Subgingival Microbiome in Rheumatoid Arthritis Patients with Periodontitis
Abstract
:1. Introduction
2. Results
2.1. Grouping of the Participants and Comparisons of Clinical Characteristics
2.2. The Alpha Diversity of Subgingival Microbiota between the RA Patients and Controls in the Three Groups
2.3. The Beta Diversity of Subgingival Microbiota between the RA Patients and Controls in the Three Groups
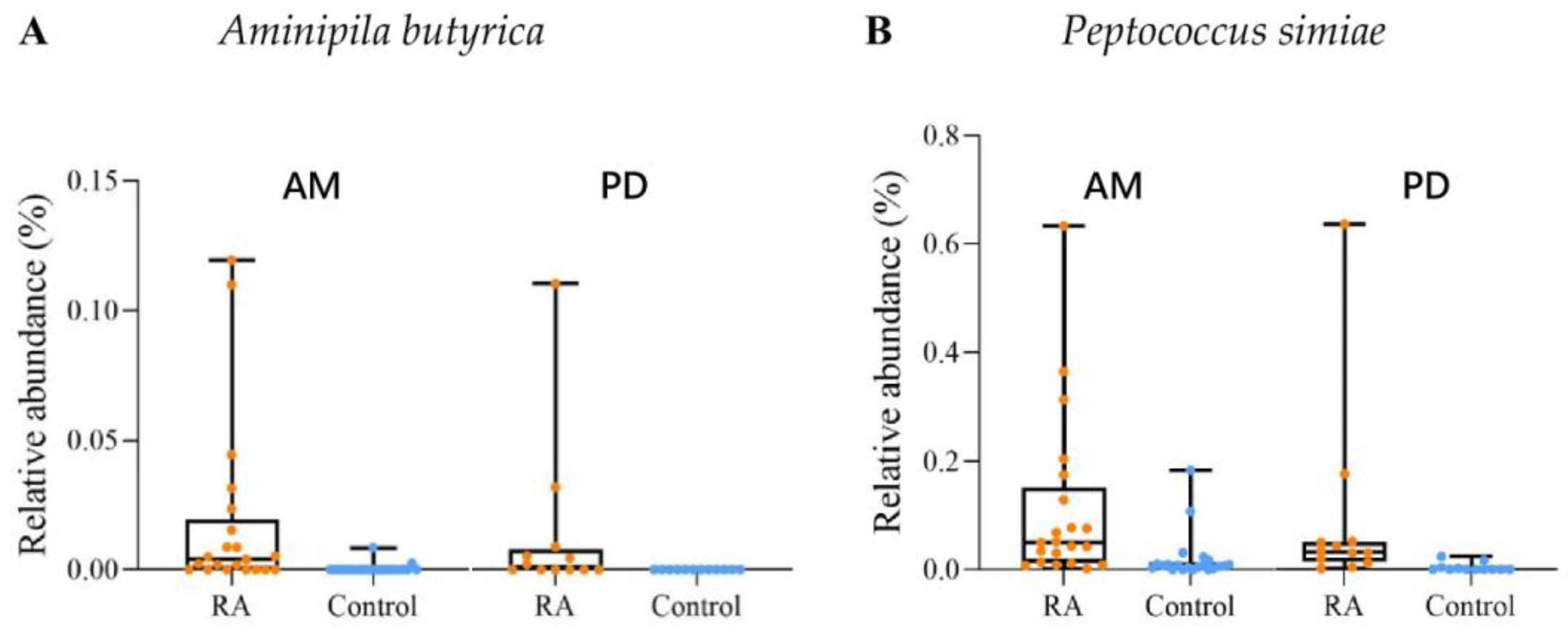
2.4. The Discriminative Taxa of the Subgingival Microbiota between the RA Patients and Controls in Groups AM and PD
2.5. The Relationships between the Abundance of Discriminative Taxa and the Concentrations of ACPAs in Groups AM and PD
2.6. Differences in the Metabolic Pathways between the RA Patients and Controls in Groups AM and PD
3. Discussion
4. Materials and Methods
4.1. Study Participants and the Assessment of RA
4.2. The Subgingival Microbiome Sampling and the Assessments of the Periodontitis Statuses in the RA Patients and Controls
4.3. Microbial DNA Extraction, 16S rRNA Gene Sequencing
4.4. Statistics and Bioinformatic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lundberg, K.; Wegner, N.; Yucel-Lindberg, T.; Venables, P.J. Periodontitis in RA—The citrullinated enolase connection. Nat. Rev. Rheumatol. 2010, 6, 727–730. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; Redwan, E.M. Interplay of Microbiota and Citrullination in the Immunopathogenesis of Rheumatoid Arthritis. Probiotics Antimicrob. Proteins 2021, 14, 99–113. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, B.; Mittereder, N.; Chaerkady, R.; Strain, M.; An, L.-L.; Rahman, S.; Ma, W.; Low, C.P.; Chan, D.; et al. Spontaneous Secretion of the Citrullination Enzyme PAD2 and Cell Surface Exposure of PAD4 by Neutrophils. Front. Immunol. 2017, 8, 1200. [Google Scholar] [CrossRef]
- Kroot, E.-J.J.A.; De Jong, B.A.W.; Van Leeuwen, M.A.; Swinkels, H.; Hoogen, F.H.J.V.D.; Hof, M.V.; Van De Putte, L.B.A.; Van Rijswijk, M.H.; Van Venrooij, W.J.; Van Riel, P.L.C.M. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2000, 43, 1831–1835. [Google Scholar] [CrossRef]
- Mankia, K.; Cheng, Z.; Do, T.; Hunt, L.; Meade, J.; Kang, J.; Clerehugh, V.; Speirs, A.; Tugnait, A.; Hensor, E.M.A.; et al. Prevalence of Periodontal Disease and Periodontopathic Bacteria in Anti–Cyclic Citrullinated Protein Antibody–Positive At-Risk Adults without Arthritis. JAMA Netw. Open 2019, 2, e195394. [Google Scholar] [CrossRef]
