dogs. Front. Vet. Sci. 2018, 5, 165. [Google Scholar] [CrossRef] [PubMed]
- Brioschi, F.A.; Di Cesare, F.; Gioeni, D.; Rabbogliatti, V.; Ferrari, F.; D’Urso, E.S.; Amari, M.; Ravasio, G. Oral Transmucosal Cannabidiol Oil Formulation as Part of a Multimodal Analgesic Regimen: Effects on Pain Relief and Quality of Life Improvement in Dogs Affected by Spontaneous Osteoarthritis. Animals 2020, 10, 1505. [Google Scholar] [CrossRef] [PubMed]
- Kogan, L.; Hellyer, P.; Downing, R. The Use of Cannabidiol-Rich Hemp Oil Extract to Treat Canine Osteoarthritis-Related Pain: A Pilot Study. AHVMA J. 2020, 58, 1–10. [Google Scholar]
- Verrico, C.D.; Wesson, S.; Konduri, V.; Hofferek, C.J.; Vazquez-Perez, J.; Blair, E.; Dunner, K., Jr.; Salimpour, P.; Decker, W.K.; Halpert, M.M. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain 2020, 161, 2191–2202. [Google Scholar] [CrossRef]
- Mejia, S.; Duerr, F.M.; Griffenhagen, G.; McGrath, S. Evaluation of the effect of cannabidiol on naturally occuring osteoarthritis-associated pain: A pilot study in dogs. J. Am. Anim. Hosp. Assoc. 2021, 57, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Price, A.K.; de Godoy, M.R.C.; Harper, T.A.; Knap, K.E.; Joslyn, S.; Pietrzkowski, Z.; Cross, B.K.; Detweiler, K.B.; Swanson, K.S. Effects of dietary calcium fructoborate supplementation on joint comfort and flexibility and serum inflammatory markers in dogs with osteoarthritis. J. Anim. Sci. 2017, 95, 2907–2916. [Google Scholar] [CrossRef][Green Version]
- Moreau, M.; Lussier, B.; Pelletier, J.P.; Martel-Pelletier, J.; Bédard, C.; Gauvin, D.; Troncy, E. A medicinal herb-based natural health product improves the condition of a canine natural osteoarthritis model: A randomized placebo-controlled trial. Res. Vet. Sci. 2014, 97, 574–581. [Google Scholar] [CrossRef]
- Musco, N.; Vassalotti, G.; Mastellone, V.; Cortese, L.; Della Rocca, G.; Molinari, M.L.; Calabro, S.; Tudisco, R.; Cutrignelli, M.I.; Lombardi, P. Effects of a nutritional supplement in dogs affected by osteoarthritis. Vet. Med. Sci. 2019, 5, 325–335. [Google Scholar] [CrossRef]
- Caterino, C.; Aragosa, F.; Della Valle, G.; Costanza, D.; Lamagna, F.; Piscitelli, A.; Nieddu, A.; Fatone, G. Clinical efficacy of Curcuvet and Boswellic acid combined with conventional nutraceutical product: An aid to canine osteoarthritis. PLoS ONE 2021, 16, e0252279. [Google Scholar] [CrossRef]
- Gingerich, D.A.; Strobel, J.D. Use of client-specific outcome measures to assess treatment effects in geriatric, arthritic dogs: Controlled clinical evaluation of a nutraceutical. Vet. Ther. 2003, 4, 56–66. [Google Scholar]
- Innes, J.F.; Fuller, C.J.; Grover, E.R.; Kelly, A.L.; Burn, J.F. Randomised, double-blind, placebo-controlled parallel group study of P54FP for the treatment of dogs with osteoarthritis. Vet. Rec. 2003, 152, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Dupuis, J.; Bonneau, N.H.; Lécuyer, M. Clinical evaluation of a powder of quality elk velvet antler for the treatment of osteoarthrosis in dogs. Can. Vet. J. 2004, 45, 133–139. [Google Scholar]
- Reichling, J.; Schmokel, H.; Fitzi, J.; Bucher, S.; Saller, R. Dietary support with Boswellia resin in canine inflammatory joint and spinal disease. Schweiz. Arch. Fur Tierheilkd. 2004, 146, 71–79. [Google Scholar] [CrossRef]
