LAG-3 as a Potent Target for Novel Anticancer Therapies of a Wide Range of Tumors
Abstract
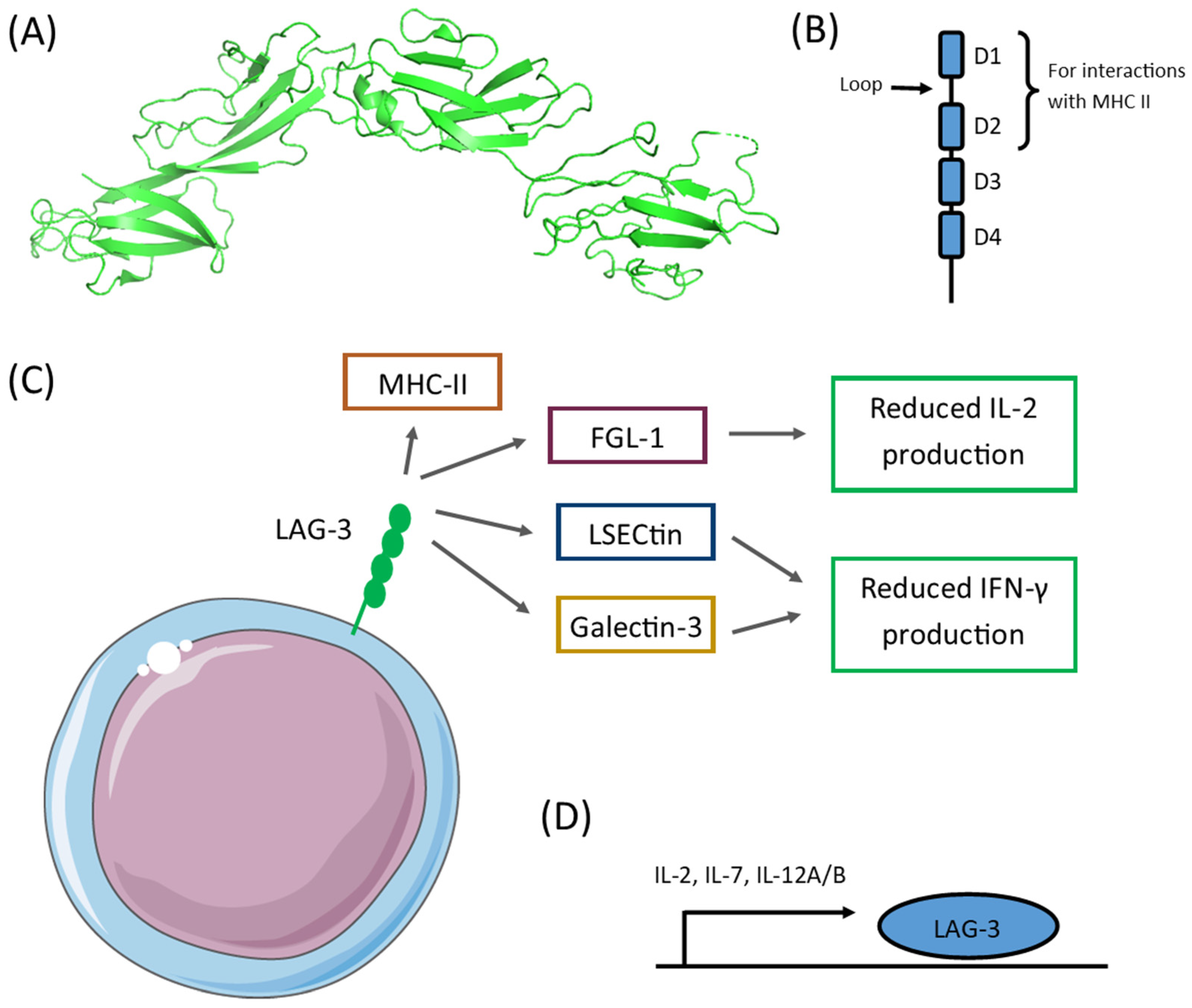
:1. Introduction
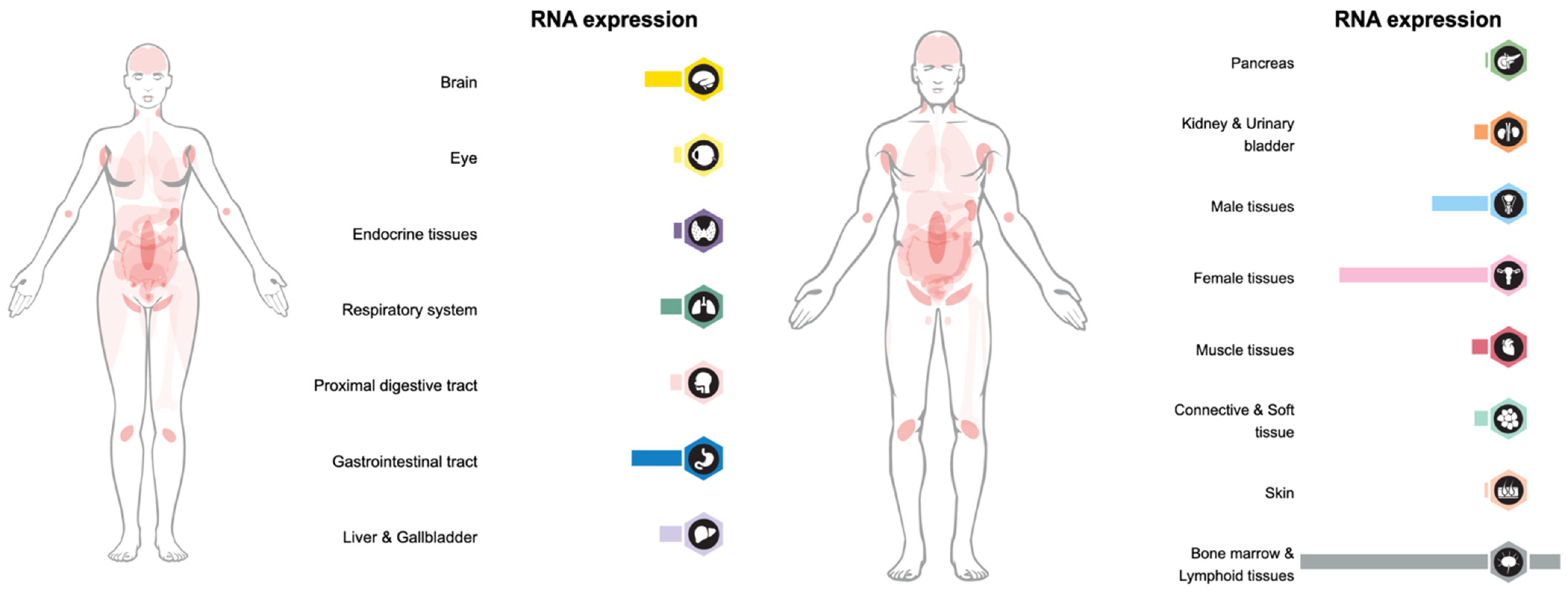
2. LAG-3 Expression
3. LAG-3 in Neoplasms
3.1. Brain Tumors
3.2. Head and Neck Tumors
3.3. Endocrine Tumors
3.4. Lung Tumors
3.5. Abdominal Tumors
3.6. Uro-Genital Tumors
3.7. Breast Tumors
3.8. Skin Tumors
3.9. Lymphoid Tumors
4. Anti-LAG-3 Antibody-Based Therapies
5. Anti-LAG-3 Cell-Based Therapies
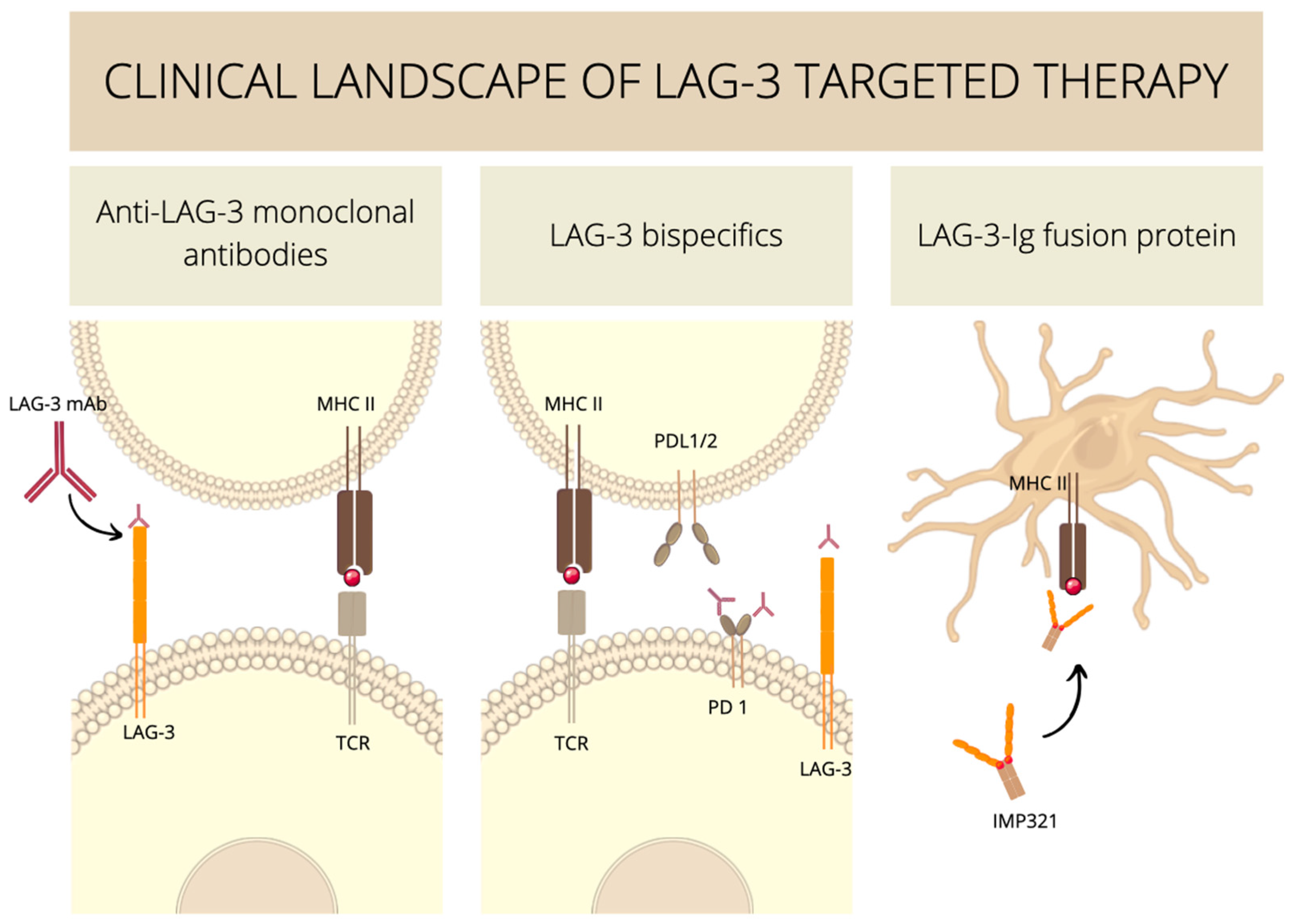
6. Experimental Medicine Involving LAG-3 Clinical Trials
6.1. Anti-LAG-3 Monoclonal Antibodies
6.2. Anti-LAG-3 Bispecifics
6.3. Soluble LAG-3–Ig Fusion Proteins
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lecocq, Q.; Keyaerts, M.; Devoogdt, N.; Breckpot, K. The Next-Generation Immune Checkpoint LAG-3 and Its Therapeutic Potential in Oncology: Third Time’s a Charm. Int. J. Mol. Sci. 2021, 22, 75. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Sugiura, D.; Okazaki, I.M.; Okazaki, T. LAG-3: From molecular functions to clinical applications. J. Immunother. Cancer 2020, 8, e001014. [Google Scholar] [CrossRef] [PubMed]
- Bruniquel, D.; Borie, N.; Hannier, S.; Triebel, F. Regulation of expression of the human lymphocyte activation gene-3 (LAG-3) molecule, a ligand for MHC class II. Immunogenetics 1998, 48, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Y.; Forbes, K.; Vignali, K.M.; Heale, B.S.; Saftig, P.; Hartmann, D.; Black, R.A.; Rossi, J.J.; Blobel, C.P.; et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J. 2007, 26, 494–504. [Google Scholar] [CrossRef]
- Huang, R.Y.; Francois, A.; McGray, A.R.; Miliotto, A.; Odunsi, K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology 2017, 6, e1249561. [Google Scholar] [CrossRef]
- Hemon, P.; Jean-Louis, F.; Ramgolam, K.; Brignone, C.; Viguier, M.; Bachelez, H.; Triebel, F.; Charron, D.; Aoudjit, F.; Al-Daccak, R.; et al. MHC Class II Engagement by Its Ligand LAG-3 (CD223) Contributes to Melanoma Resistance to Apoptosis. J. Immunol. 2011, 186, 5173–5183. [Google Scholar] [CrossRef]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG3. Cell 2019, 176, 334. [Google Scholar] [CrossRef]
- Xu, F.; Liu, J.; Liu, D.; Liu, B.; Wang, M.; Hu, Z.; Du, X.; Tang, L.; He, F. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer Res. 2014, 74, 3418–3428. [Google Scholar] [CrossRef]
- Ruvolo, P.P. Galectin 3 as a guardian of the tumor microenvironment. Biochim. Biophys. Acta 2016, 1863, 427–437. [Google Scholar] [CrossRef]
- Graydon, C.G.; Mohideen, S.; Fowke, K.R. LAG3’s Enigmatic Mechanism of Action. Front. Immunol. 2021, 11, 3444. [Google Scholar] [CrossRef]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef]
- Woo, S.R.; Li, N.; Bruno, T.C.; Forbes, K.; Brown, S.; Workman, C.; Drake, C.G.; Vignali, D.A.A. Differential subcellular localization of the regulatory T-cell protein LAG-3 and the coreceptor CD4. Eur. J. Immunol. 2010, 40, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.P.; Tang, X.Y.; Xiong, Y.L.; Zheng, K.F.; Liu, Y.J.; Shi, X.G.; Lv, Y.; Jiang, T.; Ma, N.; Zhao, J.B. Immune Checkpoint LAG3 and Its Ligand FGL1 in Cancer. Front. Immunol. 2022, 12, 5962. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Lee, S.J.; Park, C.-G.; Lee, Y.S.; Chun, T. Trafficking of LAG-3 to the surface on activated T cells via its cytoplasmic domain and protein kinase C signaling. J. Immunol. 2014, 193, 3101–3112. [Google Scholar] [CrossRef] [PubMed]
- Maçon-Lemaître, L.; Triebel, F. The negative regulatory function of the lymphocyte-activation gene-3 co-receptor (CD223) on human T cells. Immunology 2005, 115, 170–178. [Google Scholar] [CrossRef]
- Ma, C.; Sun, X.; Shen, D.; Sun, Y.; Guan, N.; Qi, C. Ectopic expression of LAG-3 in non–small-cell lung cancer cells and its clinical significance. J. Clin. Lab. Anal. 2020, 34, 23244. [Google Scholar] [CrossRef]
- Xu, J.; Shen, D.; Zhang, T.; Wang, J.; De, W.; Zhang, J. Lymphocyte-activated gene-3 (LAG3) protein expressed in tumor-infiltrating lymphocytes of colorectal cancer. Pol. J. Pathol. 2022, 72, 324–330. [Google Scholar] [CrossRef]
- Workman, C.J.; Vignali, D.A.A. Negative Regulation of T Cell Homeostasis by Lymphocyte Activation Gene-3 (CD223). J. Immunol. 2005, 174, 688–695. [Google Scholar] [CrossRef]
- Annunziato, F.; Manetti, R.; Tomasévic, I.; Giudizi, M.; Biagiotti, R.; Giannò, V.; Germano, P.; Mavilia, C.; Maggi, E.; Romagnani, S. Expression and release of LAG-3-encoded protein by human CD4+ T cells are associated with IFN-gamma production. FASEB J. 1996, 10, 769–776. [Google Scholar] [CrossRef]
- LAG-3 Modulation of Natural Killer Cell Immunoregulatory Function|The Journal of Immunology. Available online: https://www.jimmunol.org/content/202/1_Supplement/76.7 (accessed on 10 July 2022).
- Marçais, A.; Cherfils-Vicini, J.; Viant, C.; Degouve, S.; Viel, S.; Fenis, A.; Rabilloud, J.; Mayol, K.; Tavares, A.; Bienvenu, J.; et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat. Immunol. 2014, 15, 749–757. [Google Scholar] [CrossRef]
- Marhelava, K.; Pilch, Z.; Bajor, M.; Graczyk-Jarzynka, A.; Zagozdzon, R. Targeting Negative and Positive Immune Checkpoints with Monoclonal Antibodies in Therapy of Cancer. Cancers 2019, 11, 1756. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a Cancer Immunotherapy Target. Immunol. Rev. 2017, 276, 80. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, X.; Jin, T.; Tian, Y.; Dai, C.; Widarma, C.; Song, R.; Xu, F. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy. Signal Transduct. Target. Ther. 2020, 5, 250. [Google Scholar] [CrossRef]
- Workman, C.J.; Rice, D.S.; Dugger, K.J.; Kurschner, C.; Vignali, D.A.A. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3). Eur. J. Immunol. 2002, 32, 2255–2263. [Google Scholar] [CrossRef]
- Workman, C.J.; Wang, Y.; El Kasmi, K.C.; Pardoll, D.M.; Murray, P.J.; Drake, C.G.; Vignali, D.A.A. LAG-3 Regulates Plasmacytoid Dendritic Cell Homeostasis. J. Immunol. 2009, 182, 1885. [Google Scholar] [CrossRef]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2008, 10, 29–37. [Google Scholar] [CrossRef]
- Andreae, S.; Piras, F.; Burdin, N.; Triebel, F. Maturation and Activation of Dendritic Cells Induced by Lymphocyte Activation Gene-3 (CD223). J. Immunol. 2002, 168, 3874–3880. [Google Scholar] [CrossRef]
- Kisielow, M.; Kisielow, J.; Capoferri-Sollami, G.; Karjalainen, K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur. J. Immunol. 2005, 35, 2081–2088. [Google Scholar] [CrossRef]
