Anti-Citrullinated Peptide Antibodies Control Oral Porphyromonas and Aggregatibacter species in Patients with Rheumatoid Arthritis
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Two Cohorts Studied
2.2. Porphyromonas sp. Reduction Characterizes Early RA Patients
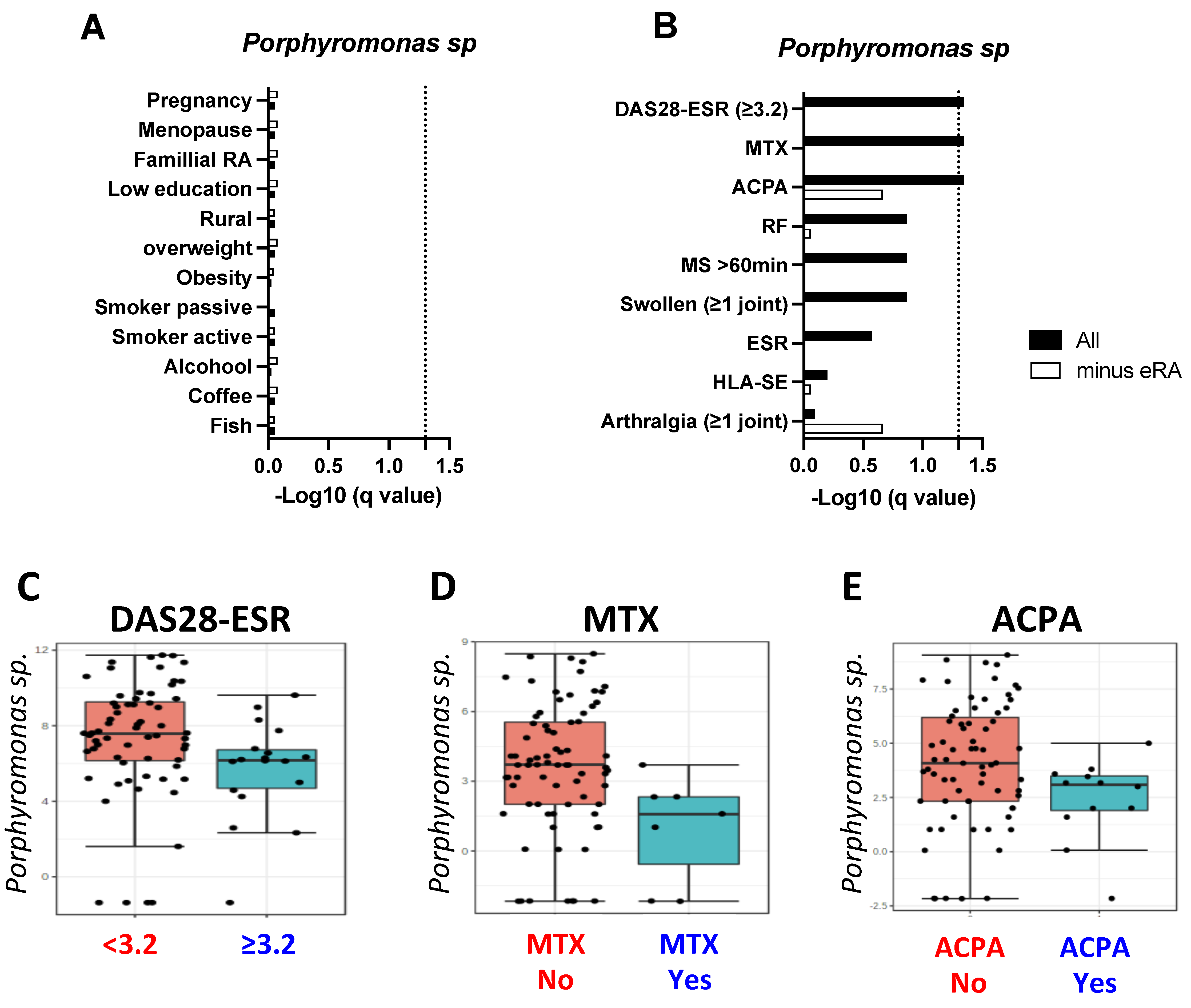
2.3. Individual, Genetic and Bio-Clinical Factors
2.4. RA Population
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Microbiome Analysis
4.3. Environmental Factors
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- Arleevskaya, M.; Takha, E.; Petrov, S.; Kazarian, G.; Novikov, A.; Larionova, R.; Valeeva, A.; Shuralev, E.; Mukminov, M.; Bost, C.; et al. Causal risk and protective factors in rheumatoid arthritis: A genetic update. J. Transl. Autoimmun. 2021, 4, 100119. [Google Scholar] [CrossRef] [PubMed]
- Quirke, A.M.; Lugli, E.B.; Wegner, N.; Hamilton, B.C.; Charles, P.; Chowdhury, M.; Ytterberg, A.J.; Zubarev, R.A.; Potempa, J.; Culshaw, S.; et al. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: A potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016, 8, 369ra176. [Google Scholar] [CrossRef]
- Giles, J.T.; Reinholdt, J.; Andrade, F.; Konig, M.F. Associations of Antibodies Targeting Periodontal Pathogens With Subclinical Coronary, Carotid, and Peripheral Arterial Atherosclerosis in Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 568–575. [Google Scholar] [CrossRef]
- Johansson, L.; Sherina, N.; Kharlamova, N.; Potempa, B.; Larsson, B.; Israelsson, L.; Potempa, J.; Rantapaa-Dahlqvist, S.; Lundberg, K. Concentration of antibodies against Porphyromonas gingivalis is increased before the onset of symptoms of rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 201. [Google Scholar] [CrossRef]
- Van Steenbergen, H.W.; Aletaha, D.; Beaart-van de Voorde, L.J.; Brouwer, E.; Codreanu, C.; Combe, B.; Fonseca, J.E.; Hetland, M.L.; Humby, F.; Kvien, T.K.; et al. EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 491–496. [Google Scholar] [CrossRef]
- Liu, X.; Tian, K.M.; Ma, X.; Wang, S.; Luo, C.; Du, Q. Analysis of subgingival microbiome of periodontal disease and rheumatoid arthritis in Chinese: A case-control study. Saudi J. Biol. Sci. 2020, 27, 1835–1842. [Google Scholar] [CrossRef]
- Cheng, Z.; Do, T.; Mankia, K.; Meade, J.; Hunt, L.; Clerehugh, V.; Speirs, A.; Tugnait, A.; Emery, P.; Devine, D. Dysbiosis in the oral microbiomes of anti-CCP positive individuals at risk of developing rheumatoid arthritis. Ann. Rheum. Dis. 2021, 80, 162–168. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Equinda, M.; Khanin, R.; Buischi, Y.; Viale, A.; Lipuma, L.; Attur, M.; Pillinger, M.H.; Weissmann, G.; et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012, 64, 3083–3094. [Google Scholar] [CrossRef]
