A Bioengineering Approach for the Development of Fibroblast Growth Factor-7-Functionalized Sericin Biomaterial Applicable for the Cultivation of Keratinocytes
Abstract
1. Introduction
2. Results
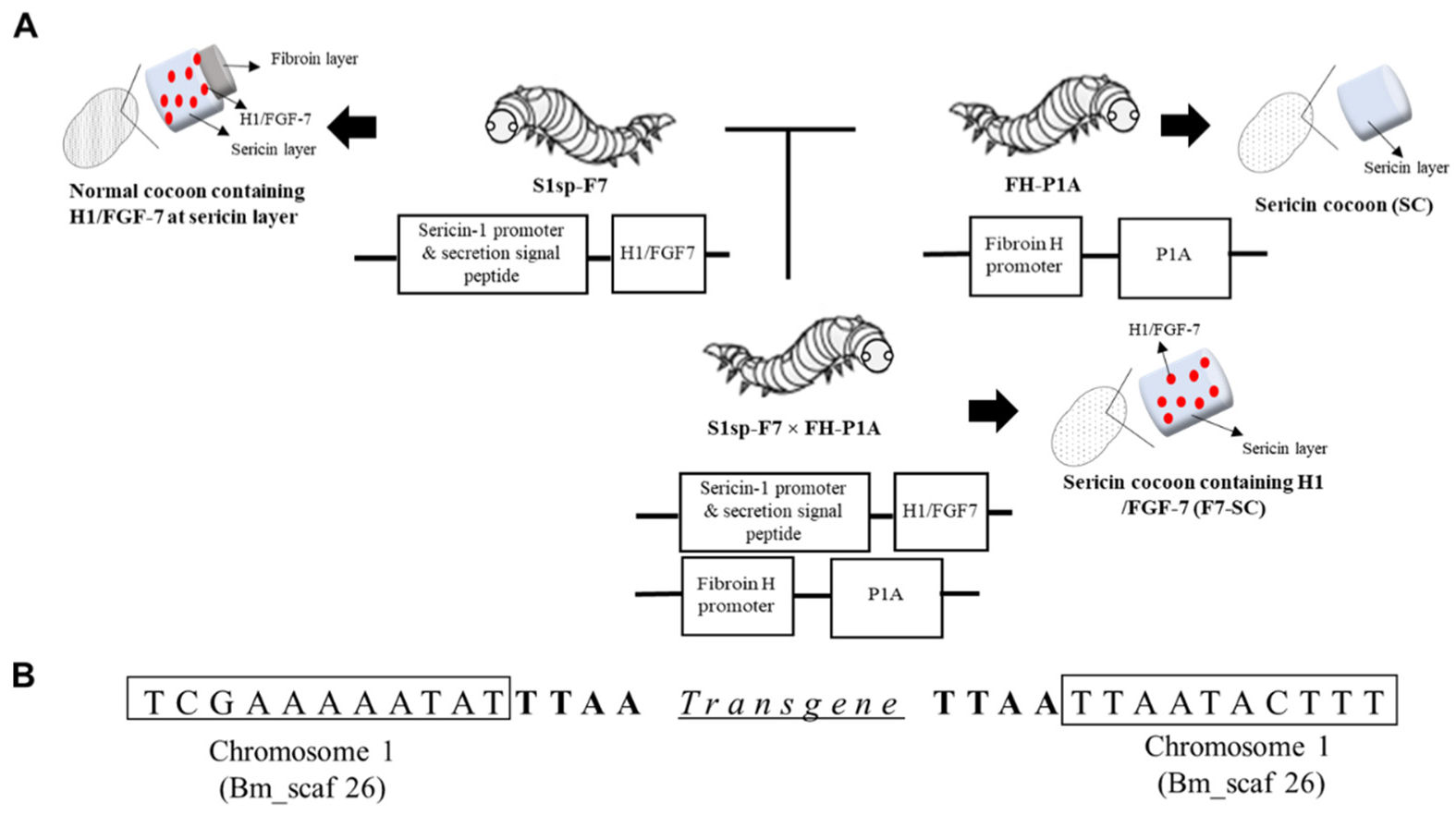
2.1. Establishment of Transgenic Silkworm Line Spinning Sericin Cocoons Incorporating H1/FGF-7
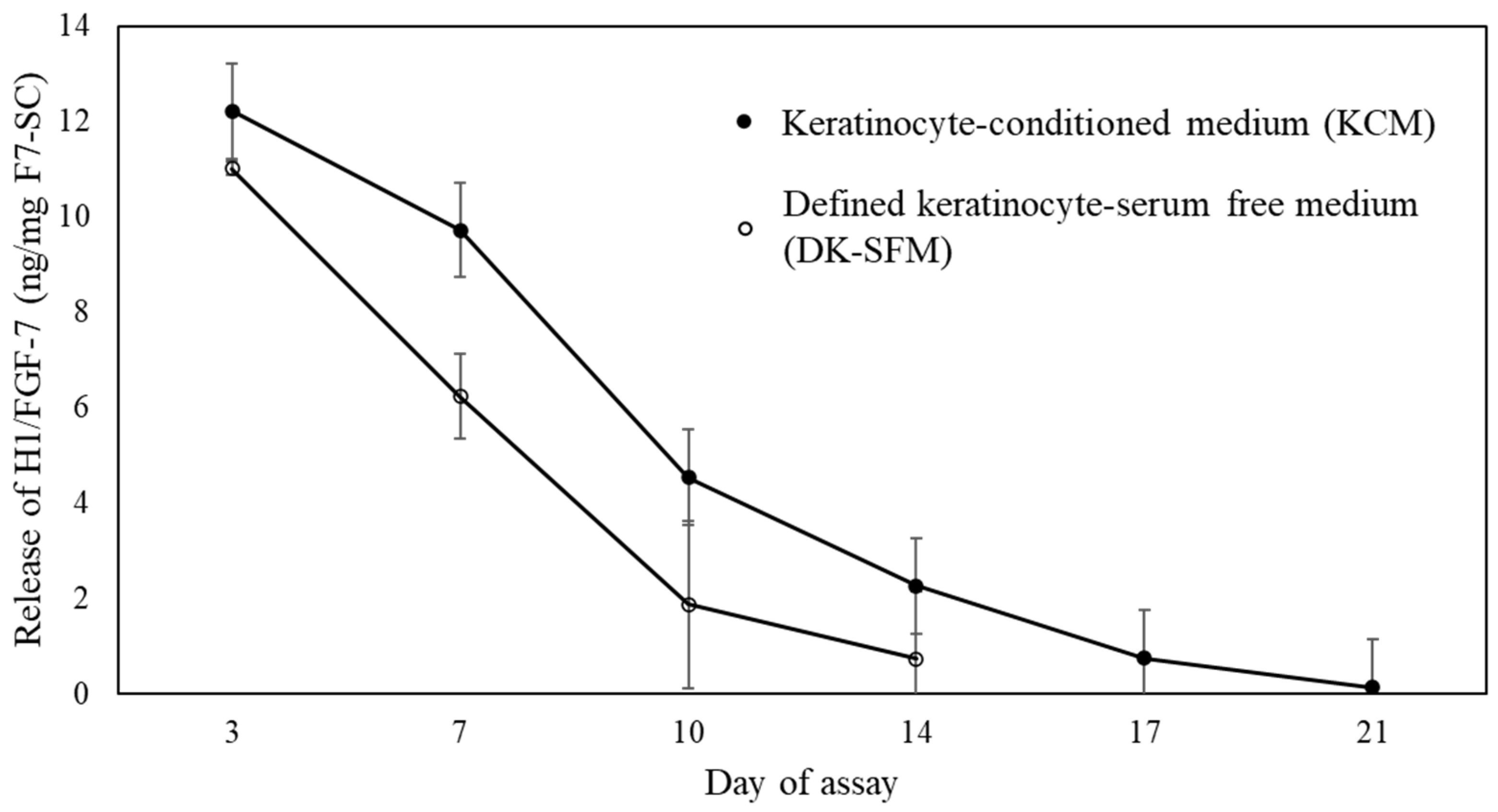
2.2. Release Profile of H1/FGF-7 from F7-SC Powders in Aqueous Media
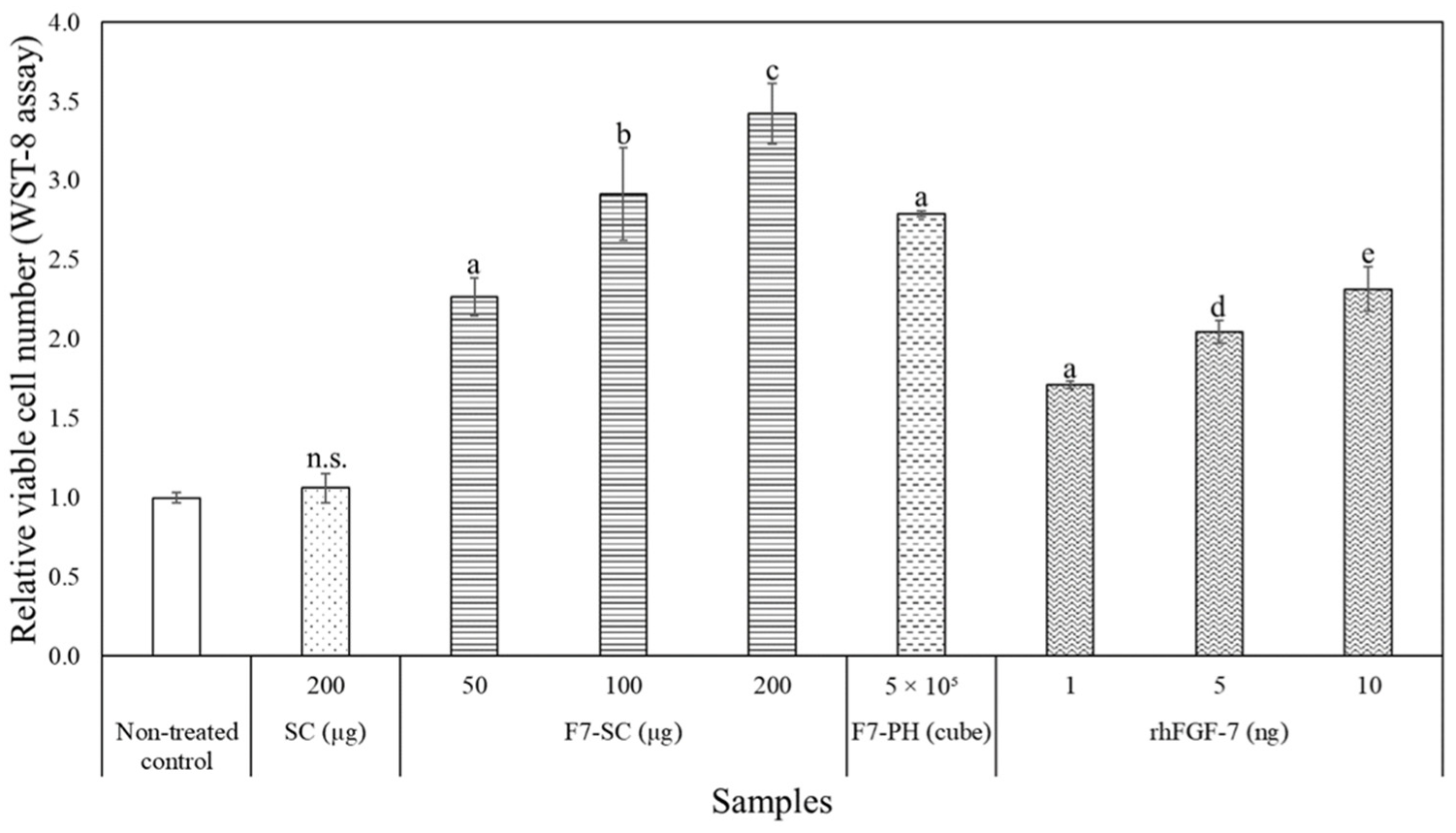
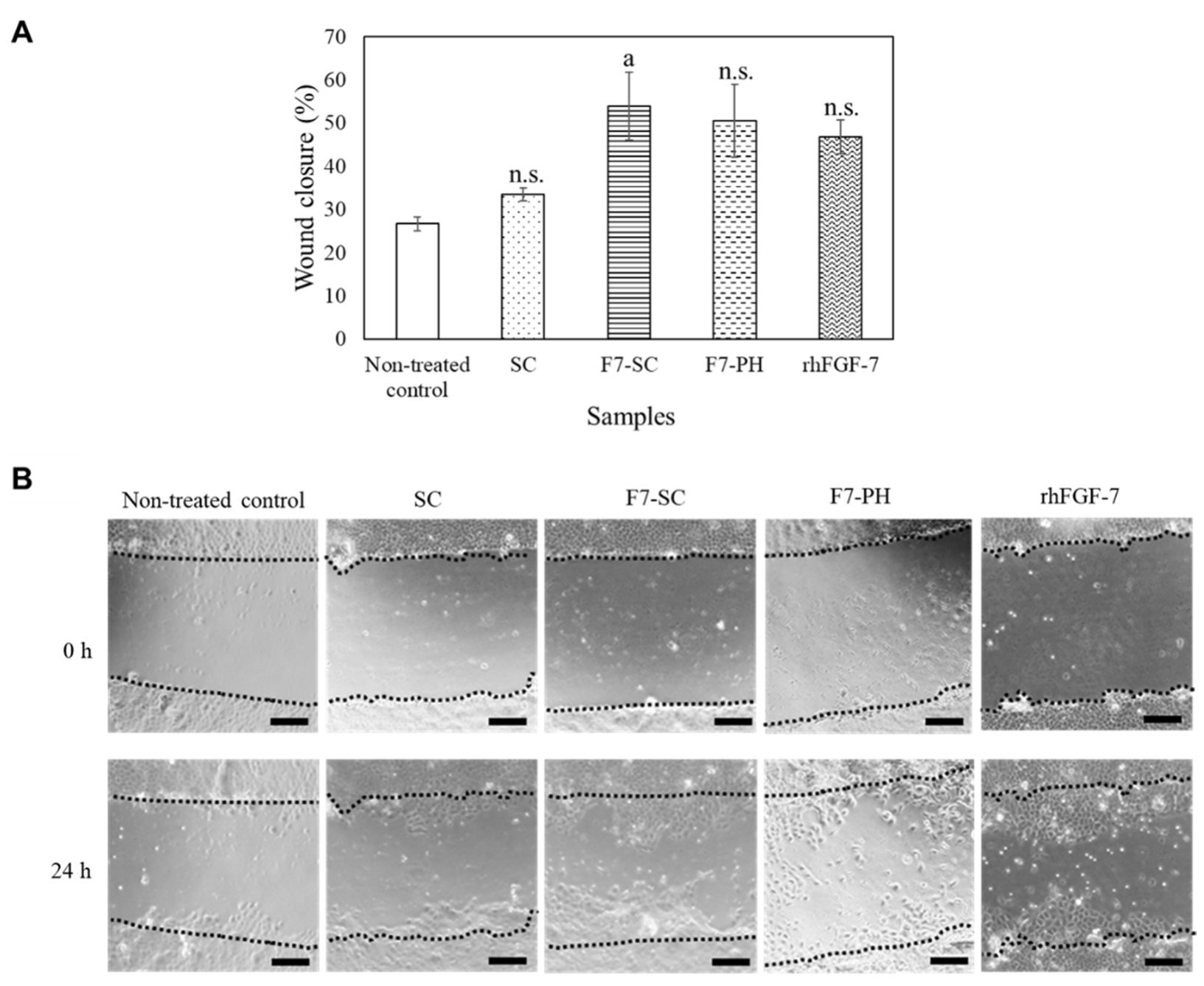
2.3. Biological Activities of H1/FGF-7 Incorporated into Sericin-Cocoon Powder
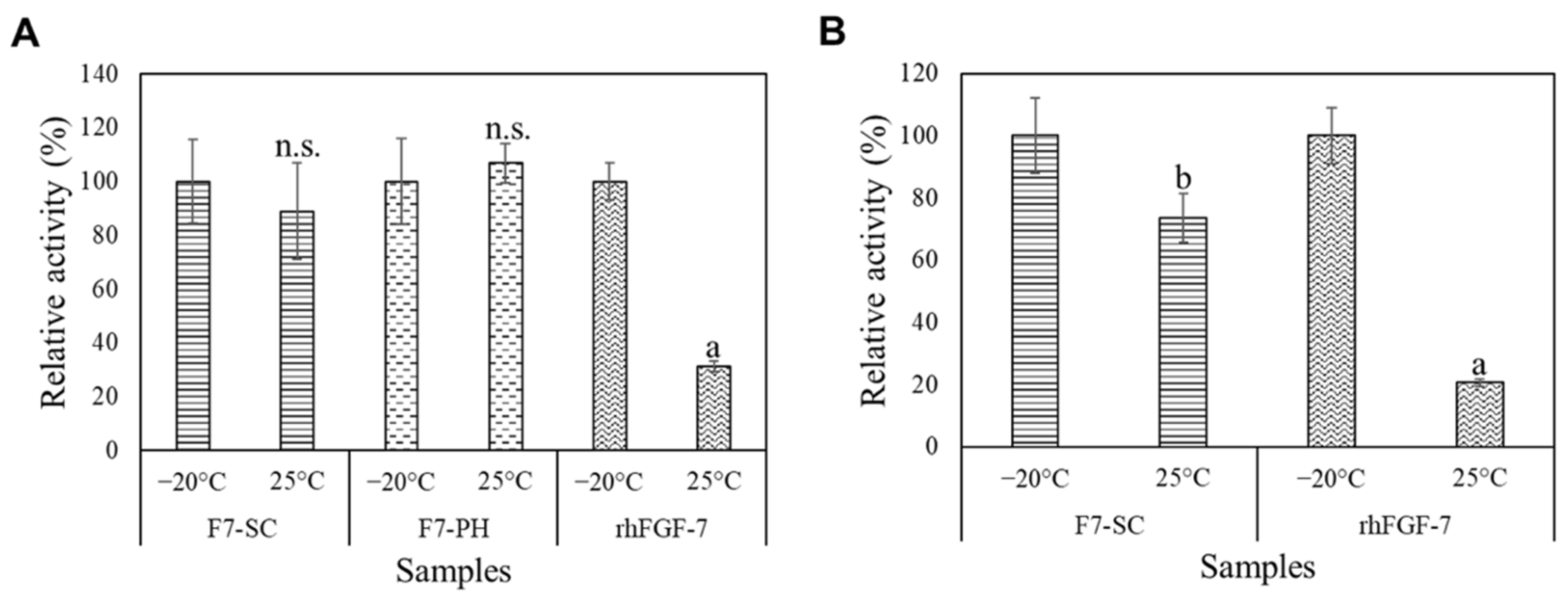
2.4. Storage Stability of H1/FGF-7 Incorporated into Sericin-Cocoon Powder
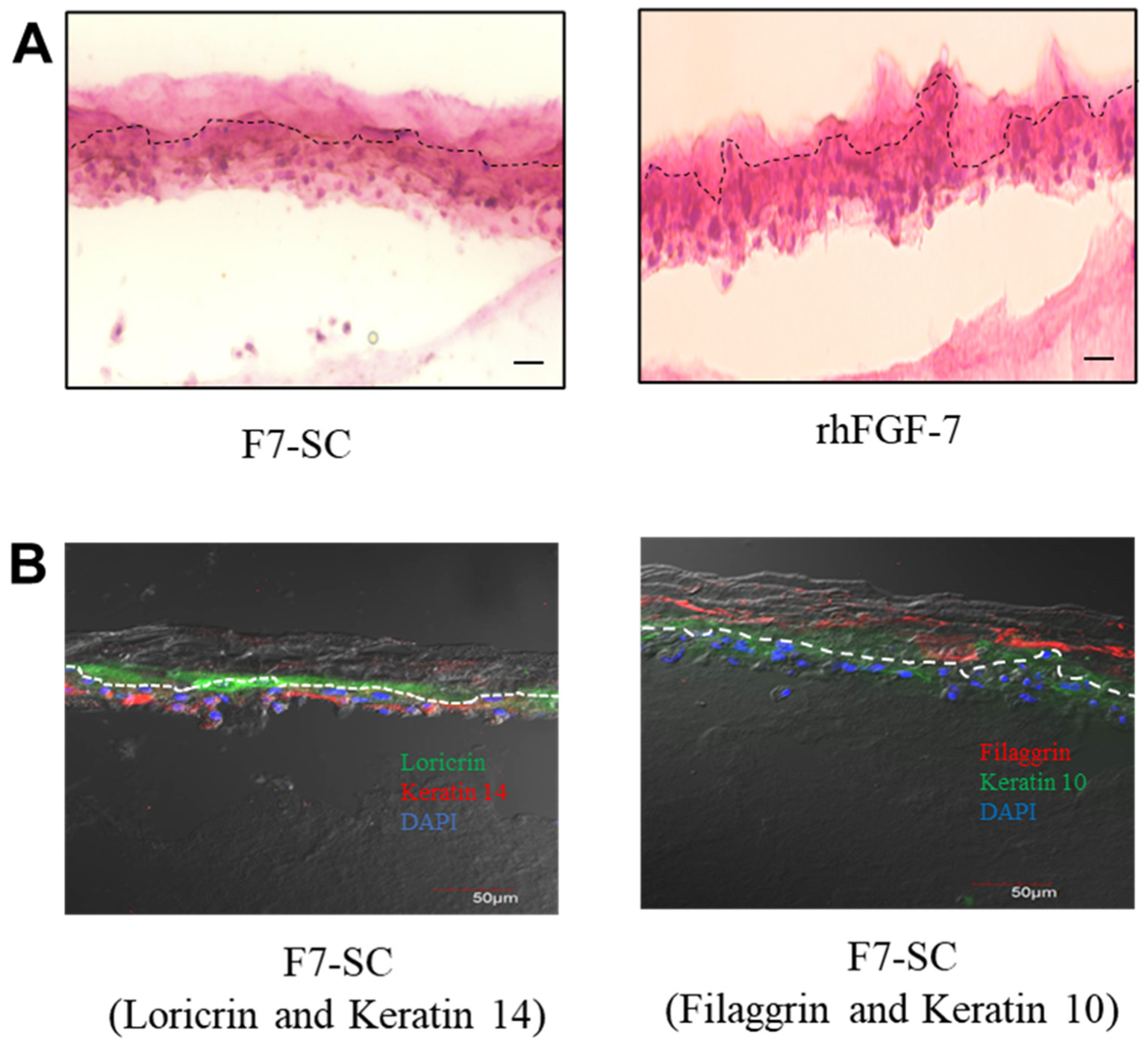
2.5. Three-Dimensional Cultivation of Keratinocytes In Vitro
3. Discussion
4. Materials and Methods
4.1. Silkworm Strains
4.2. Cultivation of NHEK Cells
4.3. Generation of Transgenic Silkworm Lines S1sp-F7 and S1sp-F7 × FH-P1A
4.4. Preparation of Sericin-Cocoon Powders
4.5. Western Blot Analysis of H1/FGF-7
4.6. Release Profile of H1/FGF-7 from F7-SC Powder
4.7. Effect of H1/FGF-7 on Proliferation of NHEK Cells
4.8. Effect of H1/FGF-7 on Migration of NHEK Cells
4.9. Storage Stability of H1/FGF-7 Incorporated into Sericin-Cocoon Powder
4.10. Three-Dimensional Cultivation of NHEK Cells
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rubin, J.S.; Bottaro, D.P.; Chedid, M.; Miki, T.; Ron, D.; Cheon, H.G.; Taylor, W.G.; Fortney, E.; Hiromi, S.; Finch, P.W.; et al. Keratinocyte growth factor. Cell Biol. Int. 1995, 19, 359–411. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Chang, Z.; Peng, B.; Xia, Q.; Lu, W.; Huang, P.; Tsao, M.-S.; Chiao, P.J. Keratinocyte growth factor/fibroblast growth factor-7-regulated cell migration and invasion through activation of NF-κB transcription factors. J. Biol. Chem. 2007, 282, 6001–6011. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Smola, H. Paracrine regulation of keratinocyte proliferation and differentiation. Trends Cell Biol. 2001, 11, 143–146. [Google Scholar] [CrossRef]
