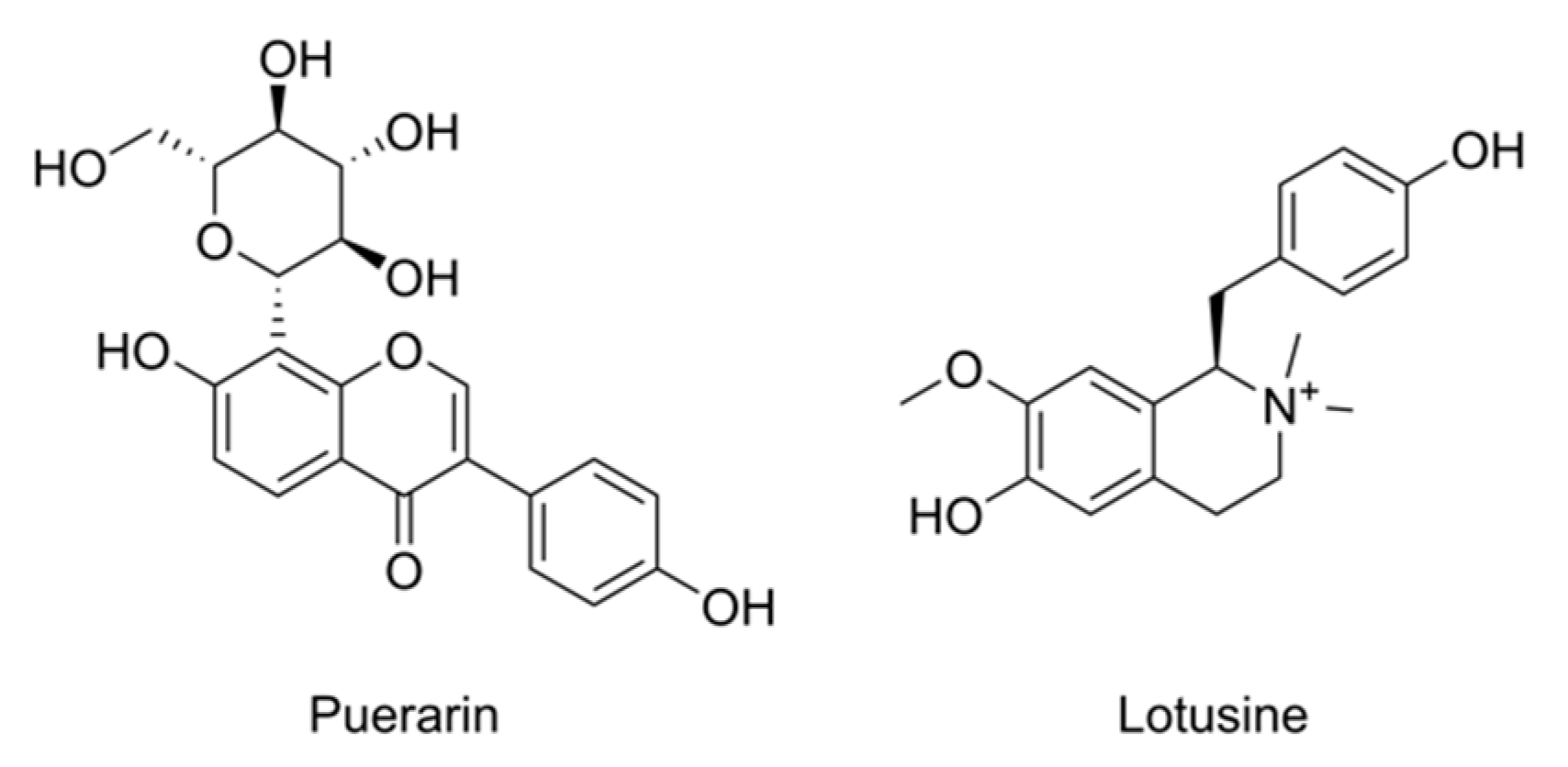
Insight into Lotusine and Puerarin in Repairing Alcohol-Induced Metabolic Disorder Based on UPLC-MS/MS
Abstract
:1. Introduction
2. Results
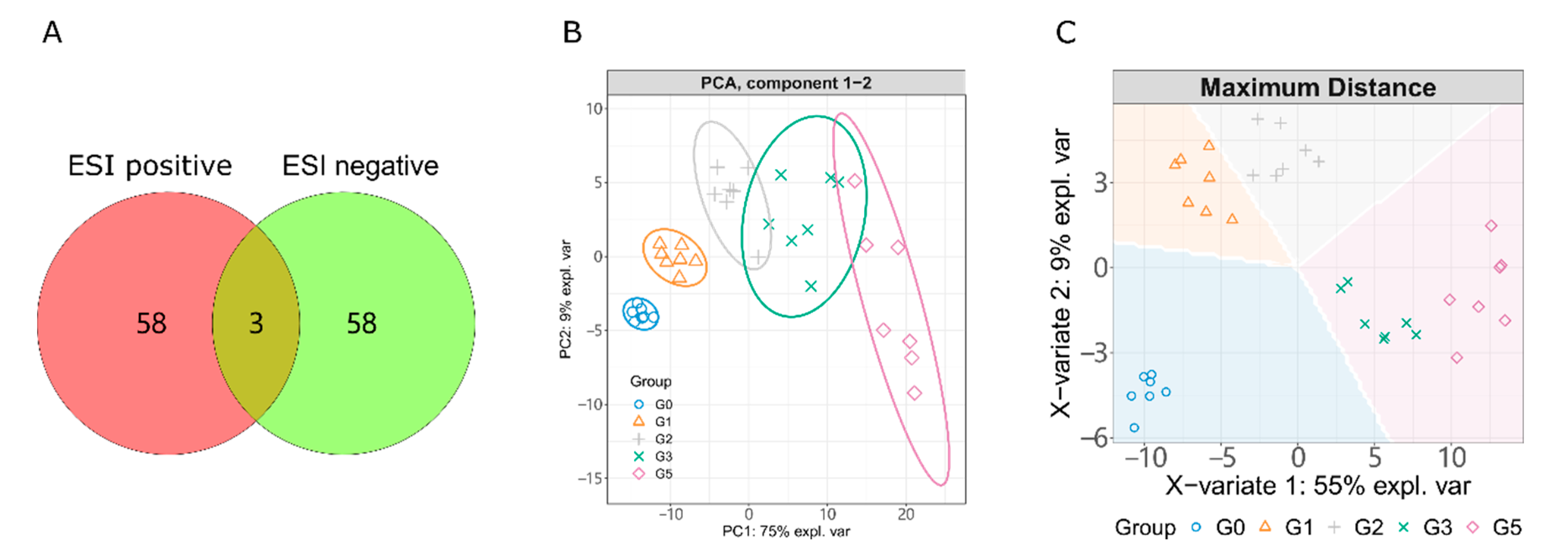
2.1. LC-MS/MS Based Metabolism Analysis
2.2. Alcohol Induced Metabolism Variation
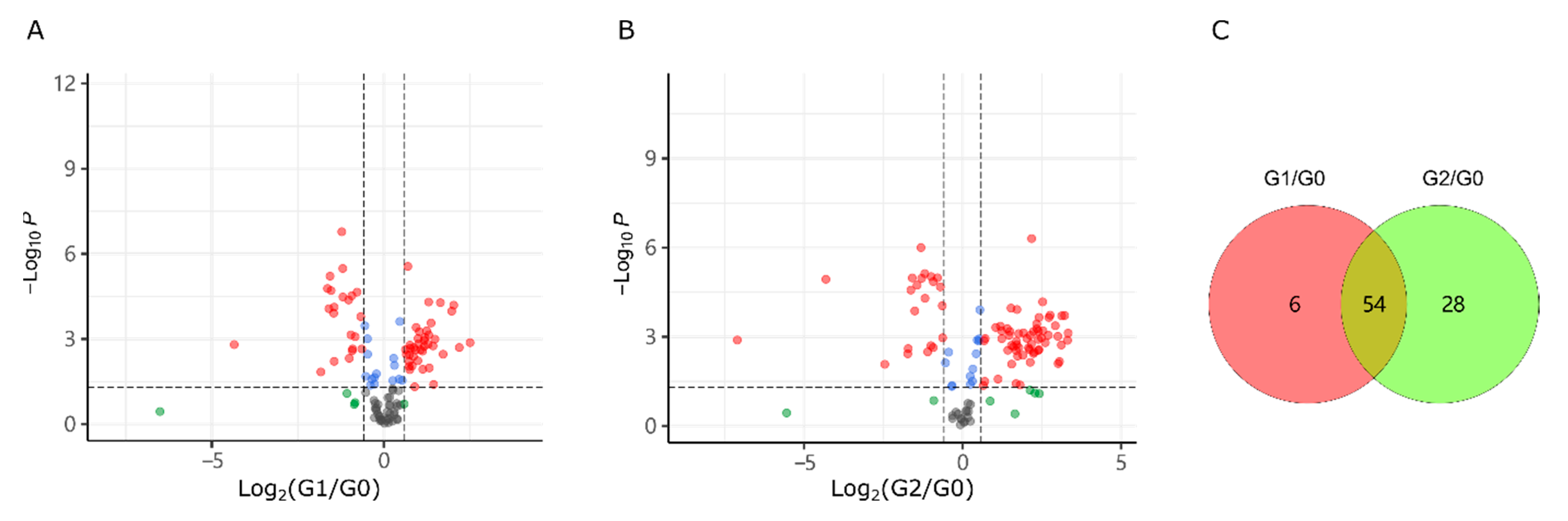
2.3. Puerarin Repair against Metabolism Disorder Induced by Alcohol
2.4. Lotusine Repair against Metabolism Disorder Induced by Alcohol
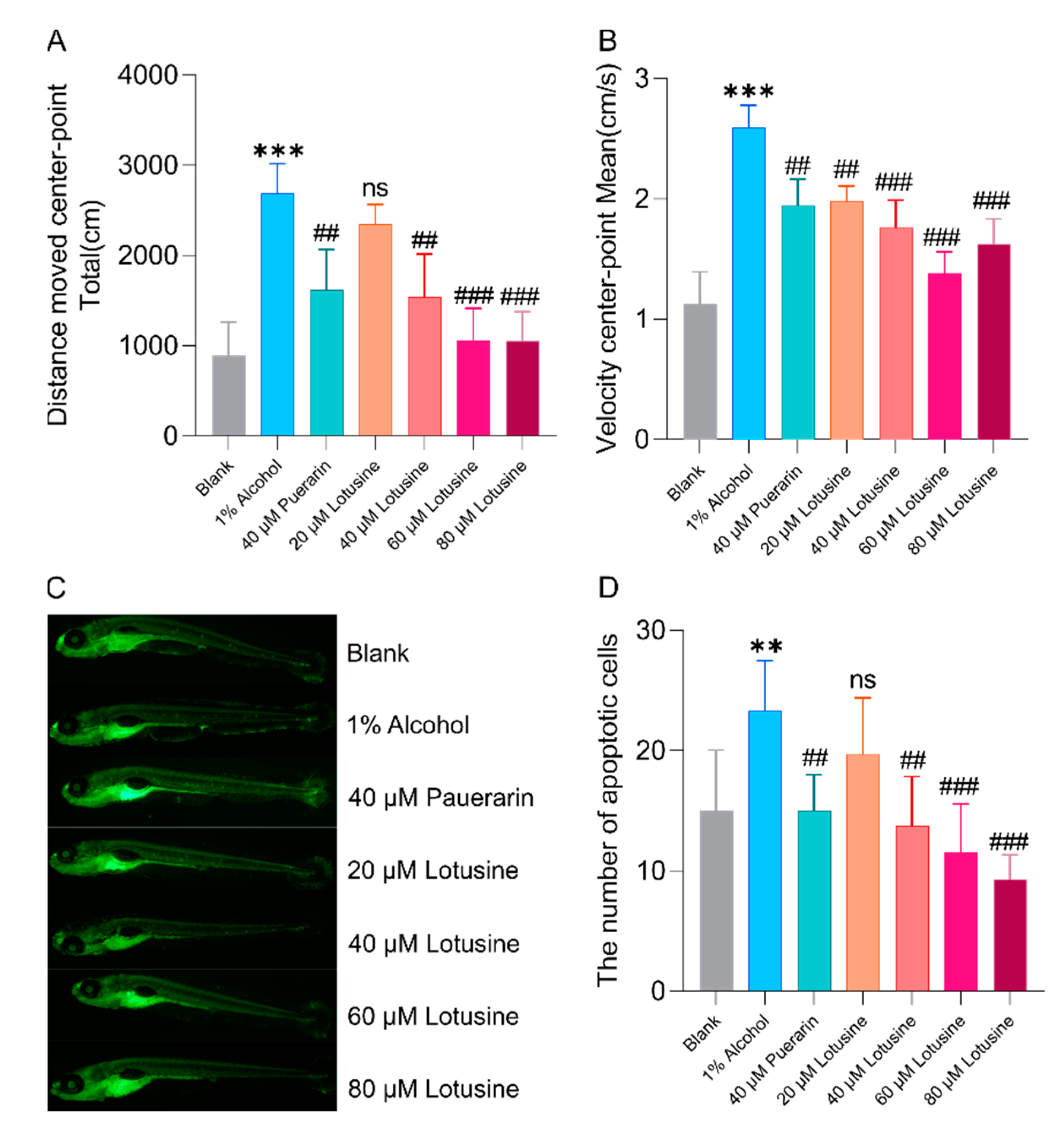
2.5. Neuroprotective Effects against Alcohol-Induced Damage
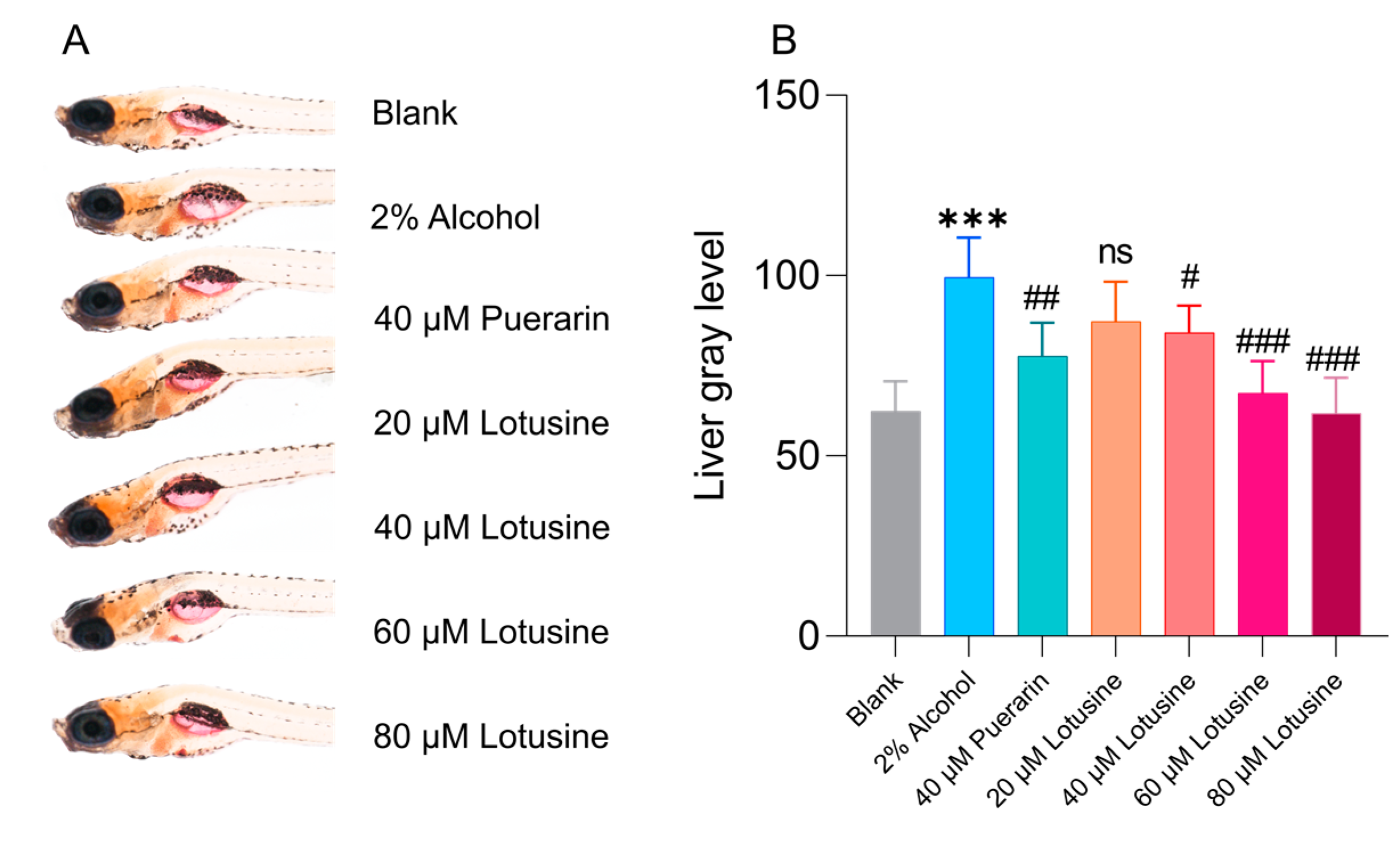
2.6. Hepatoprotective Effects against Alcohol-Induced Damage
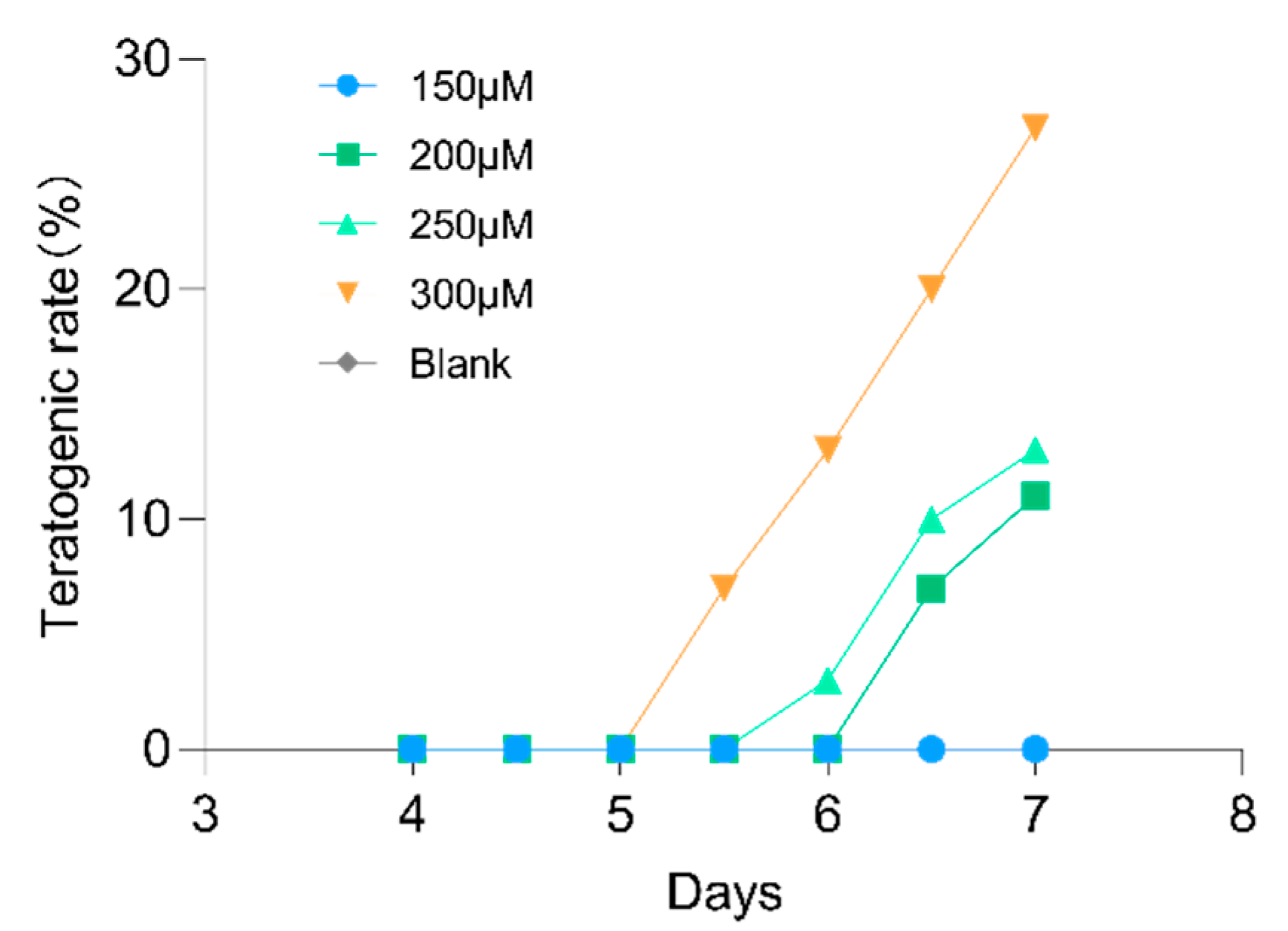
2.7. Lotusine Toxicity Evaluation
3. Discussion
3.1. The Scene of Alcohol-Induced Metabolic Disorder in Zebrafish Larvae
3.2. Puerarin Repair against Alcohol-Induced Metabolism Disorder
3.3. Lotusine Repair against Alcohol-Induced Metabolism Disorder
3.4. Neuroprotection and Hepatoprotection of Puerarin and Lostusine
3.5. Limitations
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Zebrafish Maintaince and Embryo Collection
4.3. Preparation of Metablic Samples
4.4. LC-MS/MS Analysis
4.5. LC-MS Data Analysis
4.6. Evaluation of Neuronal Apoptosis
4.7. Zebrafish Locomotor Behavior Measurements
4.8. Evaluation of Alcohol-Induced Fatty Liver
4.9. Lotusine Toxicity Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Regional Office for South-East Asia Preventing Violence against Children 2020: Report for the WHO South-East Asia Region; World Health Organization. Regional Office for South-East Asia: New Delhi, India, 2021; ISBN 978-92-9022-866-0. [Google Scholar]
- Ohashi, K.; Pimienta, M.; Seki, E. Alcoholic liver disease: A current molecular and clinical perspective. Liver Res. 2018, 2, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.D.; Parry, C.; Rehm, J. Chronic diseases and conditions related to alcohol use. Alcohol Res. Curr. Rev. 2013, 35, 155–173. [Google Scholar]