- Lopez-Oliva, I.; Paropkari, A.D.; Saraswat, S.; Serban, S.; Yonel, Z.; Sharma, P.; de Pablo, P.; Raza, K.; Filer, A.; Chapple, I.; et al. Dysbiotic Subgingival Microbial Communities in Periodontally Healthy Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Kroese, J.M.; Brandt, B.W.; Buijs, M.J.; Crielaard, W.; Lobbezoo, F.; Loos, B.G.; van Boheemen, L.; van Schaardenburg, D.; Zaura, E.; Volgenant, C.M.C. Differences in the Oral Microbiome in Patients With Early Rheumatoid Arthritis and Individuals at Risk of Rheumatoid Arthritis Compared to Healthy Individuals. Arthritis Rheumatol. 2021, 73, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zheng, L.; Qing, P.; Zhao, H.; Li, Y.; Su, L.; Zhang, Q.; Zhao, Y.; Luo, Y.; Liu, Y. Oral Microbiota Perturbations Are Linked to High Risk for Rheumatoid Arthritis. Front. Cell. Infect. Microbiol. 2020, 9, 475. [Google Scholar] [CrossRef] [PubMed]
- Esberg, A.; Johansson, L.; Johansson, I.; Dahlqvist, S.R. Oral Microbiota Identifies Patients in Early Onset Rheumatoid Arthritis. Microorganisms 2021, 9, 1657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhao, Y.; Li, S.; Yang, L.; Wang, H.; Wang, T.; Bin, S.; Gai, Z.; Heng, X.; Zhang, C.; et al. Variations in oral microbiome profiles in rheumatoid arthritis and osteoarthritis with potential biomarkers for arthritis screening. Sci. Rep. 2018, 8, 17126. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, J.D.; Fernandes, G.R.; Calderaro, D.C.; Mendonça, S.M.S.; Silva, J.M.; Albiero, M.L.; Cunha, F.Q.; Xiao, E.; Ferreira, G.A.; Teixeira, A.L.; et al. Oral microbial dysbiosis linked to worsened periodontal condition in rheumatoid arthritis patients. Sci. Rep. 2019, 9, 8379. [Google Scholar] [CrossRef]
- De Jesus, V.C.; Singh, M.; Schroth, R.J.; Chelikani, P.; Hitchon, C.A. Association of Bitter Taste Receptor T2R38 Polymorphisms, Oral Microbiota, and Rheumatoid Arthritis. Curr. Issues Mol. Biol. 2021, 43, 1460–1472. [Google Scholar] [CrossRef]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Maia, B.; Monteiro-Silva, F. Acquisition and maturation of oral microbiome throughout childhood: An update. Dent. Res. J. 2014, 11, 291–301. [Google Scholar]
- Crielaard, W.; Zaura, E.; Schuller, A.A.; Huse, S.M.; Montijn, R.C.; Keijser, B.J. Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med. Genom. 2011, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Cattoni, F.; Tetè, G.; D’orto, B.; Bergamaschi, A.; Polizzi, E.; Gastaldi, G. Comparison of hygiene levels in metal-ceramic and stratified zirconia in prosthetic rehabilitation on teeth and implants: A retrospective clinical study of a three-year follow-up. J. Biol. Regul. Homeost. Agents 2021, 35, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Tetè, G.; D’orto, B.; Ferrante, L.; Polizzi, E.; Cattoni, F. Role of mast cells in oral inflammation. J. Biol. Regul. Homeost. Agents 2021, 35, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Arleevskaya, M.I.; Albina, S.; Larionova, R.V.; Gabdoulkhakova, A.G.; Lemerle, J.; Renaudineau, Y. Prevalence and Incidence of Upper Respiratory Tract Infection Events Are Elevated Prior to the Development of Rheumatoid Arthritis in First-Degree Relatives. Front Immunol. 2018, 9, 2771. [Google Scholar] [CrossRef]