- Marchese, C.; Rubin, J.; Ron, D.; Fagcioni, A.; Torrisi, M.R.; Messina, A.; Frati, L.; Aaronson, S.A. Human keratinocyte growth factor activity on proliferation and differentiation of human keratinocytes: Differentiation response distinguishes KCF from EGF family. J. Cell Physiol. 1990, 144, 326–332. [Google Scholar] [CrossRef]
- Peplow, P.V.; Chatterjee, M.P. A review of the influence of growth factors and cytokines in in vitro human keratinocyte migration. Cytokine 2013, 62, 1–21. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Egger, B.; Tolmos, J.; Procaccino, F.; Sarosi, I.; Friess, H.; Büchler, M.W.; Stamos, M.; Eysselein, V.E. Keratinocyte growth factor promotes healing of left-sided colon anastomoses. Am. J. Surg. 1998, 176, 18–24. [Google Scholar] [CrossRef]
- Kumar, P.; Ciftci, S.; Barthes, J.; Knopf-Marques, H.; Muller, C.B.; DeBry, C.; Vrana, N.E.; Ghaemmaghami, A.M. A composite gelatin/hyaluronic acid hydrogel as an ECM mimic for developing mesenchymal stem cell-derived epithelial tissue patches. J. Tissue Eng. Regen. Med. 2019, 14, 45–57. [Google Scholar] [CrossRef]
- Tabata, Y.; Ikada, Y. Vascularization effect of basic fibroblast growth factor released from gelatin hydrogels with different biodegradabilities. Biomaterials 1999, 20, 2169–2175. [Google Scholar] [CrossRef]
- Nishida, A.; Naganuma, T.; Kanazawa, T.; Takashima, Y.; Yamada, M.; Okada, H. The characterization of protein release from sericin film in the presence of an enzyme: Towards fibroblast growth factor-2 delivery. Int. J. Pharm. 2011, 414, 193–202. [Google Scholar] [CrossRef]
- Ijiri, H.; Coulibaly, F.; Nishimura, G.; Nakai, D.; Chiu, E.; Takenaka, C.; Ikeda, K.; Nakazawa, H.; Hamada, N.; Kotani, E.; et al. Structure-based targeting of bioactive proteins into cypovirus polyhedra and application to immobilized cytokines for mammalian cell culture. Biomaterials 2009, 30, 4297–4308. [Google Scholar] [CrossRef]