- Hong, D.-G.; Song, G.Y.; Eom, C.B.; Ahn, J.-H.; Kim, S.M.; Shim, A.; Han, Y.-H.; Roh, Y.-S.; Han, C.Y.; Bae, E.J.; et al. Loss of ERdj5 exacerbates oxidative stress in mice with alcoholic liver disease via suppressing Nrf2. Free Radic. Biol. Med. 2022, 184, 42–52. [Google Scholar] [CrossRef]
- Hyun, J.; Han, J.; Lee, C.; Yoon, M.; Jung, Y. Pathophysiological aspects of alcohol metabolism in the liver. Int. J. Mol. Sci. 2021, 22, 5717. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-A.; Ke, B.-J.; Cheng, S.-C.; Lee, C.-L. Red quinoa bran extract prevented alcoholic fatty liver disease via increasing antioxidative system and repressing fatty acid synthesis factors in mice fed alcohol liquid diet. Molecules 2021, 26, 6973. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Cho, J.; Kim, A.; Eom, T.K.; Kim, J.-S.; Kim, J.-B.; Doh, Y.H.; Jee, Y. Phenolic acid and flavonoid-rich fraction of Sasa quelpaertensis Nakai leaves prevent alcohol induced fatty liver through AMPK activation. J. Ethnopharmacol. 2018, 224, 335–348. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, D.; Lin, X.-X.; Zhao, Q.; Guo, J.; Chen, L.; Zhang, W.; Xiao, J.; Lian, G.-H.; Peng, S.-F.; et al. The LRP6 functional mutation Rs2302685 contributes to individual susceptibility to alcoholic liver injury related to the Wnt/β-catenin-TCF1-CYP2E1 signaling pathway. Arch. Toxicol. 2019, 93, 1679–1695. [Google Scholar] [CrossRef]
- McIntosh, C.; Chick, J. Alcohol and the nervous system. J. Neurol. Neurosurg. Psychiatry 2004, 75, iii16–iii21. [Google Scholar] [CrossRef]
- Behl, T.; Yadav, H.N.; Sharma, P.L. Alcoholic neuropathy: Involvement of multifaceted signalling mechanisms. Curr. Mol. Pharmacol. 2021, 14, 2–10. [Google Scholar] [CrossRef]