- Lino, A.C.; Dang, V.D.; Lampropoulou, V.; Welle, A.; Joedicke, J.; Pohar, J.; Simon, Q.; Thalmensi, J.; Baures, A.; Flühler, V.; et al. LAG-3 Inhibitory Receptor Expression Identifies Immunosuppressive Natural Regulatory Plasma Cells. Immunity 2018, 49, 120–133.e9. [Google Scholar] [CrossRef]
- Huard, B.; Tournier, M.; Hercend, T.; Triebel, F.; Faure, F. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur. J. Immunol. 1994, 24, 3216–3221. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zhang, X.; Chen, F.; Pan, Q.; Phiphatwatchara, P.; Zeng, Y.; Chen, H. The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes Cancer 2018, 9, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Demeure, C.E.; Wolfers, J.; Martin-Garcia, N.; Gaulard, P.; Triebel, F. T Lymphocytes infiltrating various tumour types express the MHC class II ligand lymphocyte activation gene-3 (LAG-3): Role of LAG-3/MHC class II interactions in cell-cell contacts. Eur. J. Cancer 2001, 37, 1709–1718. [Google Scholar] [CrossRef]
- Ciraolo, E.; Althoff, S.; Ruß, J.; Rosnev, S.; Butze, M.; Pühl, M.; Frentsch, M.; Bullinger, L.; Na, I.K. Simultaneous Genetic Ablation of PD-1, LAG-3, and TIM-3 in CD8 T Cells Delays Tumor Growth and Improves Survival Outcome. Int. J. Mol. Sci. 2022, 23, 3207. [Google Scholar] [CrossRef] [PubMed]
- Grosso, J.F.; Kelleher, C.C.; Harris, T.J.; Maris, C.H.; Hipkiss, E.L.; De Marzo, A.; Anders, R.; Netto, G.; Getnet, D.; Bruno, T.C.; et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Investig. 2007, 117, 3383–3392. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Arasanz, H.; Fernández-Rubio, L.; Bocanegra, A.; Echaide, M.; Garnica, M.; Ramos, P.; Fernández-Hinojal, G.; Vera, R.; et al. Clinical landscape of LAG-3-targeted therapy. Immuno-Oncol. Technol. 2022, 14, 100079. [Google Scholar] [CrossRef]
- Galatro, T.F.; Holtman, I.R.; Lerario, A.M.; Vainchtein, I.D.; Brouwer, N.; Sola, P.R.; Veras, M.M.; Pereira, T.F.; Leite, R.E.P.; Möller, T.; et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci. 2017, 20, 1162–1171. [Google Scholar] [CrossRef]
- Tasic, B.; Menon, V.; Nguyen, T.N.; Kim, T.K.; Jarsky, T.; Yao, Z.; Levi, B.; Gray, L.T.; Sorensen, S.A.; Dolbeare, T.; et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat. Neurosci. 2016, 19, 335–346. [Google Scholar] [CrossRef]
- Mao, X.; Ou, M.T.; Karuppagounder, S.S.; Kam, T.I.; Yin, X.; Xiong, Y.; Ge, P.; Umanah, G.E.; Brahmachari, S.; Shin, J.H.; et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 2016, 353, 183776. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Mair, M.J.; Kiesel, B.; Feldmann, K.; Widhalm, G.; Dieckmann, K.; Wöhrer, A.; Müllauer, L.; Preusser, M.; Berghoff, A.S. LAG-3 expression in the inflammatory microenvironment of glioma. J. Neurooncol. 2021, 152, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.; Tomaszowski, K.H.; Marisetty, A.; Kong, L.Y.; Wei, J.; Duna, M.; Blumberg, K.; Ji, X.; Jacobs, C.; Fuller, G.N.; et al. Profiling of patients with glioma reveals the dominant immunosuppressive axis is refractory to immune function restoration. JCI Insight 2020, 5, 134386. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Du, Q.; Jin, J.; Wei, Y.; Lu, Y.; Li, Q. LAG3 and its emerging role in cancer immunotherapy. Clin. Transl. Med. 2021, 11, e365. [Google Scholar] [CrossRef]
- Harris-Bookman, S.; Mathios, D.; Martin, A.M.; Xia, Y.; Kim, E.; Xu, H.; Belcaid, Z.; Polanczyk, M.; Barberi, T.; Theodros, D.; et al. Expression of LAG-3 and efficacy of combination treatment with anti-LAG-3 and anti-PD-1 monoclonal antibodies in glioblastoma. Int. J. Cancer 2018, 143, 3201. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.W.; Mao, L.; Yu, G.T.; Bu, L.L.; Ma, S.R.; Liu, B.; Gutkind, J.S.; Kulkarni, A.B.; Zhang, W.F.; Sun, Z.J. LAG-3 confers poor prognosis and its blockade reshapes antitumor response in head and neck squamous cell carcinoma. Oncoimmunology 2016, 5, e1239005. [Google Scholar] [CrossRef]
- Botticelli, A.; Zizzari, I.G.; Scagnoli, S.; Pomati, G.; Strigari, L.; Cirillo, A.; Cerbelli, B.; Di Filippo, A.; Napoletano, C.; Scirocchi, F.; et al. The Role of Soluble LAG3 and Soluble Immune Checkpoints Profile in Advanced Head and Neck Cancer: A Pilot Study. J. Pers. Med. 2021, 11, 651. [Google Scholar] [CrossRef]
- Jie, H.B.; Gildener-Leapman, N.; Li, J.; Srivastava, R.M.; Gibson, S.P.; Whiteside, T.L.; Ferris, R.L. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br. J. Cancer 2013, 109, 2629–2635. [Google Scholar] [CrossRef]
- Camisaschi, C.; Casati, C.; Rini, F.; Perego, M.; De Filippo, A.; Triebel, F.; Parmiani, G.; Belli, F.; Rivoltini, L.; Castelli, C. LAG-3 expression defines a subset of CD4+CD25highFoxp3+ regulatory T cells that are expanded at tumor sites. J. Immunol. 2010, 184, 6545–6551. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Y.C.; Shen, C.K.; Ma, B.; Xu, W.B.; Wang, Q.F.; Zhang, Y.; Liao, T.; Wei, W.J.; Wang, Y. Immune Checkpoint Protein Expression Defines the Prognosis of Advanced Thyroid Carcinoma. Front. Endocrinol. 2022, 13, 630. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, X.; Pan, Y.; Xu, J.; Si, Y.; Min, Z.; Yu, B. A new risk factor indicator for papillary thyroid cancer based on immune infiltration. Cell Death Dis. 2021, 12, 51. [Google Scholar] [CrossRef]
- Giannini, R.; Moretti, S.; Ugolini, C.; MacErola, E.; Menicali, E.; Nucci, N.; Morelli, S.; Colella, R.; Mandarano, M.; Sidoni, A.; et al. Immune Profiling of Thyroid Carcinomas Suggests the Existence of Two Major Phenotypes: An ATC-Like and a PDTC-Like. J. Clin. Endocrinol. Metab. 2019, 104, 3557–3575. [Google Scholar] [CrossRef] [PubMed]
- Young, H.J.; Pharaon, R.; Bonjoc, K.-J.C.; Ally, F.; Yin, H.; Kang, R.; Gernon, T.; Maghami, E.; Chaudhry, A. Differential immune pathways in classic and mixed variants of anaplastic thyroid cancer. J. Clin. Oncol. 2020, 38, e18579. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Rugo, H.S.; O’neil, B.H.; Santoro, A.; Schellens, J.H.M.; Cohen, R.B.; Doi, T.; Ott, P.A.; Pishvaian, M.J.; Puzanov, I.; et al. Pembrolizumab for patients with PD-L1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Ann. Oncol. 2017, 28, v142. [Google Scholar] [CrossRef]