- Kotani, E.; Yamamoto, N.; Kobayashi, I.; Uchino, K.; Muto, S.; Ijiri, H.; Shimabukuro, J.; Tamura, T.; Sezutsu, H.; Mori, H. Cell proliferation by silk gut incorporating FGF-2 protein microcrystals. Sci. Rep. 2015, 5, 11051. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, G.; Sun, C.; Zhang, J.; Zhang, Y.; Zhang, Y.; Ye, C.; Wang, X.; Ilghari, D.; Li, X. A novel solid-phase site-specific pegylation enhances the in vitro and in vivo biostabilty of recombinant human keratinocyte growth factor 1. PLoS ONE 2012, 7, e36423. [Google Scholar] [CrossRef][Green Version]
- Nguyen, T.H.; Kim, S.-H.; Decker, C.G.; Wong, D.Y.; Loo, J.A.; Maynard, H.D. A heparin-mimicking polymer conjugate stabilizes basic fibroblast growth factor. Nat. Chem. 2013, 5, 221–227. [Google Scholar] [CrossRef]
- Jiao, Z.; Song, Y.; Jin, Y.; Zhang, C.; Peng, D.; Chen, Z.; Chang, P.; Kundu, S.C.; Wang, G.; Wang, Z.; et al. In vivo characterizations of the immune properties of sericin: An ancient material with emerging value in biomedical applications. Macromol. Biosci. 2017, 17, 170029. [Google Scholar] [CrossRef]
- Panilaitis, B.; Altman, G.H.; Chen, J.; Jin, H.-J.; Karageorgiou, V.; Kaplan, D.L. Macrophage responses to silk. Biomaterials 2003, 24, 3079–3085. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Tao, M.-L.; Shen, W.-D.; Mao, J.-P.; Chen, Y.-H. Synthesis of silk sericin peptides-L-asparaginase bioconjugates and their characterization. J. Chem. Technol. Biotechnol. 2006, 81, 136–145. [Google Scholar] [CrossRef]
- Scrivano, L.; Iacopetta, D.; Sinicropi, M.S.; Saturnino, C.; Longo, P.; Parisi, O.I.; Puoci, F. Synthesis of sericin-based conjugates by click chemistry: Enhancement of sunitinib bioavailability and cell membrane permeation. Drug Deliv. 2017, 24, 482–490. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.-Q.; Ma, Y.; Xia, Y.-Y.; Shen, W.-D.; Mao, J.-P.; Xue, R.-Y. Silk sericin–insulin bioconjugates: Synthesis, characterization and biological activity. J. Control. Release 2006, 115, 307–315. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The effect of sericin from various extraction methods on cell viability and collagen production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef]
