Effects of Heme Oxygenase-1 on c-Kit-Positive Cardiac Cells
Abstract
1. Introduction
2. Results
2.1. Characterization of Murine WT, HO-1TG, and HO-1KO CPCs
2.2. Effect of HO-1 on CPC Proliferation
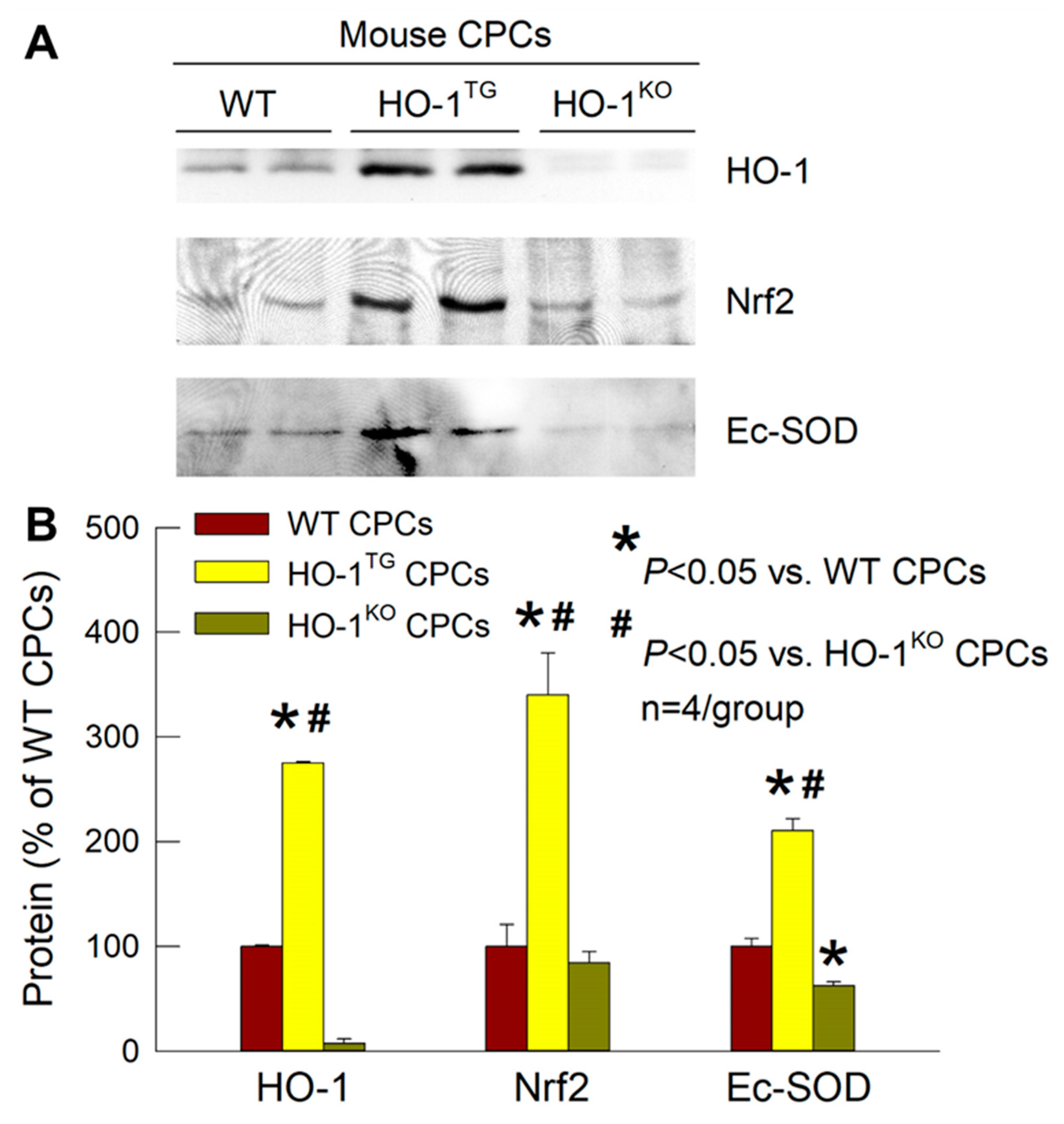
2.3. Effects of HO-1 on Nrf2 and Ec-SOD
2.4. BrdU Incorporation into CPCs under Hypoxia
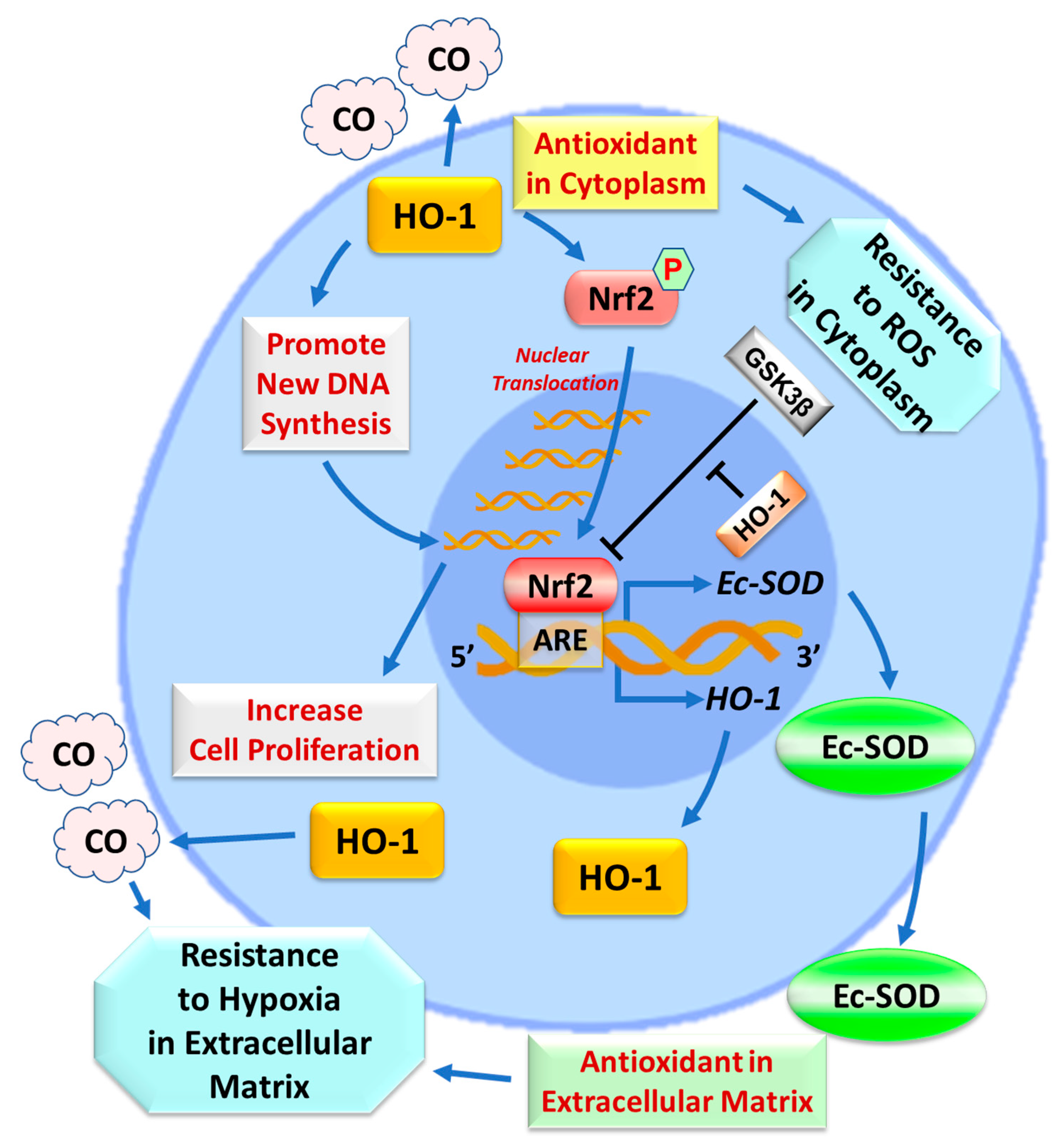
3. Discussion
4. Materials and Methods
4.1. Isolation of Murine CPCs
4.2. Mouse CPC Culture
4.3. Flow Cytometric Analysis
4.4. Immunofluorescent Staining
4.5. Cell Proliferation
4.6. Calculation of Cell Doubling Time
4.7. DNA Synthesis Assay
4.8. Western Immunoblotting Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bolli, R.; Tang, X.L.; Guo, Y.; Li, Q. After the storm: An objective appraisal of the efficacy of c-kit+ cardiac progenitor cells in preclinical models of heart disease. Can. J. Physiol. Pharmacol. 2021, 99, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, Y.; Nong, Y.; Tomlin, A.; Gumpert, A.; Zhu, X.; Hassan, S.A.; Bolli, R. Comparison of Repeated Doses of C-kit-Positive Cardiac Cells versus a Single Equivalent Combined Dose in a Murine Model of Chronic Ischemic Cardiomyopathy. Int. J. Mol. Sci. 2021, 22, 3145. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Nong, Y.; Li, Q.; Tomlin, A.; Kahlon, A.; Gumpert, A.; Slezak, J.; Zhu, X.; Bolli, R. Comparison of One and Three Intraventricular Injections of Cardiac Progenitor Cells in a Murine Model of Chronic Ischemic Cardiomyopathy. Stem Cell Rev. Rep. 2021, 17, 604–615. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Y.; Ou, Q.; Chen, N.; Wu, W.J.; Yuan, F.; O’Brien, E.; Wang, T.; Luo, L.; Hunt, G.N.; et al. Intracoronary administration of cardiac stem cells in mice: A new, improved technique for cell therapy in murine models. Basic Res. Cardiol. 2011, 106, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Ghafghazi, S. Stem cells: Cell therapy for cardiac repair: What is needed to move forward? Nat. Rev. Cardiol. 2017, 14, 257–258. [Google Scholar] [CrossRef]
- Keith, M.C.; Bolli, R. “String theory” of c-kit(pos) cardiac cells: A new paradigm regarding the nature of these cells that may reconcile apparently discrepant results. Circ. Res. 2015, 116, 1216–1230. [Google Scholar] [CrossRef]
- Wysoczynski, M.; Khan, A.; Bolli, R. New Paradigms in Cell Therapy: Repeated Dosing, Intravenous Delivery, Immunomodulatory Actions, and New Cell Types. Circ. Res. 2018, 123, 138–158. [Google Scholar] [CrossRef]