- Sun, H.; Dai, J.; Zhao, L.; Zhu, J.; Wang, H.; Chen, P.; Lu, H.; Chen, Q.; Zhang, Z.; Lu SJTU-Yale, H. Lymphocyte activation gene-3 is associated with programmed death-ligand 1 and programmed cell death protein 1 in small cell lung cancer. Ann. Transl. Med. 2021, 9, 1468. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhang, J.; Qin, Y.; Wu, Y.; Zhu, L.; Lu, L.; Tang, G.; Shen, Q. Increased expression of immunosuppressive molecules on intratumoral and circulating regulatory T cells in non-small-cell lung cancer patients. Am. J. Cancer Res. 2015, 5, 2190. [Google Scholar]
- Ma, Q.Y.; Huang, D.Y.; Zhang, H.J.; Wang, S.; Chen, X.F. Function and regulation of LAG3 on CD4+CD25- T cells in non-small cell lung cancer. Exp. Cell Res. 2017, 360, 358–364. [Google Scholar] [CrossRef]
- Datar, I.; Sanmamed, M.F.; Wang, J.; Henick, B.S.; Choi, J.; Badri, T.; Dong, W.; Mani, N.; Toki, M.; Mejías, L.D.; et al. Expression Analysis and Significance of PD-1, LAG-3, and TIM-3 in Human Non-Small Cell Lung Cancer Using Spatially Resolved and Multiparametric Single-Cell Analysis. Clin. Cancer Res. 2019, 25, 4663–4673. [Google Scholar] [CrossRef]
- Ding, L.; Getz, G.; Wheeler, D.A.; Mardis, E.R.; McLellan, M.D.; Cibulskis, K.; Sougnez, C.; Greulich, H.; Muzny, D.M.; Morgan, M.B.; et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008, 455, 1069–1075. [Google Scholar] [CrossRef]
- He, Y.; Yu, H.; Rozeboom, L.; Rivard, C.J.; Ellison, K.; Dziadziuszko, R.; Suda, K.; Ren, S.; Wu, C.; Hou, L.; et al. LAG-3 Protein Expression in Non–Small Cell Lung Cancer and Its Relationship with PD-1/PD-L1 and Tumor-Infiltrating Lymphocytes. J. Thorac. Oncol. 2017, 12, 814–823. [Google Scholar] [CrossRef]
- Song, L.; Wang, X.; Cheng, W.; Wu, Y.; Liu, M.; Liu, R.; Zhang, S.; Xia, H.; Liu, H.; Tai, X.; et al. Expression signature, prognosis value and immune characteristics of cathepsin F in non-small cell lung cancer identified by bioinformatics assessment. BMC Pulm. Med. 2021, 21, 420. [Google Scholar] [CrossRef]
- Xiong, D.; Pan, J.; Yin, Y.; Jiang, H.; Szabo, E.; Lubet, R.A.; Wang, Y.; You, M. Novel mutational landscapes and expression signatures of lung squamous cell carcinoma. Oncotarget 2018, 9, 7424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Givechian, K.B.; Garner, C.; Benz, S.; Song, B.; Rabizadeh, S.; Soon-Shiong, P. An immunogenic NSCLC microenvironment is associated with favorable survival in lung adenocarcinoma. Oncotarget 2019, 10, 1840. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, D.J.; Tabb, E.S.; Kunitoki, K.; Zhang, M.L.; Kem, M.; Barth, J.; Qualls, D.A.; Mooradian, M.J.; Gainor, J.F.; Mino-Kenudson, M.; et al. Lymphocyte-activation gene 3 in non-small-cell lung carcinomas: Correlations with clinicopathologic features and prognostic significance. Mod. Pathol. 2022, 35, 615–624. [Google Scholar] [CrossRef]
- Park, Y.; Seo, A.N.; Koh, J.; Nam, S.K.; Kwak, Y.; Ahn, S.H.; Park, D.J.; Kim, H.H.; Lee, H.S. Expression of the immune checkpoint receptors PD-1, LAG3, and TIM3 in the immune context of stage II and III gastric cancer by using single and chromogenic multiplex immunohistochemistry. Oncoimmunology 2021, 10, e1954761. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jilisihan, B.; Wang, W.; Tang, Y.; Keyoumu, S. Soluble LAG3 acts as a potential prognostic marker of gastric cancer and its positive correlation with CD8+T cell frequency and secretion of IL-12 and INF-γ in peripheral blood. Cancer Biomark. 2018, 23, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, H.; Yamaguchi, K.; Hanamura, F.; Ito, M.; Makiyama, A.; Uchino, K.; Shimokawa, H.; Tamura, S.; Esaki, T.; Mitsugi, K.; et al. OX40 and LAG3 are associated with better prognosis in advanced gastric cancer patients treated with anti-programmed death-1 antibody. Br. J. Cancer 2020, 122, 1507–1517. [Google Scholar] [CrossRef]
- Lv, K.; Li, R.; Cao, Y.; Gu, Y.; Liu, X.; He, X.; Jin, K.; Fang, H.; Fei, Y.; Shi, M.; et al. Lymphocyte-activation gene 3 expression associates with poor prognosis and immunoevasive contexture in Epstein-Barr virus-positive and MLH1-defective gastric cancer patients. Int. J. Cancer 2021, 148, 759–768. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z. The effect of immune microenvironment on the progression and prognosis of colorectal cancer. Med. Oncol. 2014, 31, 82. [Google Scholar] [CrossRef]
- Rhyner Agocs, G.; Assarzadegan, N.; Kirsch, R.; Dawson, H.; Galván, J.A.; Lugli, A.; Zlobec, I.; Berger, M.D. Lag-3 expression predicts outcome in stage ii colon cancer. J. Pers. Med. 2021, 11, 749. [Google Scholar] [CrossRef]
- Zhou, G.; Noordam, L.; Sprengers, D.; Doukas, M.; Boor, P.P.C.; van Beek, A.A.; Erkens, R.; Mancham, S.; Grünhagen, D.; Menon, A.G.; et al. Blockade of LAG3 enhances responses of tumor-infiltrating T cells in mismatch repair-proficient liver metastases of colorectal cancer. Oncoimmunology 2018, 7, e1448332. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, J.; Wu, G.; Teng, M.; Wang, S.; Cui, M.; Li, Y. Co-expression of LAG3 and TIM3 identifies a potent Treg population that suppresses macrophage functions in colorectal cancer patients. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1002–1009. [Google Scholar] [CrossRef]
- Guo, M.; Yuan, F.; Qi, F.; Sun, J.; Rao, Q.; Zhao, Z.; Huang, P.; Fang, T.; Yang, B.; Xia, J. Expression and clinical significance of LAG-3, FGL1, PD-L1 and CD8+T cells in hepatocellular carcinoma using multiplex quantitative analysis. J. Transl. Med. 2020, 18, 306. [Google Scholar] [CrossRef]
- Cheung, C.C.L.; Seah, Y.H.J.; Fang, J.; Orpilla, N.; Lee, J.N.L.W.; Toh, H.C.; Choo, S.P.; Lim, K.H.; Tai, W.M.D.; Yeong, J. 89 The immune marker LAG-3 increases the predictive value of CD38+ immune cells for survival outcome in immunotherapy-treated hepatocellular carcinoma. J. Immunother. Cancer 2021, 9, A97–A98. [Google Scholar] [CrossRef]
- Yarchoan, M.; Xing, D.; Luan, L.; Xu, H.; Sharma, R.B.; Popovic, A.; Pawlik, T.M.; Kim, A.K.; Zhu, Q.; Jaffee, E.M.; et al. Characterization of the immune microenvironment in hepatocellular carcinoma. Clin. Cancer Res. 2017, 23, 7333–7339. [Google Scholar] [CrossRef]
- Chew, V.; Lai, L.; Pan, L.; Lim, C.J.; Li, J.; Ong, R.; Chua, C.; Leong, J.Y.; Lim, K.H.; Toh, H.C.; et al. Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. Proc. Natl. Acad. Sci. USA 2017, 114, E5900–E5909. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, Z.; Rangelova, E.; Poiret, T.; Ambati, A.; Rane, L.; Xie, S.; Verbeke, C.; Dodoo, E.; Del Chiaro, M.; et al. Expansion of tumor-reactive T cells from patients with pancreatic cancer. J. Immunother. 2016, 39, 81–89. [Google Scholar] [CrossRef]