- Umehara, C.; Lian, A.A.; Funahashi, Y.; Takaki, K.; Maruta, R.; Ohmaru, Y.; Okahisa, Y.; Aoki, T.; Mori, H.; Kotani, E. Proliferation of mouse embryonic stem cells on substrate coated with intact silkworm sericin. J. Text. Inst. 2021, 113. [Google Scholar] [CrossRef]
- Tomita, M. Transgenic silkworms that weave recombinant proteins into silk cocoons. Biotechnol. Lett. 2011, 33, 645–654. [Google Scholar] [CrossRef]
- Ogawa, S.; Tomita, M.; Shimizu, K.; Yoshizato, K. Generation of a transgenic silkworm that secretes recombinant proteins in the sericin layer of cocoon: Production of recombinant human serum albumin. J. Biotechnol. 2007, 128, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, H.; Wang, Y.; Wang, R.; Yuan, L.; Ding, H.; Song, C.; Ma, S.; Peng, Z.; Peng, Z.; et al. Advanced silk material spun by a transgenic silkworm promotes cell proliferation for biomedical application. Acta Biomater. 2014, 10, 4947–4955. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Xu, S.; Wang, R.; Chen, W.; Hou, K.; Tian, C.; Wang, F.; Yu, L.; Lu, Z.; et al. Genetically engineered bi-functional silk material with improved cell proliferation and anti-inflammatory activity for medical application. Acta Biomater. 2019, 86, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, H.; Ishida, K.; Kamiishi, Y.; Yoshii, F.; Kume, T. Production of fine powder from silk by radiation. Macromol. Mater. Eng. 2000, 283, 126–131. [Google Scholar] [CrossRef]
- Otsuki, R.; Yamamoto, M.; Matsumoto, E.; Iwamoto, S.-I.; Sezutsu, H.; Suzui, M.; Takaki, K.; Wakabayashi, K.; Mori, H.; Kotani, E. Bioengineered silkworms with butterfly cytotoxin-modified silk glands produce sericin cocoons with a utility for a new biomaterial. Proc. Natl. Acad. Sci. USA 2017, 114, 6740–6745. [Google Scholar] [CrossRef]
- Maruta, R.; Takaki, K.; Yamaji, Y.; Sezutsu, H.; Mori, H.; Kotani, E. Effects of transgenic silk materials that incorporate FGF-7 protein microcrystals on the proliferation and differentiation of human keratinocytes. FASEB BioAdvances 2020, 2, 734–744. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Zhang, X.; Wang, S.; Lu, C. Recognition of signal peptide by protein translocation machinery in middle silk gland of silkworm Bombyx mori. Acta Biochim. Biophys. Sin. 2008, 40, 38–46. [Google Scholar] [CrossRef][Green Version]
