Novel Targets for a Combination of Mechanical Unloading with Pharmacotherapy in Advanced Heart Failure
Abstract
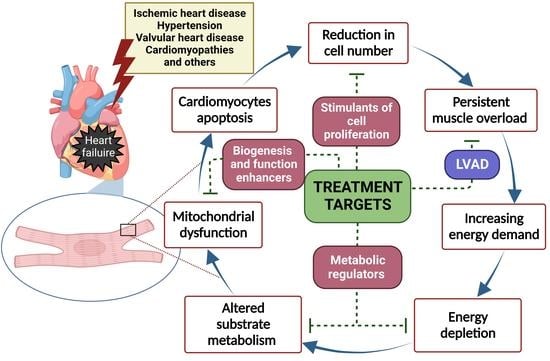
1. Introduction
2. Mechanical Unloading of Failing Heart
2.1. Ventricular Assist Devices (VADs)
2.2. The Current Status of Left Ventricular Assistance Devices (LVADs) Therapy in Heart Failure
2.3. Bridge to Recovery: A Meaningful Phenomenon in LVAD Therapy
2.4. LVAD Limitations
3. Combination of Mechanical Unloading and Pharmacotherapy for Chronic Heart Failure Treatment
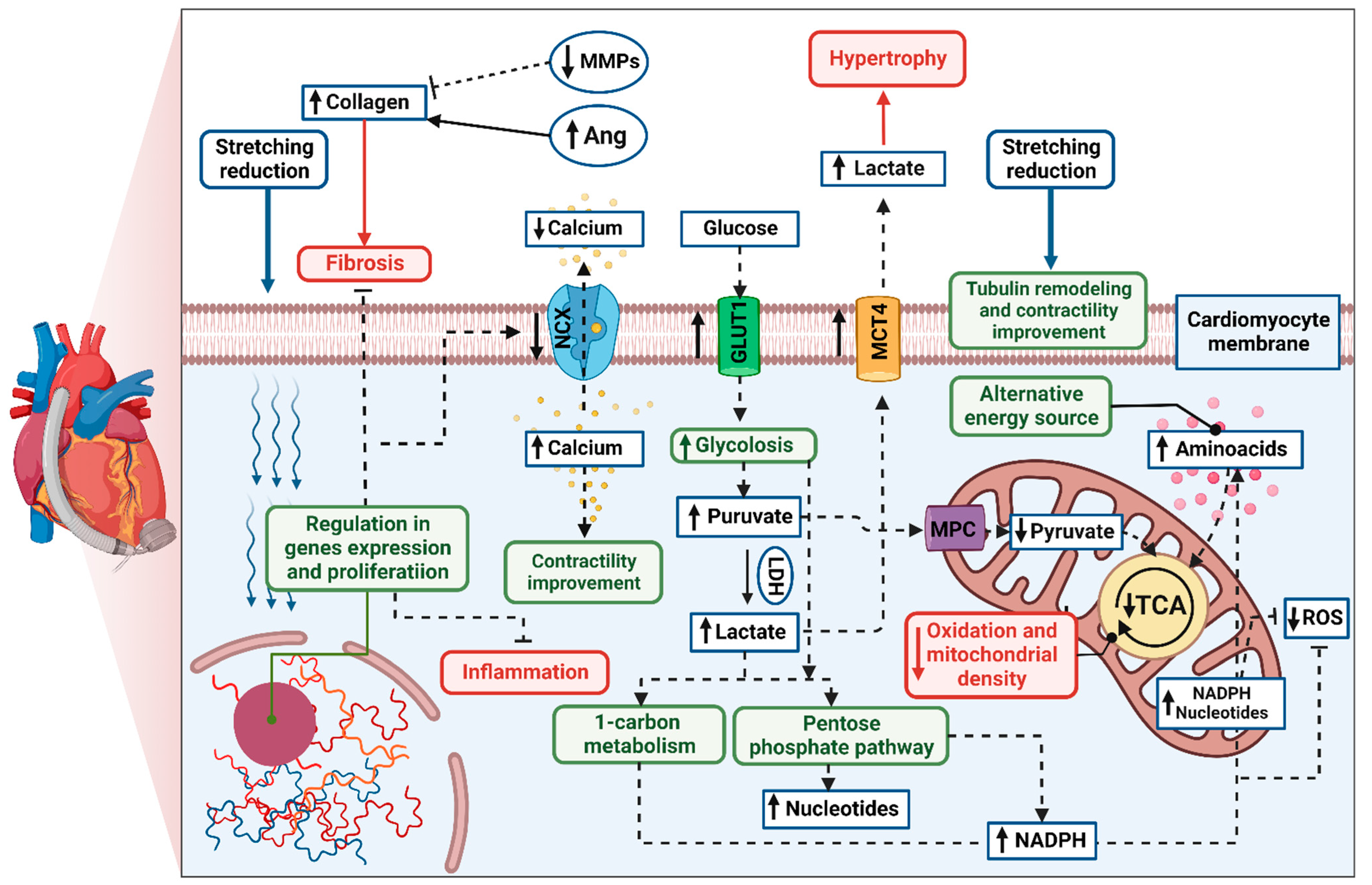
3.1. Potential Benefits
3.2. Novel Pharmacotherapies for Cardiomyocyte Regeneration during LVAD Support
3.2.1. Stimulation of Cardiomyocyte Proliferation
3.2.2. Regulators of Cardiac Substrate Metabolism
3.2.3. Mitochondria-Targeted Treatment
3.2.4. Inhibition of Inflammation
3.2.5. Other Strategies for Cardiac Regeneration
| Type of Improvement | Study Title | Design | Enrolment/Inclusion Criteria | Agent or Intervention | Time Frame | Primary Outcome | Secondary Outcomes | NCT Number |
|---|---|---|---|---|---|---|---|---|
| Device-related modifications | LVAD Conditioning for Cardiac Recovery | N/A | 100/AHF, LVAD implantation as a BTT | Reduction in LVAD speed to minimum operating setting, (during 8 visits, every 2–3 weeks) | Baseline *, 12 month | LVEF | LVEDD, LVESD, LVEDV, LVESV, Glucose 1 and 6-phosphate, Pyruvate, Lactic Acid, Acetyl Coenzyme A, GLUT1, 4, MPC1, 2, mitochondrial density | 03238690 |
| POCT to Improve Monitoring of LVAD Patients | N/A | 60/AHF, LVAD implantation | Development of a low-cost detection of LVAD-related coagulation and thrombosis | - | PT/INR LDH | - | 03555552 | |
| CYCLONE-LVAD (Role of Cytosorb in LVAD Implantation) | N/A | 60/HF, LVAD implantation | Cytokine haemoadsorption by Cytosorb® device to prevent postoperative complication | Baseline *, 6, 12, 24 h, 2, 3, 7 days | IL-6 | Prevalence of vasoplegia and organ dysfunction (RV, liver, and kidney), hospitalization, mortality | 04596813 | |
| DOAC LVAD (Evaluation of the Hemocompatibility of the Direct OralAnti-Coagulant Apixaban in LVAD) | Phase 2 | 40/HF, LVAD (HeartMate 3) implantation | Apixaban (5 mg b.i.d.) Warfarin (standard dose and titrated to obtain INR 2.0–2.5.) | 3 and 6 month | Survival free of adverse events (stroke, device thrombosis, bleeding, aortic root thrombus), mortality | - | 04865978 | |
| The ARIES HeartMate 3 Pump IDE Study | N/A | 628/AHF, LVAD (HeartMate 3) implantation | Aspirin (100 mg), vitamin K antagonist (to obtain INR 2.0–3.0) | 12, 36 month | Adverse event (stroke, pump thrombosis, bleeding) after 1 year | Rate of survival and adverse events after 3 years | 04069156 | |
| LVAD with cardiovascular drugs | HARPS ** (Harefield Recovery Protocol Study for Patients With Refractory Chronic Heart Failure) | Phase 1 | 18/Nonischaemic AHF, LVAD implantation, LVEF ≤ 40% | Clenbuterol (20 tablets mcg t.i.d., titrated to maximally tolerated dose, and then liquid 59 mcg/ml t.i.d) | Baseline *, 2, 6, 12 month | % of LVAD removal and freedom from MCS or HTx for 1 year after explantation | Time to device explant, LVEF, Creatinine, AST, quality of life | 00585546 |
| ENVAD-HF (Sacubitril/Valsartan in LVAD Recipients) | Phase 4 | 60/LVAD (HeartMate 3) recipients | Sacubitril and Valsartan (24/26 mg, 49/51 mg, 97/103 mg b.i.d.) | Baseline *, 2, 3, and 12 month | Mortality, the occurrence of renal failure, hyperkalaemia, symptomatic hypotension) | BNP, hospitalization, eGFR | 04103554 | |
| SEAL-IT (Safety and Efficacy of ARNI After LVAD Implant Study) | Phase 4 | 50/AHF, NYHA class II-IV and LVEF < 40% | Sacubitril and Valsartan (24/26 mg b.i.d. and increased every 2–4 weeks) | Baseline *, 3, 6, and 12 month | Incidence of medication-related adverse events, BNP | MAP, NYHA class, LVEDD, mitral E/A ratio, LA volume, RAP, PADP, others | 04191681 | |
| Mechanical support combined with cellular therapy | LVAD Combined With Allogeneic Mesenchymal Stem Cells Implantation in Patients With End-stage HF | Phase 2, 3 | 5/AHF due to ischemic cardiomyopathy | Allogeneic stem cells | Baseline *, 12, and 24 month | Myocardial perfusion/viability (SPECT segmental analysis) | Morbidity, LV function | 01759212 |
| ASSURANCE (Stem Cell Therapy in Patients With Severe Heart Failure & Undergoing LVAD Placement) | Phase 1, 2 | 25/LVAD implantation, NYHA class III or IV, LVEF < 30%, and cardiomyopathy | Bone-Marrow-Derived Mononuclear Cells (20 × 106 cells/400 µL) | Baseline *, 10 weeks, 24 month | Adverse events, Myocardial viability (PET/CT Scan), mortality | LV dimensions, histological assessment | 00869024 | |
| Regulation of substrate metabolism under LVAD condition | Heart Failure Patients With LVAD Being Treated With Sodium–Glucose Co-Transporter 2 Inhibitors | Phase 4 | 40/LVAD implantation, eGFR ≥ 30 | SGLT2 inhibitors (empagliflozin/dapagliflozin; 10 mg q.d.) | Baseline *, 6 month | LVEDD | - | 05278962 |
| Mitochondria target treatment in LVAD recipient | PilotNR-LVAD *** (Nicotinamide Riboside in LVAD Recipients) | Early Phase 1 | 5/AHF, planned elective LVAD implantation | Nicotinamide riboside (1000 mg b.i.d. until LVAD implantation) | - | Incidence of medication-related adverse events | Whole blood NAD+, mitochondrial respiration in isolated peripheral blood mononuclear cells (PBMCs) | 03727646 |
| Immunomodulating treatments during LVAD support | Interleukin-1 Receptor Antagonist for the Treatment of Heart Failure in Patients With Left Ventricular Assist Devices | Phase 1, 2 | 10/LVAD implantation | Anakinra (100 mg SQ q.d. for 2 weeks) | Baseline *, 6 month | CRP | Neutrophil count, EF, TNF-alpha, | 02547766 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2022, cvac013. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, E.B.; Böhm, M. Management of end stage heart failure. Heart 2007, 93, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Task, A.; Members, F.; Mcdonagh, T.A.; United, C.; Gardner, R.S.; Force, T.; United, C.; Baumbach, A.; Kingdom, U.; Bo, M.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 3599–3726. [Google Scholar] [CrossRef]
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.J.; Hsich, E.; Meiser, B.; Potena, L.; Robinson, A.; Rossano, J.W.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report-2019; focus theme: Donor and recipient size match. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2019, 38, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef]
- Siems, C.; Huddleston, S.; John, R. A Brief History of Xenotransplantation. Ann. Thorac. Surg. 2022, 113, 706–710. [Google Scholar] [CrossRef]
- Drakos, S.G.; Kfoury, A.G.; Selzman, C.H.; Verma, D.R.; Nanas, J.N.; Li, D.Y.; Stehlik, J. Left ventricular assist device unloading effects on myocardial structure and function: Current status of the field and call for action. Curr. Opin. Cardiol. 2011, 26, 245–255. [Google Scholar] [CrossRef]
- Kadakia, S.; Moore, R.; Ambur, V.; Toyoda, Y. Current status of the implantable LVAD. Gen. Thorac. Cardiovasc. Surg. 2016. [Google Scholar] [CrossRef]
- Liotta, D.; Hall, W.C.; Henly, W.S.; Beall, A.C.; Cooley, D.A.; Debakey, M.E. Prolonged assisted circulation during and after heart or aortic surgery. Trans. Am. Soc. Artif. Intern. Organs 1963, 9, 182–185. [Google Scholar]
