Changes in Radiosensitivity to Gamma-Rays of Lymphocytes from Hyperthyroid Patients Treated with I-131
Abstract
1. Introduction
2. Results
2.1. Radiation-Induced Damage in Terms of γ-H2AX
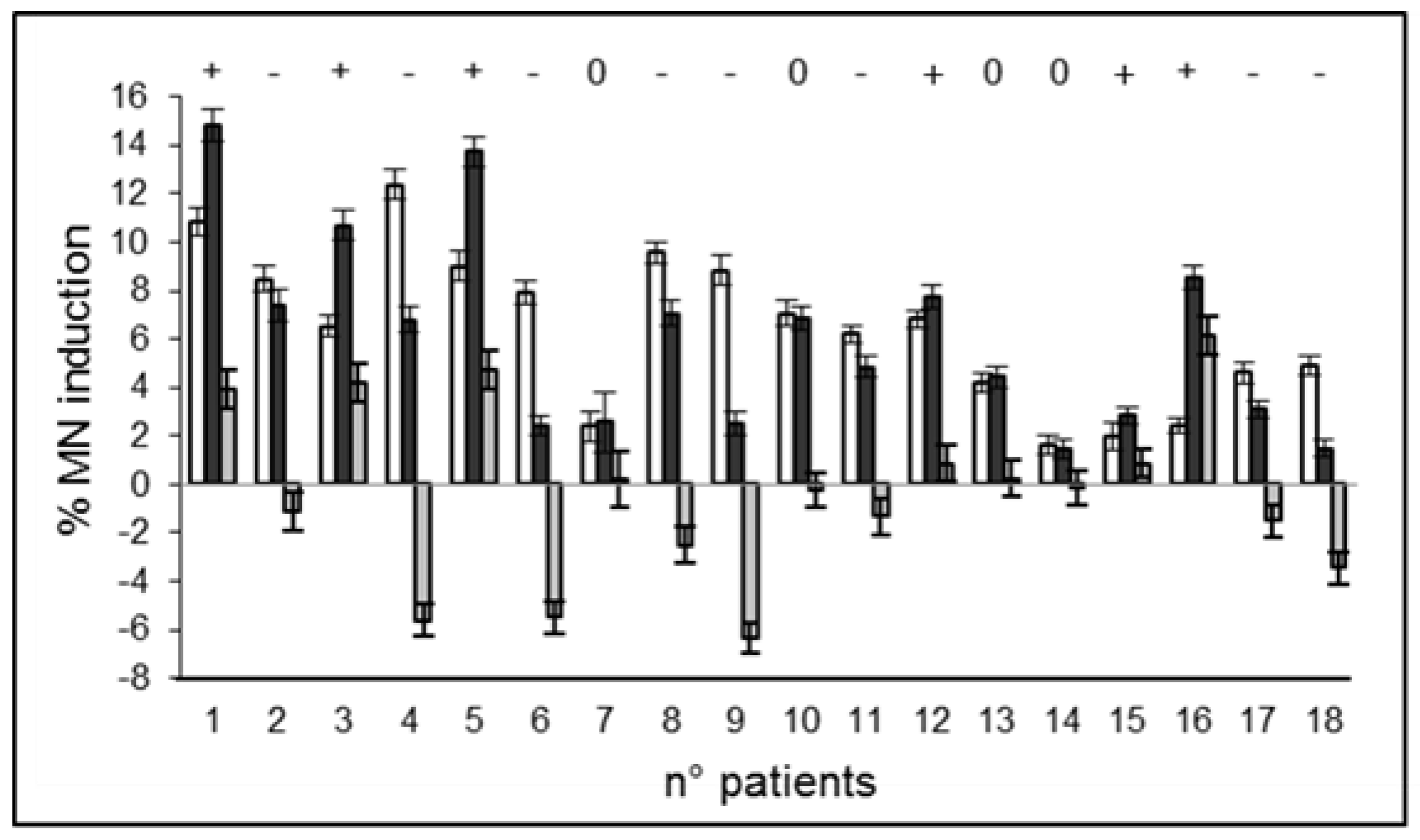
2.2. Radiation-Induced Damage in Terms of MN Induction
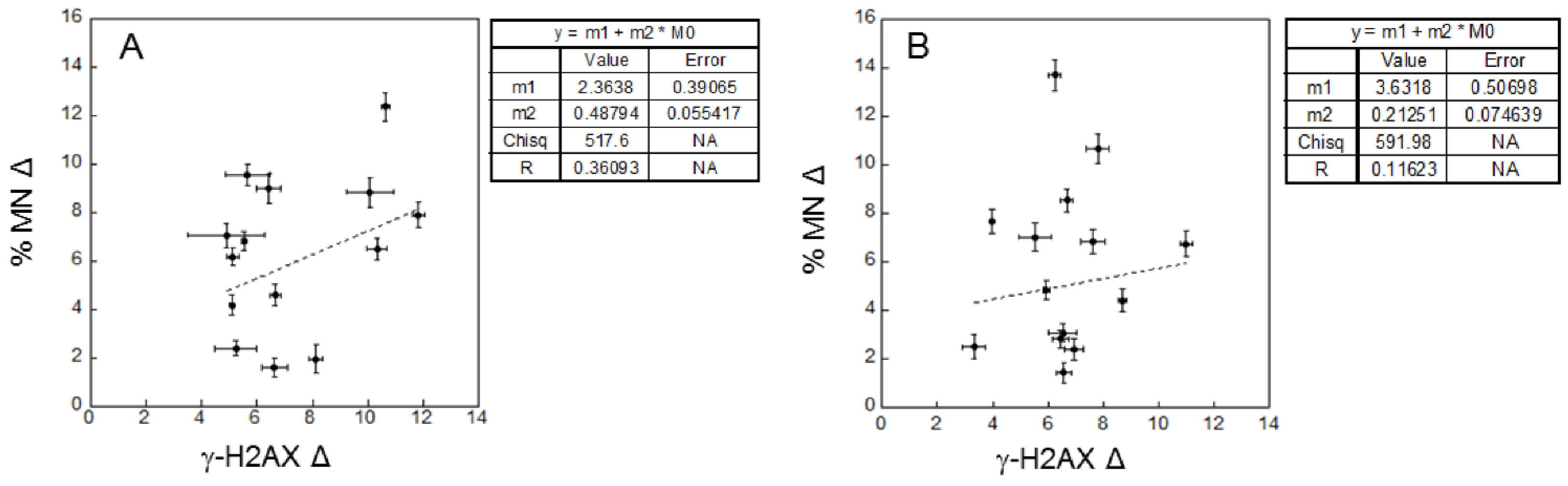
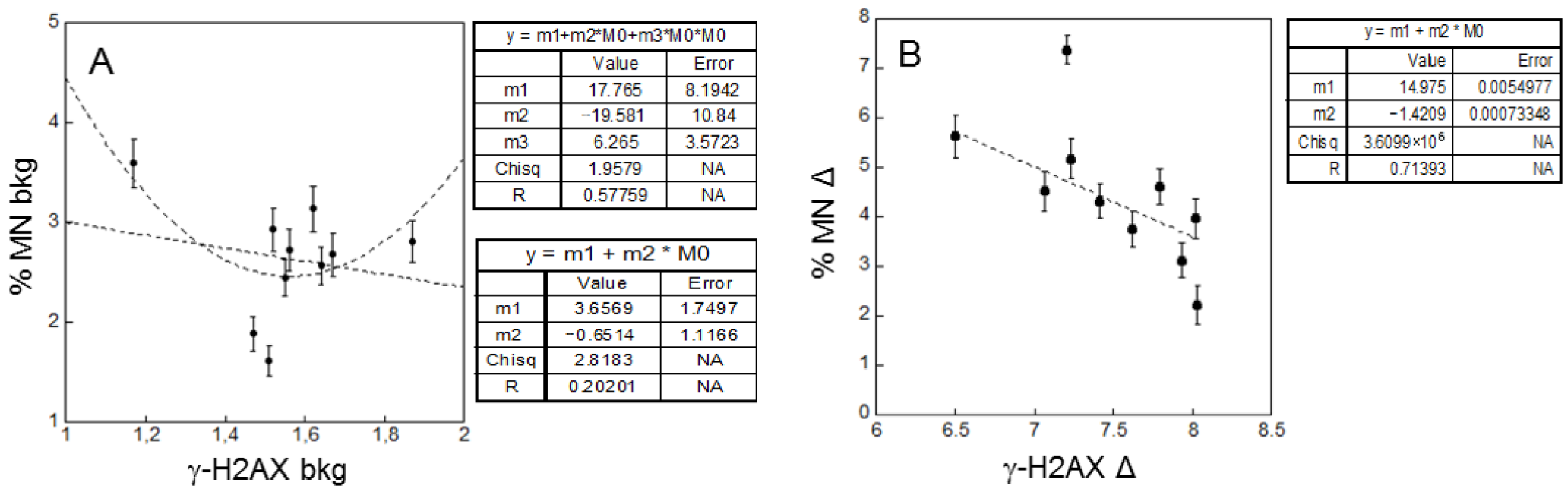
2.3. Correlations
- Correlations between background levels and correlations between radio-sensitivity in patients and healthy donors
- Correlation between radiobiological and clinical responses
3. Discussion
4. Materials and Methods
4.1. Patients and Healthy Donors
4.2. Radioiodine Treatment
4.3. Lymphocyte Isolation and Culture
4.4. Irradiation
4.5. Flow Cytometry Measurements for γ-H2AX Detection
4.6. MN Induction Assay
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| I-131 | Radioiodine |
| γ-H2AX | Phosphorylated histone H2AX variant |
| MN | Micronuclei |
| GD | Graves’ disease |
| TA | Toxic adenoma |
| TMNG | Toxic multinodular goiter |
| AR | Adaptive response |
| BE | Bystander effect |
| HRS/IRR | Low-dose hyperadiosensitivity/increased radioresistance |
| GI | Genomic instability |
| cD | Challenging dose |
| pD | Priming dose |
| PBL | Peripheral Blood Lymphocytes |
| DSB | Double Strand Breaks |
| DPBS | Dulbecco’phosphate-buffered saline |
| FBS | Foetal calf serum |
| PHA | Phytohemagglutinin |
| F-18-FDG | Fluorine 18 fluorodeoxyglucose |
| PET | Positron emission tomography |
| TRAbs | Thyrotropin receptor antibodies |
| 53BP1 | p53-binding protein 1 |
| CT | Computed Tomography |
References
- Ross, D.S. Radioiodine therapy for hyperthyroidism. N. Engl. J. Med. 2011, 364, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Bahn, R.S.; Burch, H.B.; Cooper, D.S.; Garber, J.R.; Greenlee, M.C.; Klein, I.; Laurberg, P.; McDougall, I.R.; Montori, V.M.; Rivkees, S.A.; et al. Hyperthyroidism and other causes of thyrotoxicosis: Management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid 2011, 21, 593–646. [Google Scholar] [CrossRef] [PubMed]