- Boileau, C.; Martel-Pelletier, J.; Caron, J.; Msika, P.; Guillou, G.B.; Baudouin, C.; Pelletier, J.P. Protective effects of total fraction of avocado/soybean unsaponifiables on the structural changes in experimental dog osteoarthritis: Inhibition of nitric oxide synthase and matrix metalloproteinase-13. Arthritis Res. Ther. 2009, 11, R41. [Google Scholar] [CrossRef]
- Beynen, A.C.; Legerstee, E. Influence of dietary beta-1,3/1,6-glucans on clinical signs of canine osteoarthritis in a double-blind, placebo-controlled trial. Am. J. Anim. Vet. Sci. 2010, 5, 97–101. [Google Scholar] [CrossRef]
- Boileau, C.; Martel-Pelletier, J.; Caron, J.; Paré, F.; Troncy, E.; Moreau, M.; Pelletier, J.P. Oral treatment with a Brachystemma calycinum D don plant extract reduces disease symptoms and the development of cartilage lesions in experimental dog osteoarthritis: Inhibition of protease-activated receptor 2. Ann. Rheum. Dis. 2010, 69, 1179–1184. [Google Scholar] [CrossRef]
- Moreau, M.; Lussier, B.; Pelletier, J.P.; Martel-Pelletier, J.; Bédard, C.; Gauvin, D.; Troncy, E. Brachystemma calycinum D. Don Effectively Reduces the Locomotor Disability in Dogs with Naturally Occurring Osteoarthritis: A Randomized Placebo-Controlled Trial. Evid.-Based Complement. Altern. Med. 2012, 2012, 646191. [Google Scholar] [CrossRef]
- Beynen, A.C.; Saris, D.H.J.; De Jong, L.; Staats, M.; Einerhand, A.W.C. Impact of dietary polydextrose on clinical signs of canine osteoarthritis. Am. J. Anim. Vet. Sci. 2011, 6, 93–99. [Google Scholar] [CrossRef][Green Version]
- Imhoff, D.J.; Gordon-Evans, W.J.; Evans, R.B.; Johnson, A.L.; Griffon, D.J.; Swanson, K.S. Evaluation of S-adenosyl l-methionine in a double-blinded, randomized, placebo-controlled, clinical trial for treatment of presumptive osteoarthritis in the dog. Vet. Surg. 2011, 40, 228–232. [Google Scholar] [CrossRef]
- Fleck, A.; Gupta, R.C.; Goad, J.T.; Lasher, M.A.; Canerdy, T.D.; Kalidindi, S.R. Anti-Arthritic Efficacy And Safety Of Crominex® 3+ (Trivalent Chromium, Phyllanthus emblica Extract, And Shilajit) In Moderately Arthritic Dogs. J. Vet. Sci. Anim. Husb. 2014, 2, 101. [Google Scholar] [CrossRef]
- Lawley, S.; Gupta, R.C.; Goad, J.T.; Canerdy, T.D.; Kalidindi, S.R. Anti-Inflammatory and Anti-Arthritic Efficacy and Safety of Purified Shilajit in Moderately Arthritic Dogs. J. Vet. Sci. Anim. Husb. 2013, 1, 302. [Google Scholar] [CrossRef]
- Rhouma, M.; de Oliveira El-Warrak, A.; Troncy, E.; Beaudry, F.; Chorfi, Y. Anti-inflammatory response of dietary vitamin E and its effects on pain and joint structures during early stages of surgically induced osteoarthritis in dogs. Can. J. Vet. Res. 2013, 77, 191–198. [Google Scholar]
- Murdock, N.; Gupta, R.C.; Vega, N.; Kotora, K.; Miller, J.; Goad, T.J.; Lasher, A.M.; Canerdy, D.T.; Kalidindi, S.R. Evaluation of Terminalia chebula extract for anti-arthritic efficacy and safety in osteoarthritic dogs. J. Vet. Sci. Technol. 2016, 7, 1. [Google Scholar] [CrossRef]
- Comblain, F.; Barthélémy, N.; Lefèbvre, M.; Schwartz, C.; Lesponne, I.; Serisier, S.; Feugier, A.; Balligand, M.; Henrotin, Y. A randomized, double-blind, prospective, placebo-controlled study of the efficacy of a diet supplemented with curcuminoids extract, hydrolyzed collagen and green tea extract in owner’s dogs with osteoarthritis. BMC Vet. Res. 2017, 13, 395. [Google Scholar] [CrossRef] [PubMed]