- Bolli, R.; Tang, X.L.; Sanganalmath, S.K.; Rimoldi, O.; Mosna, F.; Abdel-Latif, A.; Jneid, H.; Rota, M.; Leri, A.; Kajstura, J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation 2013, 128, 122–131. [Google Scholar] [CrossRef]
- Hong, K.U.; Guo, Y.; Li, Q.H.; Cao, P.; Al-Maqtari, T.; Vajravelu, B.N.; Du, J.; Book, M.J.; Zhu, X.; Nong, Y.; et al. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS ONE 2014, 9, e96725. [Google Scholar] [CrossRef]
- Hong, K.U.; Li, Q.H.; Guo, Y.; Patton, N.S.; Moktar, A.; Bhatnagar, A.; Bolli, R. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res. Cardiol. 2013, 108, 346. [Google Scholar] [CrossRef]
- Tang, X.L.; Li, Q.; Rokosh, G.; Sanganalmath, S.K.; Chen, N.; Ou, Q.; Stowers, H.; Hunt, G.; Bolli, R. Long-Term Outcome of Administration of c-kit(POS) Cardiac Progenitor Cells After Acute Myocardial Infarction: Transplanted Cells Do not Become Cardiomyocytes, but Structural and Functional Improvement and Proliferation of Endogenous Cells Persist for at Least One Year. Circ. Res. 2016, 118, 1091–1105. [Google Scholar] [PubMed]
- Tang, X.L.; Nakamura, S.; Li, Q.; Wysoczynski, M.; Gumpert, A.M.; Wu, W.J.; Hunt, G.; Stowers, H.; Ou, Q.; Bolli, R. Repeated Administrations of Cardiac Progenitor Cells Are Superior to a Single Administration of an Equivalent Cumulative Dose. J. Am. Heart Assoc. 2018, 7, e007400. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Rokosh, G.; Sanganalmath, S.K.; Yuan, F.; Sato, H.; Mu, J.; Dai, S.; Li, C.; Chen, N.; Peng, Y.; et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation 2010, 121, 293–305. [Google Scholar] [CrossRef]
- Sanganalmath, S.K.; Bolli, R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ. Res. 2013, 113, 810–834. [Google Scholar] [CrossRef] [PubMed]
- Tokita, Y.; Tang, X.L.; Li, Q.; Wysoczynski, M.; Hong, K.U.; Nakamura, S.; Wu, W.J.; Xie, W.; Li, D.; Hunt, G.; et al. Repeated Administrations of Cardiac Progenitor Cells Are Markedly More Effective Than a Single Administration: A New Paradigm in Cell Therapy. Circ. Res. 2016, 119, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Rokosh, G.; Sanganalmath, S.K.; Tokita, Y.; Keith, M.C.; Shirk, G.; Stowers, H.; Hunt, G.N.; Wu, W.; Dawn, B.; et al. Effects of Intracoronary Infusion of Escalating Doses of Cardiac Stem Cells in Rats With Acute Myocardial Infarction. Circ. Heart Fail. 2015, 8, 757–765. [Google Scholar] [CrossRef]