- Seifert, L.; Plesca, I.; Müller, L.; Sommer, U.; Heiduk, M.; von Renesse, J.; Digomann, D.; Glück, J.; Klimova, A.; Weitz, J.; et al. LAG-3-Expressing Tumor-Infiltrating T Cells Are Associated with Reduced Disease-Free Survival in Pancreatic Cancer. Cancers 2021, 13, 1297. [Google Scholar] [CrossRef]
- Lee, S.J.; Byeon, S.J.; Lee, J.; Park, S.H.; Park, J.O.; Park, Y.S.; Kang, W.K.; Lim, H.Y.; Kim, K.M.; Kim, S.T. LAG3 in Solid Tumors as a Potential Novel Immunotherapy Target. J. Immunother. 2019, 42, 279–283. [Google Scholar] [CrossRef]
- Chen, B.; Chen, W.; Jin, J.; Wang, X.; Cao, Y.; He, Y. Data Mining of Prognostic Microenvironment-Related Genes in Clear Cell Renal Cell Carcinoma: A Study with TCGA Database. Dis. Markers 2019, 2019, 1649. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Becht, E.; Pagès, F.; Skliris, G.; Verkarre, V.; Vano, Y.; Mejean, A.; Saint-Aubert, N.; Lacroix, L.; Natario, I.; et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin. Cancer Res. 2015, 21, 3031–3040. [Google Scholar] [CrossRef]
- Klümper, N.; Ralser, D.J.; Bawden, E.G.; Landsberg, J.; Zarbl, R.; Kristiansen, G.; Toma, M.; Ritter, M.; Hölzel, M.; Ellinger, J.; et al. LAG3 (LAG-3, CD223) DNA methylation correlates with LAG3 expression by tumor and immune cells, immune cell infiltration, and overall survival in clear cell renal cell carcinoma. J. Immunother. Cancer 2020, 8, 552. [Google Scholar] [CrossRef] [Green Version]
- Angevin, E.; Kremer, F.; Gaudin, C.; Hercend, T.; Triebel, F. Analysis of T-cell immune response in renal cell carcinoma: Polarization to type 1-like differentiation pattern, clonal T-cell expansion and tumor-specific cytotoxicity. J. Cancer 1997, 72, 431–440. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, E.; Long, J.; Hu, Z.; Peng, J.; Liu, L.; Tang, F.; Li, L.; Ouyang, Y.; Zeng, Z. Immune infiltration in renal cell carcinoma. Cancer Sci. 2019, 110, 1564–1572. [Google Scholar] [CrossRef]
- Zelba, H.; Bedke, J.; Hennenlotter, J.; Mostböck, S.; Zettl, M.; Zichner, T.; Chandran, A.; Stenzl, A.; Rammensee, H.G.; Gouttefangeas, C. PD-1 and LAG-3 dominate checkpoint receptor-mediated T-cell inhibition in renal cell carcinoma. Cancer Immunol. Res. 2019, 7, 1891–1899. [Google Scholar] [CrossRef]
- Siska, P.J.; Johnpulle, R.A.N.; Zhou, A.; Bordeaux, J.; Kim, J.Y.; Dabbas, B.; Dakappagari, N.; Rathmell, J.C.; Rathmell, W.K.; Morgans, A.K.; et al. Deep exploration of the immune infiltrate and outcome prediction in testicular cancer by quantitative multiplexed immunohistochemistry and gene expression profiling. Oncoimmunology 2017, 6, e1305535. [Google Scholar] [CrossRef]
- Colluru, V.T.; McNeel, D.G. Abstract 2502: Immunization with minicircle and mini-intronic DNA vectors induce LAG-3 expressing CD8+ T cells and inferior anti-tumor responses. Cancer Res. 2015, 75, 2502. [Google Scholar] [CrossRef]
- Jafari, S.; Molavi, O.; Kahroba, H.; Hejazi, M.S.; Maleki-Dizaji, N.; Barghi, S.; Kiaie, S.H.; Jadidi-Niaragh, F. Clinical application of immune checkpoints in targeted immunotherapy of prostate cancer. Cell. Mol. Life Sci. 2020, 77, 3693–3710. [Google Scholar] [CrossRef]
- Calagua, C.; Ficial, M.; Jansen, C.S.; Hirz, T.; Del Balzo, L.; Wilkinson, S.; Lake, R.; Ku, A.T.; Voznesensky, O.; Sykes, D.B.; et al. A Subset of Localized Prostate Cancer Displays an Immunogenic Phenotype Associated with Losses of Key Tumor Suppressor Genes. Clin. Cancer Res. 2021, 27, 4836. [Google Scholar] [CrossRef]
- Puhr, H.C.; Ilhan-Mutlu, A. New emerging targets in cancer immunotherapy: The role of LAG3. ESMO Open 2019, 4, e000482. [Google Scholar] [CrossRef]
- Westergaard, M.C.W.; Milne, K.; Pedersen, M.; Hasselager, T.; Olsen, L.R.; Anglesio, M.S.; Borch, T.H.; Kennedy, M.; Briggs, G.; Ledoux, S.; et al. Changes in the Tumor Immune Microenvironment during Disease Progression in Patients with Ovarian Cancer. Cancers 2020, 12, 3828. [Google Scholar] [CrossRef]
- Huang, R.Y.; Eppolito, C.; Lele, S.; Shrikant, P.; Matsuzaki, J.; Odunsi, K. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget 2015, 6, 27359. [Google Scholar] [CrossRef] [PubMed]
- Fucikova, J.; Rakova, J.; Hensler, M.; Kasikova, L.; Belicova, L.; Hladikova, K.; Truxova, I.; Skapa, P.; Laco, J.; Pecen, L.; et al. TIM-3 dictates functional orientation of the immune infiltrate in ovarian cancer. Clin. Cancer Res. 2019, 25, 4820–4831. [Google Scholar] [CrossRef] [PubMed]
- Rådestad, E.; Klynning, C.; Stikvoort, A.; Mogensen, O.; Nava, S.; Magalhaes, I.; Uhlin, M. Immune profiling and identification of prognostic immune-related risk factors in human ovarian cancer. Oncoimmunology 2019, 8, e1535730. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.A.; Ring, K.L.; Mills, A.M. LAG-3 and GAL-3 in endometrial carcinoma: Emerging candidates for immunotherapy. Int. J. Gynecol. Pathol. 2020, 39, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Rosenfeld, J.A.; Singer, E.A.; Bhanot, G.; Ganesan, S. Genomic and immunologic correlates of LAG-3 expression in cancer. Oncoimmunology 2020, 9, e1756116. [Google Scholar] [CrossRef]
- Sun, W.; Qin, X.; Yuan, G.; Li, N.; Liang, J.; Li, C.; Li, C.; Li, M.; Zhao, X.; Zhang, H. Aberrant Expression of Fgl-1 and Lag-3 in Adenomyosis. 2021. preprint. [Google Scholar] [CrossRef]
- Van Gool, I.C.; Eggink, F.A.; Freeman-Mills, L.; Stelloo, E.; Marchi, E.; De Bruyn, M.; Palles, C.; Nout, R.A.; De Kroon, C.D.; Osse, E.M.; et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin. Cancer Res. 2015, 21, 3347–3355. [Google Scholar] [CrossRef] [PubMed]
- Varn, F.S.; Schaafsma, E.; Wang, Y.; Cheng, C. Genomic Characterization of Six Virus-Associated Cancers Identifies Changes in the Tumor Immune Microenvironment and Altered Genetic Programs. Cancer Res. 2018, 78, 6413–6423. [Google Scholar] [CrossRef]
- Chen, F.; Sherwood, T.; De Costa, A.; Yee-Toy, N.; Lung, P.; Easton, A.; Sumrow, B.; Bonvini, E.; Moore, P.A. Immunohistochemistry analyses of LAG-3 expression across different tumor types and co-expression with PD-1. J. Clin. Oncol. 2020, 38, e15086. [Google Scholar] [CrossRef]
- Tu, L.; Guan, R.; Yang, H.; Zhou, Y.; Hong, W.; Ma, L.; Zhao, G.; Yu, M. Assessment of the expression of the immune checkpoint molecules PD-1, CTLA4, TIM-3 and LAG-3 across different cancers in relation to treatment response, tumor-infiltrating immune cells and survival. Int. J. Cancer 2020, 147, 423–439. [Google Scholar] [CrossRef]