- Renson, A.; Jones, H.E.; Beghini, F.; Segata, N.; Zolnik, C.P.; Usyk, M.; Moody, T.U.; Thorpe, L.; Burk, R.; Waldron, L.; et al. Sociodemographic variation in the oral microbiome. Ann. Epidemiol. 2019, 35, 73–80.e2. [Google Scholar] [CrossRef]
- Bae, S.C.; Lee, Y.H. Association between anti-Porphyromonas gingivalis antibody, anti-citrullinated protein antibodies, and rheumatoid arthritis: A meta-analysis. Z. Rheumatol. 2018, 77, 522–532. [Google Scholar] [CrossRef]
- Hitchon, C.A.; Chandad, F.; Ferucci, E.D.; Willemze, A.; Ioan-Facsinay, A.; van der Woude, D.; Markland, J.; Robinson, D.; Elias, B.; Newkirk, M.; et al. Antibodies to porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J. Rheumatol. 2010, 37, 1105–1112. [Google Scholar] [CrossRef]
- Kharlamova, N.; Jiang, X.; Sherina, N.; Potempa, B.; Israelsson, L.; Quirke, A.M.; Eriksson, K.; Yucel-Lindberg, T.; Venables, P.J.; Potempa, J.; et al. Antibodies to Porphyromonas gingivalis Indicate Interaction Between Oral Infection, Smoking, and Risk Genes in Rheumatoid Arthritis Etiology. Arthritis. Rheumatol. 2016, 68, 604–613. [Google Scholar] [CrossRef]
- Li, S.; Yu, Y.; Yue, Y.; Liao, H.; Xie, W.; Thai, J.; Mikuls, T.R.; Thiele, G.M.; Duryee, M.J.; Sayles, H.; et al. Autoantibodies From Single Circulating Plasmablasts React With Citrullinated Antigens and Porphyromonas gingivalis in Rheumatoid Arthritis. Arthritis. Rheumatol. 2016, 68, 614–626. [Google Scholar] [CrossRef]
- Munoz-Atienza, E.; Flak, M.B.; Sirr, J.; Paramonov, N.A.; Aduse-Opoku, J.; Pitzalis, C.; Curtis, M.A. The P. gingivalis Autocitrullinome Is Not a Target for ACPA in Early Rheumatoid Arthritis. J. Dent. Res. 2020, 99, 456–462. [Google Scholar] [CrossRef]
- Gorasia, D.G.; Veith, P.D.; Chen, D.; Seers, C.A.; Mitchell, H.A.; Chen, Y.Y.; Glew, M.D.; Dashper, S.G.; Reynolds, E.C. Porphyromonas gingivalis Type IX Secretion Substrates Are Cleaved and Modified by a Sortase-Like Mechanism. PLoS Pathog. 2015, 11, e1005152. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.J.; Ju, J.H. Impact of Posttranslational Modification in Pathogenesis of Rheumatoid Arthritis: Focusing on Citrullination, Carbamylation, and Acetylation. Int. J. Mol. Sci. 2021, 22, 10576. [Google Scholar] [CrossRef]
- Lloyd, K.A.; Wigerblad, G.; Sahlstrom, P.; Garimella, M.G.; Chemin, K.; Steen, J.; Titcombe, P.J.; Marklein, B.; Zhou, D.; Stalesen, R.; et al. Differential ACPA Binding to Nuclear Antigens Reveals a PAD-Independent Pathway and a Distinct Subset of Acetylation Cross-Reactive Autoantibodies in Rheumatoid Arthritis. Front. Immunol. 2018, 9, 3033. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, F.; Dioni, I.; Tommasi, C.; Alcaro, M.C.; Paolini, I.; Barbetti, F.; Boscaro, F.; Panza, F.; Puxeddu, I.; Rovero, P.; et al. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann. Rheum. Dis. 2014, 73, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, A.J.; Alzabin, S.; Brintnell, W.; Wilson, E.; Barra, L.; Wegner, N.; Bell, D.A.; Cairns, E.; Venables, P.J. Immunization with Porphyromonas gingivalis enolase induces autoimmunity to mammalian alpha-enolase and arthritis in DR4-IE-transgenic mice. Arthritis Rheum. 2011, 63, 3818–3823. [Google Scholar] [CrossRef]
- Haag, S.; Uysal, H.; Backlund, J.; Tuncel, J.; Holmdahl, R. Human alpha-enolase is immunogenic, but not arthritogenic, in HLA-DR4-transgenic mice: Comment on the article by Kinloch et al. Arthritis Rheum. 2012, 64, 1689–1691, author reply 1691-1682. [Google Scholar] [CrossRef]
- Chukkapalli, S.; Rivera-Kweh, M.; Gehlot, P.; Velsko, I.; Bhattacharyya, I.; Calise, S.J.; Satoh, M.; Chan, E.K.; Holoshitz, J.; Kesavalu, L. Periodontal bacterial colonization in synovial tissues exacerbates collagen-induced arthritis in B10.RIII mice. Arthritis Res. Ther. 2016, 18, 161. [Google Scholar] [CrossRef]
- Gully, N.; Bright, R.; Marino, V.; Marchant, C.; Cantley, M.; Haynes, D.; Butler, C.; Dashper, S.; Reynolds, E.; Bartold, M. Porphyromonas gingivalis peptidylarginine deiminase, a key contributor in the pathogenesis of experimental periodontal disease and experimental arthritis. PLoS ONE 2014, 9, e100838. [Google Scholar] [CrossRef]
- Maresz, K.J.; Hellvard, A.; Sroka, A.; Adamowicz, K.; Bielecka, E.; Koziel, J.; Gawron, K.; Mizgalska, D.; Marcinska, K.A.; Benedyk, M.; et al. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 2013, 9, e1003627. [Google Scholar] [CrossRef]
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the oral microbiome by whole-genome sequencing and resistome analysis: The complexity of the healthy picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef]
- Eun, Y.G.; Lee, J.W.; Kim, S.W.; Hyun, D.W.; Bae, J.W.; Lee, Y.C. Oral microbiome associated with lymph node metastasis in oral squamous cell carcinoma. Sci. Rep. 2021, 11, 23176. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef] [PubMed]
- Arleevskaya, M.I.; Kravtsova, O.A.; Lemerle, J.; Renaudineau, Y.; Tsibulkin, A.P. How Rheumatoid Arthritis Can Result from Provocation of the Immune System by Microorganisms and Viruses. Front. Microbiol. 2016, 7, 1296. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Fries, J.F.; Spitz, P.; Kraines, R.G.; Holman, H.R. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980, 23, 137–145. [Google Scholar] [CrossRef]
- Langley, G.B.; Sheppeard, H. The visual analogue scale: Its use in pain measurement. Rheumatol. Int. 1985, 5, 145–148. [Google Scholar] [CrossRef]
- Inoue, E.; Yamanaka, H.; Hara, M.; Tomatsu, T.; Kamatani, N. Comparison of Disease Activity Score (DAS)28- erythrocyte sedimentation rate and DAS28- C-reactive protein threshold values. Ann. Rheum. Dis. 2007, 66, 407–409. [Google Scholar] [CrossRef]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology 2012, 51, vi5–vi9. [Google Scholar] [CrossRef]