- Bae, S.-C.; Lee, Y.H. Association between anti-Porphyromonas gingivalis antibody, anti-citrullinated protein antibodies, and rheumatoid arthritis. Zeitschrift für Rheumatologie 2017, 77, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Buschhart, A.-L.; Bolten, L.; Volzke, J.; Ekat, K.; Kneitz, S.; Mikkat, S.; Kreikemeyer, B.; Müller-Hilke, B. Periodontal pathogens alter the synovial proteome. Periodontal pathogens do not exacerbate macroscopic arthritis but alter the synovial proteome in mice. PLoS ONE 2020, 15, e0242868. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, J.-M.; Bandiaky, O.N.; Le Goff, B.; Amador, G.; Chaux, A.-G.; Soueidan, A.; Denis, F. Another Look at the Contribution of Oral Microbiota to the Pathogenesis of Rheumatoid Arthritis: A Narrative Review. Microorganisms 2021, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.S.; Hayward, M.R.; Coelho, L.P.; Li, S.S.; Costea, P.I.; Voigt, A.Y.; Wirbel, J.; Maistrenko, O.M.; Alves, R.J.; Bergsten, E.; et al. Extensive transmission of microbes along the gastrointestinal tract. eLife 2019, 8, e42693. [Google Scholar] [CrossRef]
- Hamamoto, Y.; Ouhara, K.; Munenaga, S.; Shoji, M.; Ozawa, T.; Hisatsune, J.; Kado, I.; Kajiya, M.; Matsuda, S.; Kawai, T.; et al. Effect of Porphyromonas gingivalis infection on gut dysbiosis and resultant arthritis exacerbation in mouse model. Arthritis Res. Ther. 2020, 22, 249. [Google Scholar] [CrossRef]
- Lehenaff, R.; Tamashiro, R.; Nascimento, M.M.; Lee, K.; Jenkins, R.; Whitlock, J.; Li, E.C.; Sidhu, G.; Anderson, S.; Progulske-Fox, A.; et al. Subgingival microbiome of deep and shallow periodontal sites in patients with rheumatoid arthritis: A pilot study. BMC Oral Health 2021, 21, 248. [Google Scholar] [CrossRef]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef]
- Pérez-Chaparro, P.J.; McCulloch, J.A.; Mamizuka, E.M.; Moraes, A.D.C.L.; Faveri, M.; Figueiredo, L.C.; Duarte, P.M.; Feres, M. Do different probing depths exhibit striking differences in microbial profiles? J. Clin. Periodontol. 2018, 45, 26–37. [Google Scholar] [CrossRef]
- Ueki, A.; Goto, K.; Kaku, N.; Ueki, K. Aminipila butyrica gen. nov., sp. nov., a strictly anaerobic, arginine-decomposing bacterium isolated from a methanogenic reactor of cattle waste. Int. J. Syst. Evol. Microbiol. 2018, 68, 443–448. [Google Scholar] [CrossRef]
