The TaWRKY22–TaCOPT3D Pathway Governs Cadmium Uptake in Wheat
Abstract
:1. Introduction
2. Results
2.1. TaCOPT3D Expression in Wheat under Cadmium Stress
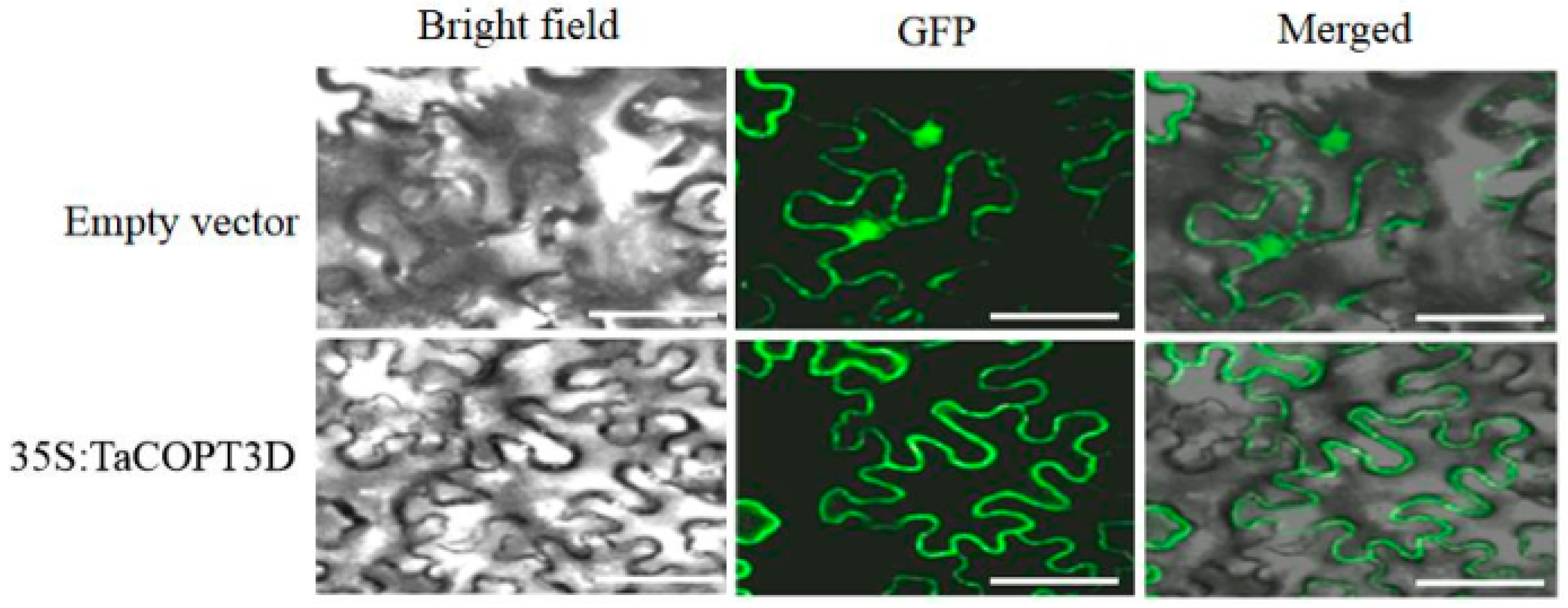
2.2. Subcellular Localization of TaCOPT3D
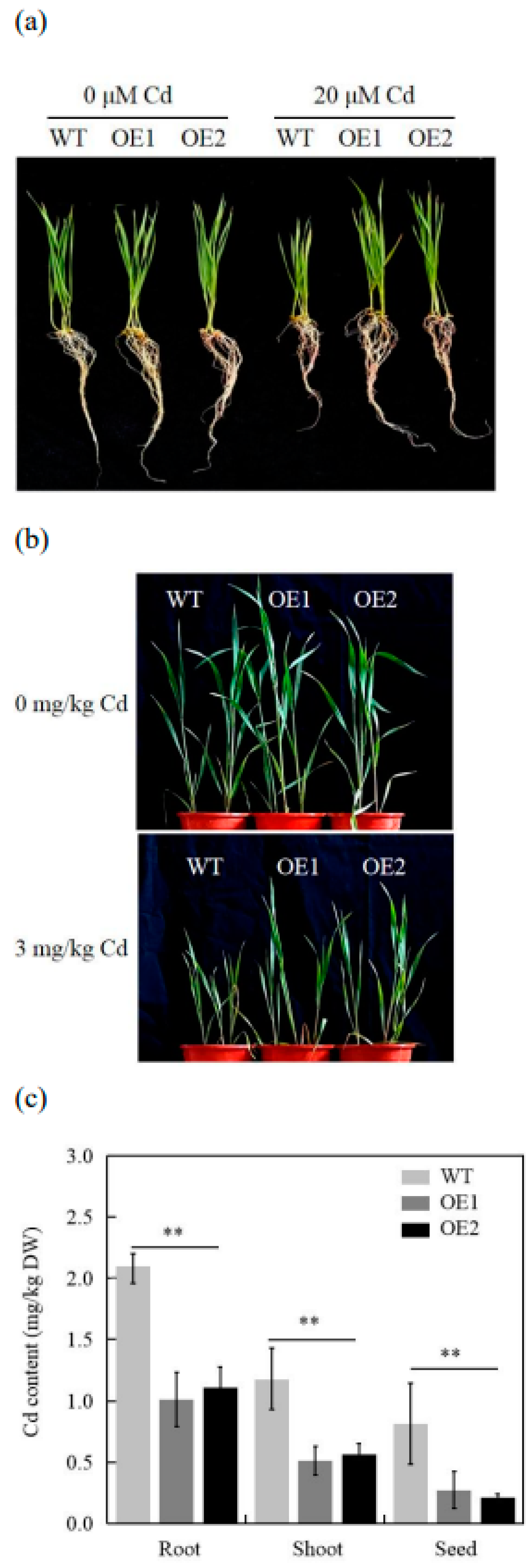
2.3. Overexpression of TaCOPT3D in Wheat Increased Cd Tolerance
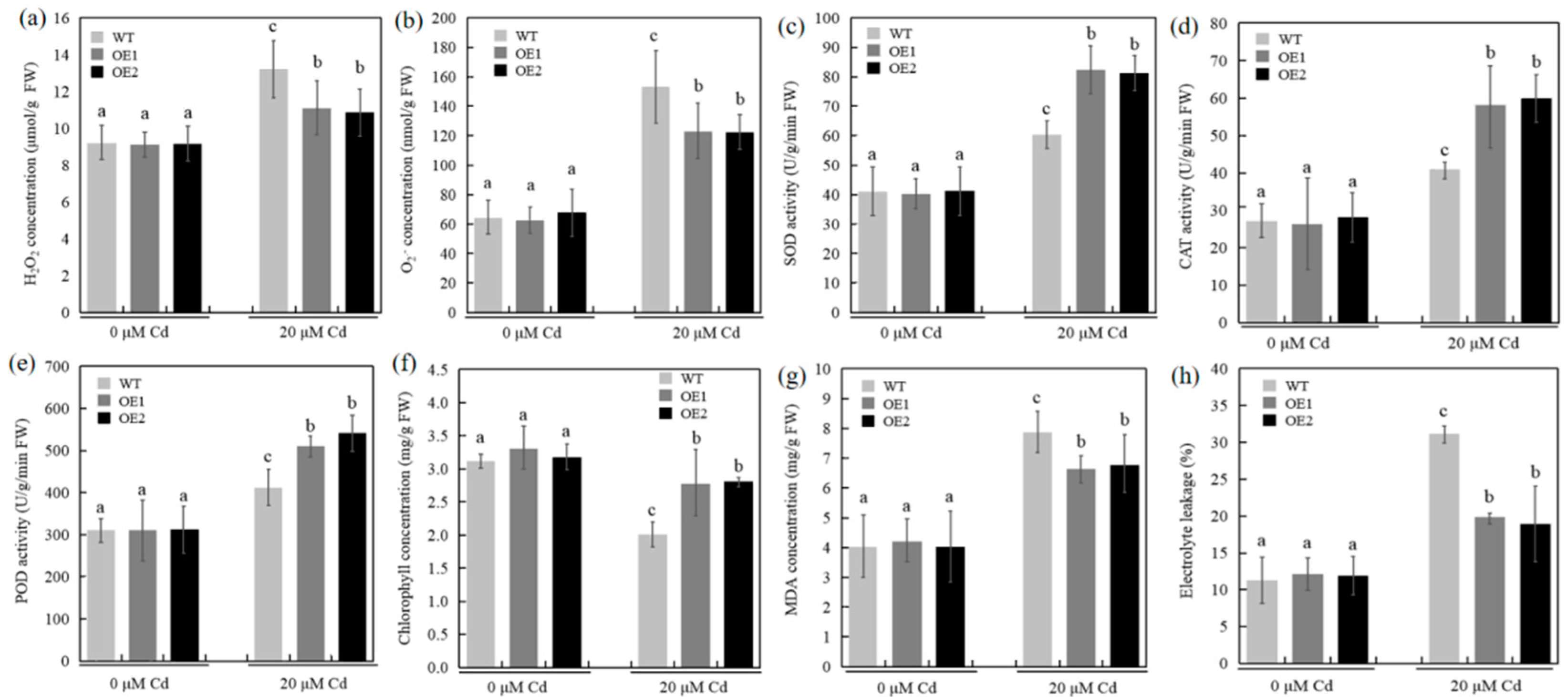
2.4. Overexpression of TaCOPT3D Enhanced the Antioxidant Capacity in Transgenic Wheat under Cd Stress
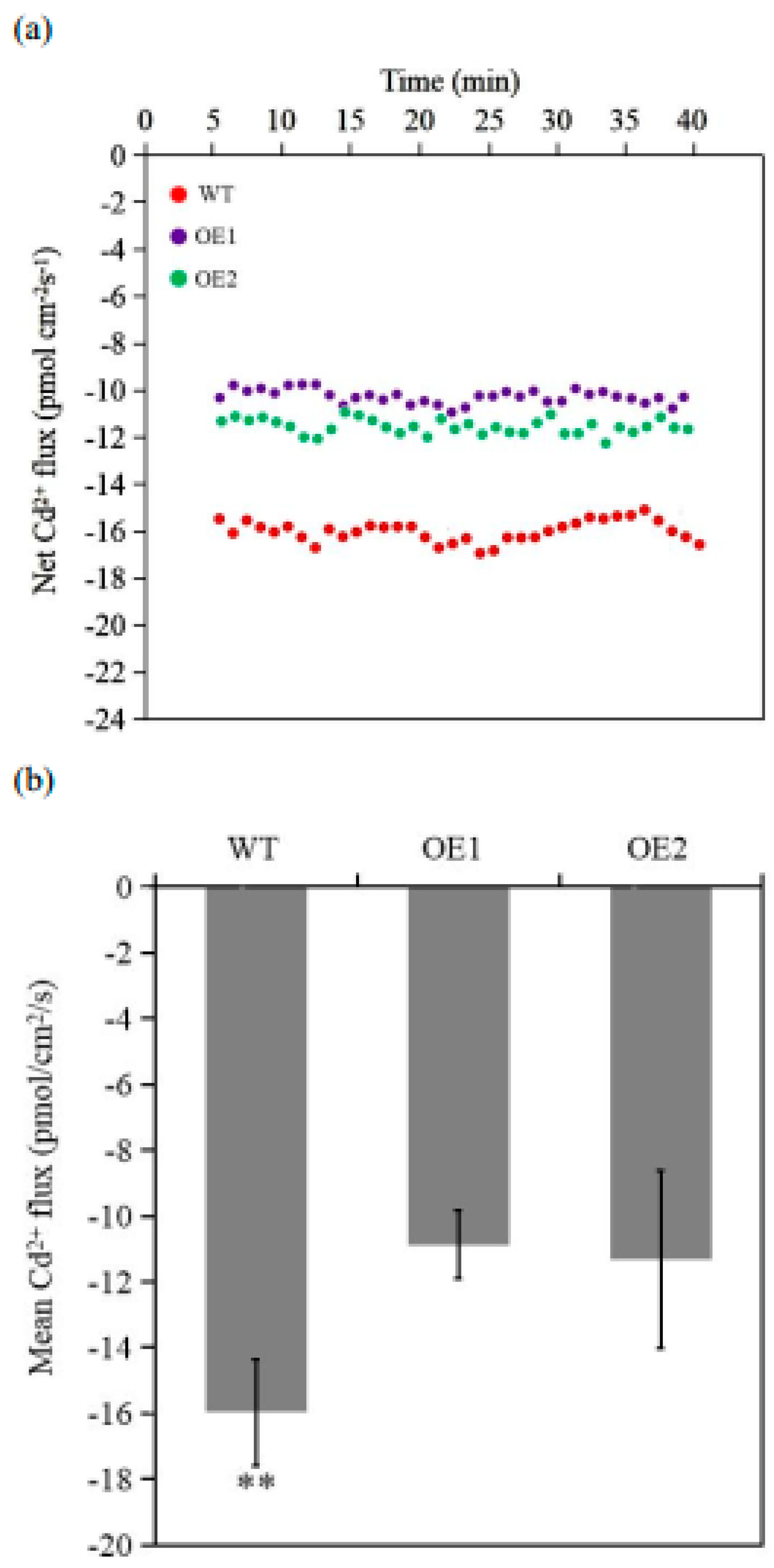
2.5. TaCPOT3D Influences the Cd2+ Flux in Wheat Roots
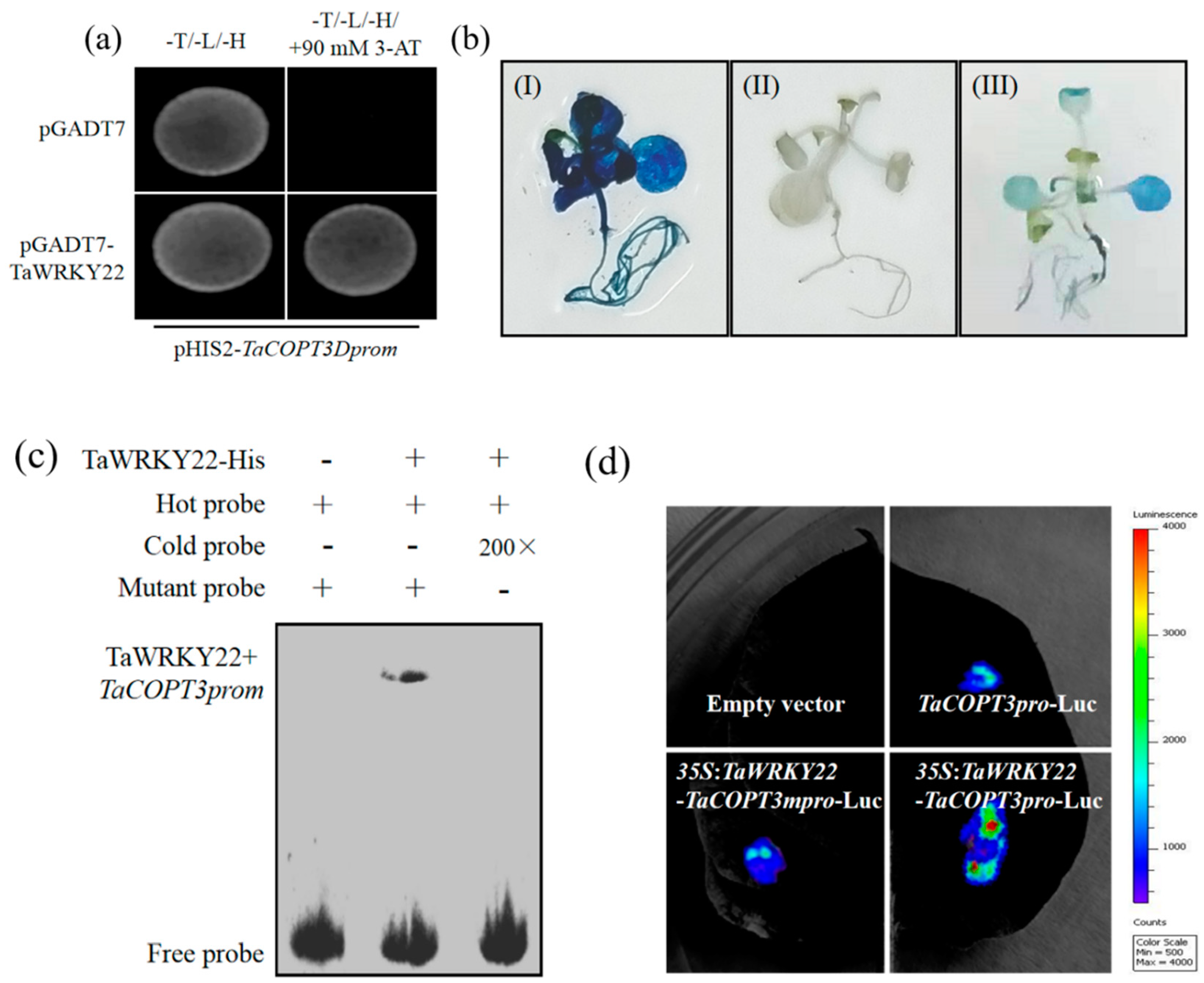
2.6. TaWRKY22 Binds to TaCPOT3D Promoter
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Cloning of Coding Sequence of TaCOPT3D
4.3. Analysis of Gene Expression
4.4. Gene Cloning and Plant Transformation
4.5. Detection of Antioxidant Enzyme Activity
4.6. Measurements of Cd2+ Flux
4.7. Measurement of Cd Contents
4.8. Yeast One-Hybrid (Y1H) Assay
4.9. Electrophoretic Mobility Shift Assay (EMSA)
4.10. Transient Expression Assays in Tobacco Leaves
4.11. Statistical Analysis
4.12. Primers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akindele, E.O.; Omisakin, D.A.; Oni, O.A.; Aliu, O.O.; Omoniyi, G.E.; Akinpelu, O.T. Heavy metal toxicity in the water column and benthic sediments of a degraded tropical stream. Ecotoxicol. Environ. Saf. 2020, 190, 110153. [Google Scholar] [CrossRef]