- Beths, T.; Munn, R.; Bauquier, S.H.; Mitchell, P.; Whittem, T. A pilot study of 4CYTETM Epiitalis Forte, a novel nutraceutical, in the management of naturally occurring osteoarthritis in dogs. Aust. Vet. J. 2020, 98, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Steagall, P.V. Analgesia: What Makes Cats Different/Challenging and What Is Critical for Cats? Vet. Clin. Small Anim. Pract. 2020, 50, 749–767. [Google Scholar] [CrossRef]
- Roberts, P.; Priest, H. Reliability and validity in research. Nurs. Stand 2006, 20, 41–45. [Google Scholar] [CrossRef]
- Gagnon, A.; Brown, D.; Moreau, M.; Lussier, B.; Otis, C.; Troncy, E. Therapeutic response analysis in dogs with naturally occurring osteoarthritis. Vet. Anaesth. Analg. 2017, 44, 1373–1381. [Google Scholar] [CrossRef]
- Mitchell, V.A.; Harley, J.; Casey, S.L.; Vaughan, A.C.; Winters, B.L.; Vaughan, C.W. Oral efficacy of Δ(9)-tetrahydrocannabinol and cannabidiol in a mouse neuropathic pain model. Neuropharmacology 2021, 189, 108529. [Google Scholar] [CrossRef]
- Urits, I.; Gress, K.; Charipova, K.; Habib, K.; Lee, D.; Lee, C.; Jung, J.W.; Kassem, H.; Cornett, E.; Paladini, A.; et al. Use of cannabidiol (CBD) for the treatment of chronic pain. Best Pract. Res. Clin. Anaesthesiol. 2020, 34, 463–477. [Google Scholar] [CrossRef]
- Wandel, S.; Jüni, P.; Tendal, B.; Nüesch, E.; Villiger, P.M.; Welton, N.J.; Reichenbach, S.; Trelle, S. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: Network meta-analysis. BMJ 2010, 341, c4675. [Google Scholar] [CrossRef] [PubMed]
- Bhathal, A.; Spryszak, M.; Louizos, C.; Frankel, G. Glucosamine and chondroitin use in canines for osteoarthritis: A review. Open Vet. J. 2017, 7, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Leong, D.J.; Cardoso, L.; Sun, H.B. Nutraceuticals and osteoarthritis pain. Pharmacol. Ther. 2018, 187, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Colletti, A.; Cicero, A.F.G. Nutraceutical Approach to Chronic Osteoarthritis: From Molecular Research to Clinical Evidence. Int. J. Mol. Sci. 2021, 22, 12920. [Google Scholar] [CrossRef] [PubMed]
- Bernal del Nozal, J.; Mendiola, J.; Ibáñez, E.; Cifuentes, A. Advanced analysis of nutraceuticals. J. Pharm. Biomed. Anal. 2010, 55, 758–774. [Google Scholar] [CrossRef]
- Grundmann, O.; Kumar, P.; Rogge, M.; Committee, A.P.P. Regulation of Dietary Supplements and Nutraceutical Products in the United States: An Argument for Greater Oversight and Uniform Standards. J. Clin. Pharmacol. 2022, 62, 14–16. [Google Scholar] [CrossRef]
- Leong, D.J.; Choudhury, M.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; Sun, H.B. Nutraceuticals: Potential for chondroprotection and molecular targeting of osteoarthritis. Int. J. Mol. Sci. 2013, 14, 23063–23085. [Google Scholar] [CrossRef]
- Largo, R.; Alvarez-Soria, M.A.; Díez-Ortego, I.; Calvo, E.; Sánchez-Pernaute, O.; Egido, J.; Herrero-Beaumont, G. Glucosamine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthr. Cartil. 2003, 11, 290–298. [Google Scholar] [CrossRef]
- Chan, P.S.; Caron, J.P.; Orth, M.W. Short-term gene expression changes in cartilage explants stimulated with interleukin beta plus glucosamine and chondroitin sulfate. J. Rheumatol. 2006, 33, 1329–1340. [Google Scholar] [PubMed]
- Wen, Z.H.; Tang, C.C.; Chang, Y.C.; Huang, S.Y.; Hsieh, S.P.; Lee, C.H.; Huang, G.S.; Ng, H.F.; Neoh, C.A.; Hsieh, C.S.; et al. Glucosamine sulfate reduces experimental osteoarthritis and nociception in rats: Association with changes of mitogen-activated protein kinase in chondrocytes. Osteoarthr. Cartil. 2010, 18, 1192–1202. [Google Scholar] [CrossRef]