- Stokkel, M.P.M.; Junak, D.H.; Lassmann, M.; Dietlein, M.; Luster, M. EANM procedure guidelines for therapy of benign thyroid disease. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2218–2228. [Google Scholar] [CrossRef] [PubMed]
- Verburg, F.A.; Luster, M.; Lassmann, M.; Reiners, C. I-131 therapy in patients with benign thyroid disease does not conclusively lead to a higher risk of subsequent malignancies. Nuklearmedizin 2011, 50, 93–99. [Google Scholar] [CrossRef]
- Dimova, E.G.; Bryant, P.E.; Chankova, S.G. “Adaptive response”—Some underlying mechanisms and open questions. Genet. Mol. Biol. 2008, 31, 396–408. [Google Scholar] [CrossRef]
- Monsieurs, M.A.; Thierens, H.M.; Vral, A.M.; Van De Wiele, C.; De Ridder, L.I.; Dierckx, R.A. Adaptive response in patients treated with I-131. J. Nucl. Med. 2000, 41, 17–22. [Google Scholar]
- Bonner, W.M.; Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Sedelnikova, O.A.; Solier, S.; Pommier, Y. γ-H2AX and cancer. Nat. Rev. Cancer 2008, 8, 957–967. [Google Scholar] [CrossRef]
- Denoyer, D.; Lobachevsky, P.; Jackson, P.; Thompson, M.; Martin, O.A.; Hicks, R.J. Analysis of 177Lu-DOTA-octreotate therapy-induced DNA damage in peripheral blood lymphocytes of patients with neuroendocrine tumors. J. Nucl. Med. 2015, 56, 505–511. [Google Scholar] [CrossRef]
- Schnarr, K.; Carter, T.F.; Gillis, D.; Webber, C.; Lemon, J.A.; Dayes, I.; Dolling, J.A.; Gulenchyn, K.; Boreham, D.R. Biological Response of Positron Emission Tomography Scan Exposure and Adaptive Response in Humans. Dose Response 2015, 13. [Google Scholar] [CrossRef]
- Komova, O.; Krasavin, E.; Nasonova, E.; Mel’nikova, L.; Shmakova, N.; Cunha, M.; Testa, E.; Beuve, M. Relationship between radioadaptive response and individual radiosensitivity to low doses of gamma radiation: An extended study of chromosome damage in blood lymphocytes of three donors. Int. J. Radiat. Biol. 2018, 94, 54–61. [Google Scholar] [CrossRef]