- Eschenhagen, T.; Bolli, R.; Braun, T.; Field, L.J.; Fleischmann, B.K.; Frisén, J.; Giacca, M.; Hare, J.M.; Houser, S.; Lee, R.T.; et al. Cardiomyocyte Regeneration: A Consensus Statement. Circulation 2017, 136, 680–686. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Heme oxygenase and the cardiovascular-renal system. Free Radic. Biol. Med. 2005, 39, 1–25. [Google Scholar] [CrossRef]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Y.; Ou, Q.; Wu, W.J.; Chen, N.; Zhu, X.; Tan, W.; Yuan, F.; Dawn, B.; Luo, L.; et al. Gene transfer as a strategy to achieve permanent cardioprotection II: rAAV-mediated gene therapy with heme oxygenase-1 limits infarct size 1 year later without adverse functional consequences. Basic Res. Cardiol. 2011, 106, 1367–1377. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Y.; Ou, Q.; Cui, C.; Wu, W.J.; Tan, W.; Zhu, X.; Lanceta, L.B.; Sanganalmath, S.K.; Dawn, B.; et al. Gene transfer of inducible nitric oxide synthase affords cardioprotection by upregulating heme oxygenase-1 via a nuclear factor-{kappa}B-dependent pathway. Circulation 2009, 120, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Yet, S.F.; Tian, R.; Layne, M.D.; Wang, Z.Y.; Maemura, K.; Solovyeva, M.; Ith, B.; Melo, L.G.; Zhang, L.; Ingwall, J.S.; et al. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ. Res. 2001, 89, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.X.; Teng, L.; Vu, D.; He, J.Q.; Guo, Y.R.; Li, Q.H.; Tang, X.L.; Rokosh, G.; Bhatnagar, A.; Bolli, R. The Heme Oxygenase 1 Inducer (CoPP) Protects Human Cardiac Stem Cells against Apoptosis through Activation of the Extracellular Signal-regulated Kinase (ERK)/NRF2 Signaling Pathway and Cytokine Release. J. Biol. Chem. 2012, 287, 33720–33732. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Kang, T.C. Nuclear Factor-Erythroid 2-Related Factor 2 (Nrf2) and Mitochondrial Dynamics/Mitophagy in Neurological Diseases. Antioxidants 2020, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Hull, T.D.; Bolisetty, S.; DeAlmeida, A.C.; Litovsky, S.H.; Prabhu, S.D.; Agarwal, A.; George, J.F. Heme oxygenase-1 expression protects the heart from acute injury caused by inducible Cre recombinase. Lab. Investig. J. Tech. Methods Pathol. 2013, 93, 868–879. [Google Scholar] [CrossRef]