- Imagawa, K.; de Andrés, M.C.; Hashimoto, K.; Pitt, D.; Itoi, E.; Goldring, M.B.; Roach, H.I.; Oreffo, R.O. The epigenetic effect of glucosamine and a nuclear factor-kappa B (NF-kB) inhibitor on primary human chondrocytes--implications for osteoarthritis. Biochem. Biophys. Res. Commun. 2011, 405, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Waly, N.E.; Refaiy, A.; Aborehab, N.M. IL-10 and TGF-β: Roles in chondroprotective effects of Glucosamine in experimental Osteoarthritis? Pathophysiology 2017, 24, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, L.; Liu, Y.; Zhang, Y.; Liang, Y.; Mei, Y. Anti-inflammatory effects in a mouse osteoarthritis model of a mixture of glucosamine and chitooligosaccharides produced by bi-enzyme single-step hydrolysis. Sci. Rep. 2018, 8, 5624. [Google Scholar] [CrossRef]
- Kuptniratsaikul, V.; Dajpratham, P.; Taechaarpornkul, W.; Buntragulpoontawee, M.; Lukkanapichonchut, P.; Chootip, C.; Saengsuwan, J.; Tantayakom, K.; Laongpech, S. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: A multicenter study. Clin. Interv. Aging 2014, 9, 451–458. [Google Scholar] [CrossRef]
- Lo, Y.Y.; Wong, J.M.; Cruz, T.F. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J. Biol. Chem. 1996, 271, 15703–15707. [Google Scholar] [CrossRef]
- Chan, P.S.; Caron, J.P.; Orth, M.W. Effect of glucosamine and chondroitin sulfate on regulation of gene expression of proteolytic enzymes and their inhibitors in interleukin-1-challenged bovine articular cartilage explants. Am. J. Vet. Res. 2005, 66, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Neil, K.M.; Orth, M.W.; Coussens, P.M.; Chan, P.S.; Caron, J.P. Effects of glucosamine and chondroitin sulfate on mediators of osteoarthritis in cultured equine chondrocytes stimulated by use of recombinant equine interleukin-1beta. Am. J. Vet. Res. 2005, 66, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Oesser, S.; Seifert, J. Stimulation of type II collagen biosynthesis and secretion in bovine chondrocytes cultured with degraded collagen. Cell Tissue Res 2003, 311, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Isaka, S.; Someya, A.; Nakamura, S.; Naito, K.; Nozawa, M.; Inoue, N.; Sugihara, F.; Nagaoka, I.; Kaneko, K. Evaluation of the effect of oral administration of collagen peptides on an experimental rat osteoarthritis model. Exp. Ther. Med. 2017, 13, 2699–2706. [Google Scholar] [CrossRef]
- Bourdon, B.; Contentin, R.; Cassé, F.; Maspimby, C.; Oddoux, S.; Noël, A.; Legendre, F.; Gruchy, N.; Galéra, P. Marine Collagen Hydrolysates Downregulate the Synthesis of Pro-Catabolic and Pro-Inflammatory Markers of Osteoarthritis and Favor Collagen Production and Metabolic Activity in Equine Articular Chondrocyte Organoids. Int. J. Mol. Sci. 2021, 22, 580. [Google Scholar] [CrossRef]