- Russo, G.L.; Tedesco, I.; Russo, M.; Cioppa, A.; Andreassi, M.G.; Picano, E. Cellular adaptive response to chronic radiation exposure in interventional cardiologists. Eur. Heart J. 2012, 33, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.J.; Cameron, J.R.; Niroomand-rad, A. Adaptive response studies may help choose astronauts for long-term space travel. Adv. Space Res. 2003, 31, 1543–1551. [Google Scholar] [CrossRef]
- Ramachandran, E.N.; Karuppasamy, C.V.; Anil Kumar, V.; Soren, D.C.; Vivek, K.P.R.; Koya, P.K.M.; Jaikrishan, G.; Das, B. Radio-adaptive response in peripheral blood lymphocytes of individuals residing in high-level natural radiation areas of Kerala in the southwest coast of India. Mutagenesis 2016, 32, 267–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Syaifudin, M.; Defiyandra, V.P.; Nurhayati, S.; Purnami, S.; Pudjadi, E. Micronucleus assay-based evaluation of radiosensitivity of lymphocytes among inhabitants living in high background radiation area of Mamuju; West Sulawesi; Indonesia. Genome Integr. 2018, 9, 2. [Google Scholar] [CrossRef]
- Pernot, E.; Hall, J.; Baatout, S.; Benotmane, M.A.; Blanchardon, E.; Bouffler, S.; El Saghire, H.; Gomolka, M.; Guertler, A.; Harms-Ringdahl, M.; et al. Ionizing radiation biomarkers for potential use in epidemiological studies. Mutat. Res. 2012, 751, 258–286. [Google Scholar] [CrossRef] [PubMed]
- Ivashkevich, A.; Redon, C.E.; Nakamura, A.J.; Martin, R.F.; Martin, O.A. Use of the gamma-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 2012, 327, 123–133. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Mah, L.J.; El-Osta, A.; Karagiannis, T.C. H2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef]
- Prasad, A.; Visweswaran, S.; Kanagaraj, K.; Raavi, V.; Arunan, M.; Venkatachalapathy, E.; Paneerselvam, S.; Jose, M.; Ozhimuthu, A.; Perumal, V. F-18-FDG PET/CT scanning: Biological effects on patients: Entrance surface dose, DNA damage, and chromosome aberrations in lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2019, 838, 59–66. [Google Scholar] [CrossRef]