- Lopez-Oliva, I.; Paropkari, A.D.; Saraswat, S.; Serban, S.; Yonel, Z.; Sharma, P.; de Pablo, P.; Raza, K.; Filer, A.; Chapple, I.; et al. Dysbiotic Subgingival Microbial Communities in Periodontally Healthy Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 1008–1013. [Google Scholar] [CrossRef]
- Shkoporov, A.; Efimov, B.A.; Kondova, I.; Ouwerling, B.; Chaplin, A.; Shcherbakova, V.; Langermans, J.A.M. Peptococcus simiae sp. nov., isolated from rhesus macaque faeces and emended description of the genus Peptococcus. Int. J. Syst. Evol. Microbiol. 2016, 66, 5187–5191. [Google Scholar] [CrossRef]
- Shi, M.; Wei, Y.; Hu, W.; Nie, Y.; Wu, X.; Lu, R. The Subgingival Microbiome of Periodontal Pockets with Different Probing Depths in Chronic and Aggressive Periodontitis: A Pilot Study. Front. Cell. Infect. Microbiol. 2018, 8, 124. [Google Scholar] [CrossRef]
- Zhao, H.; Chu, M.; Huang, Z.; Yang, X.; Ran, S.; Hu, B.; Zhang, C.; Liang, J. Variations in oral microbiota associated with oral cancer. Sci. Rep. 2017, 7, 11773. [Google Scholar] [CrossRef]
- Cheah, C.W.; Al-Maleki, A.R.; Vaithilingam, R.D.; Vadivelu, J.; Sockalingam, S.; Baharuddin, N.A.; Bartold, P.M. Associations between inflammation-related LL-37 with subgingival microbial dysbiosis in rheumatoid arthritis patients. Clin. Oral Investig. 2022, 26, 4161–4172. [Google Scholar] [CrossRef] [PubMed]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter actinomycetemcomitans–induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016, 8, 369ra176. [Google Scholar] [CrossRef] [Green Version]
- Los, F.C.O.; Randis, T.; Aroian, R.V.; Ratner, A. Role of Pore-Forming Toxins in Bacterial Infectious Diseases. Microbiol. Mol. Biol. Rev. 2013, 77, 173–207. [Google Scholar] [CrossRef] [PubMed]
- Linhartová, I.; Bumba, L.; Mašín, J.; Basler, M.; Osicka, R.; Kamanova, J.; Procházková, K.; Adkins, I.; Hejnová-Holubová, J.; Sadílková, L.; et al. RTX proteins: A highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 2010, 34, 1076–1112. [Google Scholar] [CrossRef] [PubMed]
- Downes, J.; Wade, W. Peptostreptococcus stomatis sp. nov., isolated from the human oral cavity. Int. J. Syst. Evol. Microbiol. 2006, 56, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Chaparro, P.J.; Gonçalves, C.; Figueiredo, L.C.; Faveri, M.; Lobão, E.; Tamashiro, N.; Duarte, P.; Feres, M. Newly identified pathogens associated with periodontitis: A systematic review. J. Dent. Res. 2014, 93, 846–858. [Google Scholar] [CrossRef]