- Ye, Y.; Li, Z.; Xing, D. Nitric oxide promotes MPK6–mediated caspase–3–like activation in cadmium–induced Arabidopsis thaliana programmed cell death. Plant Cell Environ. 2013, 36, 1–15. [Google Scholar] [CrossRef]
- Yuan, H.M.; Huang, X. Inhibition of root meristem growth by cadmium involves nitric oxide–mediated repression of auxin accumulation and signalling in Arabidopsis. Plant Cell Environ. 2016, 39, 120–135. [Google Scholar] [CrossRef]
- Dias, M.; Monteiro, C.; Moutinho–Pereira, J.; Correia, C.; Gonçalves, B.; Santos, C. Cadmium toxicity affects photosynthesis and plant growth at different levels. Acta Physiol. Plant. 2014, 35, 1281–1289. [Google Scholar] [CrossRef]
- Nada, E.; Ferjani, B.A.; Ali, R.; Imed, M.; Makki, B. Cadmium–induced growth inhibition and extension of biochemical parameters in Almond seedlings grown solution culture. Acta Physiol. Plant. 2007, 29, 57–62. [Google Scholar] [CrossRef]
- Ogawa, I.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Time course analysis of gene regulation under cadmium stress in rice. Plant Soil 2007, 325, 97. [Google Scholar] [CrossRef]
- Xu, J.; Sun, J.H.; Du, L.G.; Liu, X.J. Comparative transcriptome analysis of cadmium responses in Solanum nigrum and Solanum torvum. New Phytol. 2012, 196, 110–124. [Google Scholar] [CrossRef]
- Peng, F.; Wang, C.; Zhu, J.S.; Zeng, J.; Kang, H.Y.; Fan, X.; Sha, L.N.; Zhang, H.Q.; Zhou, Y.H.; Wang, Y. Expression of TpNRAMP5; a metal transporter from Polish wheat (Triticum polonicum L.), enhances the accumulation of Cd, Co and Mn in transgenic Arabidopsis plants. Planta 2018, 247, 1395–1406. [Google Scholar] [CrossRef]