- Liotta, D.; Hall, C.W.; Akers, W.W.; Villanueva, A.; O’Neal, R.M.; DeBakey, M.E. A pseudoendocardium for implantable blood pumps. Trans. Am. Soc. Artif. Intern. Organs 1966, 12, 129–138. [Google Scholar]
- DeBakey, M.E. Left ventricular bypass pump for cardiac assistance. Clinical experience. Am. J. Cardiol. 1971, 27, 3–11. [Google Scholar] [CrossRef]
- Norman, J.C.; Bernhard, W.F. Criteria, Protocols And Reporting Forms For Initial Left Ventricular Assist Device Clinical Trials. Cardiovasc. Dis. 1975, 2, 438–445. [Google Scholar] [PubMed]
- Dean, D.; Kallel, F.; Ewald, G.A.; Tatooles, A.; Sheridan, B.C.; Brewer, R.J.; Caldeira, C.; Farrar, D.J.; Akhter, S.A. Reduction in driveline infection rates: Results from the HeartMate II Multicenter Driveline Silicone Skin Interface (SSI) Registry. J. Heart lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2015, 34, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.M.; Aikens, J.E.; Craddock, H.; Aldrich, M.W.; Pagani, F.D. Development and Feasibility of Self-Management Application in Left-Ventricular Assist Devices. ASAIO J. 2018, 64, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, D.J.; Williams, C.T.; Ehrman, J.K.; Saval, M.A.; Bronsteen, K.; Schairer, J.R.; Swaffer, M.; Brawner, C.A.; Lanfear, D.E.; Selektor, Y.; et al. Cardiac rehabilitation improves functional capacity and patient-reported health status in patients with continuous-flow left ventricular assist devices: The Rehab-VAD randomized controlled trial. JACC. Heart Fail. 2014, 2, 653–659. [Google Scholar] [CrossRef]
- Levine, A.; Gass, A. Third-Generation LVADs: Has Anything Changed? Cardiol. Rev. 2019, 27, 293–301. [Google Scholar] [CrossRef]
- Iii, W.K.C.; Copeland, H.; Takeda, K.; Fernandez, F.G.; Badhwar, V.; Habib, R.H.; Jacobs, J.P.; Koehl, D.; Kirklin, J.K.; Pagani, F.D.; et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann. Thorac. Surg. 2021, 111, 778–792. [Google Scholar] [CrossRef]
- Kirklin, J.K.; Pagani, F.D.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B.; Naftel, D.C. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J. Heart Lung Transplant. 2017, 36, 1080–1086. [Google Scholar] [CrossRef]
- Rose, E.A.; Moskowitz, A.J.; Packer, M.; Sollano, J.A.; Williams, D.L.; Tierney, A.R.; Heitjan, D.F.; Meier, P.; Ascheim, D.D.; Levitan, R.G.; et al. The REMATCH trial: Rationale, design, and end points. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure. Ann. Thorac. Surg. 1999, 67, 723–730. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Böhm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Hear. Eur. Heart J. 2012, 33, 1787–1847. [Google Scholar] [CrossRef]
- McCarthy, P.M.; Nakatani, S.; Vargo, R.; Kottke-Marchant, K.; Harasaki, H.; James, K.B.; Savage, R.M.; Thomas, J.D. Structural and left ventricular histologic changes after implantable LVAD insertion. Ann. Thorac. Surg. 1995, 59, 609–613. [Google Scholar] [CrossRef]
- Frazier, O.H. First use of an untethered, vented electric left ventricular assist device for long-term support. Circulation 1994, 89, 2908–2914. [Google Scholar] [CrossRef] [PubMed]
- Loebe, M.; Weng, Y.; Müller, J.; Dandel, M.; Halfmann, R.; Spiegelsberger, S.; Hetzer, R. Successful mechanical circulatory support for more than two years with a left ventricular assist device in a patient with dilated cardiomyopathy. J. Heart lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 1997, 16, 1176–1179. [Google Scholar]
- Frazier, O.H.; Benedict, C.R.; Radovancevic, B.; Bick, R.J.; Capek, P.; Springer, W.E.; Macris, M.P.; Delgado, R.; Buja, L.M. Improved left ventricular function after chronic left ventricular unloading. Ann. Thorac. Surg. 1996, 62, 672–675. [Google Scholar] [CrossRef]
- Frazier, O.H.; Myers, T.J. Left ventricular assist system as a bridge to myocardial recovery. Ann. Thorac. Surg. 1999, 68, 734–741. [Google Scholar] [CrossRef]
- Monteagudo Vela, M.; Rial Bastón, V.; Panoulas, V.; Riesgo Gil, F.; Simon, A. A detailed explantation assessment protocol for patients with left ventricular assist devices with myocardial recovery. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 298–305. [Google Scholar] [CrossRef]
- Mancini, D.M.; Beniaminovitz, A.; Levin, H.; Catanese, K.; Flannery, M.; DiTullio, M.; Savin, S.; Cordisco, M.E.; Rose, E.; Oz, M. Low incidence of myocardial recovery after left ventricular assist device implantation in patients with chronic heart failure. Circulation 1998, 98, 2383–2389. [Google Scholar] [CrossRef]
- Farrar, D.J.; Holman, W.R.; McBride, L.R.; Kormos, R.L.; Icenogle, T.B.; Hendry, P.J.; Moore, C.H.; Loisance, D.Y.; El-Banayosy, A.; Frazier, H. Long-term follow-up of Thoratec ventricular assist device bridge-to-recovery patients successfully removed from support after recovery of ventricular function. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2002, 21, 516–521. [Google Scholar] [CrossRef]
- Maybaum, S.; Mancini, D.; Xydas, S.; Starling, R.C.; Aaronson, K.; Pagani, F.D.; Miller, L.W.; Margulies, K.; McRee, S.; Frazier, O.H.; et al. Cardiac improvement during mechanical circulatory support: A prospective multicenter study of the LVAD working group. Circulation 2007, 115, 2497–2505. [Google Scholar] [CrossRef]
- Liden, H.; Karason, K.; Bergh, C.-H.; Nilsson, F.; Koul, B.; Wiklund, L. The feasibility of left ventricular mechanical support as a bridge to cardiac recovery. Eur. J. Heart Fail. 2007, 9, 525–530. [Google Scholar] [CrossRef]
- Dandel, M.; Weng, Y.; Siniawski, H.; Potapov, E.; Drews, T.; Lehmkuhl, H.B.; Knosalla, C.; Hetzer, R. Prediction of cardiac stability after weaning from left ventricular assist devices in patients with idiopathic dilated cardiomyopathy. Circulation 2008, 118, S94–S105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simon, M.A.; Primack, B.A.; Teuteberg, J.; Kormos, R.L.; Bermudez, C.; Toyoda, Y.; Shah, H.; Gorcsan, J., 3rd; McNamara, D.M. Left ventricular remodeling and myocardial recovery on mechanical circulatory support. J. Card. Fail. 2010, 16, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, Y.; Kearns, M.; Josan, K.; Bashir, J.; Ignaszewski, A.; Kaan, A.; Kealy, J.; Moss, R.; Cheung, A. Successful weaning and explantation of the Heartmate II left ventricular assist device. Can. J. Cardiol. 2011, 27, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, E.F.; Wang, L.; Singh, R.; Schueler, S.; Genovese, L.D.; Woods, A.; Tang, D.; Smith, N.R.; Psotka, M.A.; Tovey, S.; et al. LVAD decommissioning for myocardial recovery: Long-term ventricular remodeling and adverse events. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2021, 40, 1560–1570. [Google Scholar] [CrossRef]
- Birks, E.J.; George, R.S.; Hedger, M.; Bahrami, T.; Wilton, P.; Bowles, C.T.; Webb, C.; Bougard, R.; Amrani, M.; Yacoub, M.H.; et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: A prospective study. Circulation 2011, 123, 381–390. [Google Scholar] [CrossRef]