- Lourenço, T.; Heller, D.; Silva-Boghossian, C.M.; Cotton, S.L.; Paster, B.J.; Colombo, A.P.V. Microbial signature profiles of periodontally healthy and diseased patients. J. Clin. Periodontol. 2014, 41, 1027–1036. [Google Scholar] [CrossRef]
- Suárez, L.J.; Garzón, H.; Arboleda, S.; Rodríguez, A. Oral Dysbiosis and Autoimmunity: From Local Periodontal Responses to an Imbalanced Systemic Immunity. A Review. Front. Immunol. 2020, 11, 591255. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell. Infect. Microbiol. 2020, 9, 476. [Google Scholar] [CrossRef]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J.Y.; et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2017, 67, 1024–1032. [Google Scholar] [CrossRef]
- Uchino, Y.; Goto, Y.; Konishi, Y.; Tanabe, K.; Toda, H.; Wada, M.; Kita, Y.; Beppu, M.; Mori, S.; Hijioka, H.; et al. Colorectal Cancer Patients Have Four Specific Bacterial Species in Oral and Gut Microbiota in Common—A Metagenomic Comparison with Healthy Subjects. Cancers 2021, 13, 3332. [Google Scholar] [CrossRef]
- Dai, W.; Li, C.; Li, T.; Hu, J.; Zhang, H. Super-taxon in human microbiome are identified to be associated with colorectal cancer. BMC Bioinform. 2022, 23, 243. [Google Scholar] [CrossRef]
- Ternes, D.; Karta, J.; Tsenkova, N.; Wilmes, P.; Haan, S.; Letellier, E. Microbiome in Colorectal Cancer: How to Get from Meta-omics to Mechanism? Trends Microbiol. 2020, 28, 401–423. [Google Scholar] [CrossRef]
- Tong, Y.; Zheng, L.; Qing, P.; Zhao, H.; Li, Y.; Su, L.; Zhang, Q.; Zhao, Y.; Luo, Y.; Liu, Y. Oral Microbiota Perturbations Are Linked to High Risk for Rheumatoid Arthritis. Front. Cell. Infect. Microbiol. 2020, 9, 475. [Google Scholar] [CrossRef]
- Patini, R.; Staderini, E.; Lajolo, C.; Lopetuso, L.; Mohammed, H.; Rimondini, L.; Rocchetti, V.; Franceschi, F.; Cordaro, M.; Gallenzi, P. Relationship between oral microbiota and periodontal disease: A systematic review. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 5775–5788. [Google Scholar]
- Shi, B.; Chang, M.; Martin, J.; Mitreva, M.; Lux, R.; Klokkevold, P.; Sodergren, E.; Weinstock, G.; Haake, S.K.; Li, H. Dynamic Changes in the Subgingival Microbiome and Their Potential for Diagnosis and Prognosis of Periodontitis. mBio 2015, 6, e01926-14. [Google Scholar] [CrossRef]
- Cheng, Z.; Do, T.; Mankia, K.; Meade, J.; Hunt, L.; Clerehugh, V.; Speirs, A.; Tugnait, A.; Emery, P.; Devine, D. Dysbiosis in the oral microbiomes of anti-CCP positive individuals at risk of developing rheumatoid arthritis. Ann. Rheum. Dis. 2020, 80, 162–168. [Google Scholar] [CrossRef]