- Cocks, M.M.; Mills, A.M. The Immune Checkpoint Inhibitor LAG-3 and Its Ligand GAL-3 in Vulvar Squamous Neoplasia. Int. J. Gynecol. Pathol. 2022, 41, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Burugu, S.; Gao, D.; Leung, S.; Chia, S.K.; Nielsen, T.O.; Nielsen, T.O. LAG-3þ tumor infiltrating lymphocytes in breast cancer: Clinical correlates and association with PD-1/ PD-L1þ tumors. Ann. Oncol. 2017, 28, 2977–2984. [Google Scholar] [CrossRef]
- Liu, Q.; Qi, Y.; Zhai, J.; Kong, X.; Wang, X.; Wang, Z.; Fang, Y.; Wang, J. Molecular and Clinical Characterization of LAG3 in Breast Cancer Through 2994 Samples. Front. Immunol. 2021, 12, 2420. [Google Scholar] [CrossRef] [PubMed]
- Bottai, G.; Raschioni, C.; Losurdo, A.; Di Tommaso, L.; Tinterri, C.; Torrisi, R.; Reis-Filho, J.S.; Roncalli, M.; Sotiriou, C.; Santoro, A.; et al. An immune stratification reveals a subset of PD-1/LAG-3 double-positive triple-negative breast cancers. Breast Cancer Res. 2016, 18, 121. [Google Scholar] [CrossRef]
- Sobottka, B.; Moch, H.; Varga, Z. Differential PD-1/LAG-3 expression and immune phenotypes in metastatic sites of breast cancer. Breast Cancer Res. 2021, 23, 4. [Google Scholar] [CrossRef]
- Wu, S.; Shi, X.; Wang, J.; Wang, X.; Liu, Y.; Luo, Y.; Mao, F.; Zeng, X. Triple-Negative Breast Cancer: Intact Mismatch Repair and Partial Co-Expression of PD-L1 and LAG-3. Front. Immunol. 2021, 12, 561793. [Google Scholar] [CrossRef]
- Du, H.; Yi, Z.; Wang, L.; Li, Z.; Niu, B.; Ren, G. The co-expression characteristics of LAG3 and PD-1 on the T cells of patients with breast cancer reveal a new therapeutic strategy. Int. Immunopharmacol. 2020, 78, 106113. [Google Scholar] [CrossRef]
- Stovgaard, E.S.; Kümler, I.; List-Jensen, K.; Roslind, A.; Christensen, I.J.; Høgdall, E.; Nielsen, D.; Balslev, E. Prognostic and Clinicopathologic Associations of LAG-3 Expression in Triple-negative Breast Cancer. Appl. Immunohistochem. Mol. Morphol. AIMM 2022, 30, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.; Andersen, R.; Kjeldsen, J.W.; Fagone, P.; Munir, S.; Nicoletti, F.; Andersen, M.H.; Straten, P.T.; Svane, I.M. Aberrant Expression of MHC Class II in Melanoma Attracts Inflammatory Tumor-Specific CD4+ T- Cells, Which Dampen CD8+ T-cell Antitumor Reactivity. Cancer Res. 2015, 75, 3747–3759. [Google Scholar] [CrossRef] [PubMed]
- Camisaschi, C.; De Filippo, A.; Beretta, V.; Vergani, B.; Villa, A.; Vergani, E.; Santinami, M.; Cabras, A.D.; Arienti, F.; Triebel, F.; et al. Alternative activation of human plasmacytoid DCs in vitro and in melanoma lesions: Involvement of LAG-3. J. Investig. Dermatol. 2014, 134, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Okazaki, I.M.; Sugiura, D.; Takahashi, S.; Maeda, T.K.; Shimizu, K.; Okazaki, T. LAG-3 inhibits the activation of CD4 + T cells that recognize stable pMHCII through its conformation-dependent recognition of pMHCII. Nat. Immunol. 2018, 19, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lei, Z.; Zhao, J.; Gong, W.; Liu, J.; Chen, Z.; Liu, Y.; Li, D.; Yuan, Y.; Zhang, G.M.; et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007, 252, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Souri, Z.; Wierenga, A.P.A.; Kroes, W.G.M.; van der Velden, P.A.; Verdijk, R.M.; Eikmans, M.; Luyten, G.P.M.; Jager, M.J. LAG3 and Its Ligands Show Increased Expression in High-Risk Uveal Melanoma. Cancers 2021, 13, 4445. [Google Scholar] [CrossRef]
- Taube, J.M.; Young, G.D.; McMiller, T.L.; Chen, S.; Salas, J.T.; Pritchard, T.S.; Xu, H.; Meeker, A.K.; Fan, J.; Cheadle, C.; et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin. Cancer Res. 2015, 21, 3969–3976. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Burger, P.; Taube, J.; Soni, A.; Chaichana, K.; Sheu, M.; Belcaid, Z.; Jackson, C.; Lim, M. PD-L1, PD-1, LAG-3, and TIM-3 in Melanoma: Expression in Brain Metastases Compared to Corresponding Extracranial Tumors. Cureus 2019, 11, e6352. [Google Scholar] [CrossRef]
- Baitsch, L.; Baumgaertner, P.; Devêvre, E.; Raghav, S.K.; Legat, A.; Barba, L.; Wieckowski, S.; Bouzourene, H.; Deplancke, B.; Romero, P.; et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Investig. 2011, 121, 2350. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Melero, I.; Bhatia, S.; Bono, P.; Sanborn, R.E.; Lipson, E.J.; Callahan, M.K.; Gajewski, T.; Gomez-Roca, C.A.; Hodi, F.S.; et al. Initial efficacy of anti-lymphocyte activation gene-3 (anti–LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti–PD-1/PD-L1 therapy. J. Clin. Oncol. 2017, 35, 9520. [Google Scholar] [CrossRef]
- Shen, R.; Postow, M.A.; Adamow, M.; Arora, A.; Hannum, M.; Maher, C.; Wong, P.; Curran, M.A.; Hollmann, T.J.; Jia, L.; et al. LAG-3 expression on peripheral blood cells identifies patients with poorer outcomes after immune checkpoint blockade. Sci. Transl. Med. 2021, 13, eabf5107. [Google Scholar] [CrossRef]
- Machiraju, D.; Wiecken, M.; Lang, N.; Hülsmeyer, I.; Roth, J.; Schank, T.E.; Eurich, R.; Halama, N.; Enk, A.; Hassel, J.C. Soluble immune checkpoints and T-cell subsets in blood as biomarkers for resistance to immunotherapy in melanoma patients. Oncoimmunology 2021, 10, e1926762. [Google Scholar] [CrossRef]
- Gandhi, M.K.; Lambley, E.; Duraiswamy, J.; Dua, U.; Smith, C.; Elliott, S.; Gill, D.; Marlton, P.; Seymour, J.; Khanna, R. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood 2006, 108, 2280–2289. [Google Scholar] [CrossRef]
- El Halabi, L.; Adam, J.; Gravelle, P.; Marty, V.; Danu, A.; Lazarovici, J.; Ribrag, V.; Bosq, J.; Camara-Clayette, V.; Laurent, C.; et al. Expression of the Immune Checkpoint Regulators LAG-3 and TIM-3 in Classical Hodgkin Lymphoma. Clin. Lymphoma Myeloma Leuk. 2021, 21, 257–266.e3. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-Z.; Price-troska, T.; Novak, A.J.; Ansell, S.M. The Exhausted Intratumoral T Cell Population in B-Cell Non-Hodgkin Lymphoma Is Defined By LAG-3, PD-1 andtim-3 Expression. Blood 2015, 126, 2661. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Kim, H.J.; Villasboas, J.C.; Chen, Y.P.; Price-Troska, T.P.; Jalali, S.; Wilson, M.; Novak, A.J.; Ansell, S.M. Expression of LAG-3 defines exhaustion of intratumoral PD-1 + T cells and correlates with poor outcome in follicular lymphoma. Oncotarget 2017, 8, 61425–61439. [Google Scholar] [CrossRef]
- Lymphocyte Activation Gene-3, a MHC Class II Ligand Expressed on Activated T Cells, Stimulates TNF-Alpha and IL-12 Production by Monocytes and Dendritic Cells—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/10072520/ (accessed on 10 July 2022).