- Yamaji, N.; Sasaki, A.; Xia, J.; Yokosho, K.; Ma, J. A node–based switch for preferential distribution of manganese in rice. Nat. Commun. 2013, 4, 2442. [Google Scholar] [CrossRef]
- Castaings, L.; Caquot, A.; Loubet, S.; Curie, C. The high–affinity metal transporters NRAMP1 and IRT1 team up to take up iron under sufcient metal provision. Sci. Rep. 2016, 6, 37222. [Google Scholar] [CrossRef] [Green Version]
- Nocito, F.F.; Lancilli, C.; Dendena, B.; Lucchini, G.; Sacchi, G.A. Cadmium retention in rice roots is influenced by cadmium availability, chelation and translocation. Plant Cell Environ. 2011, 34, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Inoue, H.; Mizuno, D.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. OsYSL2 is a rice metal–nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 2004, 39, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Alam, M.M.; Nahar, K.; Mohsin, S.M.; Bhuyan, M.H.M.B.; Parvin, K.; Hawrylak–Nowak, B.; Fujit, M. Silicon–induced antioxidant defense and methylglyoxal detoxification works coordinately in alleviating nickel toxicity in Oryza sativa L. Ecotoxicology 2019, 28, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; Laohavisit, A.; Mortimer, J.C.; Shabala, L.; Swarbreck, S.M.; Shabala, S.; Davies, J.M. Annexin 1 regulates the H2O2–induced calcium signature