- Kapturczak, M.H.; Wasserfall, C.; Brusko, T.; Campbell-Thompson, M.; Ellis, T.M.; Atkinson, M.A.; Agarwal, A. Heme oxygenase-1 modulates early inflammatory responses: Evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 2004, 165, 1045–1053. [Google Scholar] [CrossRef]
- Nath, K.A. Heme oxygenase-1: A provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006, 70, 432–443. [Google Scholar] [CrossRef]
- Li, Y.; Ma, K.; Han, Z.; Chi, M.; Sai, X.; Zhu, P.; Ding, Z.; Song, L.; Liu, C. Immunomodulatory Effects of Heme Oxygenase-1 in Kidney Disease. Front. Med. 2021, 8, 708453. [Google Scholar] [CrossRef]
- Cai, C.; Guo, Y.; Teng, L.; Nong, Y.; Tan, M.; Book, M.J.; Zhu, X.; Wang, X.L.; Du, J.; Wu, W.J.; et al. Preconditioning Human Cardiac Stem Cells with an HO-1 Inducer Exerts Beneficial Effects After Cell Transplantation in the Infarcted Murine Heart. Stem Cells 2015, 33, 3596–3607. [Google Scholar] [CrossRef]
- Crane, A.M.; Bhattacharya, S.K. The use of bromodeoxyuridine incorporation assays to assess corneal stem cell proliferation. Methods Mol. Biol. 2013, 1014, 65–70. [Google Scholar]
- Sharma, R.B.; Darko, C.; Zheng, X.; Gablaski, B.; Alonso, L.C. DNA Damage Does Not Cause BrdU Labeling of Mouse or Human β-Cells. Diabetes 2019, 68, 975–987. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme Oxygenase-1 Signaling and Redox Homeostasis in Physiopathological Conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef]
- Call, J.A.; Chain, K.H.; Martin, K.S.; Lira, V.A.; Okutsu, M.; Zhang, M.; Yan, Z. Enhanced skeletal muscle expression of extracellular superoxide dismutase mitigates streptozotocin-induced diabetic cardiomyopathy by reducing oxidative stress and aberrant cell signaling. Circ. Heart Fail. 2015, 8, 188–197. [Google Scholar] [CrossRef]
- Li, Q.; Bolli, R.; Qiu, Y.; Tang, X.L.; Guo, Y.; French, B.A. Gene therapy with extracellular superoxide dismutase protects conscious rabbits against myocardial infarction. Circulation 2001, 103, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Bolli, R.; Qiu, Y.; Tang, X.L.; Murphree, S.S.; French, B.A. Gene therapy with extracellular superoxide dismutase attenuates myocardial stunning in conscious rabbits. Circulation 1998, 98, 1438–1448. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Ucar, B.I.; Ucar, G.