- Birks, E.J.; Drakos, S.G.; Patel, S.R.; Lowes, B.D.; Selzman, C.H.; Starling, R.C.; Trivedi, J.; Slaughter, M.S.; Alturi, P.; Goldstein, D.; et al. Prospective Multicenter Study of Myocardial Recovery Using Left Ventricular Assist Devices (RESTAGE-HF [Remission from Stage D Heart Failure]): Medium-Term and Primary End Point Results. Circulation 2020, 142, 2016–2028. [Google Scholar] [CrossRef]
- Matkovich, S.J.; Van Booven, D.J.; Youker, K.A.; Torre-Amione, G.; Diwan, A.; Eschenbacher, W.H.; Dorn, L.E.; Watson, M.A.; Margulies, K.B.; Dorn, G.W. 2nd Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation 2009, 119, 1263–1271. [Google Scholar] [CrossRef]
- Canseco, D.C.; Kimura, W.; Garg, S.; Mukherjee, S.; Bhattacharya, S.; Abdisalaam, S.; Das, S.; Asaithamby, A.; Mammen, P.P.A.; Sadek, H.A. Human ventricular unloading induces cardiomyocyte proliferation. J. Am. Coll. Cardiol. 2015, 65, 892–900. [Google Scholar] [CrossRef]
- Wohlschlaeger, J.; Schmitz, K.J.; Schmid, C.; Schmid, K.W.; Keul, P.; Takeda, A.; Weis, S.; Levkau, B.; Baba, H.A. Reverse remodeling following insertion of left ventricular assist devices (LVAD): A review of the morphological and molecular changes. Cardiovasc. Res. 2005, 68, 376–386. [Google Scholar] [CrossRef]
- Manginas, A.; Tsiavou, A.; Sfyrakis, P.; Giamouzis, G. Increased Number of Circulating Progenitor Cells After Methods: Results. J. Heart Lung Transplant. 2009, 28, 710–717. [Google Scholar] [CrossRef]
- Ivak, P.; Netuka, I.; Kralova-Lesna, I.; Wohlfahrt, P.; Pitha, J. Changes in circulating stem cells and endothelial progenitor cells over a 12-month period after implantation of a continuous-flow left ventricular assist device. Arch. Med. Sci. 2020, 16, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Farris, S.D.; Don, C.; Helterline, D.; Costa, C.; Plummer, T.; Steffes, S.; Mahr, C.; Mokadam, N.A.; Stempien-Otero, A. Cell-Specific Pathways Supporting Persistent Fibrosis in Heart Failure. J. Am. Coll. Cardiol. 2017, 70, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Dhar, K.; Moulton, A.M.; Rome, E.; Qiu, F.; Kittrell, J.; Raichlin, E.; Zolty, R.; Um, J.Y.; Moulton, M.J.; Basma, H.; et al. Targeted myocardial gene expression in failing hearts by RNA sequencing. J. Transl. Med. 2016, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Heerdt, P.M.; Holmes, J.W.; Cai, B.; Barbone, A.; Madigan, J.D.; Reiken, S.; Lee, D.L.; Oz, M.C.; Marks, A.R.; Burkhoff, D. Chronic unloading by left ventricular assist device reverses contractile dysfunction and alters gene expression in end-stage heart failure. Circulation 2000, 102, 2713–2719. [Google Scholar] [CrossRef]
- Diakos, N.A.; Taleb, I.; Kyriakopoulos, C.P.; Shah, K.S.; Javan, H.; Richins, T.J.; Yin, M.Y.; Yen, C.-G.; Dranow, E.; Bonios, M.J.; et al. Circulating and Myocardial Cytokines Predict Cardiac Structural and Functional Improvement in Patients With Heart Failure Undergoing Mechanical Circulatory Support. J. Am. Heart Assoc. 2021, 10, e020238. [Google Scholar] [CrossRef] [PubMed]
- Torre-Amione, G.; Stetson, S.J.; Youker, K.A.; Durand, J.B.; Radovancevic, B.; Delgado, R.M.; Frazier, O.H.; Entman, M.L.; Noon, G.P. Decreased expression of tumor necrosis factor-alpha in failing human myocardium after mechanical circulatory support: A potential mechanism for cardiac recovery. Circulation 1999, 100, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.W.; Rossman, E.I.; Piacentino, V., 3rd; Kenessey, A.; Weber, C.; Gaughan, J.P.; Ojamaa, K.; Klein, I.; Bers, D.M.; Houser, S.R.; et al. Altered myocardial Ca2+ cycling after left ventricular assist device support in the failing human heart. J. Am. Coll. Cardiol. 2004, 44, 837–845. [Google Scholar] [CrossRef]
- Terracciano, C.M.N.; Harding, S.E.; Adamson, D.; Koban, M.; Tansley, P.; Birks, E.J.; Barton, P.J.R.; Yacoub, M.H. Changes in sarcolemmal Ca entry and sarcoplasmic reticulum Ca content in ventricular myocytes from patients with end-stage heart failure following myocardial recovery after combined pharmacological and ventricular assist device therapy. Eur. Heart J. 2003, 24, 1329–1339. [Google Scholar] [CrossRef]
- Ibrahim, M.; Navaratnarajah, M.; Siedlecka, U.; Rao, C.; Dias, P.; Moshkov, A.V.; Gorelik, J.; Yacoub, M.H.; Terracciano, C.M. Mechanical unloading reverses transverse tubule remodelling and normalizes local Ca2+-induced Ca2+ release in a rodent model of heart failure. Eur. J. Heart Fail. 2012, 14, 571–580. [Google Scholar] [CrossRef]
- Seidel, T.; Navankasattusas, S.; Ahmad, A.; Diakos, N.A.; Xu, W.D.; Tristani-Firouzi, M.; Bonios, M.J.; Taleb, I.; Li, D.Y.; Selzman, C.H.; et al. Sheet-Like Remodeling of the Transverse Tubular System in Human Heart Failure Impairs Excitation-Contraction Coupling and Functional Recovery by Mechanical Unloading. Circulation 2017, 135, 1632–1645. [Google Scholar] [CrossRef]
- Thohan, V.; Stetson, S.J.; Nagueh, S.F.; Rivas-Gotz, C.; Koerner, M.M.; Lafuente, J.A.; Loebe, M.; Noon, G.P.; Torre-Amione, G. Cellular and hemodynamics responses of failing myocardium to continuous flow mechanical circulatory support using the DeBakey-Noon left ventricular assist device: A comparative analysis with pulsatile-type devices. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2005, 24, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Wallukat, G.; Weng, Y.G.; Dandel, M.; Spiegelsberger, S.; Semrau, S.; Brandes, K.; Theodoridis, V.; Loebe, M.; Meyer, R.; et al. Weaning from mechanical cardiac support in patients with idiopathic dilated cardiomyopathy. Circulation 1997, 96, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Feng, Y.; McTiernan, C.F.; Pei, W.; Moravec, C.S.; Wang, P.; Rosenblum, W.; Kormos, R.L.; Feldman, A.M. Downregulation of matrix metalloproteinases and reduction in collagen damage in the failing human heart after support with left ventricular assist devices. Circulation 2001, 104, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.; Foronjy, R.F.; Dickstein, M.L.; Gu, A.; Garrelds, I.M.; Danser, A.H.J.; Oz, M.C.; D’Armiento, J.; Burkhoff, D. Mechanical unloading during left ventricular assist device support increases left ventricular collagen cross-linking and myocardial stiffness. Circulation 2005, 112, 364–374. [Google Scholar] [CrossRef]
- Miyagawa, S.; Toda, K.; Nakamura, T.; Yoshikawa, Y.; Fukushima, S.; Saito, S.; Yoshioka, D.; Saito, T.; Sawa, Y. Building a bridge to recovery: The pathophysiology of LVAD-induced reverse modeling in heart failure. Surg. Today 2016, 46, 149–154. [Google Scholar] [CrossRef]
- Diakos, N.A.; Navankasattusas, S.; Abel, E.D.; Rutter, J.; McCreath, L.; Ferrin, P.; McKellar, S.H.; Miller, D.V.; Park, S.Y.; Richardson, R.S.; et al. Evidence of Glycolysis Up-Regulation and Pyruvate Mitochondrial Oxidation Mismatch During Mechanical Unloading of the Failing Human Heart: Implications for Cardiac Reloading and Conditioning. JACC. Basic to Transl. Sci. 2016, 1, 432–444. [Google Scholar] [CrossRef]
- Badolia, R.; Ramadurai, D.K.A.; Abel, E.D.; Ferrin, P.; Taleb, I.; Shankar, T.S.; Krokidi, A.T.; Navankasattusas, S.; McKellar, S.H.; Yin, M.; et al. The Role of Nonglycolytic Glucose Metabolism in Myocardial Recovery Upon Mechanical Unloading and Circulatory Support in Chronic Heart Failure. Circulation 2020, 142, 259–274. [Google Scholar] [CrossRef]