in Arabidopsis thaliana roots. Plant J. 2014, 77, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Gayomba, S.R.; Rutzke, M.A.; Craft, E.; Kochian, L.V.; Vatamaniuk, O.K. COPT6 is a plasma membrane transporter that functions in copper homeostasis in Arabidopsis and is a novel target of SQUAMOSA promoter–binding protein–like 7. J. Biol. Chem. 2012, 287, 33252–33267. [Google Scholar] [CrossRef]
- Sanz, A.; Pike, S.; Khan, M.A.; Carrió–Seguí, À.; Mendoza–Cózatl, D.G.; Peñarrubia, L.; Gassmann, W. Copper uptake mechanism of Arabidopsis thaliana high–affinity COPT transporters. Protoplasma 2019, 256, 161–170. [Google Scholar] [CrossRef]
- Tiwari, M.; Venkatachalam, P.; Penarrubia, L.; Sahi, S.V. COPT2, a plasma membrane located copper transporter; is involved in the uptake of Au in Arabidopsis. Sci. Rep. 2017, 7, 11430. [Google Scholar] [CrossRef]
- Perea–García, A.; García–Molina, N.; Andrés–Colás, N.; Vera–Sirera, F.; Pérez–Amador, M.A.; Puig, S.; Peñarrubia, L. Arabidopsis copper transport protein COPT2 participates in the cross talk between iron deficiency responses and low–phosphate signaling. Plant Physiol. 2013, 162, 180–194. [Google Scholar] [CrossRef]
- Yuan, M.; Li, X.H.; Xiao, J.H.; Wang, S.P. Molecular and functional analyses of COPT/Ctr–type copper transporter–like gene family in rice. BMC Plant Biol. 2011, 11, 69. [Google Scholar] [CrossRef]