- Julian, T.; Glascow, N.; Syeed, R.; Zis, P. Alcohol-related peripheral neuropathy: A systematic review and meta-analysis. J. Neurol. 2019, 266, 2907–2919. [Google Scholar] [CrossRef]
- Madaan, P.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Yadav, S.; Kaur, S.; Bhatia, S.; Al-Harrasi, A.; Abdellatif, A.A.H.; et al. Exploring the therapeutic potential of targeting purinergic and orexinergic receptors in alcoholic neuropathy. Neurotox. Res. 2022, 40, 646–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Yang, L.-F.; Lai, C.-L.; Yin, S.-J. Acetaldehyde enhances alcohol sensitivity and protects against alcoholism: Evidence from alcohol metabolism in subjects with variant ALDH2*2 gene allele. Biomolecules 2021, 11, 1183. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, X.; Shen, Y.; Hao, Y.; Cui, W.; Li, W.; Zhang, X.; Lv, H.; Li, X.; Hou, Y.; et al. Altered gut microbiota and metabolites profile are associated with reduced bone metabolism in ethanol-induced osteoporosis. Cell Prolif. 2022, 55, e13245. [Google Scholar] [CrossRef]
- Irwin, C.; van Reenen, M.; Mason, S.; Mienie, L.J.; Wevers, R.A.; Westerhuis, J.A.; Reinecke, C.J. The 1H-NMR-based metabolite profile of acute alcohol consumption: A metabolomics intervention study. PLoS ONE 2018, 13, e0196850. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.C.; Guthrie, S.; Xie, C.-Y.; Mai, K.; Lee, D.Y.; Lumeng, L.; Li, T.-K. Isoflavonoid compounds extracted from pueraria lobata suppress alcohol preference in a pharmacogenetic Rat model of alcoholism. Alcohol. Clin. Exp. Res. 1996, 20, 659–663. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Yuan, M.-H.; Zhang, C.-Y.; Liu, H.-M.; Liu, J.-R.; Wei, A.-L.; Ye, Q.; Zeng, B.; Li, M.-F.; Guo, Y.-P.; et al. Puerariae Lobatae radix flavonoids and puerarin alleviate alcoholic liver injury in zebrafish by regulating alcohol and lipid metabolism. Biomed. Pharmacother. 2021, 134, 111121. [Google Scholar] [CrossRef] [PubMed]