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12, 1555. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yuan, Y.; Jiang, X. Antibody and antibody fragments for cancer immunotherapy. J. Control. Release 2020, 328, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Nurgalieva, A.K.; Safina, S.; Shakirova, E.; Filonenko, V.; Skripova, V.; Bulatova, L.; Savenkova, D.; Bogdanov, M.; Kiyamova, R. 53P Expression of sodium-dependent phosphate transporter NaPi2b is downregulated in malignant ovarian tumors after neoadjuvant chemotherapy. Ann. Oncol. 2021, 32, S1361–S1362. [Google Scholar] [CrossRef]
- Nagasaki, J.; Togashi, Y.; Sugawara, T.; Itami, M.; Yamauchi, N.; Yuda, J.; Sugano, M.; Ohara, Y.; Minami, Y.; Nakamae, H.; et al. The critical role of CD41 T cells in PD-1 blockade against MHC-II–expressing tumors such as classic Hodgkin lymphoma. Blood Adv. 2020, 4, 4069–4082. [Google Scholar] [CrossRef]
- Dirix, L.; Triebel, F. AIPAC: A Phase IIb study of eftilagimod alpha (IMP321 or LAG-3Ig) added to weekly paclitaxel in patients with metastatic breast cancer. Future Oncol. 2019, 15, 1963–1973. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Cheng, C.; Mu, W.; Liu, X.; Li, N.; Wei, X.; Liu, X.; Xia, C.; Wang, H. CRISPR-Cas9 mediated LAG-3 disruption in CAR-T cells. Front. Med. 2017, 11, 554–562. [Google Scholar] [CrossRef]
- Poorebrahim, M.; Melief, J.; Pico de Coaña, Y.; Wickström, S.L.; Cid-Arregui, A.; Kiessling, R. Counteracting CAR T cell dysfunction. Oncogene 2021, 40, 421–435. [Google Scholar] [CrossRef]
- Wang, J.; Asch, A.S.; Hamad, N.; Weickhardt, A.; Tomaszewska-Kiecana, M.; Dlugosz-Danecka, M.; Pylypenko, H.; Bahadur, S.; Ulahannan, S.; Koucheki, J.; et al. A Phase 1, Open-Label Study of MGD013, a Bispecific DART® Molecule Binding PD-1 and LAG-3 in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Blood 2020, 136, 21–22. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; González-Rodríguez, A.P.; Payer, Á.R.; González-García, E.; López-Soto, A.; Gonzalez, S. LAG-3 Blockade with Relatlimab (BMS-986016) Restores Anti-Leukemic Responses in Chronic Lymphocytic Leukemia. Cancers 2021, 13, 2112. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Burova, E.; Hermann, A.; Dai, J.; Ullman, E.; Halasz, G.; Potocky, T.; Hong, S.; Liu, M.; Allbritton, O.; Woodruff, A.; et al. Preclinical Development of the Anti-LAG-3 Antibody REGN3767: Characterization and Activity in Combination with the Anti-PD-1 Antibody Cemiplimab in Human PD-1xLAG-3-Knockin Mice. Mol. Cancer Ther. 2019, 18, 2051–2062. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Lakhani, N.J.; Johnson, M.L.; Park, H.; Wang, D.; Yap, T.A.; Dowlati, A.; Maki, R.G.; Lynce, F.; Ulahannan, S.V.; et al. First-in-human study of REGN3767 (R3767), a human LAG-3 monoclonal antibody (mAb), ± cemiplimab in patients (pts) with advanced malignancies. J. Clin. Oncol. 2019, 37, 2508. [Google Scholar] [CrossRef]
- Hamid, O.; Wang, D.; Kim, T.M.; Kim, S.-W.; Lakhani, N.J.; Johnson, M.L.; Groisberg, R.; Papadopoulos, K.P.; Kaczmar, J.M.; Middleton, M.R.; et al. Clinical activity of fianlimab (REGN3767), a human anti-LAG-3 monoclonal antibody, combined with cemiplimab (anti-PD-1) in patients (pts) with advanced melanoma. J. Clin. Oncol. 2021, 39, 9515. [Google Scholar] [CrossRef]
- Klingler, S.; Fay, R.; Holland, J.P. Light-Induced Radiosynthesis of 89 Zr-DFO-Azepin-Onartuzumab for Imaging the Hepatocyte Growth Factor Receptor. J. Nucl. Med. 2020, 61, 1072–1078. [Google Scholar] [CrossRef]
- Grandal, M.M.; Melander, M.C.; Bhatia, V.K.; Gjetting, T.; Lindsted, T.; Fröhlich, C.; Lantto, J.; Horak, I.D.; Kragh, M.; Kofoed, K.; et al. Abstract 5626: Preclinical characterization of Sym022, a novel anti-LAG3 antibody. Cancer Res. 2018, 78, 5626. [Google Scholar] [CrossRef]
- Spreafico, A.; Janku, F.; Rodon, J.A.; Tolcher, A.W.; Chandana, S.R.; Oliva, M.; Musalli, S.; Knauss, L.; Kragh, M.; Alifrangis, L.; et al. A phase I study of Sym021, an anti-PD-1 antibody (Ab), alone and in combination with Sym022 (anti-LAG-3) or Sym023 (anti-TIM-3). Ann. Oncol. 2019, 30, v488–v489. [Google Scholar] [CrossRef]
- Pereira, N.A.; Chan, K.F.; Lin, P.C.; Song, Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs 2018, 10, 693. [Google Scholar] [CrossRef]
- Ellis, J.; Marks, D.J.B.; Srinivasan, N.; Barrett, C.; Hopkins, T.G.; Richards, A.; Fuhr, R.; Albayaty, M.; Coenen, M.; Liefaard, L.; et al. Depletion of LAG-3+ T Cells Translated to Pharmacology and Improvement in Psoriasis Disease Activity: A Phase I Randomized Study of mAb GSK2831781. Clin. Pharmacol. Ther. 2021, 109, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Slevin, S.M.; Garner, L.C.; Lahiff, C.; Tan, M.; Wang, L.M.; Ferry, H.; Greenaway, B.; Lynch, K.; Geremia, A.; Hughes, S.; et al. Lymphocyte Activation Gene (LAG)-3 Is Associated with Mucosal Inflammation and Disease Activity in Ulcerative Colitis. J. Crohn’s Colitis 2020, 14, 1446–1461. [Google Scholar] [CrossRef] [PubMed]
- Savitsky, D.; Ward, R.; Riordan, C.; Mundt, C.; Jennings, S.; Connolly, J.; Findeis, M.; Sanicola, M.; Underwood, D.; Nastri, H.; et al. Abstract 3819: INCAGN02385 is an antagonist antibody targeting the co-inhibitory receptor LAG-3 for the treatment of human malignancies. Cancer Res. 2018, 78, 3819. [Google Scholar] [CrossRef]
- Ghosh, S.; Sharma, G.; Travers, J.; Kumar, S.; Choi, J.; Toni Jun, H.; Kehry, M.; Ramaswamy, S.; Jenkins, D. TSR-033, a novel therapeutic antibody targeting LAG-3, enhances T-cell function and the activity of PD-1 blockade in vitro and in vivo. Mol. Cancer Ther. 2019, 18, 632–641. [Google Scholar] [CrossRef]
- Sullivan, M.R.; Ugolini, G.S.; Sarkar, S.; Kang, W.; Smith, E.C.; Mckenney, S.; Konry, T. Quantifying the efficacy of checkpoint inhibitors on CD8+ cytotoxic T cells for immunotherapeutic applications via single-cell interaction. Cell Death Dis. 2020, 11, 979. [Google Scholar] [CrossRef]
- Hong, D.S.; Schoffski, P.; Calvo, A.; Sarantopoulos, J.; De Olza, M.O.; Carvajal, R.D.; Prawira, A.; Kyi, C.; Esaki, T.; Akerley, W.L.; et al. Phase I/II study of LAG525 ± spartalizumab (PDR001) in patients (pts) with advanced malignancies. J. Clin. Oncol. 2018, 36, 3012. [Google Scholar] [CrossRef]
- Uboha, N.V.; Milhem, M.M.; Kovacs, C.; Amin, A.; Magley, A.; Das Purkayastha, D.; Piha-Paul, S.A. Phase II study of spartalizumab (PDR001) and LAG525 in advanced solid tumors and hematologic malignancies. J. Clin. Oncol. 2019, 37, 2553. [Google Scholar] [CrossRef]
- Schöffski, P.; Tan, D.S.W.; Martín, M.; Ochoa-De-Olza, M.; Sarantopoulos, J.; Carvajal, R.D.; Kyi, C.; Esaki, T.; Prawira, A.; Akerley, W.; et al. Original research: Phase I/II study of the LAG-3 inhibitor ieramilimab (LAG525) ± anti-PD-1 spartalizumab (PDR001) in patients with advanced malignancies. J. Immunother. Cancer 2022, 10, 3776. [Google Scholar] [CrossRef]
- Lin, C.-C.; Garralda, E.; Schöffski, P.; Hong, D.; Siu, L.; Martin, M.; Maur, M.; Hui, R.; Soo, R.; Chiu, J.; et al. 387 A Phase II, multicenter study of the safety and efficacy of LAG525 in combination with spartalizumab in patients with advanced malignancies. J. Immunother. Cancer 2020, 8, A235. [Google Scholar] [CrossRef]