- Werner, S. Keratinocyte growth factor: A unique player in epithelial repair processes. Cytokine Growth Factor Rev. 1998, 9, 153–165. [Google Scholar] [CrossRef]
- Finch, P.W.; Rubin, J.S. Keratinocyte growth factor/fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. In Advances in Cancer Research; Woude, G.V., Klein, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 91, pp. 70–137. [Google Scholar] [CrossRef]
- Radek, K.A.; Taylor, K.R.; Gallo, R.L. FGF-10 and specific structural elements of dermatan sulfate size and sulfation promote maximal keratinocyte migration and cellular proliferation. Wound Repair Regen. 2009, 17, 118–126. [Google Scholar] [CrossRef]
- Koivisto, L.; Jiang, G.; Häkkinen, L.; Chan, B.; Larjava, H. HaCaT keratinocyte migration is dependent on epidermal growth factor receptor signaling and glycogen synthase kinase-3α. Exp. Cell Res. 2006, 312, 2791–2805. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Wen, J.; Arakawa, T.; Prestrelski, S.J. A new strategy for enhancing the stability of lyophilized protein: The effect of the reconstitution medium on keratinocyte growth factor. Pharm. Res. 1995, 12, 1447–1452. [Google Scholar] [CrossRef]
- Ikeda, K.; Nakazawa, H.; Shimo-Oka, A.; Ishio, K.; Miyata, S.; Hosokawa, Y.; Matsumura, S.; Masuhara, H.; Belloncik, S.; Alain, R.; et al. Immobilization of diverse foreign proteins in viral polyhedra and potential application for protein microarrays. Proteomics 2006, 6, 54–66. [Google Scholar] [CrossRef]
- Rosdy, M.; Clauss, L.-C. Terminal epidermal differentiation of human keratinocytes grown in chemically defined medium on inert filter substrates at the air-liquid interface. J. Investig. Dermatol. 1990, 95, 409–414. [Google Scholar] [CrossRef]
- Freddi, G.; Mossotti, R.; Innocenti, R. Degumming of silk fabric with several proteases. J. Biotechnol. 2003, 106, 101–112. [Google Scholar] [CrossRef]