- Noh, B.-K.; Lee, J.K.; Jun, H.; Lee, J.H.; Jia, Y.; Hoang, M.-H.; Kim, J.-W.; Park, K.-H.; Lee, S.-J. Restoration of autophagy by puerarin in ethanol-treated hepatocytes via the activation of AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2011, 414, 361–366. [Google Scholar] [CrossRef]
- Cui, S.Q.; Wang, Q.; Zheng, Y.; Xiao, B.; Sun, H.W.; Gu, X.L.; Zhang, Y.C.; Fu, C.H.; Dong, P.X.; Wang, X.M. Puerarin protects against damage to spatial learning and memory ability in mice with chronic alcohol poisoning. Braz. J. Med. Biol. Res. 2015, 48, 515–522. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, T.; Guo, M. Current Advances in the Metabolomics Study on Lotus Seeds. Front. Plant Sci. 2016, 7, 891. [Google Scholar] [CrossRef]
- Harishkumar, R.; Selvaraj, C.I. Lotusine, an alkaloid from Nelumbo nucifera (Gaertn.), attenuates doxorubicin-induced toxicity in embryonically derived H9c2 cells. Vitro Cell. Dev. Biol.-Anim. 2020, 56, 367–377. [Google Scholar] [CrossRef]
- Ryu, T.-K.; Roh, E.; Shin, H.-S.; Kim, J.-E. Inhibitory effect of lotusine on solar UV-induced matrix metalloproteinase-1 expression. Plants 2022, 11, 773. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, P.-D.; Quetin-Leclercq, J.; Muller, M.; Ly Huong, D.T.; Pham, H.T.; Kestemont, P. Developmental toxicity of clerodendrum cyrtophyllum turcz ethanol extract in zebrafish embryo. J. Ethnopharmacol. 2021, 267, 113538. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, Q.; Wang, J.; Zhuang, K.; Jin, H.; Liu, K. Progress in using zebrafish as a toxicological model for traditional chinese medicine. J. Ethnopharmacol. 2022, 282, 114638. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kang, H.; Kwon, B.; Lee, J.P.; Lee, J.; Choi, K. Zebrafish (Danio rerio) as a model organism for screening nephrotoxic chemicals and related mechanisms. Ecotoxicol. Environ. Saf. 2022, 242, 113842. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Han, X.; Yu, T.; Liu, Y.; Zhang, H.; Mao, H.; Hu, C.; Xu, X. Isoprocarb causes neurotoxicity of zebrafish embryos through oxidative stress-induced apoptosis. Ecotoxicol. Environ. Saf. 2022, 242, 113870. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef]