- Bhagwat, B.; Cherwinski, H.; Sathe, M.; Seghezzi, W.; McClanahan, T.K.; de Waal Malefyt, R.; Willingham, A. Establishment of engineered cell-based assays mediating LAG3 and PD1 immune suppression enables potency measurement of blocking antibodies and assessment of signal transduction. J. Immunol. Methods 2018, 456, 7–14. [Google Scholar] [CrossRef]
- Plimack, E.R.; Hammers, H.J.; Choueiri, T.K.; Rini, B.I.; Motzer, R.J.; Suttner, L.; Perini, R.F.; Rogerio, J.W.; Albiges, L. A phase 1b/2 umbrella study of investigational immune and targeted combination therapies as first-line therapy for patients with advanced renal cell carcinoma (RCC). J. Clin. Oncol. 2021, 39, TPS4594. [Google Scholar] [CrossRef]
- Haines, B.B.; Javaid, S.; Cui, L.; Hirsch, H.; Cemerski, S.; McClanahan, T.; Sathe, M.; Zhang, S.; Rosenzweig, M.; Long, B.; et al. Abstract 4714: Blockade of LAG-3 amplifies immune activation signatures and augments curative antitumor responses to anti-PD-1 therapy in immune competent mouse models of cancer. Cancer Res. 2017, 77, 4714. [Google Scholar] [CrossRef]
- Garralda, E.; Sukari, A.; Lakhani, N.J.; Patnaik, A.; Lou, Y.; Im, S.-A.; Golan, T.; Geva, R.; Wermke, M.; De Miguel, M.; et al. A phase 1 first-in-human study of the anti-LAG-3 antibody MK4280 (favezelimab) plus pembrolizumab in previously treated, advanced microsatellite stable colorectal cancer. J. Clin. Oncol. 2021, 39, 3584. [Google Scholar] [CrossRef]
- Jenkins, S.; Wesolowski, R.; Gatti-Mays, M.E. Improving Breast Cancer Responses to Immunotherapy—A Search for the Achilles Heel of the Tumor Microenvironment. Curr. Oncol. Rep. 2021, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Kraman, M.; Faroudi, M.; Allen, N.L.; Kmiecik, K.; Gliddon, D.; Seal, C.; Koers, A.; Wydro, M.M.; Batey, S.; Winnewisser, J.; et al. FS118, a bispecific antibody targeting lag-3 and PD-L1, enhances T-cell activation resulting in potent antitumor activity. Clin. Cancer Res. 2020, 26, 3333–3344. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.; Wong, D.; Hu-Lieskovan, S.; Papadopoulos, K.; Morrow, M.; Grabowska, U.; Gliddon, D.; Holz, J.-B.; LoRusso, P. 395 A first-in-human study of FS118, a tetravalent bispecific antibody targeting LAG-3 and PD-L1, in patients with advanced cancer and resistance to PD-(L)1 therapy. J. Immunother. Cancer 2020, 8, A240. [Google Scholar] [CrossRef]
- Jiang, H.; Ni, H.; Zhang, P.; Guo, X.; Wu, M.; Shen, H.; Wang, J.; Wu, W.; Wu, Z.; Ding, J.; et al. PD-L1/LAG-3 bispecific antibody enhances tumor-specific immunity. Oncoimmunology 2021, 10, e1943180. [Google Scholar] [CrossRef]
- Brignone, C.; Grygar, C.; Marcu, M.; Schäkel, K.; Triebel, F. A Soluble Form of Lymphocyte Activation Gene-3 (IMP321) Induces Activation of a Large Range of Human Effector Cytotoxic Cells. J. Immunol. 2007, 179, 4202–4211. [Google Scholar] [CrossRef]
- Brignone, C.; Gutierrez, M.; Mefti, F.; Brain, E.; Jarcau, R.; Cvitkovic, F.; Bousetta, N.; Medioni, J.; Gligorov, J.; Grygar, C.; et al. First-line chemoimmunotherapy in metastatic breast carcinoma: Combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. J. Transl. Med. 2010, 8, 71. [Google Scholar] [CrossRef] [Green Version]


| Identifier | Patients Number | Recruitment Status | Condition or Disease | Target | Therapy Protocol | Short Description |
|---|---|---|---|---|---|---|
| NCT01968109 | 1499 | Active, not recruiting | Neoplasms by Site | LAG-3 PD-1 | Biological: Relatlimab Biological: Nivolumab Biological: BMS-986213 | Anti-LAG-3 Monoclonal Antibody (BMS-986016) Administered Alone and in Combination with Anti-PD-1 Monoclonal Antibody (Nivolumab, BMS-936558) in Advanced Solid Tumors |
| NCT03662659 | 274 | Active, not recruiting | Gastric Cancer Cancer of the Stomach Esophagogastric Junction | LAG-3 PD-1 | Biological: BMS-986213 Biological: Nivolumab Drug: XELOX Drug: FOLFOX Drug: SOX | Relatlimab and Nivolumab in Combination with Chemotherapy Versus Nivolumab in Combination with Chemotherapy as First-Line Treatment in Patients with Gastric or Gastroesophageal Junction Adenocarcinoma |
| NCT02061761 | 107 | Active, not recruiting | Hematologic Neoplasms | LAG-3 PD-1 | Biological: BMS-986016 Biological: BMS-936558 | Anti-LAG-3 Monoclonal Antibody (Relatlimab, BMS-986016) Administered Alone and in Combination with Anti-PD-1 Monoclonal Antibody (Nivolumab, BMS-936558) in Relapsed or Refractory B-Cell Malignancies |
| NCT03493932 | 20 | Active, not recruiting | Glioblastoma | LAG-3 | Drug: Nivolumab Drug: BMS-986016 | Nivolumab, together with an anti-LAG-3 antibody BMS-986016 in Patients with Glioblastoma |
| NCT04150965 | 104 | Active, Recruiting | Multiple Myeloma Relapsed Refractory Multiple Myeloma | LAG-3 TIGIT | Drug: Elotuzumab, pomalidomide, dexamethasone Drug: Anti-LAG-3 Drug: Anti-LAG-3 + Pomalidimide + Dexamethasone Drug: Anti-TIGIT Drug: Anti-TIGIT + Pomalidimide + Dexamethasone | Combination Immuno-Oncology Drugs Elotuzumab, Anti-LAG-3 (BMS-986016) and Anti-TIGIT (BMS-986207) in Patients with Multiple Myeloma |
| NCT03044613 | 32 | Active, not recruiting | Gastric Cancer Esophageal Cancer Gastroesophageal Cancer | LAG-3 PD-1 | Drug: Nivolumab Drug: Relatlimab Drug: Carboplatin Drug: Paclitaxel Radiation: Radiation | Nivolumab or Nivolumab/Relatlimab Prior to Concurrent Chemoradiation in Patients with Operable Stage II/III Esophageal/ Gastroesophageal Junction Cancer |
| NCT04080804 | 60 | Active, Recruting | Head and Neck Squamous Cell | LAG-3 PD-1 CTLA-4 | Drug: Nivolumab Drug: Relatlimab | Anti-PD1 (Nivolumab) Administered Alone or in Combination with Anti-LAG3 (Relatlimab) or Anti-CTLA4 (Ipilimumab) in Resectable Head and Neck Cancer |
| NCT03459222 | 255 | Active, Recruiting | Advanced Cancer | LAG-3 PD-1 CTLA-4 | Biological: Relatlimab Biological: Nivolumab Drug: BMS-986205 Biological: Ipilimumab | Relatlimab (Anti-LAG-3 Monoclonal Antibody) Administered in Combination with Both Nivolumab (Anti-PD-1 Monoclonal Antibody) and BMS-986205 (IDO1 Inhibitor) or in Combination with Both Nivolumab and Ipilimumab (Anti-CTLA-4 Monoclonal Antibody) in Advanced Malignant Tumours |
| NCT03005782 | 669 | Active, Recruting | Malignancies | LAG-3 PD-1 | Drug: REGN3767 Drug: cemiplimab | REGN3767 (Anti-LAG-3 mAb) Administered Alone or in Combination with REGN2810 (Anti-PD-1 mAb) in Patients with Advanced Malignancies |
| NCT04566978 | 20 | Active Recruiting | Large B-cell Lymphoma DLBCL | LAG-3 | Drug: 89Zr-DFO-REGN3767 Diagnostic Test: PET/CT | 89Zr-DFO-REGN3767 Anti LAG-3 Antibody Positron Emission Tomography in Patients with Relapsed/Refractory DLBCL |
| NCT03489369 | 15 | Completed, Phase 1 | Metastatic Cancer Solid Tumor Lymphoma | LAG-3 | Experimental: Sym022 | Antineoplastic Activity of Sym022 (Anti-LAG-3) in Patients with Advanced Solid Tumor Malignancies or Lymphomas |
| NCT03311412 | 91 | Completed, Phase 1 | Metastatic Cancer Solid Tumor Lymphoma | LAG-3, PD-1, TIM-3 | Drug: Sym021 Drug: Sym022 Drug: Sym023 | Activity of Sym021 (Anti-PD-1) as Monotherapy, in Combination with Either Sym022 (Anti-LAG-3) or Sym023 (Anti-TIM-3), and in Combination with Both Sym022 and Sym023 in Patients with Advanced Solid Tumor Malignancies or Lymphomas |
