Efficacy of Combination Therapy with Lenvatinib and Radioactive Iodine in Thyroid Cancer Preclinical Model
Abstract
:1. Introduction
2. Results
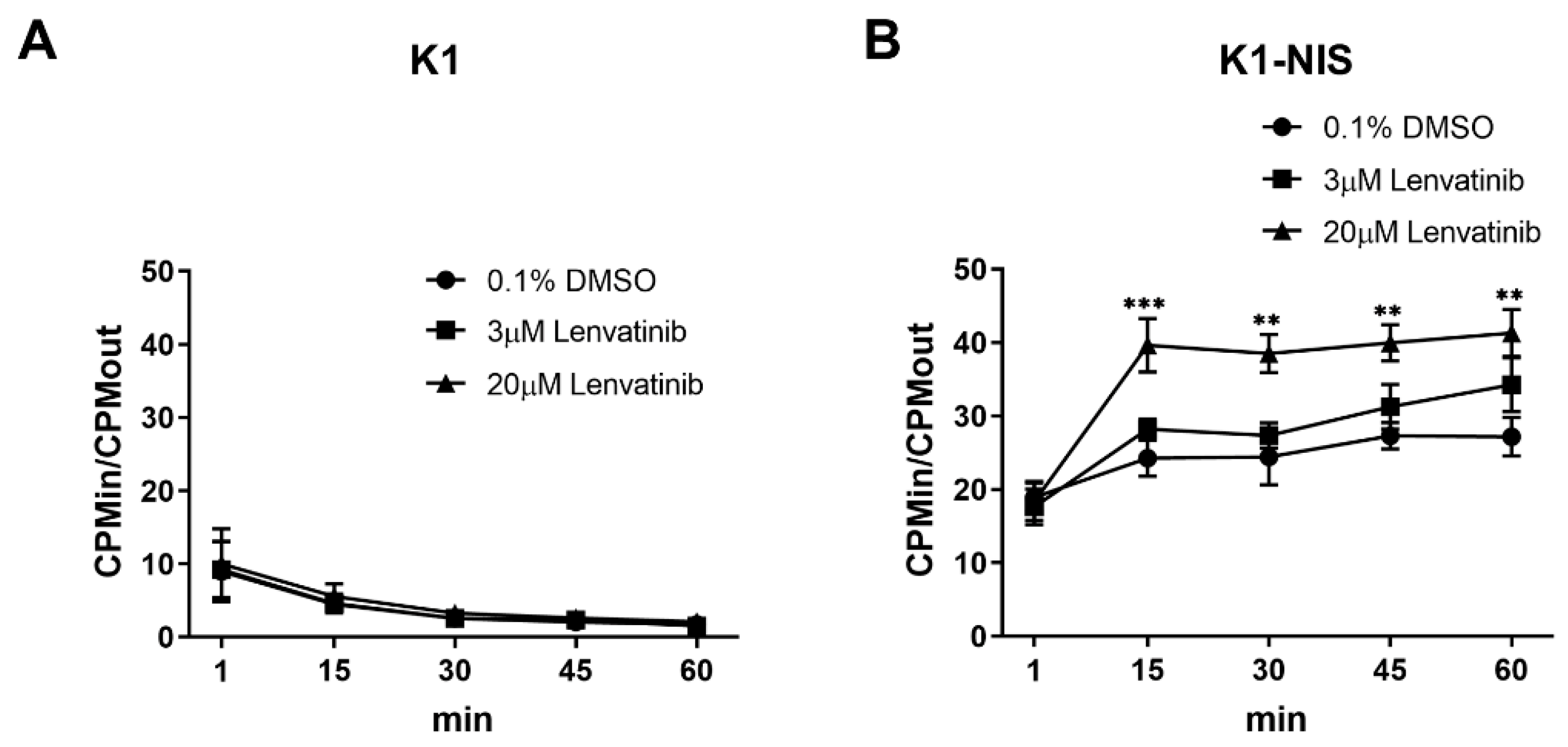