- Chavez-Muñoz, C.; Kilani, R.T.; Ghahary, A. Profile of exosomes related proteins released by differentiated and undifferentiated human keratinocytes. J. Cell. Physiol. 2009, 221, 221–231. [Google Scholar] [CrossRef]
- Martínez-Mora, C.; Mrowiec, A.; García-Vizcaíno, E.M.; Alcaraz, A.; Cenis, J.L.; Nicolás, F.J. Fibroin and sericin from bombyx mori silk stimulate cell migration through upregulation and phosphorylation of C-Jun. PLoS ONE 2012, 7, e42271. [Google Scholar] [CrossRef]
- Seeger, M.A.; Paller, A.S. The roles of growth factors in keratinocyte migration. Adv. Wound Care 2015, 4, 213–224. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Tao, M.-L.; Shen, W.-D.; Zhou, Y.-Z.; Ding, Y.; Ma, Y.; Zhou, W.-L. Immobilization of l-asparaginase on the microparticles of the natural silk sericin protein and its characters. Biomaterials 2004, 25, 3751–3759. [Google Scholar] [CrossRef]
- Sumi, T.; Imamura, H. Water-mediated interactions destabilize proteins. Protein Sci. 2021, 30, 2132–2143. [Google Scholar] [CrossRef] [PubMed]
- Dellambra, E.; Odorisio, T.; D’Arcangelo, D.; Failla, C.M.; Facchiano, A. Non-animal models in dermatological research. Altex 2019, 36, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, T.D.N.; Catarino, C.M.; Pennacchi, P.C.; de Assis, S.R.; Gimenes, F.; Consolaro, M.E.L.; Barros, S.B.D.M.; Maria-Engler, S.S. A new reconstructed human epidermis for in vitro skin irritation testing. Toxicol. Vitr. 2017, 42, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, M.; Jiang, S.; Zhang, Y.; Li, R.; Lu, Y.; Liu, L.; Wu, G.; Liu, Y.; Xie, L.; et al. Skin Toxicity Assessment of Silver Nanoparticles in a 3D Epidermal Model Compared to 2D Keratinocytes. Int. J. Nanomed. 2019, 14, 9707–9719. [Google Scholar] [CrossRef]
- Neves, C.R.; Gibbs, S. Progress on reconstructed human skin models for allergy research and identifying contact sensitizers. In Current Topics in Microbiology and Immunology; Springer Science and Business Media Deutschland GmbH: Basel, Switzerland, 2018; pp. 103–129. [Google Scholar]