- Gao, X.; Gazit, E.; Livneh, A.; Stastny, P. Rheumatoid arthritis in Israeli Jews: Shared sequences in the third hypervariable region of DRB1 alleles are associated with susceptibility. J. Rheumatol. 1991, 18, 801–803. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Arleevskaya, M.I.; Shafigullina, A.Z.; Filina, Y.V.; Lemerle, J.; Renaudineau, Y. Associations between Viral Infection History Symptoms, Granulocyte Reactive Oxygen Species Activity, and Active Rheumatoid Arthritis Disease in Untreated Women at Onset: Results from a Longitudinal Cohort Study of Tatarstan Women. Front. Immunol. 2017, 8, 1725. [Google Scholar] [CrossRef] [PubMed]
- Stepaniak, U.; Szafraniec, K.; Kubinova, R.; Malyutina, S.; Peasey, A.; Pikhart, H.; Pajak, A.; Bobak, M. Age at natural menopause in three central and eastern European urban populations: The HAPIEE study. Maturitas 2013, 75, 87–93. [Google Scholar] [CrossRef] [PubMed]


| Technique (OTU/ASV) | Oral Location | Population | Alpha/Beta Diversity | P (Pg) and A (Aa) | Clinical Associations | References |
|---|---|---|---|---|---|---|
| 16S (OTU) | Subgingival | 54 RA, 45 PD, 44 HC | Increased richness (RA) | Similar | - | [8] |
| shotgun | Subgingival | 48 ACPA+, 26 RA, 32 HC | Decreased richness (ACPA+) | P species (ACPA+ > RA/HC) | ACPA | [9] |
| 16S (OTU) | Subgingival | 31 eRA, 34 RA, 18 HC | Similar | P species (HC > RA) | PD (all ACPA/RF+) | [10] |
| 16S (OTU) | Subgingival | 22 RA, 19 controls | Unknown | Similar | - | [11] |
| 16S (OTU) | Subgingival, saliva, mucosa | 50 RA, 50 ACPA/RF+, 50 HC | Similar | Similar (OTU116) | - | [12] |
| 16S (OTU) | Saliva | 29 ACPA+, 27 RA, 23 HC | Decreased richness (ACPA+) | Pg (HC > ACPA+) | - | [13] |
| 16S (ASV) | Saliva | 61 eRA, 59 HC | Richness: eRA > HC | Pg and Aa (eRA > HC) | [14] | |
| shotgun | Subgingival, saliva | 54 RA, 51 HC | Unknown | Pg and A (HC > RA) | DMARDs (A species) | [15] |
| 16S (OTU) | Saliva | 110 RA, 67 OA, 155 Hc | Richness: eRA/OA > HC | Pg (RA + OA > HC) | - | [16] |
| 16S (OTU) | Subgingival | 42 RA, 47 HC | Richness: RA > HC | Aa (RA-PD > HC-PD) | PD | [17] |
| 16S (ASV) | Mucosa | 35 RA, 64 non-RA | Similar | P and A species (non-RA > RA) | - | [18] |
| 16S (ASV) | Mucosa | 15 eRA, 43 RA, 69 non-RA | Similar | P and A species (non-RA > RA) | ACPA | current study |
| Early RA | CSA = 0 | CSA = 1 | CSA = 2 | CSA ≥ 3 | q Values | RA | |
|---|---|---|---|---|---|---|---|
| Cohort 1 | Cohort 2 | ||||||
| Number | 15 | 22 | 19 | 11 | 17 | - | 42 |
| Age, median (IQ) | 50 (32–58) | 58 (32–63) | 46 (31–56) | 52 (30–62) | 44 (36–59) | 0.561 | 57 (43–78) |
| RA in FDR | 8/15 | 0/22 | 13/19 | 10/11 | 15/17 | <10−4 | 25/42 |
| DAS28-ESR > 3.2 | 13/15 | 0/22 | 0/19 | 1/11 | 5/17 | <10−4 | 32/42 |
| ESR >30 mm/hr | 10/15 | 2/22 | 1/19 | 1/11 | 5/17 | <10−4 | 17/38 |