- Tong, T.; Zhao, W.; Wu, Y.-Q.; Chang, Y.; Wang, Q.-T.; Zhang, L.-L.; Wei, W. Chicken type II collagen induced immune balance of main subtype of helper T cells in mesenteric lymph node lymphocytes in rats with collagen-induced arthritis. Inflamm. Res. 2010, 59, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Li, X.Y.; Wang, H.K.; Jia, J.F.; Zheng, Z.H.; Ding, J.; Fan, C.M. Oral administration of type-II collagen peptide 250-270 suppresses specific cellular and humoral immune response in collagen-induced arthritis. Clin. Immunol. 2007, 122, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Park, M.J.; Cho, M.L.; Kwok, S.K.; Ju, J.H.; Ko, H.J.; Park, S.H.; Kim, H.Y. Type II collagen oral tolerance; mechanism and role in collagen-induced arthritis and rheumatoid arthritis. Mod. Rheumatol. 2009, 19, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, M.; Barati, M.; Khodaei, M.; Babashahi, M.; Kalhori, A.; Tahmassian, A.H.; Mosharkesh, E.; Arzhang, P.; Eini-Zinab, H. Is collagen supplementation friend or foe in rheumatoid arthritis and osteoarthritis? A comprehensive systematic review. Int. J. Rheum. Dis. 2022, 25, 973–981. [Google Scholar] [CrossRef]
- Zainal, Z.; Longman, A.J.; Hurst, S.; Duggan, K.; Caterson, B.; Hughes, C.E.; Harwood, J.L. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthr. Cartil. 2009, 17, 896–905. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Jia, M.-R.; Sun, T. The roles of special proresolving mediators in pain relief. Rev. Neurosci. 2018, 29, 645–660. [Google Scholar] [CrossRef]
- Fattori, V.; Pinho-Ribeiro, F.A.; Staurengo-Ferrari, L.; Borghi, S.M.; Rossaneis, A.C.; Casagrande, R.; Verri, W.A., Jr. The specialised pro-resolving lipid mediator maresin 1 reduces inflammatory pain with a long-lasting analgesic effect. Br. J. Pharmacol. 2019, 176, 1728–1744. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- Sakata, S.; Hayashi, S.; Fujishiro, T.; Kawakita, K.; Kanzaki, N.; Hashimoto, S.; Iwasa, K.; Chinzei, N.; Kihara, S.; Haneda, M.; et al. Oxidative stress-induced apoptosis and matrix loss of chondrocytes is inhibited by eicosapentaenoic acid. J. Orthop. Res. 2015, 33, 359–365. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, A.; Ma, L.; Yu, H.; Zhang, L.; Meng, H.; Cui, Y.; Yu, F.; Yang, B. Docosahexenoic acid treatment ameliorates cartilage degeneration via a p38 MAPK-dependent mechanism. Int. J. Mol. Med. 2016, 37, 1542–1550. [Google Scholar] [CrossRef]
- Matta, J.A.; Miyares, R.L.; Ahern, G.P. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J. Physiol. 2007, 578, 397–411. [Google Scholar] [CrossRef]
- Kelly, S.; Chapman, R.J.; Woodhams, S.; Sagar, D.R.; Turner, J.; Burston, J.J.; Bullock, C.; Paton, K.; Huang, J.; Wong, A.; et al. Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann. Rheum. Dis. 2015, 74, 252–259. [Google Scholar] [CrossRef]
- Kalogerou, M.; Ioannou, S.; Kolovos, P.; Prokopiou, E.; Potamiti, L.; Kyriacou, K.; Panagiotidis, M.; Ioannou, M.; Fella, E.; Worth, E.P.; et al. Omega-3 fatty acids promote neuroprotection, decreased apoptosis and reduced glial cell activation in the retina of a mouse model of OPA1-related autosomal dominant optic atrophy. Exp. Eye Res. 2022, 215, 108901. [Google Scholar] [CrossRef]
- Vučković, S.; Srebro, D.; Vujović, K.S.; Vučetić, Č.; Prostran, M. Cannabinoids and Pain: New Insights From Old Molecules. Front. Pharmacol. 2018, 9, 1259. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef]
- Fahmi, H.; Martel-Pelletier, J.; Pelletier, J.P.; Kapoor, M. Peroxisome proliferator-activated receptor gamma in osteoarthritis. Mod. Rheumatol. 2011, 21, 1–9. [Google Scholar] [CrossRef]
- Malan, T.P.; Ibrahim, M.M.; Lai, J.; Vanderah, T.W.; Makriyannis, A.; Porreca, F. CB2 cannabinoid receptor agonists: Pain relief without psychoactive effects? Curr. Opin. Pharmacol. 2003, 3, 62–67. [Google Scholar] [CrossRef]