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Pharmacological Protection against Ischemia-Reperfusion Injury by Regulating the Nrf2-Keap1-ARE Signaling Pathway. Antioxidants 2021, 10, 823. [Google Scholar] [CrossRef]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Folz, R.J.; Crapo, J.D. Extracellular superoxide dismutase (SOD3): Tissue-specific expression, genomic characterization, and computer-assisted sequence analysis of the human EC SOD gene. Genomics 1994, 22, 162–171. [Google Scholar] [CrossRef]
- Biswas, C.; Shah, N.; Muthu, M.; La, P.; Fernando, A.P.; Sengupta, S.; Yang, G.; Dennery, P.A. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. J. Biol. Chem. 2014, 289, 26882–26894. [Google Scholar] [CrossRef]
- Yet, S.F.; Perrella, M.A.; Layne, M.D.; Hsieh, C.M.; Maemura, K.; Kobzik, L.; Wiesel, P.; Christou, H.; Kourembanas, S.; Lee, M.E. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J. Clin. Investig. 1999, 103, R23–R29. [Google Scholar] [CrossRef]
- Bolli, R.A.; Dasari, C.; Arshia, A.; Devadoss, D.; Guo, Y.; Ashraf, U.; Li, Q. Physiological Oxygen Tension Enhances Competence and Functional Properties of Murine Cardiac Mesenchymal Cells. Stem Cell Rev. Rep. 2021, 17, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.-S.; El-Ashram, S.; Luo, D.-Z.; Luo, H.-N.; Wang, B.-Y.; Chen, S.-F.; Bai, Y.-S.; Chen, Z.-S.; Liu, C.-Y.; Ji, H.-Q. A Comparative Study of Biological Characteristics and Transcriptome Profiles of Mesenchymal Stem Cells from Different Canine Tissues. Int. J. Mol. Sci. 2019, 20, 1485. [Google Scholar] [CrossRef]
- Öfner, D.; Hittmair, A.; Marth, C.; Öfner, C.; Totsch, M.; Daxenbichler, G.; Mikuz, G.; Margreiter, R.; Schmid, K.W. Relationship Between Quantity of Silver Stained Nucleolar Organizer Regions Associated Proteins (Ag-NORs) and Population Doubling Time in Ten Breast Cancer Cell Lines. Pathol.-Res. Pract. 1992, 188, 742–746. [Google Scholar] [CrossRef]
- Bar, E.E.; Lin, A.; Mahairaki, V.; Matsui, W.; Eberhart, C.G. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am. J. Pathol. 2010, 177, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, Y.; Tan, W.; Ou, Q.; Wu, W.J.; Sturza, D.; Dawn, B.; Hunt, G.; Cui, C.; Bolli, R. Cardioprotection afforded by inducible nitric oxide synthase gene therapy is mediated by cyclooxygenase-2 via a nuclear factor-kappaB dependent pathway. Circulation 2007, 116, 1577–1584. [Google Scholar] [CrossRef][Green Version]