- Cluntun, A.A.; Badolia, R.; Lettlova, S.; Mckellar, S.H.; Rutter, J.; Drakos, S.G. Article The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure Article The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab. 2021, 1–20. [Google Scholar] [CrossRef]
- Diakos, N.A.; Selzman, C.H.; Sachse, F.B.; Stehlik, J.; Kfoury, A.G.; Wever-Pinzon, O.; Catino, A.; Alharethi, R.; Reid, B.B.; Miller, D.V.; et al. Myocardial atrophy and chronic mechanical unloading of the failing human heart: Implications for cardiac assist device-induced myocardial recovery. J. Am. Coll. Cardiol. 2014, 64, 1602–1612. [Google Scholar] [CrossRef]
- Margulies, K.B.; Matiwala, S.; Cornejo, C.; Olsen, H.; Craven, W.A.; Bednarik, D. Mixed messages: Transcription patterns in failing and recovering human myocardium. Circ. Res. 2005, 96, 592–599. [Google Scholar] [CrossRef]
- Hall, J.L.; Grindle, S.; Han, X.; Fermin, D.; Park, S.; Chen, Y.; Bache, R.J.; Mariash, A.; Guan, Z.; Ormaza, S.; et al. Genomic profiling of the human heart before and after mechanical support with a ventricular assist device reveals alterations in vascular signaling networks. Physiol. Genom. 2004, 17, 283–291. [Google Scholar] [CrossRef]
- Shen, S.; Sewanan, L.R.; Campbell, S.G. Evidence for synergy between sarcomeres and fibroblasts in an in vitro model of myocardial reverse remodeling. J. Mol. Cell. Cardiol. 2021, 158, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Oriyanhan, W.; Tsuneyoshi, H.; Nishina, T.; Matsuoka, S.; Ikeda, T.; Komeda, M. Determination of optimal duration of mechanical unloading for failing hearts to achieve bridge to recovery in a rat heterotopic heart transplantation model. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2007, 26, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, N.; Jouan, J.; Gourjault, M.; Diebold, B.; Ortuno, S.; Hamdan, A.; Latremouille, C.; Pirracchio, R.; Morshuis, M. Understanding left ventricular assist devices. Blood Purif. 2018, 46, 292–300. [Google Scholar] [CrossRef]
- Zimpfer, D.; Strueber, M.; Aigner, P.; Schmitto, J.D.; Fiane, A.E.; Larbalestier, R.; Tsui, S.; Jansz, P.; Simon, A.; Schueler, S.; et al. Evaluation of the HeartWare ventricular assist device Lavare cycle in a particle image velocimetry model and in clinical practice. Eur. J. Cardio-Thoracic Surg. Off. J. Eur. Assoc. Cardio-Thoracic Surg. 2016, 50, 839–848. [Google Scholar] [CrossRef]
- Mariani, S.; Hanke, J.S.; Dogan, G.; Schmitto, J.D. Out of hospital management of LVAD patients during COVID-19 outbreak. Artif. Organs 2020, 44, 873–876. [Google Scholar] [CrossRef]
- Kataria, R.; Jorde, U.P. Gastrointestinal Bleeding During Continuous-Flow Left Ventricular Assist Device Support: State of the Field. Cardiol. Rev. 2019, 27, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Frankfurter, C.; Molinero, M.; Vishram-Nielsen, J.K.K.; Foroutan, F.; Mak, S.; Rao, V.; Billia, F.; Orchanian-Cheff, A.; Alba, A.C. Predicting the Risk of Right Ventricular Failure in Patients Undergoing Left Ventricular Assist Device Implantation: A Systematic Review. Circ. Heart Fail. 2020, 13, e006994. [Google Scholar] [CrossRef] [PubMed]
- Cowger, J.A.; Romano, M.A.; Shah, P.; Shah, N.; Mehta, V.; Haft, J.W.; Aaronson, K.D.; Pagani, F.D. Hemolysis: A harbinger of adverse outcome after left ventricular assist device implant. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2014, 33, 35–43. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Lertjitbanjong, P.; Cheungpasitporn, W.; Hansrivijit, P.; Fülöp, T.; Kovvuru, K.; Kanduri, S.R.; Davis, P.W.; Vallabhajosyula, S.; Bathini, T.; et al. Incidence and impact of acute kidney injury on patients with implantable left ventricular assist devices: A Meta-analysis. Ren. Fail. 2020, 42, 495–512. [Google Scholar] [CrossRef]
- Zinoviev, R.; Lippincott, C.K.; Keller, S.C.; Gilotra, N.A. In Full Flow: Left Ventricular Assist Device Infections in the Modern Era. Open Forum. Infect. Dis. 2020, 7, ofaa124. [Google Scholar] [CrossRef] [PubMed]
- Gopinathannair, R.; Cornwell, W.K.; Dukes, J.W.; Ellis, C.R.; Hickey, K.T.; Joglar, J.A.; Pagani, F.D.; Roukoz, H.; Slaughter, M.S.; Patton, K.K. Device Therapy and Arrhythmia Management in Left Ventricular Assist Device Recipients: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e967–e989. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, K.; Kluis, A.; Alexy, T.; John, R.; Voeller, R. Neurological complications associated with left ventricular assist device therapy. Expert Rev. Cardiovasc. Ther. 2018, 16, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Kormos, R.L.; McCall, M.; Althouse, A.; Lagazzi, L.; Schaub, R.; Kormos, M.A.; Zaldonis, J.A.; Sciortino, C.; Lockard, K.; Kuntz, N.; et al. Left Ventricular Assist Device Malfunctions: It Is More Than Just the Pump. Circulation 2017, 136, 1714–1725. [Google Scholar] [CrossRef]
- Antonides, C.F.J.; Schoenrath, F.; De By, T.M.M.H.; Muslem, R.; Yalcin, Y.C.; Netuka, I.; Gummert, J.; Potapov, E.V.; Meyns, B.; Özbaran, M.; et al. Outcomes of patients after successful left ventricular assist device explantation: A EUROMACS study. ESC Heart Fail. 2020. [Google Scholar] [CrossRef]
- Yacoub, M.H. A novel strategy to maximize the efficacy of left ventricular assist devices as a bridge to recovery. Eur. Heart J. 2001, 22, 534–540. [Google Scholar] [CrossRef]
- Hon, J.K.F.; Yacoub, M.H. Bridge to recovery with the use of left ventricular assist device and clenbuterol. Ann. Thorac. Surg. 2003, 75, S36–S41. [Google Scholar] [CrossRef]
- Bowles, C.T.; Ph, D.; Burke, M.; Path, F.R.C.; Banner, N.R.; Khaghani, A. Left Ventricular Assist Device and Drug Therapy for the Reversal of Heart Failure. N. Engl. J. Med. 2006, 1873–1884. [Google Scholar]
- Groenning, B.A.; Nilsson, J.C.; Sondergaard, L.; Fritz-hansen, T.; Larsson, H.B.W.; Dms, C.; Hildebrandt, P.R.; Dms, C. Antiremodeling Effects on the Left Ventricle During Beta-Blockade With Metoprolol in the Treatment of Chronic Heart Failure. Clin. Trial 2000, 36, 2072–2080. [Google Scholar] [CrossRef]
- Greenberg, B.; Quinones, M.A.; Koilpillai, C.; Limacher, M.; Shindler, D.; Benedict, C.; Shelton, B. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation 1995, 91, 2573–2581. [Google Scholar] [CrossRef]
- Wong, M.; Staszewsky, L.; Latini, R.; Barlera, S.; Volpi, A.; Chiang, Y.; Benza, R.L.; Gottlieb, S.O.; Kleemann, T.D.; Rosconi, F.; et al. Valsartan Benefits Left Ventricular Structure and Function in Heart Failure: Val-HeFT Echocardiographic Study. Clin. Trial 2002, 40, 970–975. [Google Scholar] [CrossRef]
- Grupper, A.; Zhao, Y.M.; Sajgalik, P.; Joyce, L.D.; Park, S.J.; Pereira, N.L.; Stulak, J.M.; Burnett, J.C.; Edwards, B.S.; Daly, R.C.; et al. Effect of Neurohormonal Blockade Drug Therapy on Outcomes and Left Ventricular Function and Structure After Left Ventricular Assist Device Implantation. Am. J. Cardiol. 2016, 117, 1765–1770. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Saeed, O.; Murthy, S.; Bhatia, V.; Shin, J.J.; Wang, D.; Negassa, A.; Pullman, J.; Goldstein, D.J.; Maybaum, S. Combining neurohormonal blockade with continuous-flow left ventricular assist device support for myocardial recovery: A single-arm prospective study. J. Heart. Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2013, 32, 305–312. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.; Caraballo, C.; Ravindra, N.G.; Miller, P.E.; Mezzacappa, C.; Levin, A.; Gruen, J.; Rodwin, B.; Reinhardt, S.; van Dijk, D.; et al. Neurohormonal Blockade and Clinical Outcomes in Patients With Heart Failure Supported by Left Ventricular Assist Devices. JAMA Cardiol. 2020, 5, 175–182. [Google Scholar] [CrossRef]