- Mikuls, T.R.; Walker, C.; Qiu, F.; Yu, F.; Thiele, G.M.; Alfant, B.; Li, E.C.; Zhao, L.Y.; Wang, G.P.; Datta, S.; et al. The subgingival microbiome in patients with established rheumatoid arthritis. Rheumatology 2018, 57, 1162–1172. [Google Scholar] [CrossRef]
- Esteves, G.; Pereira, J.; Azevedo, N.; Azevedo, A.; Mendes, L. Friends with Benefits: An Inside Look of Periodontal Microbes’ Interactions Using Fluorescence In Situ Hybridization—Scoping Review. Microorganisms 2021, 9, 1504. [Google Scholar] [CrossRef]
- Mark Welch, J.; Rossetti, B.; Rieken, C.; Dewhirst, F.; Borisky, G. Biogeography of a human oral microbiome at the micron scale. Proc. Natl. Acad. Sci. USA 2016, 113, E791–E800. [Google Scholar] [CrossRef]
- Tseng, Y.-C.; Yang, H.-Y.; Lin, W.-T.; Chang, C.-B.; Chien, H.-C.; Wang, H.-P.; Chen, C.-M.; Wang, J.-T.; Li, C.; Wu, S.-F.; et al. Salivary dysbiosis in Sjögren’s syndrome and a commensal-mediated immunomodulatory effect of salivary gland epithelial cells. npj Biofilms Microbiomes 2021, 7, 21. [Google Scholar] [CrossRef]
- A Kiprianova, E.; Klochko, V.V.; Zelena, L.B.; Churkina, L.N.; Avdeeva, L.V. Pseudomonas batumici sp. nov., the antibiotic-producing bacteria isolated from soil of the Caucasus Black Sea coast. Mikrobiolohichnyi Zhurnal 2011, 73, 3–8. [Google Scholar]
- Klochko, V.V.; Zelena, L.B.; Kim, J.Y.; Avdeeva, L.V.; Reva, O.N. Prospects of a new antistaphylococcal drug batumin revealed by molecular docking and analysis of the complete genome sequence of the batumin-producer Pseudomonas batumici UCM B-321. Int. J. Antimicrob. Agents 2015, 47, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, J.D.; Fernandes, G.R.; Calderaro, D.C.; Mendonça, S.M.S.; Silva, J.M.; Albiero, M.L.; Cunha, F.Q.; Xiao, E.; Ferreira, G.A.; Teixeira, A.L.; et al. Oral microbial dysbiosis linked to worsened periodontal condition in rheumatoid arthritis patients. Sci. Rep. 2019, 9, 8379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scher, J.U.; Ubeda, C.; Equinda, M.; Khanin, R.; Buischi, Y.; Viale, A.; Lipuma, L.; Attur, M.; Pillinger, M.; Weissmann, G.; et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Care Res. 2012, 64, 3083–3094. [Google Scholar] [CrossRef] [PubMed]