- Chen, C.-J.; Lee, D.-Y.; Yu, J.; Lin, Y.-N.; Lin, T.-M. Recent advances in LC-MS-based metabolomics for clinical biomarker discovery. Mass Spectrom. Rev. 2022, 41, e21785. [Google Scholar] [CrossRef]
- Hu, L.; Liu, J.; Zhang, W.; Wang, T.; Zhang, N.; Lee, Y.H.; Lu, H. Functional metabolomics decipher biochemical functions and associated mechanisms underlie small-molecule metabolism. Mass Spectrom. Rev. 2020, 39, 417–433. [Google Scholar] [CrossRef]
- Picart-Armada, S.; Fernández-Albert, F.; Vinaixa, M.; Rodríguez, M.A.; Aivio, S.; Stracker, T.H.; Yanes, O.; Perera-Lluna, A. Null diffusion-based enrichment for metabolomics data. PLoS ONE 2017, 12, e0189012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.; Cheng, J.; Leng, X.; Hu, X.; Ao, Y. The potential role of necroptosis in clinical diseases (review). Int. J. Mol. Med. 2021, 47, 89. [Google Scholar] [CrossRef]
- Nangle, M.; Keast, J. Semaphorin 3A inhibits growth of adult sympathetic and parasympathetic neurones via distinct cyclic nucleotide signalling pathways. Br. J. Pharmacol. 2011, 162, 1083–1095. [Google Scholar] [CrossRef]
- Li, M.; Lu, Y.; Li, Y.; Tong, L.; Gu, X.; Meng, J.; Zhu, Y.; Wu, L.; Feng, M.; Tian, N.; et al. Transketolase deficiency protects the liver from DNA damage by increasing levels of ribose 5-phosphate and nucleotides. Cancer Res. 2019, 79, 3689–3701. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Xue, Y.; Yu, H.; Zhang, Y.; Tao, L.; Yang, Y.; Qian, Z. Effects of puerarin on spatial Learning and memory function in mice with acute alcohol consumption: An evaluation based upon firing rate and oxygen saturation analysis. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2019, 28, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Penetar, D.M.; Toto, L.H.; Farmer, S.L.; Lee, D.Y.-W.; Ma, Z.; Liu, Y.; Lukas, S.E. The isoflavone puerarin reduces alcohol intake in heavy drinkers: A pilot study. Drug Alcohol Depend. 2012, 126, 251–256. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, R.; Wang, X.; Bao, X.; Lu, C. Roles of necroptosis in alcoholic liver disease and hepatic pathogenesis. Cell Prolif. 2022, 55, e13193. [Google Scholar] [CrossRef]
- Roychowdhury, S.; McMullen, M.R.; Pisano, S.G.; Liu, X.; Nagy, L.E. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology 2013, 57, 1773–1783. [Google Scholar] [CrossRef]
- Régnier, M.; Polizzi, A.; Guillou, H.; Loiseau, N. Sphingolipid metabolism in non-alcoholic fatty liver diseases. Biochimie 2019, 159, 9–22. [Google Scholar] [CrossRef]
- Jun-Long, H.; Yi, L.; Bao-Lian, Z.; Jia-Si, L.; Ning, Z.; Zhou-Heng, Y.; Xue-Jun, S.; Wen-Wu, L. Necroptosis signaling pathways in stroke: From mechanisms to therapies. Curr. Neuropharmacol. 2018, 16, 1327–1339. [Google Scholar] [CrossRef]
- Blot, F.G.C.; Krijnen, W.H.J.J.; Den Hoedt, S.; Osório, C.; White, J.J.; Mulder, M.T.; Schonewille, M. Sphingolipid metabolism governs purkinje cell patterned degeneration in Atxn1[82Q]/+ mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2016969118. [Google Scholar] [CrossRef]
- Villanueva, J.A.; Esfandiari, F.; Wong, D.H.; Ahmad, I.; Melnyk, S.; James, S.J.; Halsted, C.H. Abnormal transsulfuration and glutathione metabolism in the micropig model of alcoholic liver disease. Alcohol. Clin. Exp. Res. 2006, 30, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K.; Watabe, M.; Nakaki, T. Regulation of neuronal glutathione synthesis. J. Pharmacol. Sci. 2008, 108, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The human metabolome database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liigand, J.; Tian, S.; Arndt, D.; Greiner, R.; Wishart, D.S. CFM-ID 4.0: More accurate ESI-MS/MS spectral prediction and compound identification. Anal. Chem. 2021, 93, 11692–11700. [Google Scholar] [CrossRef]