- Haider, L.; Hugon-Vallet, E.; Constantin, J.P.; Riad, Z.; Sebbag, L.; Mewton, N. ARNI Pre-Operative Use and Vasoplegic Syndrome in Patients Undergoing Heart Transplantation or Left Ventricular Assist Device Surgery. Med. Sci. 2021, 10, 2. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Morrow, D.A.; DeVore, A.D.; Duffy, C.I.; Ambrosy, A.P.; McCague, K.; Rocha, R.; Braunwald, E. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N. Engl. J. Med. 2019, 380, 539–548. [Google Scholar] [CrossRef]
- Navaratnarajah, M.; Ibrahim, M.; Siedlecka, U.; van Doorn, C.; Shah, A.; Gandhi, A.; Dias, P.; Sarathchandra, P.; Yacoub, M.H.; Terracciano, C.M. Influence of ivabradine on reverse remodelling during mechanical unloading. Cardiovasc. Res. 2013, 97, 230–239. [Google Scholar] [CrossRef]
- Fang, Y.; Debunne, M.; Vercauteren, M.; Brakenhielm, E.; Richard, V.; Lallemand, F.; Henry, J.P.; Mulder, P.; Thuillez, C. Heart rate reduction induced by the if current inhibitor ivabradine improves diastolic function and attenuates cardiac tissue hypoxia. J. Cardiovasc. Pharmacol. 2012, 59, 260–267. [Google Scholar] [CrossRef]
- Ma̧czewski, M.; Mackiewicz, U. Effect of metoprolol and ivabradine on left ventricular remodelling and Ca2+ handling in the post-infarction rat heart. Cardiovasc. Res. 2008, 79, 42–51. [Google Scholar] [CrossRef]
- Navaratnarajah, M.; Siedlecka, U.; Ibrahim, M.; Van Doorn, C.; Soppa, G.; Gandhi, A.; Shah, A.; Kukadia, P.; Yacoub, M.H.; Terracciano, C.M. Impact of Combined Clenbuterol and Metoprolol Therapy on Reverse Remodelling during Mechanical Unloading. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.F.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C. Metabolic remodelling in heart failure. Nat. Rev. Cardiol. 2018, 15, 457–470. [Google Scholar] [CrossRef]
- Ambardekar, A.V.; Dorosz, J.L.; Cleveland, J.C.; Lindenfeld, J.; Buttrick, P.M. Longitudinal left ventricular structural and functional imaging during full support with continuous-flow ventricular assist devices: A retrospective, preliminary analysis. J. Heart Lung Transplant. 2012, 31, 1311–1313. [Google Scholar] [CrossRef]
- Drakos, S.G.; Kfoury, A.G.; Hammond, E.H.; Reid, B.B.; Revelo, M.P.; Rasmusson, B.Y.; Whitehead, K.J.; Salama, M.E.; Selzman, C.H.; Stehlik, J.; et al. Impact of mechanical unloading on microvasculature and associated central remodeling features of the failing human heart. J. Am. Coll. Cardiol. 2010, 56, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.A.; Silva Enciso, J.; Adler, E.D. Often talked about, seldom seen: Promoting myocardial recovery with ventricular assist device. J. Am. Coll. Cardiol. 2014, 64, 1613–1614. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Michler, R.E. The current status of stem cell therapy in ischemic heart disease. J. Card. Surg. 2018, 33, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-B.; Huang, H.; Sun, P.; Ma, S.-Z.; Liu, A.-H.; Xue, J.; Fu, J.-H.; Liang, Y.-Q.; Liu, B.; Wu, D.-Y.; et al. Human Umbilical Cord-Derived Mesenchymal Stromal Cells Improve Left Ventricular Function, Perfusion, and Remodeling in a Porcine Model of Chronic Myocardial Ischemia. Stem Cells Transl. Med. 2016, 5, 1004–1013. [Google Scholar] [CrossRef]
- Evers, K.S.; Dawoud, F.; George, R.T.; Lima, J.A.C.; Lardo, A.C. CT for Evaluation of Myocardial Cell Therapy in Heart Failure. JCMG 2011, 4, 1284–1293. [Google Scholar] [CrossRef]
- Schuleri, K.H.; Amado, L.C.; Boyle, A.J.; Centola, M.; Saliaris, A.P.; Gutman, M.R.; Hatzistergos, K.E.; Oskouei, B.N.; Zimmet, J.M.; Young, R.G.; et al. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2002-11. [Google Scholar] [CrossRef]
- Psaltis, P.J.; Carbone, A.; Nelson, A.J.; Lau, D.H.; Jantzen, T.; Manavis, J.; Williams, K.; Itescu, S.; Sanders, P.; Gronthos, S.; et al. Reparative effects of allogeneic mesenchymal precursor cells delivered transendocardially in experimental nonischemic cardiomyopathy. JACC. Cardiovasc. Interv. 2010, 3, 974–983. [Google Scholar] [CrossRef]
- Martens, T.P.; See, F.; Schuster, M.D.; Sondermeijer, H.P.; Hefti, M.M.; Zannettino, A.; Gronthos, S.; Seki, T.; Itescu, S. Mesenchymal lineage precursor cells induce vascular network formation in ischemic myocardium. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3 (Suppl. 1), S18-22. [Google Scholar] [CrossRef]
- Dooley, L.M.; Abdalmula, A.; Washington, E.A.; Kaufman, C.; Tudor, E.M.; Ghosh, P.; Itescu, S.; Kimpton, W.G.; Bailey, S.R. Effect of mesenchymal precursor cells on the systemic inflammatory response and endothelial dysfunction in an ovine model of collagen-induced arthritis. PLoS ONE 2015, 10, e0124144. [Google Scholar] [CrossRef][Green Version]
- Borow, K.M.; Yaroshinsky, A.; Greenberg, B.; Perin, E.C. Phase 3 DREAM-HF Trial of Mesenchymal Precursor Cells in Chronic Heart Failure. Circ. Res. 2019, 125, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal. Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Lunde, K.; Solheim, S.; Aakhus, S.; Arnesen, H.; Abdelnoor, M.; Egeland, T.; Endresen, K.; Ilebekk, A.; Mangschau, A.; Fjeld, J.G.; et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N. Engl. J. Med. 2006, 355, 1199–1209. [Google Scholar] [CrossRef]
- Stempien-Otero, A.; Helterline, D.; Plummer, T.; Farris, S.; Prouse, A.; Polissar, N.; Stanford, D.; Mokadam, N.A. Mechanisms of bone marrow-derived cell therapy in ischemic cardiomyopathy with left ventricular assist device bridge to transplant. J. Am. Coll. Cardiol. 2015, 65, 1424–1434. [Google Scholar] [CrossRef]
- Yau, T.M.; Pagani, F.D.; Mancini, D.M.; Chang, H.L.; Lala, A.; Woo, Y.J.; Acker, M.A.; Selzman, C.H.; Soltesz, E.G.; Kern, J.A.; et al. Intramyocardial Injection of Mesenchymal Precursor Cells and Successful Temporary Weaning From Left Ventricular Assist Device Support in Patients With Advanced Heart Failure: A Randomized Clinical Trial. JAMA 2019, 321, 1176–1186. [Google Scholar] [CrossRef]
- Ascheim, D.D.; Gelijns, A.C.; Goldstein, D.; Moye, L.A.; Smedira, N.; Lee, S.; Klodell, C.T.; Szady, A.; Parides, M.K.; Jeffries, N.O.; et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation 2014, 129, 2287–2296. [Google Scholar] [CrossRef]
- Lenneman, A.J.; Birks, E.J. Treatment strategies for myocardial recovery in heart failure. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 287. [Google Scholar] [CrossRef]
- Eulalio, A.; Mano, M.; Ferro, M.D.; Zentilin, L.; Sinagra, G.; Zacchigna, S.; Giacca, M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 2012, 492, 376–381. [Google Scholar] [CrossRef]
- Gao, F.; Kataoka, M.; Liu, N.; Liang, T.; Huang, Z.; Gu, F.; Ding, J.; Liu, J.; Zhang, F.; Ma, Q.; et al. Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction. Nat. Commun. 2019. [Google Scholar] [CrossRef] [PubMed]