- Wei, J.L.; Liao, S.S.; Li, M.Z.; Zhu, B.; Wang, H.C.; Gu, L.; Yin, H.Y.; Du, X.Y. AetSRG1 contributes to the inhibition of wheat Cd accumulation by stabilizing phenylalanine ammonia lyase. J. Hazard. Mater. 2022, 428, 128226. [Google Scholar] [CrossRef]
- Sui, F.Q.; Chang, J.D.; Tang, Z.; Liu, W.J.; Huang, X.Y.; Zhao, F.J. Nramp5 expression and functionality likely explain higher cadmium uptake in rice than in wheat and maize. Plant Soil 2018, 433, 377–389. [Google Scholar] [CrossRef]
- Pedas, P.; Ytting, C.K.; Fuglsang, A.T.; Jahn, T.P.; Schjoerring, J.K.; Husted, S. Manganese efficiency in barley: Identification and characterization of the metal ion transporter HvIRT1. Plant Physiol. 2008, 148, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Gaedeke, N.; Klein, M.; Kolukisaoglu, U.; Forestier, C.; Müller, A.; Ansorge, M.; Becker, D.; Mamnun, Y.; Kuchler, K.; Schulz, B.; et al. The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J. 2001, 20, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Q.; Liu, L.F.; Jiang, Y.J.; Zhang, B.C.; Gao, Y.P.; Liu, X.L.; Lin, Q.S.; Ling, H.Q.; Zhou, Y.H. Disruption of secondary wall cellulose biosynthesis alters cadmium translocation and tolerance in rice plants. Mol. Plant 2013, 6, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef]
- Carrió–Seguí, A.; Garcia–Molina, A.; Sanz, A.; Peñarrubia, L. Defective copper transport in the copt5 mutant affects cadmium tolerance. Plant Cell Physiol. 2015, 56, 442–454. [Google Scholar] [CrossRef]