- Ismail, I.H.; Wadhra, T.I.; Hammarsten, O. An optimized method for detecting gamma-H2AX in blood cells reveals a significant interindividual variation in the gamma-H2AX response among humans. Nucleic Acids Res. 2007, 35, e36. [Google Scholar] [CrossRef]
- Horn, S.; Barnard, S.; Rothkamm, K. Gamma-H2AX-based dose estimation for whole and partial body radiation exposure. PLoS ONE 2011, 6, e25113. [Google Scholar] [CrossRef] [PubMed]
- Vral, A.; Fenech, M.; Thierens, H. The micronucleus assay as a biological dosimeter of in vivo ionizing radiation exposure. Mutagenesis 2011, 26, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Surowy, H.; Rinckleb, A.; Luedeke, M.; Stuber, M.; Wecker, A.; Varga, D.; Maier, C.; Hoegel, J.; Vogel, W. Heritability of baseline and induced micronucleus frequencies. Mutagenesis 2011, 26, 111–117. [Google Scholar] [CrossRef] [PubMed]
- El-Zein, R.; Vral, A.; Etzel, C.J. Cytokinesis-blocked micronucleus assay and cancer risk assessment. Mutagenesis 2011, 26, 101–106. [Google Scholar] [CrossRef]
- Lassmann, M.; Hänscheid, H.; Gassen, D.; Biko, J.; Meineke, V.; Reiners, C.; Scherthan, H. In vivo formation of γ-H2AX and 53BP1 DNA repair foci in blood cells after radioiodine therapy of differentiated thyroid cancer. J. Nucl. Med. 2010, 51, 1318–1325. [Google Scholar] [CrossRef]
- Eberlein, U.; Scherthan, H.; Bluemel, C.; Peper, M.; Lapa, C.; Buck, A.K.; Port, M.; Lassmann, M. DNA Damage in Peripheral Blood Lymphocytes of Thyroid Cancer Patients After Radioiodine Therapy. J. Nucl. Med. 2016, 57, 173–179. [Google Scholar] [CrossRef]
- May, M.S.; Brand, M.; Wuest, W.; Anders, K.; Kuwert, T.; Prante, O.; Schmidt, D.; Maschauer, S.; Semelka, R.C.; Uder, M.; et al. Induction and repair of DNA double-strand breaks in blood lymphocytes of patients undergoing ¹⁸F-FDG PET/CT examinations. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1712–1719. [Google Scholar] [CrossRef]