- Vegter, E.L.; van der Meer, P.; de Windt, L.J.; Pinto, Y.M.; Voors, A.A. MicroRNAs in heart failure: From biomarker to target for therapy. Eur. J. Heart Fail. 2016, 18, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Dlouha, D.; Ivak, P.; Netuka, I.; Benesova, S.; Tucanova, Z.; Hubacek, J.A. An Integrative Study of Aortic mRNA/miRNA Longitudinal Changes in Long-Term LVAD Support. Int. J. Mol. Sci. 2021, 22, 7414. [Google Scholar] [CrossRef] [PubMed]
- Sansone, R.; Stanske, B.; Keymel, S.; Schuler, D.; Horn, P.; Saeed, D.; Boeken, U.; Westenfeld, R.; Lichtenberg, A.; Kelm, M.; et al. Macrovascular and microvascular function after implantation of left ventricular assist devices in end-stage heart failure: Role of microparticles. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2015, 34, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef]
- Huss, J.M.; Kelly, D.P. Mitochondrial energy metabolism in heart failure: A question of balance. J. Clin. Investig. 2005, 115, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Grynberg, A.; Demaison, L. Fatty acid oxidation in the heart. J. Cardiovasc. Pharmacol. 1996, 28 (Suppl. 1), S11-7. [Google Scholar] [CrossRef]
- Dodd, M.S.; Ball, D.R.; Schroeder, M.A.; Le Page, L.M.; Atherton, H.J.; Heather, L.C.; Seymour, A.-M.; Ashrafian, H.; Watkins, H.; Clarke, K.; et al. In vivo alterations in cardiac metabolism and function in the spontaneously hypertensive rat heart. Cardiovasc. Res. 2012, 95, 69–76. [Google Scholar] [CrossRef]
- Osorio, J.C.; Stanley, W.C.; Linke, A.; Castellari, M.; Diep, Q.N.; Panchal, A.R.; Hintze, T.H.; Lopaschuk, G.D.; Recchia, F.A. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation 2002, 106, 606–612. [Google Scholar] [CrossRef]
- Neubauer, S. The Failing Heart—An Engine Out of Fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, H.; Horowitz, J.D.; Frenneaux, M.P. Perhexiline. Cardiovasc. Drug Rev. 2007, 25, 76–97. [Google Scholar]
- Cho, Y.W.; Belej, M.; Aviado, D.M. Pharmacology of a new antianginal drug: Perhexiline. I. Coronary circulation and myocardial metabolism. Chest 1970, 58, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Hudak, W.J.; Lewis, R.E.; Kuhn, W.L. Cardiovascular pharmacology of perhexiline. J. Pharmacol. Exp. Ther. 1970, 173, 371–382. [Google Scholar] [PubMed]
- Cappola, T.P. Perhexiline: Lessons for heart failure therapeutics. JACC Heart Fail. 2015, 3, 212–213. [Google Scholar] [CrossRef] [PubMed]
- Beadle, R.M.; Williams, L.K.; Kuehl, M.; Bowater, S.; Abozguia, K.; Leyva, F.; Yousef, Z.; Wagenmakers, A.J.M.; Thies, F.; Horowitz, J.; et al. Improvement in cardiac energetics by perhexiline in heart failure due to dilated cardiomyopathy. JACC Heart Fail. 2015, 3, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Peng, Y.; Hang, W.; Zhou, N.; Wang, D.W. Trimetazidine in Heart Failure. Front. Pharmacol. 2020, 11, 569132. [Google Scholar] [CrossRef]
- Ruixing, Y.; Wenwu, L.; Al-Ghazali, R. Trimetazidine inhibits cardiomyocyte apoptosis in a rabbit model of ischemia-reperfusion. Transl. Res. 2007, 149, 152–160. [Google Scholar] [CrossRef]
- Zhang, J.; He, X.; Bai, X.; Sun, Y.; Jiang, P.; Wang, X.; Li, W.; Zhang, Y. Protective effect of trimetazidine in radiation-induced cardiac fibrosis in mice. J. Radiat. Res. 2020, 61, 657–665. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Li, L.; Su, Q.; Liu, T.; Tang, Z. Trimetazidine pretreatment inhibits myocardial apoptosis and improves cardiac function in a Swine model of coronary microembolization. Cardiology 2015, 130, 130–136. [Google Scholar] [CrossRef]
- Randle, P.J. Endocrine control of metabolism. Annu. Rev. Physiol. 1963, 25, 291–324. [Google Scholar] [CrossRef]
- Tuunanen, H.; Engblom, E.; Naum, A.; Någren, K.; Scheinin, M.; Hesse, B.; Juhani Airaksinen, K.E.; Nuutila, P.; Iozzo, P.; Ukkonen, H.; et al. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation 2008, 118, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Fragasso, G.; Palloshi, A.; Puccetti, P.; Silipigni, C.; Rossodivita, A.; Pala, M.; Calori, G.; Alfieri, O.; Margonato, A. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J. Am. Coll. Cardiol. 2006, 48, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; DeMets, D.L.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; Bengtsson, O.; Ponikowski, P.; Sabatine, M.S.; et al. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur. J. Heart Fail. 2019, 21, 665–675. [Google Scholar] [CrossRef]
- Rådholm, K.; Figtree, G.; Perkovic, V.; Solomon, S.D.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Barrett, T.D.; Shaw, W.; Desai, M.; et al. Canagliflozin and Heart Failure in Type 2 Diabetes Mellitus: Results From the CANVAS Program. Circulation 2018, 138, 458–468. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Empagliflozin’s Fuel Hypothesis: Not so Soon. Cell Metab. 2016, 24, 200–202. [Google Scholar] [CrossRef]
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Krüger, M.; Hoppel, C.L.; et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 2016, 133, 698–705. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 6736, 1–11. [Google Scholar] [CrossRef]
- Petrie, M.C.; Verma, S.; Docherty, K.F.; Inzucchi, S.E.; Anand, I.; Belohlávek, J.; Böhm, M.; Chiang, C.-E.; Chopra, V.K.; de Boer, R.A.; et al. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes. JAMA 2020, 323, 1353–1368. [Google Scholar] [CrossRef]
- Trial, E.O.; Inzucchi, S.E.; Zinman, B.; Fitchett, D.; Wanner, C.; Ferrannini, E.; Schumacher, M.; Schmoor, C.; Ohneberg, K.; Johansen, O.E.; et al. How Does Empagliflozin Reduce Cardiovascular Mortality ? Insights From a Mediation Analysis of the EMPA-REG OUTCOME Trial. Randomized Control. Trial 2018, 41, 356–363. [Google Scholar] [CrossRef]
- Meineri, M.; Van Rensburg, A.E.; Vegas, A. Right ventricular failure after LVAD implantation: Prevention and treatment. Best Pract. Res. Clin. Anaesthesiol. 2012, 26, 217–229. [Google Scholar] [CrossRef]
- Sanz, J.; Sánchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1463–1482. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, S.G. Fibrates Revisited: Potential Role in Cardiovascular Risk Reduction. Diabetes Metab. J. 2020, 44, 213–221. [Google Scholar] [CrossRef]
- Okopień, B.; Bułdak, Ł.; Bołdys, A. Expert Review of Clinical Pharmacology Benefits and risks of the treatment with fibrates–A comprehensive summary. Expert Rev. Clin. Pharmacol. 2018, 11, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Task, A.; Members, F.; Mach, F.; Baigent, C.; Catapano, A.L.; Koskina, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; et al. 2019 ESC / EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 1–66. [Google Scholar] [CrossRef]
- Khuchua, Z.; Glukhov, A.I.; Strauss, A.W.; Javadov, S. Elucidating the beneficial role of ppar agonists in cardiac diseases. Int. J. Mol. Sci. 2018, 19, 3464. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Foufelle, F.; Scotto, C.; Dauça, M.; Wahli, W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 1996, 137, 354–366. [Google Scholar] [CrossRef]
- Han, L.; Shen, W.J.; Bittner, S.; Kraemer, F.B.; Azhar, S. PPARs: Regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-β/δ and PPAR-γ. Future Cardiol. 2017, 13, 279–296. [Google Scholar] [CrossRef]