- Heo, D.H.; Baek, I.J.; Kang, H.J.; Kim, J.H.; Chang, M.; Kang, C.M.; Yun, C.W. Cd2+ binds to Atx1 and affects the physical interaction between Atx1 and Ccc2 in Saccharomyces cerevisiae. Biotechnol. Lett. 2012, 34, 303–307. [Google Scholar] [CrossRef]
- Han, Y.; Ling, Q.; Dong, F.Q.; de Dios, V.R.; Li, Z.; Zhang, W.; Huo, T.T.; Chen, Y.; Hu, X.Y.; Wang, X.H.; et al. Iron and copper micronutrients influences cadmium accumulation in rice grains by altering its transport and allocation. Sci. Total Environ. 2021, 777, 146118. [Google Scholar] [CrossRef]
- Liang, S.; Xiong, W.; Yin, C.; Xie, X.; Jin, Y.J.; Zhang, S.; Yang, B.; Ye, G.; Chen, S.; Luan, W.J. Overexpression of OsARD1 improves submergence; drought; and salt tolerances of seedling through the enhancement of ethylene synthesis in rice. Front. Plant Sci. 2019, 10, 1088. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, Z.Q.; Yokosho, K.; Ding, B.; Fan, W.; Gong, Q.Q.; Xin, G.; Wu, Y.R.; Yang, J.L.; Ma, J.F.; et al. Transcription factor WRKY22 promotes aluminum tolerance via activation of OsFRDL4 expression and enhancement of citrate secretion in rice (Oryza sativa). New Phytol. 2018, 219, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Brand, L.H.; Fischer, N.M.; Harter, K.; Kohlbacher, O.; Wanke, D. Elucidating the evolutionary conserved DNA–binding specificities of WRKY transcription factors by molecular dynamics and in vitro binding assays. Nucleic Acids Res. 2013, 41, 9764–9778. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.Z.; Li, M.Z.; Wang, H.C.; Zhu, B.; Gu, L.; Du, X.Y.; Ren, M.J. TaWRKY70 positively regulates TaCAT5 enhanced Cd tolerance in transgenic Arabidopsis. Environ. Exp. Bot. 2021, 190, 104591. [Google Scholar] [CrossRef]
- Sheng, Y.B.; Yan, X.X.; Huang, Y.; Han, Y.Y.; Zhang, C.; Ren, Y.B.; Fan, T.T.; Xiao, F.M.; Liu, Y.S.; Cao, S.Q. The WRKY transcription factor; WRKY13, activates PDR8 expression to positively regulate cadmium tolerance in Arabidopsis. Plant Cell Environ. 2019, 42, 891–903. [Google Scholar] [CrossRef]
- Wang, J.; Duan, X.C.; Wang, Y.Z.; Sheng, J.J. Transcriptomic and physiological analyses of Miscanthus lutarioriparius in response to plumbum stress. Ind. Crop Prod. 2022, 176, 114305. [Google Scholar] [CrossRef]
- Shim, D.; Hwang, J.; Lee, J.; Lee, S.; Choi, Y.; An, G.; Martinoia, E.; Lee, Y. Orthologs of the class A4 heat shock transcription factor HsfA4a confer cadmium tolerance in wheat and rice. Plant Cell 2009, 21, 4031–4043. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real–time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Wang, H.C.; Wei, J.L.; Li, M.Z.; Liao, S.S.; Zhu, B.; Yin, H.Y.; Du, X.Y. TaHSP17.8 regulates TaJUB1D in the management of low cadmium accumulation in wheat. J. Exp. Bot. 2020, 184, 104374. [Google Scholar] [CrossRef]
- Zhang, W.W.; Song, J.F.; Yue, S.Q.; Duan, K.X.; Yang, H.Q. MhMAPK4 from Malus hupehensis Rehd. decreases cell death in tobacco roots by controlling Cd2+ uptake. Ecotox. Environ. Safe. 2019, 168, 230–240. [Google Scholar] [CrossRef]