- Thierens, H.; Vral, A.; de Ridder, L. Biological dosimetry using the micronucleus assay for lymphocytes: Interindividual differences in dose response. Health Phys. 1991, 61, 623–630. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Biological Mechanisms of Radiation Actions at Low Doses; United Nations: New York, NY, USA, 2012; pp. 1–29. [Google Scholar]
- Blyth, B.J.; Sykes, P.J. Radiation-induced bystander effects: What are they; and how relevant are they to human radiation exposures? Radiat. Res. 2011, 176, 139–157. [Google Scholar] [CrossRef]
- Kadim, M.; Salomaa, S.; Wright, E.; Hildebrandt, G.; Belyakov, O.V.; Prise, K.M.; Little, M.P. Non-targeted effects of ionising radiation-implications for low dose risk. Mutat. Res. 2013, 752, 84–98. [Google Scholar] [CrossRef]
- Fratini, E.; Carbone, C.; Capece, D.; Esposito, G.; Simone, G.; Tabocchini, M.A.; Tomasi, M.; Belli, M.; Satta, L. Low-radiation environment affects the development of protection mechanisms in V79 cells. Radiat. Environ. Biophys. 2015, 54, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Hänscheid, H.; Canzi, C.; Eschner, W.; Flux, G.; Luster, M.; Strigari, L.; Lassmann, M. EANM Dosimetry Committee Series on Standard Operational Procedures for Pre-Therapeutic Dosimetry II. Dosimetry prior to radioiodine therapy of benign thyroid diseases. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Bal, C.; Damle, N.A.; Ballal, S.; Goswami, R.; Hari, S.; Kumar, P. Comparison, of, clinical, outcome, after, a, fixed, dose, versus, dosimetry-based, radioiodine, treatment, of, Graves’, disease: Results of a randomized controlled trial in Indian population. Indian J. Endocrinol. Metab. 2014, 18, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Canto, A.U.; Dominguez, P.N.; Jimeno, C.A.; Obaldo, J.M.; Ogbac, R.V. Comparison of Fixed versus Calculated Activity of Radioiodine for the Treatment of Graves Disease in Adults. Endocrinol. Metab. 2016, 31, 168–173. [Google Scholar] [CrossRef]