- Koh, J.-H.; Hancock, C.R.; Terada, S.; Higashida, K.; Holloszy, J.O.; Han, D.-H. PPARβ Is Essential for Maintaining Normal Levels of PGC-1α and Mitochondria and for the Increase in Muscle Mitochondria Induced by Exercise. Cell Metab. 2017, 25, 1176–1185.e5. [Google Scholar] [CrossRef]
- Chandra, M.; Miriyala, S.; Panchatcharam, M. PPAR γ and Its Role in Cardiovascular Diseases. PPAR Res. 2017, 2017, 6404638. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.A.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Ihm, S.-H.; Chang, K.; Kim, H.-Y.; Baek, S.H.; Youn, H.-J.; Seung, K.-B.; Kim, J.-H. Peroxisome proliferator-activated receptor-gamma activation attenuates cardiac fibrosis in type 2 diabetic rats: The effect of rosiglitazone on myocardial expression of receptor for advanced glycation end products and of connective tissue growth factor. Basic Res. Cardiol. 2010, 105, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.D.; Park, C.; March, K.; Coady, E.; Khir, A.; Chaturvedi, N.; Thom, S.A.M. A randomized placebo controlled double blind crossover study of pioglitazone on left ventricular diastolic function in type 2 diabetes. Int. J. Cardiol. 2013, 167, 1329–1332. [Google Scholar] [CrossRef]
- Kim, S.K.; Zhao, Z.S.; Lee, Y.J.; Lee, K.E.; Kang, S.M.; Choi, D.; Lim, S.-K.; Chung, N.; Lee, H.C.; Cha, B.S. Left-ventricular diastolic dysfunction may be prevented by chronic treatment with PPAR-alpha or -gamma agonists in a type 2 diabetic animal model. Diabetes. Metab. Res. Rev. 2003, 19, 487–493. [Google Scholar] [CrossRef]
- Nesti, L.; Tricò, D.; Mengozzi, A.; Natali, A. Rethinking pioglitazone as a cardioprotective agent: A new perspective on an overlooked drug. Cardiovasc. Diabetol. 2021, 1–17. [Google Scholar] [CrossRef]
- Javadov, S.; Kuznetsov, A.V. Mitochondria: The cell powerhouse and nexus of stress. Front. Physiol. 2013, 4, 207. [Google Scholar] [CrossRef]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC. Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef]
- Lei, L.; Liu, Y. Efficacy of coenzyme Q10 in patients with cardiac failure: A meta-analysis of clinical trials. BMC Cardiovasc. Disord. 2017, 17, 196. [Google Scholar] [CrossRef]
- Ribeiro Junior, R.F.; Dabkowski, E.R.; Shekar, K.C.O.; Connell, K.A.; Hecker, P.A.; Murphy, M.P. MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic. Biol. Med. 2018, 117, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Kalén, A.; Appelkvist, E.L.; Dallner, G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989, 24, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Dahlström, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 2013, 167, 1860–1866. [Google Scholar] [CrossRef]
- Szeto, H.H. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br. J. Pharmacol. 2014, 171, 2029–2050. [Google Scholar] [CrossRef] [PubMed]
- Daubert, M.A.; Yow, E.; Dunn, G.; Marchev, S.; Barnhart, H.; Douglas, P.S.; O’Connor, C.; Goldstein, S.; Udelson, J.E.; Sabbah, H.N. Novel Mitochondria-Targeting Peptide in Heart Failure Treatment: A Randomized, Placebo-Controlled Trial of Elamipretide. Circ. Heart Fail. 2017, 10. [Google Scholar] [CrossRef]
- Gibson, C.M.; Giugliano, R.P.; Kloner, R.A.; Bode, C.; Tendera, M.; Jánosi, A.; Merkely, B.; Godlewski, J.; Halaby, R.; Korjian, S.; et al. EMBRACE STEMI study: A Phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention. Eur. Heart J. 2016, 37, 1296–1303. [Google Scholar] [CrossRef]
- Heerdt, P.M.; Schlame, M.; Jehle, R.; Barbone, A.; Burkhoff, D.; Blanck, T.J.J. Disease-specific remodeling of cardiac mitochondria after a left ventricular assist device. Ann. Thorac. Surg. 2002, 73, 1216–1221. [Google Scholar] [CrossRef]
- Wang, W.; Karamanlidis, G.; Tian, R. Novel targets for mitochondrial medicine. Sci. Transl. Med. 2016, 8, 326rv3. [Google Scholar] [CrossRef]
- Lee, C.F.; Chavez, J.D.; Garcia-Menendez, L.; Choi, Y.; Roe, N.D.; Chiao, Y.A.; Edgar, J.S.; Goo, Y.A.; Goodlett, D.R.; Bruce, J.E.; et al. Normalization of NAD+ Redox Balance as a Therapy for Heart Failure. Circulation 2016, 134, 883–894. [Google Scholar] [CrossRef]
- Kutryb-Zajac, B.; Koszalka, P.; Slominska, E.M.; Smolenski, R.T. The effects of pro- and anti-atherosclerotic factors on intracellular nucleotide concentration in murine endothelial cells. Nucleosides. Nucleotides Nucleic Acids 2018, 37, 645–652. [Google Scholar] [CrossRef]
- Airhart, S.E.; Shireman, L.M.; Risler, L.J.; Anderson, G.D.; Nagana Gowda, G.A.; Raftery, D.; Tian, R.; Shen, D.D.; O’Brien, K.D. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS ONE 2017, 12, e0186459. [Google Scholar] [CrossRef] [PubMed]
- Guyton, J.R.; Bays, H.E. Safety considerations with niacin therapy. Am. J. Cardiol. 2007, 99, 22C–31C. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, D.D.-H.; Qiu, Y.; Airhart, S.; Liu, Y.; Stempien-Otero, A.; O’Brien, K.D.; Tian, R. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Investig. 2020, 130, 6054–6063. [Google Scholar] [CrossRef] [PubMed]
- Dick, S.A.; Epelman, S. Chronic heart failure and inflammation. Circ. Res. 2016, 119, 159–176. [Google Scholar] [CrossRef]
- Torre-Amione, G.; Kapadia, S.; Benedict, C.; Oral, H.; Young, J.B.; Mann, D.L. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the Studies of Left Ventricular Dysfunction (SOLVD). J. Am. Coll. Cardiol. 1996, 27, 1201–1206. [Google Scholar] [CrossRef]
- Testa, M.; Yeh, M.; Lee, P.; Fanelli, R.; Loperfido, F.; Berman, J.W.; LeJemtel, T.H. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J. Am. Coll. Cardiol. 1996, 28, 964–971. [Google Scholar] [CrossRef]
- Mann, D.L. Past, Present, and the Foreseeable Future Scientific Rationale for Studying. Circ. Res. 2002, 91, 988–999. [Google Scholar] [CrossRef]
- Tabit, C.E.; Coplan, M.J.; Chen, P.; Jeevanandam, V.; Uriel, N.; Liao, J.K. Tumor necrosis factor-α levels and non-surgical bleeding in continuous-flow left ventricular assist devices. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2018, 37, 107–115. [Google Scholar] [CrossRef]
- Chung, E.S.; Packer, M.; Lo, K.H.; Fasanmade, A.A.; Willerson, J.T. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF Therapy Against Congestive Heart Failure. Circulation 2003, 107, 3133–3140. [Google Scholar] [CrossRef]
- Sliwa, K.; Woodiwiss, A.; Kone, V.N.; Candy, G.; Badenhorst, D.; Norton, G.; Zambakides, C.; Peters, F.; Essop, R. Therapy of ischemic cardiomyopathy with the immunomodulating agent pentoxifylline: Results of a randomized study. Circulation 2004, 109, 750–755. [Google Scholar] [CrossRef]
- Lecour, S.; Smith, R.M.; Woodward, B.; Opie, L.H.; Rochette, L.; Sack, M.N. Identification of a novel role for sphingolipid signaling in TNFα and ischemic preconditioning mediated cardioprotection. J. Mol. Cell. Cardiol. 2002, 34, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Van Tassell, B.W.; Biondi-Zoccai, G.; Kontos, M.C.; Grizzard, J.D.; Spillman, D.W.; Oddi, C.; Roberts, C.S.; Melchior, R.D.; Mueller, G.H.; et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am. J. Cardiol. 2013, 111, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Abouzaki, N.A.; Oddi Erdle, C.; Carbone, S.; Trankle, C.R.; Melchior, R.D.; Turlington, J.S.; Thurber, C.J.; Christopher, S.; Dixon, D.L.; et al. Interleukin-1 Blockade in Acute Decompensated Heart Failure: A Randomized, Double-Blinded, Placebo-Controlled Pilot Study. J. Cardiovasc. Pharmacol. 2016, 67, 544–551. [Google Scholar] [CrossRef]