- Djoumbou-Feunang, Y.; Pon, A.; Karu, N.; Zheng, J.; Li, C.; Arndt, D.; Gautam, M.; Allen, F.; Wishart, D.S. CFM-ID 3.0: Significantly improved ESI-MS/MS prediction and compound identification. Metabolites 2019, 9, 72. [Google Scholar] [CrossRef]
- Rohart, F.; Gautier, B.; Singh, A.; Cao, K.-A.L. MixOmics: An R package for ′omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef]
- Picart-Armada, S.; Fernández-Albert, F.; Vinaixa, M.; Yanes, O.; Perera-Lluna, A. FELLA: An R package to enrich metabolomics data. BMC Bioinform. 2018, 19, 538. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Zhu, Z.-J.; Ren, S.-P.; Deng, Y.-C.; Xu, J.-Y.; Zhang, S.-M.; Gao, J.-M.; Zhang, Q. Metabolomic navigated citrus waste repurposing to restore amino acids disorder in neural lesion. Food Chem. 2022, 387, 132933. [Google Scholar] [CrossRef]
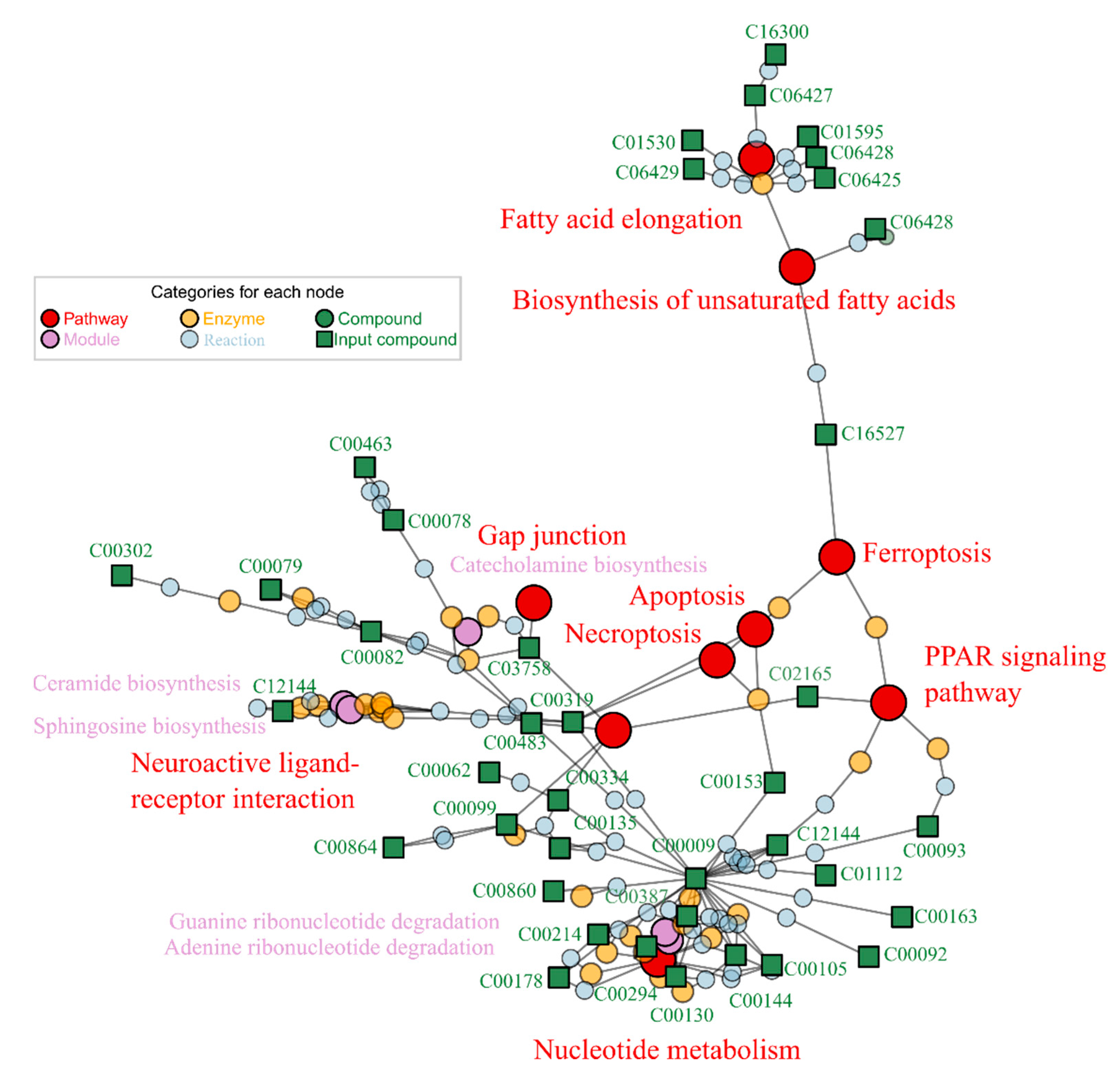
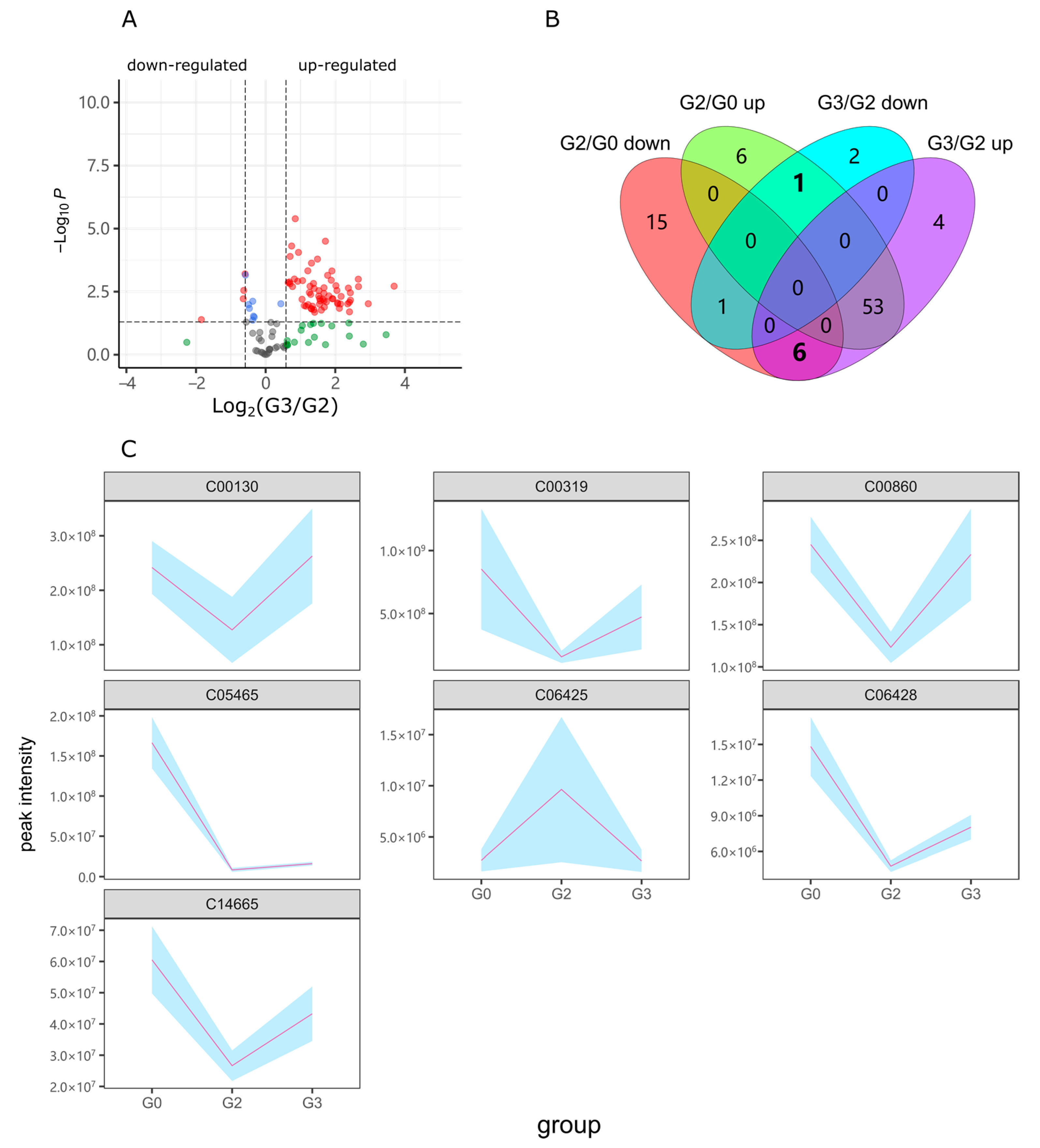
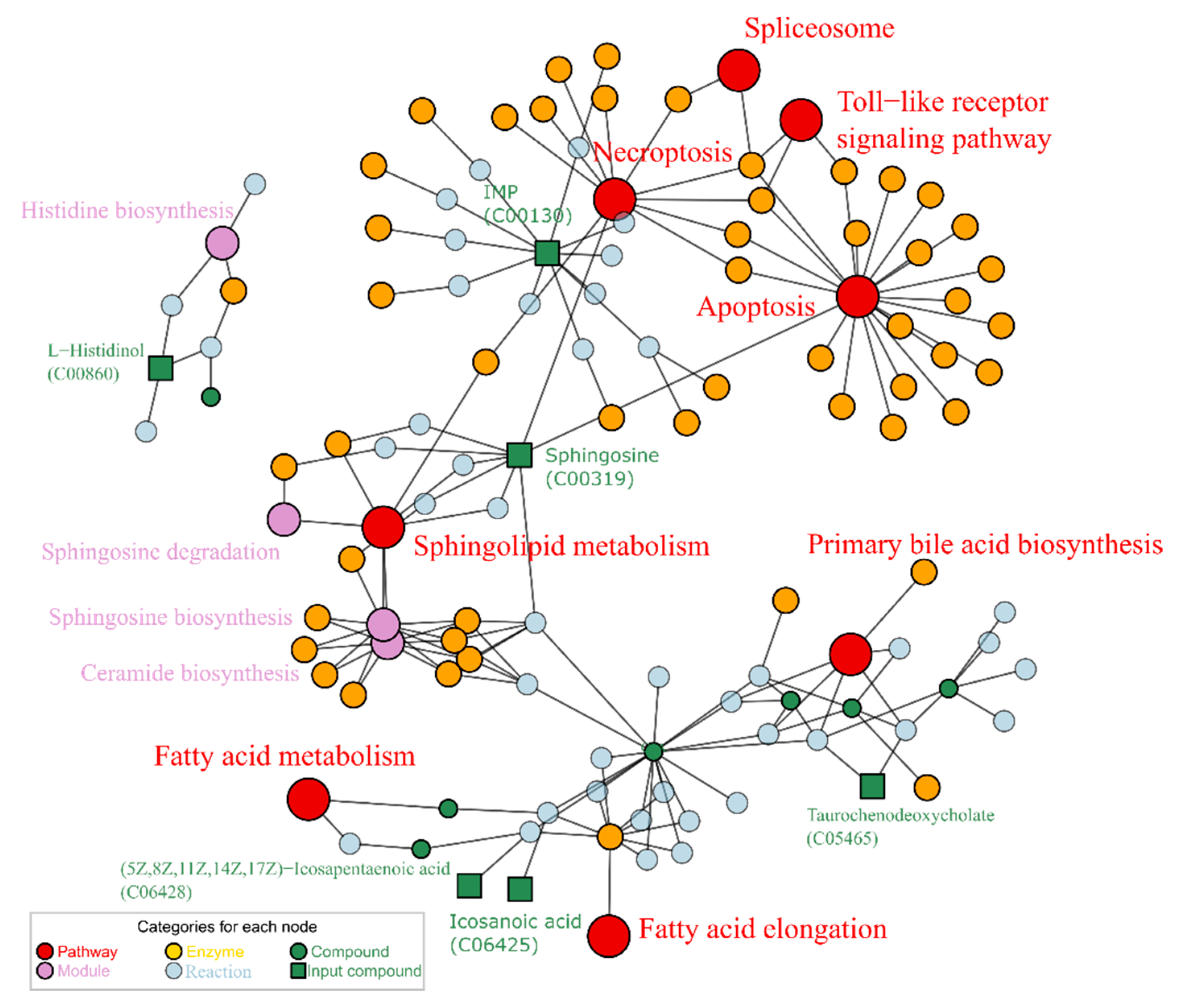

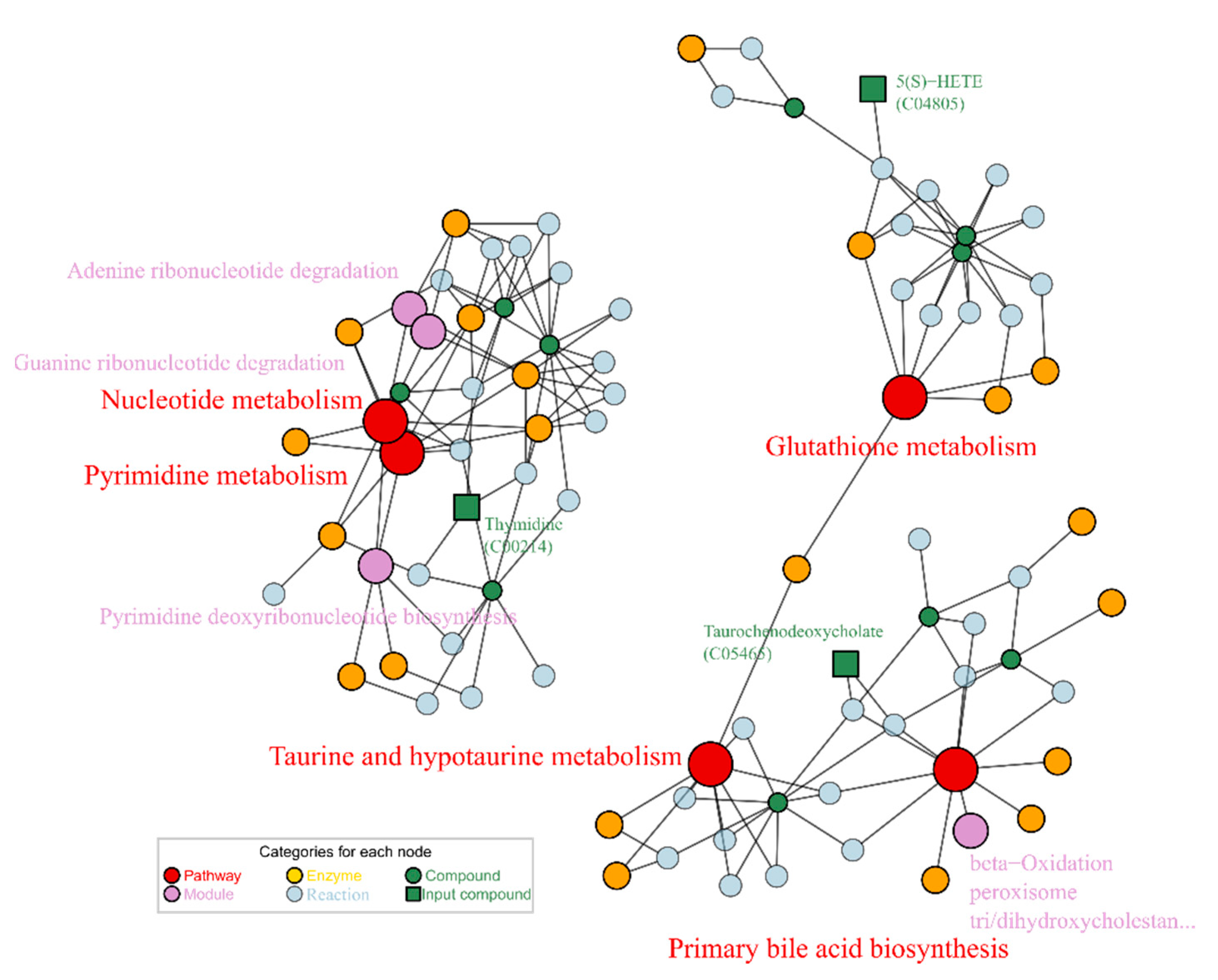
| KEGG ID | KEGG Name | −log (p) G2/G0 | −log (p) G1/G0 |
|---|---|---|---|
| map00062 | Fatty acid elongation | 6.00 | 4.19 |
| map04210 | Apoptosis | 6.00 | 6.00 |
| map04216 | Ferroptosis | 6.00 | Not hit |
| map04217 | Necroptosis | 6.00 | 6.00 |
| map04310 | Wnt signaling pathway | 6.00 | Not hit |
| map03013 | Nucleocytoplasmic transport | 5.28 | Not hit |
| map01040 | Biosynthesis of unsaturated fatty acids | 4.95 | 2.7 |
| map01232 | Nucleotide metabolism | 3.81 | 5.65 |
| map04080 | Neuroactive ligand-receptor interaction | 3.03 | Not hit |
| map00730 | Thiamine metabolism | 2.67 | 4.21 |
| map04540 | Gap junction | 2.56 | Not hit |
| map03320 | PPAR signaling pathway | 2.09 | Not hit |