| RF (>3 ULN) | 11 (6)/15 | 0 (0)/22 | 1 (0)/19 | 1 (0)/11 | 4 (1)/17 | <10−4 | 26 (15)/42 |
| ACPA (>3 ULN) | 10 (10)/15 | 0 (0)/22 | 1 (0)/19 | 1 (0)/11 | 1 (0)/17 | <10−4 | 32 (24)/42 |
| HLA-DRB1 SE | 5/12 | 7/16 | 9/15 | 5/7 | 6/13 | 0.697 | 20/30 |
| MS > 60 min | 11/15 | 0/22 | 0/19 | 2/11 | 6/17 | <10−4 | 21/42 |
| Tender arthralgia | 14/15 | 0/22 | 1/19 | 6/11 | 12/17 | <10−4 | 35/42 |
| Swollen | 12/15 | 0/22 | 0/19 | 0/11 | 4/17 | <10−4 | 24/42 |
| MTX:GC:biologics:no | 7:3:0:6 | 0:0:0:22 | 0:0:0:19 | 0:0:0:11 | 0:0:0:17 | <10−42 | 32:19:3:5 |
| Childbirth | 13/15 | 18/22 | 15/19 | 10/11 | 13/17 | 0.882 | 35/42 |
| Menopause (≥50 years) | 9/15 | 16/22 | 8/19 | 7/11 | 5/17 | 0.105 | 27/42 |
| Low education | 8/15 | 12/22 | 9/19 | 4/11 | 6/17 | 0.697 | 23/42 |
| Fish | 9/15 | 19/22 | 14/19 | 4/11 | 12/17 | 0.093 | 31/42 |
| Coffee | 7/15 | 9/22 | 10/19 | 3/11 | 7/17 | 0.787 | 22/42 |
| Alcohol | 11/15 | 14/22 | 9/19 | 7/11 | 10/17 | 0.698 | 29/42 |
| Smoker (active) | 1/15 | 1/22 | 3/19 | 1/11 | 0/17 | 0.561 | 1/42 |
| Smoker (passive) | 2/15 | 8/22 | 9/19 | 4/11 | 3/17 | 0.250 | 15/42 |
| Overweight | 6/13 | 14/20 | 10/18 | 3/10 | 7/16 | 0.158 | 20/37 |
| Obesity | 4/13 | 8/20 | 2/18 | 3/10 | 4/16 | 0.533 | 7/37 |
| Rural | 5/15 | 10/22 | 6/18 | 6/11 | 11/17 | 0.093 | 27/42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arleevskaya, M.I.; Boulygina, E.A.; Larionova, R.; Validov, S.; Kravtsova, O.; Shagimardanova, E.I.; Velo, L.; Hery-Arnaud, G.; Carlé, C.; Renaudineau, Y. Anti-Citrullinated Peptide Antibodies Control Oral Porphyromonas and Aggregatibacter species in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 12599. https://doi.org/10.3390/ijms232012599
Arleevskaya MI, Boulygina EA, Larionova R, Validov S, Kravtsova O, Shagimardanova EI, Velo L, Hery-Arnaud G, Carlé C, Renaudineau Y. Anti-Citrullinated Peptide Antibodies Control Oral Porphyromonas and Aggregatibacter species in Patients with Rheumatoid Arthritis. International Journal of Molecular Sciences. 2022; 23(20):12599. https://doi.org/10.3390/ijms232012599
Chicago/Turabian StyleArleevskaya, Marina I., Eugenia A. Boulygina, Regina Larionova, Shamil Validov, Olga Kravtsova, Elena I. Shagimardanova, Lourdes Velo, Geneviève Hery-Arnaud, Caroline Carlé, and Yves Renaudineau. 2022. "Anti-Citrullinated Peptide Antibodies Control Oral Porphyromonas and Aggregatibacter species in Patients with Rheumatoid Arthritis" International Journal of Molecular Sciences 23, no. 20: 12599. https://doi.org/10.3390/ijms232012599
APA StyleArleevskaya, M. I., Boulygina, E. A., Larionova, R., Validov, S., Kravtsova, O., Shagimardanova, E. I., Velo, L., Hery-Arnaud, G., Carlé, C., & Renaudineau, Y. (2022). Anti-Citrullinated Peptide Antibodies Control Oral Porphyromonas and Aggregatibacter species in Patients with Rheumatoid Arthritis. International Journal of Molecular Sciences, 23(20), 12599. https://doi.org/10.3390/ijms232012599