- Buckley, L.F.; Abbate, A. Interleukin-1 Blockade in Cardiovascular Diseases: From Bench to Bedside. BioDrugs 2018, 32, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Elzman, C.R.H.S.; Al, H.E.T. Interleukin-1 Receptor Antagonism as Adjunct Therapy for Heart Failure Patients with Left Ventricular Assist Devices. Clin. Trial 2021, 67, 145–147. [Google Scholar] [CrossRef]
- Samuel, T.J.; Rosenberry, R.P.; Lee, S.; Pan, Z. Correcting Calcium Dysregulation in Chronic Heart Failure Using SERCA2a Gene Therapy. Int. J. Mol. Sci. 2018, 19, 1086. [Google Scholar] [CrossRef] [PubMed]
- del Monte, F.; Harding, S.E.; Schmidt, U.; Matsui, T.; Kang, Z.B.; Dec, G.W.; Gwathmey, J.K.; Rosenzweig, A.; Hajjar, R.J. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation 1999, 100, 2308–2311. [Google Scholar] [CrossRef]
- Zsebo, K.; Yaroshinsky, A.; Rudy, J.J.; Wagner, K.; Greenberg, B.; Jessup, M.; Hajjar, R.J. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: Analysis of recurrent cardiovascular events and mortality. Circ. Res. 2014, 114, 101–108. [Google Scholar] [CrossRef]
- Lyon, A.R.; Babalis, D.; Morley-Smith, A.C.; Hedger, M.; Suarez Barrientos, A.; Foldes, G.; Couch, L.S.; Chowdhury, R.A.; Tzortzis, K.N.; Peters, N.S.; et al. Investigation of the safety and feasibility of AAV1/SERCA2a gene transfer in patients with chronic heart failure supported with a left ventricular assist device-the SERCA-LVAD TRIAL. Gene Ther. 2020, 27, 579–590. [Google Scholar] [CrossRef]
- Chioncel, O.; Collins, S.P.; Butler, J. Istaroxime in acute heart failure: The holy grail is at HORIZON? Eur. J. Heart Fail. 2020, 22, 1694–1697. [Google Scholar] [CrossRef]
- Carubelli, V.; Zhang, Y.; Metra, M.; Lombardi, C.; Felker, G.M.; Filippatos, G.; O’Connor, C.M.; Teerlink, J.R.; Simmons, P.; Segal, R.; et al. Treatment with 24 hour istaroxime infusion in patients hospitalised for acute heart failure: A randomised, placebo-controlled trial. Eur. J. Heart Fail. 2020, 22, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Metra, M.; Chioncel, O.; Cotter, G.; Davison, B.; Filippatos, G.; Mebazaa, A.; Novosadova, M.; Ponikowski, P.; Simmons, P.; Soffer, J.; et al. Safety and Efficacy of Istaroxime for Patients with Acute-Heart-Failure-Related Pre-cardiogenic Shock—A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study (SEISMiC). Eur. J. Heart Fail. 2022. [Google Scholar] [CrossRef] [PubMed]
- Racioppi, M.F.; Burgos, J.I.; Morell, M.; Gonano, L.A.; Vila Petroff, M. Cellular Mechanisms Underlying the Low Cardiotoxicity of Istaroxime. J. Am. Heart Assoc. 2021, 10, e018833. [Google Scholar] [CrossRef] [PubMed]
- Aryan, L.; Younessi, D.; Zargari, M.; Banerjee, S.; Agopian, J.; Rahman, S.; Borna, R.; Ruffenach, G.; Umar, S.; Eghbali, M. The Role of Estrogen Receptors in Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 4314. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Fliegner, D.; Schubert, C.; Penkalla, A.; Witt, H.; Kararigas, G.; Dworatzek, E.; Staub, E.; Martus, P.; Ruiz Noppinger, P.; Kintscher, U.; et al. Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1597-606. [Google Scholar] [CrossRef]
- Iorga, A.; Li, J.; Sharma, S.; Umar, S.; Bopassa, J.C.; Nadadur, R.D.; Centala, A.; Ren, S.; Saito, T.; Toro, L.; et al. Rescue of Pressure Overload-Induced Heart Failure by Estrogen Therapy. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Lagranha, C.J.; Deschamps, A.; Aponte, A.; Steenbergen, C.; Murphy, E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ. Res. 2010, 106, 1681–1691. [Google Scholar] [CrossRef]
- Gardner, J.D.; Murray, D.B.; Voloshenyuk, T.G.; Brower, G.L.; Bradley, J.M.; Janicki, J.S. Estrogen attenuates chronic volume overload induced structural and functional remodeling in male rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H497–H504. [Google Scholar] [CrossRef]
- Frump, A.L.; Albrecht, M.; Yakubov, B.; Breuils-Bonnet, S.; Nadeau, V.; Tremblay, E.; Potus, F.; Omura, J.; Cook, T.; Fisher, A.; et al. 17β-Estradiol and estrogen receptor α protect right ventricular function in pulmonary hypertension via BMPR2 and apelin. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
| Adverse Events | Reported Frequency, % | Ref. |
|---|---|---|
| Bleeding | 30–70 | [67] |
| Right heart failure | 20–22 | [68] |
| Haemolysis | 18–37 | [69] |
| Acute kidney injury | 25–37 | [70] |
| Infection | 19–39 | [71] |
| Ventricular arrhythmias | 20–50 | [72] |
| Stroke | 8–25 | [73] |
| Device malfunction (system failure) | 36–51 | [74] |
| Study | Treatment | Number | Main Outcomes | |||
|---|---|---|---|---|---|---|
| Cardiac | Clinical | Survival | Recovery * | |||
| Yacoub [76] | MU + clenbuterol | 17 | ↑EF, ↓LV volume | ↓IL-6, ↓ANP, BNP | N/A | 4 (24%) |
| Birks et al. [78] | MU + LI + CAR+ SPL + LST + clenbuterol | 15 | ↑LVEF, ↓LVED/S Dimension, | ↓BNP | 91% (1 yr.), 82% (4 yr.) | 11 (73%) |
| Birks et al. [35] | MU + LI + CAR + SPL + LST + DGX + clenbuterol | 19 | ↑EF, ↑FS, ↓LVEDD, ↓LVESD | N/A | 83.3% (1–3 yr.) | 12 (63.2%) |
| Grupper et al. [82] | MU | 33 | ↑LVEF (~5%), ↓LVEDD (~4.5%), ↓LVMI (~10%) | ↓BNP (~42%) | N/A | N/A |
| MU + NHB | 31 | ↑LVEF (~12%), ↓LVEDD (~7.5%), ↓LVMI (~31%) | ↓BNP (~88%) | N/A | N/A | |
| Patel et al. [83] | MU + NHB | 21 | ↑LVEF (~90%), ↓LVIDD (~10%), ↓LVMI (~29%) | N/A | N/A | 3 (14.3%) |
| McCullough et al. [84] | MU | 1725 | N/A | KCCQ = 64.9, 987 ft | 44% (4 yr.) | 17 (0.99%) |
| MU + NHB | 10,419 | N/A | KCCQ = 68.8, 1103 ft | 56% (4 yr.) | 169 (1.62%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jedrzejewska, A.; Braczko, A.; Kawecka, A.; Hellmann, M.; Siondalski, P.; Slominska, E.; Kutryb-Zajac, B.; Yacoub, M.H.; Smolenski, R.T. Novel Targets for a Combination of Mechanical Unloading with Pharmacotherapy in Advanced Heart Failure. Int. J. Mol. Sci. 2022, 23, 9886. https://doi.org/10.3390/ijms23179886
Jedrzejewska A, Braczko A, Kawecka A, Hellmann M, Siondalski P, Slominska E, Kutryb-Zajac B, Yacoub MH, Smolenski RT. Novel Targets for a Combination of Mechanical Unloading with Pharmacotherapy in Advanced Heart Failure. International Journal of Molecular Sciences. 2022; 23(17):9886. https://doi.org/10.3390/ijms23179886
Chicago/Turabian StyleJedrzejewska, Agata, Alicja Braczko, Ada Kawecka, Marcin Hellmann, Piotr Siondalski, Ewa Slominska, Barbara Kutryb-Zajac, Magdi H. Yacoub, and Ryszard T. Smolenski. 2022. "Novel Targets for a Combination of Mechanical Unloading with Pharmacotherapy in Advanced Heart Failure" International Journal of Molecular Sciences 23, no. 17: 9886. https://doi.org/10.3390/ijms23179886
APA StyleJedrzejewska, A., Braczko, A., Kawecka, A., Hellmann, M., Siondalski, P., Slominska, E., Kutryb-Zajac, B., Yacoub, M. H., & Smolenski, R. T. (2022). Novel Targets for a Combination of Mechanical Unloading with Pharmacotherapy in Advanced Heart Failure. International Journal of Molecular Sciences, 23(17), 9886. https://doi.org/10.3390/ijms23179886