- Chung, E.; Choi, H.; Lim, J.E.; Son, Y. Development of skin inflammation test model by co-culture of reconstituted 3D skin and RAW264.7 cells. Tissue Eng. Regen. Med. 2014, 11, 87–92. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, L.; Wu, Y.; Xia, Q.; Chen, J.; Roux, K.H. Identification and characterization of an arginine kinase as a major allergen from silkworm (bombyx mori) larvae. Int. Arch. Allergy Immunol. 2009, 150, 8–14. [Google Scholar] [CrossRef]
- Berardo, A.; Pantano, M.F.; Pugno, N.M. Slip knots and unfastening topologies enhance toughness without reducing strength of silk fibroin fibres. Interface Focus 2016, 6, 20150060. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Kuwabara, N.; Uchino, K.; Kobayashi, I.; Kanda, T. An improved DNA injection method for silkworm eggs drastically increases the efficiency of producing transgenic silkworms. J. Insect Biotechnonol. Sericol. 2007, 76, 155–159. [Google Scholar] [CrossRef]
- Yamano, M.; Hirose, R.; Lye, P.Y.; Takaki, K.; Maruta, R.; Liew, M.W.O.; Sakurai, S.; Mori, H.; Kotani, E. Bioengineered silkworm for producing cocoons with high fibroin content for regenerated fibroin biomaterial-based applications. Int. J. Mol. Sci. 2022, 23, 7433. [Google Scholar] [CrossRef]
- Tamura, T.; Thibert, C.; Royer, C.; Kanda, T.; Eappen, A.; Kamba, M.; Kômoto, N.; Thomas, J.-L.; Mauchamp, B.; Chavancy, G.; et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat. Biotechnol. 2000, 18, 81–84. [Google Scholar] [CrossRef]
- Chiu, E.; Coulibaly, F.; Metcalf, P. Insect virus polyhedra, infectious protein crystals that contain virus particles. Curr. Opin. Struct. Biol. 2012, 22, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, H.; Yuan, L.; Ma, S.; Wang, Y.; Duan, X.; Duan, J.; Xiang, Z.; Xia, Q. An optimized sericin-1 expression system for mass-producing recombinant proteins in the middle silk glands of transgenic silkworms. Transgenic Res. 2013, 22, 925–938. [Google Scholar] [CrossRef]
- Tatematsu, K.-I.; Kobayashi, I.; Uchino, K.; Sezutsu, H.; Iizuka, T.; Yonemura, N.; Tamura, T. Construction of a binary transgenic gene expression system for recombinant protein production in the middle silk gland of the silkworm Bombyx mori. Transgenic Res. 2010, 19, 473–487. [Google Scholar] [CrossRef]