- Rooij, A.; Vandenbroucke, J.P.; Smit, J.W.; Stokkel, M.P.; Dekkers, O.M. Clinical outcomes after estimated versus calculated activity of radioiodine for the treatment of hyperthyroidism: Systematic review and meta-analysis. Eur. J. Endocrinol. 2009, 161, 771–777. [Google Scholar] [CrossRef]
- Hamasaki, K.; Imai, K.; Nakachi, K.; Takahashi, N.; Kodama, Y.; Kusunoki, Y. Short-term culture and γ-H2AX flow cytometry determine differences in individual radiosensitivity in human peripheral T lymphocytes. Environ. Mol. Mutagen. 2007, 48, 38–47. [Google Scholar] [CrossRef]
- Fenech, M. The in vitro micronucleus technique. Mutat. Res. 2000, 455, 81–95. [Google Scholar] [CrossRef]
- Bevington, P.R.; Robinson, D.K. Data Reduction and Error Analysis for the Physical Sciences, 3rd ed.; In McGraw-Hill Education: New York, NY, USA, 2002; pp. 1–320. [Google Scholar]
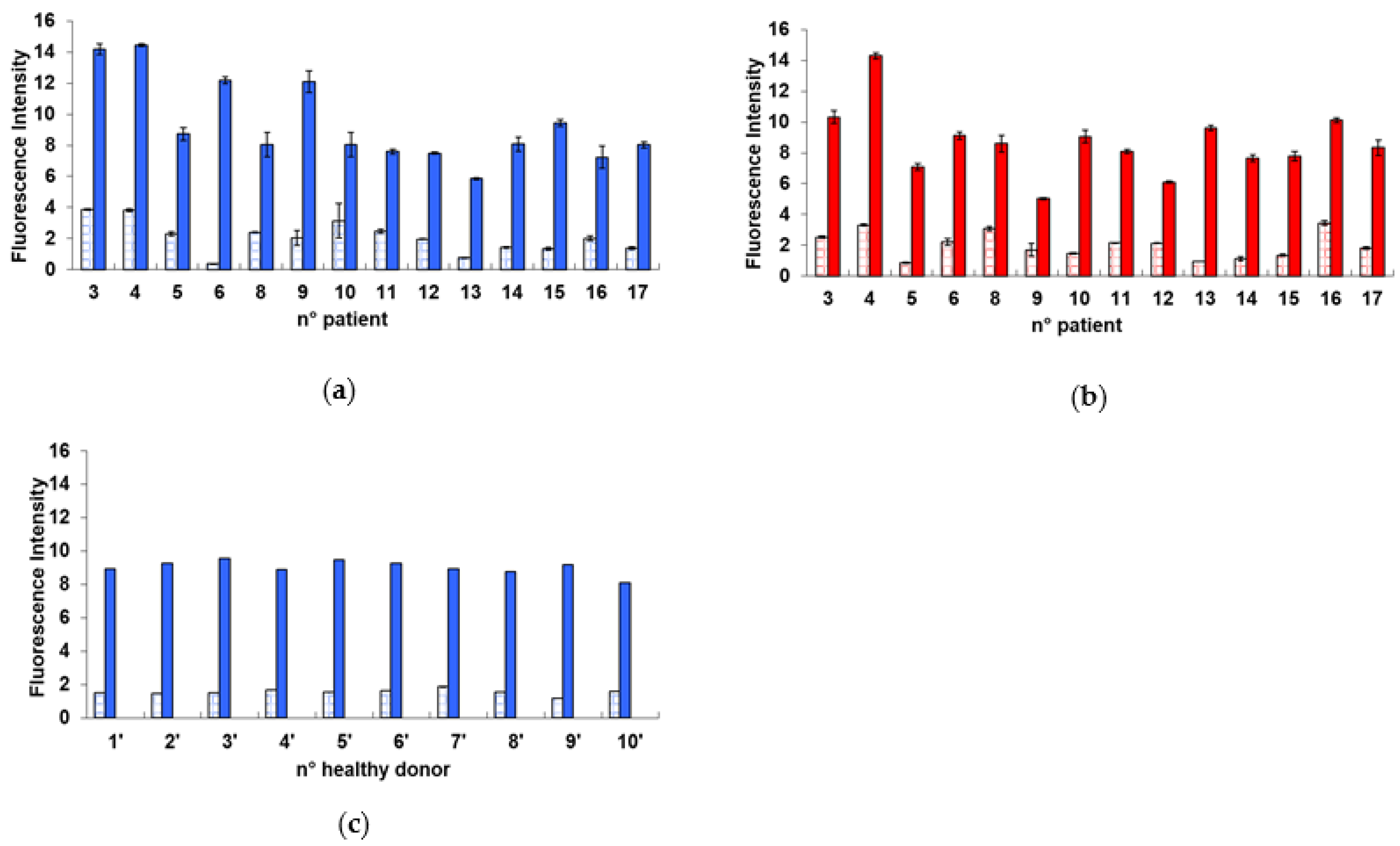




| Mean Fluorescence Intensity | Mean Delta Fluorescence Intensity | Mean MN Percentage | Mean Delta MN Percentage | |
|---|---|---|---|---|
| Patients | ||||
| before I-131, background | 2.08 ± 1.03 | 7.31 ± 2.42 | 3.84 ± 1.99 | 6.43 ± 3.17 |
| before I-131, after cD * | 9.39 ± 2.71 | 10.26 ± 3.51 | ||
| after I-131, background | 1.96 ± 0.87 | 6.66 ± 1.87 | 4.67 ± 2.39 | 6.07 ± 4.01 |
| after I-131, after cD * | 8.53 ± 2.23 | 10.74 ± 5.07 | ||
| Healthy donors | ||||
| no I-131, background | 1.56 ± 0.18 | 7.48 ± 0.49 | 2.64 ± 0.57 | 4.48 ± 1.41 |
| no I-131, after cD * | 9.04 ± 0.41 | 7.12 ± 1.53 |
| Patient No | Radiosensitivity before I−131 (γ−H2AX Assay) | Change in Radiosensitivity (γ−H2AX Assay) | Radiosensitivity before I−131 (MN Assay) | Change in Radiosensitivity (MN Assay) | Outcome at 6 Months |
|---|---|---|---|---|---|
| 1 | NE | NE | 10.860 | + | Hypo |
| 2 | NE | NE | 8.4800 | − | Hyper |
| 3 | 10.332 | − | 6.5100 | + | Hypo |
| 4 | 10.639 | + | 12.360 | − | Hypo |
| 5 | 6.4320 | 0 | 9.0000 | + | Eu |
| 6 | 11.824 | − | 7.9100 | − | Hypo |
| 7 | NE | NE | 2.3800 | 0 | NE |