- Kroese, J.M.; Brandt, B.W.; Buijs, M.J.; Crielaard, W.; Lobbezoo, F.; Loos, B.G.; van Boheemen, L.; van Schaardenburg, D.; Zaura, E.; Volgenant, C.M. The oral microbiome in early rheumatoid arthritis patients and individuals at risk differs from healthy controls. Arthritis Rheumatol. 2021, 73, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Malmström, V.; Catrina, A.I.; Klareskog, L. The immunopathogenesis of seropositive rheumatoid arthritis: From triggering to targeting. Nat. Rev. Immunol. 2016, 17, 60–75. [Google Scholar] [CrossRef]
- Willis, V.C.; Demoruelle, M.K.; Derber, L.A.; Chartier-Logan, C.J.; Parish, M.C.; Pedraza, I.F.; Weisman, M.H.; Norris, J.M.; Holers, V.M.; Deane, K.D. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum. 2013, 65, 2545–2554. [Google Scholar]
- Reynisdottir, G.; Karimi, R.; Joshua, V.; Olsen, H.; Hensvold, A.H.; Harju, A.; Engström, M.; Grunewald, J.; Nyren, S.; Eklund, A.; et al. Structural Changes and Antibody Enrichment in the Lungs Are Early Features of Anti-Citrullinated Protein Antibody-Positive Rheumatoid Arthritis. Arthritis Rheumatol. 2013, 66, 31–39. [Google Scholar] [CrossRef]
- Reynisdottir, G.; Olsen, H.; Joshua, V.; Engström, M.; Forsslund, H.; Karimi, R.; Sköld, C.M.; Nyren, S.; Eklund, A.; Grunewald, J.; et al. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann. Rheum. Dis. 2015, 75, 1722–1727. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S162–S170. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Mitra, A.; Skrzypczak, M.; Ginalski, K.; Rowicka, M. Strategies for Achieving High Sequencing Accuracy for Low Diversity Samples and Avoiding Sample Bleeding Using Illumina Platform. PLoS ONE 2015, 10, e0120520. [Google Scholar] [CrossRef]
- Willis, A.D. Rarefaction, Alpha Diversity, and Statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J. Hypothesis testing and statistical analysis of microbiome. Genes Dis. 2017, 4, 138–148. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [Green Version]
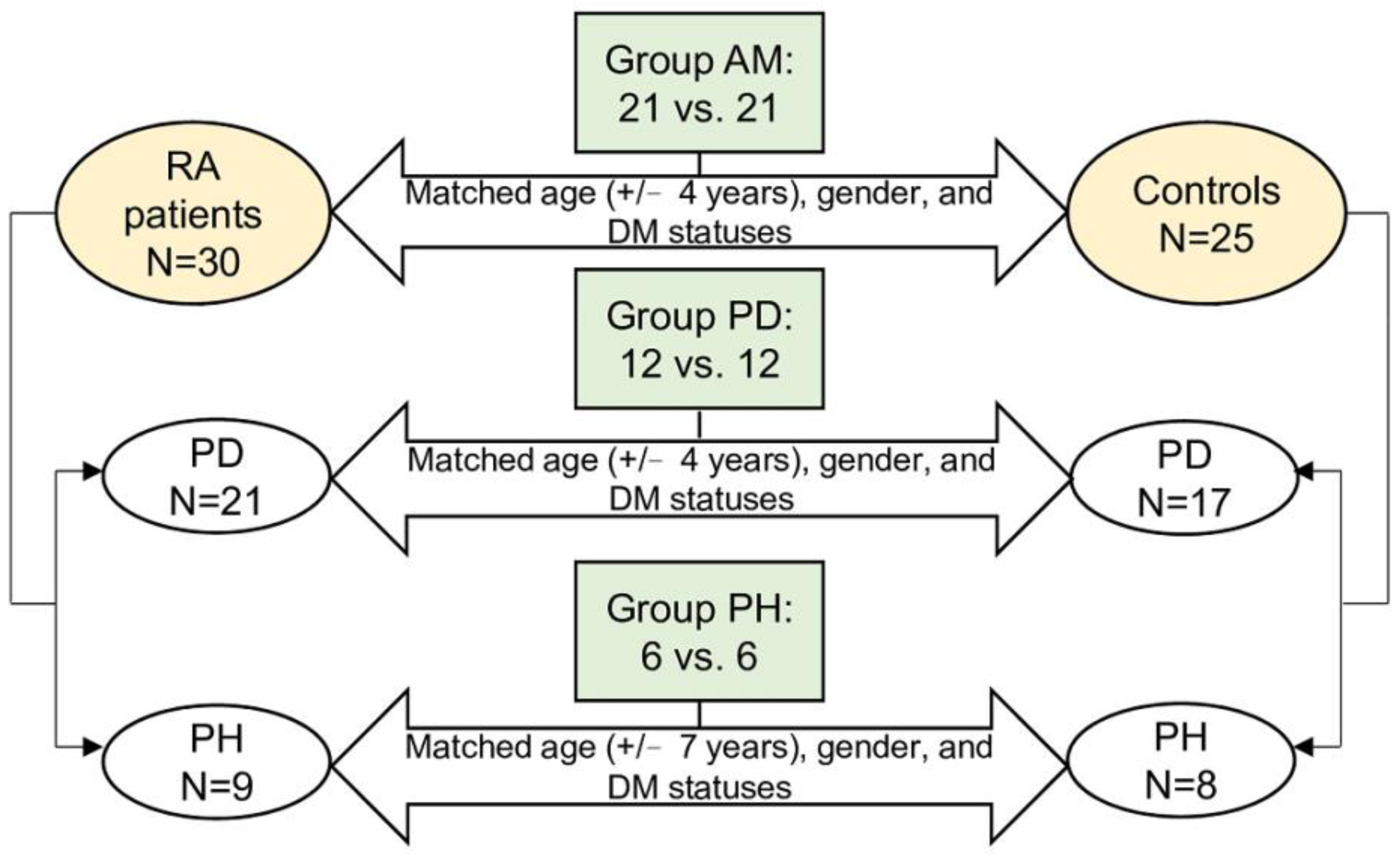


| AM | PD | PH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RA | Control | p-Value | RA | Control | p-Value | RA | Control | p-Value | |
| N | 21 | 21 | 12 | 12 | 6 | 6 | |||
| Age | 57.57 ± 8.06 | 57.19 ± 8.73 | 0.884 | 59.00 ± 7.80 | 58.50 ± 7.86 | 0.877 | 54.50 ± 11.66 | 53.33 ± 11.04 | 0.862 |
| Female, % | 16 (76.19) | 16 (76.19) | 1 | 9 (75) | 9 (75) | 1 | 5 (83.33) | 5 (83.33) | 1 |
| Diabetes mellitus, % | 4 (19.05) | 4 (19.05) | 1 | 2 (16.67) | 2 (16.67) | 1 | 1 (16.67) | 1 (16.67) | 1 |
| Autoantibody status | |||||||||
| RF, positive | 17 (80.95) | 2 (9.52) | <0.001 | 11 (91.67) | 1 (8.33) | <0.001 | 5 (83.33) | 1 (16.67) | 0.021 |