- Sakudoh, T.; Sezutsu, H.; Nakashima, T.; Kobayashi, I.; Fujimoto, H.; Uchino, K.; Banno, Y.; Iwano, H.; Maekawa, H.; Tamura, T.; et al. Carotenoid silk coloration is controlled by a carotenoid-binding protein, a product of the yellow blood gene. Proc. Natl. Acad. Sci. USA 2007, 104, 8941–8946. [Google Scholar] [CrossRef]
- Yonemura, N.; Tamura, T.; Uchino, K.; Kobayashi, I.; Tatematsu, K.; Iizuka, T.; Sezutsu, H.; Muthulakshmi, M.; Nagaraju, J.; Kusakabe, T. PhiC31 integrase-mediated cassette exchange in silkworm embryos. Mol. Genet. Genom. 2012, 287, 731–739. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, A.A.; Yamaji, Y.; Kajiwara, K.; Takaki, K.; Mori, H.; Liew, M.W.O.; Kotani, E.; Maruta, R. A Bioengineering Approach for the Development of Fibroblast Growth Factor-7-Functionalized Sericin Biomaterial Applicable for the Cultivation of Keratinocytes. Int. J. Mol. Sci. 2022, 23, 9953. https://doi.org/10.3390/ijms23179953
Lian AA, Yamaji Y, Kajiwara K, Takaki K, Mori H, Liew MWO, Kotani E, Maruta R. A Bioengineering Approach for the Development of Fibroblast Growth Factor-7-Functionalized Sericin Biomaterial Applicable for the Cultivation of Keratinocytes. International Journal of Molecular Sciences. 2022; 23(17):9953. https://doi.org/10.3390/ijms23179953
Chicago/Turabian StyleLian, Ai Ai, Yuka Yamaji, Kazuki Kajiwara, Keiko Takaki, Hajime Mori, Mervyn Wing On Liew, Eiji Kotani, and Rina Maruta. 2022. "A Bioengineering Approach for the Development of Fibroblast Growth Factor-7-Functionalized Sericin Biomaterial Applicable for the Cultivation of Keratinocytes" International Journal of Molecular Sciences 23, no. 17: 9953. https://doi.org/10.3390/ijms23179953
APA StyleLian, A. A., Yamaji, Y., Kajiwara, K., Takaki, K., Mori, H., Liew, M. W. O., Kotani, E., & Maruta, R. (2022). A Bioengineering Approach for the Development of Fibroblast Growth Factor-7-Functionalized Sericin Biomaterial Applicable for the Cultivation of Keratinocytes. International Journal of Molecular Sciences, 23(17), 9953. https://doi.org/10.3390/ijms23179953