| 8 | 5.6650 | 0 | 9.5600 | − | Hypo |
| 9 | 10.069 | − | 8.8400 | − | Hypo |
| 10 | 4.9050 | + | 7.0600 | 0 | Hypo |
| 11 | 5.1320 | + | 6.1900 | − | Hypo |
| 12 | 5.5300 | − | 6.8300 | 0 | Hypo |
| 13 | 5.1010 | + | 4.1800 | 0 | Eu |
| 14 | 6.6370 | 0 | 1.6200 | 0 | Hypo |
| 15 | 8.1250 | − | 1.9700 | 0 | Eu |
| 16 | 5.2400 | + | 2.4000 | + | Hypo |
| 17 | 6.6590 | 0 | 4.6000 | − | Hyper |
| 18 | NE | NE | 4.9200 | − | Eu |
| Mean value | 7.31 | 5.53 |
| Conditional Probability of an Outcome Given a Certain Level of Radio-Sensitivity | γ-H2AX Assay | MN Assay |
|---|---|---|
| p(Hypo/low radio-sensitivity) | 6/9 (67%) | 3/8 (38%) |
| p(Eu/low radio-sensitivity) | 2/9 (22%) | 4/8 (50%) |
| p(Hyper/low radio-sensitivity) | 1/9 (11%) | 1/8 (13%) |
| p(Hypo/high radio-sensitivity) | 4/5 (80%) | 9/11 (82%) |
| p(Eu/high radio-sensitivity) | 1/5 (20%) | 1/11 (9%) |
| p(Hyper/high radio-sensitivity) | 0/5 (0%) | 1/11 (9%) |
| Conditional Probability of a Level of Radio-Sensitivity Given a Certain Outcome | γ-H2AX assay | MN assay |
| p(low radio-sensitivity/Hypo) | 6/10 (60%) | 2/11 (18%) |
| p(high radio-sensitivity/Hypo) | 4/10 (40%) | 9/11 (82%) |
| p(low radio-sensitivity/Eu) | 2/3 (67%) | 3/4 (80%) |
| p(high radio-sensitivity/Eu) | 1/3 (33%) | 1/4 (25%) |
| p(low radio-sensitivity/Hyper) | 1/1 (100%) | 1/2 (50%) |
| p(high radio-sensitivity/Hyper) | 0/1 (0%) | 1/2 (50%) |
| Conditional Probability of High Radio-Sensitivity from Both Assays Given a Certain Outcome | ||
| p(Hypo/2xhigh radio-sensitivity) | 4/4 (100)% | |
| p(Eu/2xhigh radio- sensitivity) | 0/4 (0%) | |
| p(Hyper/2xhigh radio-sensitivity) | 0/4 (0%) | |
| Patient No. | Sex | Age | Pathology | Administered I-131 Activity (MBq) |
|---|---|---|---|---|
| 1 | F | 43 | GD | 296 |
| 2 | M | 80 | TA | 600 |
| 3 | F | 30 | GD | 370 |
| 4 | M | 62 | GD | 370 |
| 5 | F | 65 | TMNG | 600 |
| 6 | M | 71 | TMNG | 600 |
| 7 | M | 70 | TA | 600 |
| 8 | F | 66 | TA | 600 |
| 9 | M | 58 | TMNG | 600 |
| 10 | M | 78 | TA | 600 |