- Manzanares, J.; Julian, M.; Carrascosa, A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr. Neuropharmacol. 2006, 4, 239–257. [Google Scholar] [CrossRef]
- Starowicz, K.; Malek, N.; Przewlocka, B. Cannabinoid receptors and pain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2013, 2, 121–132. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Panichi, V.; Mariani, E.; Flamigni, F.; Borzì, R.M. Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells 2020, 9, 1232. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef]
- Klinck, M.P.; Mogil, J.S.; Moreau, M.; Lascelles, B.D.X.; Flecknell, P.A.; Poitte, T.; Troncy, E. Translational pain assessment: Could natural animal models be the missing link? Pain 2017, 158, 1633–1646. [Google Scholar] [CrossRef]
- Adebowale, A.; Du, J.; Liang, Z.; Leslie, J.L.; Eddington, N.D. The bioavailability and pharmacokinetics of glucosamine hydrochloride and low molecular weight chondroitin sulfate after single and multiple doses to beagle dogs. Biopharm. Drug Dispos. 2002, 23, 217–225. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Qian, J.; Liang, Q.; Wang, Z.; Xu, J.; He, S.; Ma, H. Bioavailability and Bioavailable Forms of Collagen after Oral Administration to Rats. J. Agric. Food Chem. 2015, 63, 3752–3756. [Google Scholar] [CrossRef]
- Paul-Murphy, J.; Ludders, J.W.; Robertson, S.A.; Gaynor, J.S.; Hellyer, P.W.; Wong, P.L. The need for a cross-species approach to the study of pain in animals. J. Am. Vet. Med. Assoc. 2004, 224, 692–697. [Google Scholar] [CrossRef]
- Epstein, M.E.; Rodanm, I.; Griffenhagen, G.; Kadrlik, J.; Petty, M.C.; Robertson, S.A.; Simpson, W. 2015 AAHA/AAFP pain management guidelines for dogs and cats. J. Feline Med. Surg. 2015, 17, 251–272. [Google Scholar] [CrossRef]
- Rialland, P.; Bichot, S.; Moreau, M.; Guillot, M.; Lussier, B.; Gauvin, D.; Martel-Pelletier, J.; Pelletier, J.P.; Troncy, E. Clinical validity of outcome pain measures in naturally occurring canine osteoarthritis. BMC Vet. Res. 2012, 8, 162. [Google Scholar] [CrossRef]
- Caldwell, J.; Gardner, I.; Swales, N. An introduction to drug disposition: The basic principles of absorption, distribution, metabolism, and excretion. Toxicol. Pathol. 1995, 23, 102–114. [Google Scholar] [CrossRef]
- Johnston, S.A. Osteoarthritis. Joint anatomy, physiology, and pathobiology. Vet. Clin. N. Am. Small Anim. Pract. 1997, 27, 699–723. [Google Scholar] [CrossRef]
- Martinez, N.; McDonald, B. A study into the fatty acid content of selected veterinary diets, supplements and fish oil capsules in Australia. Vet. Dermatol. 2021, 32, 256-e69. [Google Scholar] [CrossRef]
- Moreau, M.; Troncy, E. Review of Fortified Foods and Natural Medicinal Products in Companion Animals Afflicted by Naturally Occurring Osteoarthritis. In Nutritional Modulators of Pain in the Aging Population, 1st ed.; Watson, R., Zibadi, S., Eds.; Academic press: London, UK, 2017; pp. 281–291. [Google Scholar] [CrossRef]
- Williams, P.; Pettitt, R. Nutraceutical use in osteoarthritic canines: A review. Companion Anim. 2021, 26, 1–5. [Google Scholar] [CrossRef]

| Criterion | Sub-Criteria (Score) |
|---|---|
| Risk of bias | 1. Randomisation: Non-randomised (0), Not mentioned (0) or Randomised (2) |