| NCT04641871 | 100 | Active Recruiting | Metastatic Cancer Solid Tumour | LAG-3 PD-1 TIM-3 | Drug: Sym021 Drug: Sym022 Drug: Sym023 Drug: Irinotecan Hydrochloride | Sym021 (Anti-PD 1) in Combination with Either Sym022 (Anti-LAG-3) or Sym023 (Anti-TIM-3) or Sym023 and Irinotecan in Patients with Recurrent Advanced Biliary Tract Carcinomas |
| NCT03250832 | 111 | Active, not recruiting | Neoplasms | LAG-3 PD-1 | Drug: TSR-033 Drug: Dostarlimab Drug: mFOLFOX6 Drug: FOLFIRI Drug: Bevacizumab | TSR-033, an Anti-LAG-3 Monoclonal Antibody, Alone and in Combination with an Anti-PD-1 in Patients with Advanced Solid Tumours |
| NCT03499899 | 88 | Completed | Triple-negative Breast Cancer | LAG-3 PD-1 | Drug: LAG525 Drug: spartalizumab Drug: carboplatin | LAG525 in Combination with Spartalizumab, or with Spartalizumab and Carboplatin, or with Carboplatin, in Patients with Advanced Triple-negative Breast Cancer |
| NCT02460224 | 490 | Completed | Advanced Solid Tumours | LAG-3 PD-1 | Drug: LAG525 Drug: PDR001 | LAG525 Single Agent and in Combination with PDR001 Administered to Patients with Advanced Malignancies |
| NCT03484923 | 196 | Active, not recruiting | Melanoma | LAG-3 PD-1 MET IL-1β CDK4/6 | Drug: Spartalizumab Drug: LAG525 Drug: Capmatinib Drug: Canakinumab Drug: Ribociclib | Spartalizumab (PDR001) Combinations in Previously Treated Unresectable or Metastatic Melanoma |
| NCT05064059 | 432 | Active, recruiting | Colorectal Cancer | LAG-3 PD-1 | Biological: favezelimab/pembrolizumab Drug: regorafenib Drug: TAS-102 | Favezelimab/Pembrolizumab (MK-4280A) in participants with metastatic colorectal cancer |
| NCT03598608 | 154 | Active, recruiting | Hodgkin Disease Lymphoma, Non-Hodgkin Lymphoma, B-Cell | LAG-3 PD-1 | Biological: pembrolizumab Biological: Favezelimab | Combination of MK-4280 and Pembrolizumab (MK-3475) in Participants with Hematologic Malignancies |
| Identifier | Patients Number | Recruitment Status | Condition or Disease | Target Antigen | Therapy Protocol | Short Description |
|---|---|---|---|---|---|---|
| NCT03219268 | 353 | Active, not recruiting | Advanced Solid Tumors Hematologic Neoplasms Ovarian Cancer HER2-positive Advanced Solid Tumors Non-Small Cell Lung Cancer Small-cell Lung Cancer Squamous Cell Carcinoma of Head and Neck Cholangiocarcinoma Cervical Cancer TNBC-Triple-Negative Breast Cancer | LAG-3 PD-1 | Biological: tebotelimab Biological: margetuximab | MGD013, A Bispecific DART® Protein Binding PD-1 and LAG-3 in Patients with Unresectable or Metastatic Neoplasms |
| NCT04140500 | 320 | Active, Recruiting | Solid Tumors Metastatic Melanoma Non-small Cell Lung Cancer Esophageal Squamous Cell Carcinoma | LAG-3 PD-1 | Drug: RO7247669 | RO7247669, a PD1-LAG3 Bispecific Antibody, in Patients with Advanced and/or Metastatic Solid Tumours |
| NCT03440437 | 80 | Active, Recruiting | Advanced Cancer Metastatic Cancer Squamous Cell Carcinoma of Head and Neck | LAG-3 PD-1 | Drug: FS118 | FS118, a LAG-3/PD-L1 Bispecific Antibody, in Patients with Advanced Malignancies |
| NCT04618393 | 43 | Active, Recruting | Advanced Solid Tumor | LAG-3 PD-1 | Biological: EMB-02 | EMB-02, a Bi-specific Antibody Against PD-1 and LAG-3, in Patients with Advanced Solid Tumors |
| NCT04916119 | 322 | Active Recruiting | Advanced Malignancies | LAG-3 PD-1 | Drug: IBI323 | IBI323(anti-LAG-3/PD-L1) or in combination with chemotherapy in participants with advanced malignancies |
| NCT03849469 | 242 | Active Recruiting | Solid tumors | LAG-3 CTLA-4 | Biological: XmAb®22841 Biological: Pembrolizumab (Keytruda®) | XmAb22841 monotherapy and in combination with pembrolizumab in Patients with Solid tumors |
| Identifier | Patients Number | Recruitment Status | Condition or Disease | Target Antigen | Therapy Protocol | Short Description |
|---|---|---|---|---|---|---|
| NCT00349934 | 33 | Completed, Phase 1 | Metastatic Breast Cancer | LAG-3 | Biological: IMP321 | IMP321 in Metastatic Breast Carcinoma Patients Receiving First-line Paclitaxel |
| NCT02614833 | 242 | Completed | Adenocarcinoma Breast Stage IV | LAG-3 | Biological: IMP321 (eftilagimod alpha) Drug: Placebo Drug: Paclitaxel | Study in Hormone Receptor-positive Metastatic Breast Carcinoma Patients Receiving IMP321 (LAG-3Ig Fusion Protein) or Placebo as Adjunctive to a Standard Chemotherapy Treatment Regimen of Paclitaxel |
| NCT00351949 | 24 | Completed | Stage IV Renal Cell Carcinoma | LAG-3 | Biological: IMP321 | IMP321 in Advanced or Metastatic Renal Cell Carcinoma Patients |
| NCT03252938 | 45 | Active, Recruiting | Solid Tumors Peritoneal Carcinomatosis | LAG-3 | Drug: IMP321 Drug: Avelumab | MP321 (LAG-3Ig Fusion Protein) in Patients with Advanced Stage Solid Tumor Entities |
| NCT00351949 | 24 | Completed | Stage IV Renal Cell Carcinoma | LAG-3 | Biological: IMP321 | IMP321 in Patients with Metastatic Renal Cell Carcinoma (MRCC) |
| NCT02676869 | 24 | Completed | Stage IV Melanoma Stage III Melanoma | LAG-3 PD-1 | Drug: IMP321 (eftilagimod alpha) Drug: Pembrolizumab | MP321 in Patients in Combination with Pembrolizumab in Patients with Unresectable or Metastatic Melanoma |
| NCT01968109 | 1499 | Active, not recruiting | Neoplasms by Site | LAG-3 PD-1 | Biological: Relatlimab Biological: Nivolumab Biological: BMS-986213 | Anti-LAG-3 Monoclonal Antibody (BMS-986016) Administered Alone and in Combination with Anti-PD-1 Monoclonal Antibody (Nivolumab, BMS-936558) in Advanced Solid Tumors |
| NCT03044613 | 32 | Active, not recruiting | Gastric Cancer Esophageal Cancer Gastroesophageal Cancer | LAG-3 PD-1 | Drug: Nivolumab Drug: Relatlimab Drug: Carboplatin Drug: Paclitaxel Radiation: Radiation | Nivolumab or Nivolumab/Relatlimab Prior to Concurrent Chemoradiation in Patients with Operable Stage II/III Esophageal/ Gastroesophageal Junction Cancer |
| NCT04370704 | 144 | Active, Recruiting | Melanoma | LAG-3 PD-1 TIM-3 | Drug: INCAGN02385 Drug: INCAGN02390 Drug: INCMGA00012. | Combination Therapy with INCMGA00012 (Anti-PD-1), INCAGN02385 (Anti-LAG-3), and INCAGN02390 (Anti-TIM-3) in Participants with Select Advanced Malignancies |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauer, N.; Szlasa, W.; Jonderko, L.; Oślizło, M.; Kunachowicz, D.; Kulbacka, J.; Karłowicz-Bodalska, K. LAG-3 as a Potent Target for Novel Anticancer Therapies of a Wide Range of Tumors. Int. J. Mol. Sci. 2022, 23, 9958. https://doi.org/10.3390/ijms23179958
Sauer N, Szlasa W, Jonderko L, Oślizło M, Kunachowicz D, Kulbacka J, Karłowicz-Bodalska K. LAG-3 as a Potent Target for Novel Anticancer Therapies of a Wide Range of Tumors. International Journal of Molecular Sciences. 2022; 23(17):9958. https://doi.org/10.3390/ijms23179958
Chicago/Turabian StyleSauer, Natalia, Wojciech Szlasa, Laura Jonderko, Małgorzata Oślizło, Dominika Kunachowicz, Julita Kulbacka, and Katarzyna Karłowicz-Bodalska. 2022. "LAG-3 as a Potent Target for Novel Anticancer Therapies of a Wide Range of Tumors" International Journal of Molecular Sciences 23, no. 17: 9958. https://doi.org/10.3390/ijms23179958
APA StyleSauer, N., Szlasa, W., Jonderko, L., Oślizło, M., Kunachowicz, D., Kulbacka, J., & Karłowicz-Bodalska, K. (2022). LAG-3 as a Potent Target for Novel Anticancer Therapies of a Wide Range of Tumors. International Journal of Molecular Sciences, 23(17), 9958. https://doi.org/10.3390/ijms23179958