- Triana, J.F.; Li, X.Y.; Jamaluddin, U.; Thornby, J.I.; Bolli, R. Postischemic myocardial “stunning”. Identification of major differences between the open-chest and the conscious dog and evaluation of the oxygen radical hypothesis in the conscious dog. Circ. Res. 1991, 69, 731–747. [Google Scholar] [CrossRef]
- Shinmura, K.; Bolli, R.; Liu, S.-Q.; Tang, X.-L.; Kodani, E.; Xuan, Y.-T.; Srivastava, S.; Bhatnagar, A. Aldose Reductase Is an Obligatory Mediator of the Late Phase of Ischemic Preconditioning. Circ. Res. 2002, 91, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Bolli, R.; Black, R.G., Jr.; Kodani, E.; Tang, X.L.; Yang, Z.; Bhattacharya, S.; Auchampach, J.A. A(1) or A(3) adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ. Res. 2001, 88, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Leesar, M.A.; Stoddard, M.F.; Manchikalapudi, S.; Bolli, R. Bradykinin-induced preconditioning in patients undergoing coronary angioplasty. J. Am. Coll. Cardiol. 1999, 34, 639–650. [Google Scholar] [CrossRef]
- Bolli, R.A.R.; Arshia, A.; Hassan, S.A.; Dasari, C.; Nong, Y.; Guo, Y.; Tomlin, A.A.; Li, Q. Cardiac Mesenchymal Cells Cultured at Physiologic Oxygen Tension Have Superior Therapeutic Efficacy in Heart Failure Caused by Myocardial Infarction. Front. Cell Dev. Biol. 2021, 9, 662415. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Dasari, C.; Li, D.; Arshia, A.; Umer, A.M.; Abouzid, M.R.A.; Guo, Y.; Bolli, R. Effects of Heme Oxygenase-1 on c-Kit-Positive Cardiac Cells. Int. J. Mol. Sci. 2021, 22, 13448. https://doi.org/10.3390/ijms222413448
Li Q, Dasari C, Li D, Arshia A, Umer AM, Abouzid MRA, Guo Y, Bolli R. Effects of Heme Oxygenase-1 on c-Kit-Positive Cardiac Cells. International Journal of Molecular Sciences. 2021; 22(24):13448. https://doi.org/10.3390/ijms222413448
Chicago/Turabian StyleLi, Qianhong, Chandrashekhar Dasari, Ding Li, Asma Arshia, Ahmed Muaaz Umer, Mohamed Riad Abdelgawad Abouzid, Yiru Guo, and Roberto Bolli. 2021. "Effects of Heme Oxygenase-1 on c-Kit-Positive Cardiac Cells" International Journal of Molecular Sciences 22, no. 24: 13448. https://doi.org/10.3390/ijms222413448
APA StyleLi, Q., Dasari, C., Li, D., Arshia, A., Umer, A. M., Abouzid, M. R. A., Guo, Y., & Bolli, R. (2021). Effects of Heme Oxygenase-1 on c-Kit-Positive Cardiac Cells. International Journal of Molecular Sciences, 22(24), 13448. https://doi.org/10.3390/ijms222413448