- Lin, T.T.; Yang, W.N.; Lu, W.; Wang, Y.; Qi, X.T. Transcription factors PvERF15 and PvMTF–1 form a cadmium stress transcriptional pathway. Plant Physiol. 2017, 173, 1565–1573. [Google Scholar] [CrossRef]
- Shang, Y.; Yan, L.; Liu, Z.Q.; Cao, Z.; Mei, C.; Xin, Q.; Wu, F.Q.; Wang, X.F.; Du, S.Y.; Jiang, T.; et al. The Mg–chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA–responsive genes of inhibition. Plant Cell 2010, 22, 1909–1935. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, H.; He, F.; Du, X.; Ren, M.; Bao, Y. The TaWRKY22–TaCOPT3D Pathway Governs Cadmium Uptake in Wheat. Int. J. Mol. Sci. 2022, 23, 10379. https://doi.org/10.3390/ijms231810379
Liu X, Wang H, He F, Du X, Ren M, Bao Y. The TaWRKY22–TaCOPT3D Pathway Governs Cadmium Uptake in Wheat. International Journal of Molecular Sciences. 2022; 23(18):10379. https://doi.org/10.3390/ijms231810379
Chicago/Turabian StyleLiu, Xiaojuan, Hongcheng Wang, Fang He, Xuye Du, Mingjian Ren, and Yinguang Bao. 2022. "The TaWRKY22–TaCOPT3D Pathway Governs Cadmium Uptake in Wheat" International Journal of Molecular Sciences 23, no. 18: 10379. https://doi.org/10.3390/ijms231810379
APA StyleLiu, X., Wang, H., He, F., Du, X., Ren, M., & Bao, Y. (2022). The TaWRKY22–TaCOPT3D Pathway Governs Cadmium Uptake in Wheat. International Journal of Molecular Sciences, 23(18), 10379. https://doi.org/10.3390/ijms231810379