| 2. Type of study: Single cohort (0), Cross-over (1) or Parallel (2) 3. Controlled study: No control group (0), Positive control (1*) or Placebo (1*) 4. Blinding procedure: Non-blinded (0), Single-blinded (1) or Double-blinded (2) | |
| Methodological quality | 5. Inclusion criteria: None (0), Other (1*), Experimental induction of OA in healthy animals (2), Owner-reported lameness (2*), Veterinary orthopaedic examination (2*), Inclusion grid (2*) or X-rays (2*) 6. Non-inclusion criteria: None (0), Weaning period too short (1*), Adequate weaning period (2*) or Description of non-inclusion criteria (2*) |
| 7. Exclusion criteria: None (0) or Description of exclusion criteria (2) 8. Control of possible bias: Non-randomised, or non-blinded, study with subjective assessment (0), Non-randomised, or non-blinded, study with objective assessments (1*), Research hypotheses and objectives clearly stated (0.5/each*), Ethics committee approval indicated (1*), Manuscript edited according to ARRIVE or CONSORT criteria (1*), Declaration of any conflict of interest (1*), Randomised, blinded study (2*) or No indication of the dose used (−5*) 9. Data collection and analysis: No information (0), Electronic collection, or methods already used (1), Quality assurance control (2*), Statistical analyses clearly described (1 or 2*) | |
| Strengths of the scientific evidence | 10. Sample size: <10 per group (0), Between 10 and 20 per group (2) or >20 per group (4) 11. Nature of data: Non-validated subjective (0*), Validated subjective (2*), Non-validated objective (1*) or Validated objective (4*) outcomes 12. Repetition of results obtained (according to the level of risk of bias): Only one study carried out (except if [A]) (0), Several studies [C] or [D] (1), One study [A] (2), Several studies [B or less] (3), Several studies [A and/or less] (4) or Several studies of level [A] (6) |
| Quality of Trial | Level | Effect | Improvement | Non-Effect |
|---|---|---|---|---|
| Very high | A | +5 | +3 | −5 |
| Good | B | +4 | +2 | −4 |
| Medium | C | +2 | +1 | −2 |
| Low | D | +1 | +1 | −1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbeau-Grégoire, M.; Otis, C.; Cournoyer, A.; Moreau, M.; Lussier, B.; Troncy, E. A 2022 Systematic Review and Meta-Analysis of Enriched Therapeutic Diets and Nutraceuticals in Canine and Feline Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 10384. https://doi.org/10.3390/ijms231810384
Barbeau-Grégoire M, Otis C, Cournoyer A, Moreau M, Lussier B, Troncy E. A 2022 Systematic Review and Meta-Analysis of Enriched Therapeutic Diets and Nutraceuticals in Canine and Feline Osteoarthritis. International Journal of Molecular Sciences. 2022; 23(18):10384. https://doi.org/10.3390/ijms231810384
Chicago/Turabian StyleBarbeau-Grégoire, Maude, Colombe Otis, Antoine Cournoyer, Maxim Moreau, Bertrand Lussier, and Eric Troncy. 2022. "A 2022 Systematic Review and Meta-Analysis of Enriched Therapeutic Diets and Nutraceuticals in Canine and Feline Osteoarthritis" International Journal of Molecular Sciences 23, no. 18: 10384. https://doi.org/10.3390/ijms231810384
APA StyleBarbeau-Grégoire, M., Otis, C., Cournoyer, A., Moreau, M., Lussier, B., & Troncy, E. (2022). A 2022 Systematic Review and Meta-Analysis of Enriched Therapeutic Diets and Nutraceuticals in Canine and Feline Osteoarthritis. International Journal of Molecular Sciences, 23(18), 10384. https://doi.org/10.3390/ijms231810384