| map03410 | Base excision repair | Not hit | 2.33 |
| M00099 | Sphingosine biosynthesis | 4.56 | 6.00 |
| M00958 | Adenine ribonucleotide degradation, AMP => Urate | 4.20 | 6.00 |
| M00094 | ceramide biosynthesis | 4.06 | 6.00 |
| M00959 | Guanine ribonucleotide degradation, GMP => Urate | 3.32 | 5.15 |
| M00042 | Catecholamine biosynthesis, tyrosine => dopamine => noradrenaline => adrenaline | 2.83 | Not hit |
| M00892 | UDP-N-acetyl-D-glucosamine biosynthesis, eukaryotes, glucose => UDP-GlcNAc | 2.48 | Not hit |
| M00096 | C5 isoprenoid biosynthesis, non-mevalonate pathway | Not hit | 3.44 |
| M00170 | C4-dicarboxylic acid cycle, phosphoenolpyruvate carboxykinase type | Not hit | 2.69 |
| M00014 | Glucuronate pathway (uronate pathway) | Not hit | 2.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhang, X.; Yan, L.; Zhang, Z.; Wei, J.; Li, L.; Zhang, Q. Insight into Lotusine and Puerarin in Repairing Alcohol-Induced Metabolic Disorder Based on UPLC-MS/MS. Int. J. Mol. Sci. 2022, 23, 10385. https://doi.org/10.3390/ijms231810385
Xu J, Zhang X, Yan L, Zhang Z, Wei J, Li L, Zhang Q. Insight into Lotusine and Puerarin in Repairing Alcohol-Induced Metabolic Disorder Based on UPLC-MS/MS. International Journal of Molecular Sciences. 2022; 23(18):10385. https://doi.org/10.3390/ijms231810385
Chicago/Turabian StyleXu, Jiayang, Xiaoyue Zhang, Lili Yan, Zhichao Zhang, Jing Wei, Luqi Li, and Qiang Zhang. 2022. "Insight into Lotusine and Puerarin in Repairing Alcohol-Induced Metabolic Disorder Based on UPLC-MS/MS" International Journal of Molecular Sciences 23, no. 18: 10385. https://doi.org/10.3390/ijms231810385
APA StyleXu, J., Zhang, X., Yan, L., Zhang, Z., Wei, J., Li, L., & Zhang, Q. (2022). Insight into Lotusine and Puerarin in Repairing Alcohol-Induced Metabolic Disorder Based on UPLC-MS/MS. International Journal of Molecular Sciences, 23(18), 10385. https://doi.org/10.3390/ijms231810385