| ACPA, positive | 15 (71.43) | 0 (0) | <0.001 | 10 (83.33) | 0 (0) | <0.001 | 4 (66.67) | 0 (0) | 0.558 |
| ACPA, level | 54.50 (3.9, 223.00) | 1.00 (0.60, 1.40) | <0.001 | 97.50 (35.75, 338.50) | 0.80 (0.47, 1.18) | <0.001 | 142.50 (28.20, 191.25) | 1.20 (0.85, 1.85) | 0.336 |
| Periodontitis, % | 14 (66.67) | 14 (66.67) | 1 | 12 (100) | 12 (100) | 1 | 0 (0) | 0 (0) | 1 |
| Group | ||
|---|---|---|
| AM | PD | |
| Aminipila butyrica | 0.360 (0.019) | 0.440 (0.032) |
| Peptococcus simiae | 0.464 (0.002) | 0.435 (0.033) |
| Bacteroides ovatus | 0.313 (0.044) | - |
| Bulleidia extructa | 0.316 (0.041) | - |
| Genus_Parvimonas sp. | 0.330 (0.033) | - |
| Peptostreptococcus stomatis | 0.617 (<0.001) | - |
| Genus_Acidovorax sp. | - | 0.440 (0.032) |
| Family_Actinomycetaceae sp. | - | 0.441 (0.031) |
| Genus_Actinomyces sp. | - | 0.438 (0.032) |
| Cloacibacterium haliotis | - | 0.524 (0.009) |
| Flexilinea flocculi | - | 0.445 (0.029) |
| Genus_Lactobacillus sp. | - | 0.456 (0.025) |
| Leptotrichia hofstadii | - | 0.440 (0.031) |
| Rivicola pingtungensis | - | 0.416 (0.043) |
| Rothia mucilaginosa | - | 0.542 (0.006) |
| Streptococcus sanguinis | - | 0.476 (0.019) |
| Pseudomonas batumici | - | −0.436 (0.033) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-J.; Hung, W.-C.; Chou, Y.-H.; Lai, C.-H.; Peng, P.; Jhou, P.-S.; Tsai, M.-R.; Sheu, J.J.-C.; Yen, J.-H. Subgingival Microbiome in Rheumatoid Arthritis Patients with Periodontitis. Int. J. Mol. Sci. 2022, 23, 9883. https://doi.org/10.3390/ijms23179883
Chen Y-J, Hung W-C, Chou Y-H, Lai C-H, Peng P, Jhou P-S, Tsai M-R, Sheu JJ-C, Yen J-H. Subgingival Microbiome in Rheumatoid Arthritis Patients with Periodontitis. International Journal of Molecular Sciences. 2022; 23(17):9883. https://doi.org/10.3390/ijms23179883
Chicago/Turabian StyleChen, Yi-Jing, Wei-Chun Hung, Yu-Hsiang Chou, Chern-Hsiung Lai, Po Peng, Pei-Syuan Jhou, Min-Ru Tsai, Jim Jinn-Chyuan Sheu, and Jeng-Hsien Yen. 2022. "Subgingival Microbiome in Rheumatoid Arthritis Patients with Periodontitis" International Journal of Molecular Sciences 23, no. 17: 9883. https://doi.org/10.3390/ijms23179883
APA StyleChen, Y.-J., Hung, W.-C., Chou, Y.-H., Lai, C.-H., Peng, P., Jhou, P.-S., Tsai, M.-R., Sheu, J. J.-C., & Yen, J.-H. (2022). Subgingival Microbiome in Rheumatoid Arthritis Patients with Periodontitis. International Journal of Molecular Sciences, 23(17), 9883. https://doi.org/10.3390/ijms23179883