| 11 | F | 78 | TMNG | 600 |
| 12 | F | 69 | TMNG | 600 |
| 13 | F | 73 | TA | 600 |
| 14 | F | 59 | GD | 222 |
| 15 | M | 74 | TA | 600 |
| 16 | M | 27 | GD | 222 |
| 17 | M | 47 | TA | 600 |
| 18 | M | 69 | TA | 600 |
| Age Group | Patients (γ-H2AX) | Patients (MN Induction) | Donors (γ-H2AX and MN Induction) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M | F | Tot (%) | M | F | Tot (%) | M | F | Tot (%) | |
| ≤55 | 2 | 1 | 3 (21%) | 2 | 2 | 4 (22%) | 1 | 1 | 2 (20%) |
| 56–69 | 2 | 4 | 6 (43%) | 3 | 4 | 7 (39%) | 2 | 2 | 4 (40%) |
| ≥70 | 3 | 2 | 5 (36%) | 5 | 2 | 7 (39%) | 2 | 2 | 4 (40%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dini, V.; Salvatori, M.; Belli, M.; Lago, M.E.; Nosdeo, A.; Dambra, D.P.; Lo Conte, L.; Pecchia, I.; Giordano, A. Changes in Radiosensitivity to Gamma-Rays of Lymphocytes from Hyperthyroid Patients Treated with I-131. Int. J. Mol. Sci. 2022, 23, 10156. https://doi.org/10.3390/ijms231710156
Dini V, Salvatori M, Belli M, Lago ME, Nosdeo A, Dambra DP, Lo Conte L, Pecchia I, Giordano A. Changes in Radiosensitivity to Gamma-Rays of Lymphocytes from Hyperthyroid Patients Treated with I-131. International Journal of Molecular Sciences. 2022; 23(17):10156. https://doi.org/10.3390/ijms231710156
Chicago/Turabian StyleDini, Valentina, Massimo Salvatori, Mauro Belli, Maria Elena Lago, Alessandra Nosdeo, Donatella Pia Dambra, Luisa Lo Conte, Ilaria Pecchia, and Alessandro Giordano. 2022. "Changes in Radiosensitivity to Gamma-Rays of Lymphocytes from Hyperthyroid Patients Treated with I-131" International Journal of Molecular Sciences 23, no. 17: 10156. https://doi.org/10.3390/ijms231710156
APA StyleDini, V., Salvatori, M., Belli, M., Lago, M. E., Nosdeo, A., Dambra, D. P., Lo Conte, L., Pecchia, I., & Giordano, A. (2022). Changes in Radiosensitivity to Gamma-Rays of Lymphocytes from Hyperthyroid Patients Treated with I-131. International Journal of Molecular Sciences, 23(17), 10156. https://doi.org/10.3390/ijms231710156