2.1. Increased Intracellular Uptake of Radioiodine through Sodium Iodide Symporter after Lenvatinib Treatment In Vitro
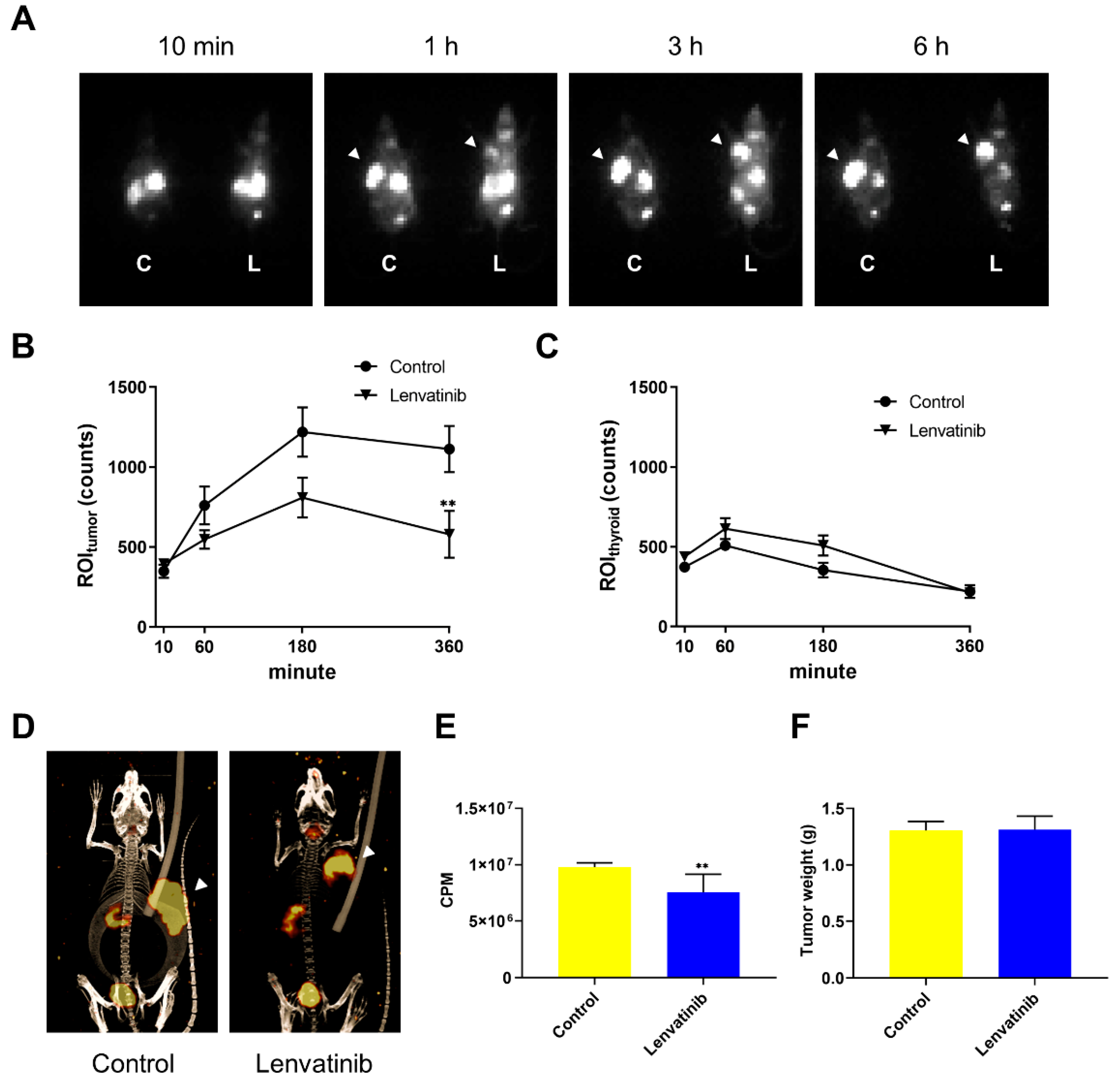
2.2. In Vivo DTC Radioiodine Avidity after Lenvatinib Treatment
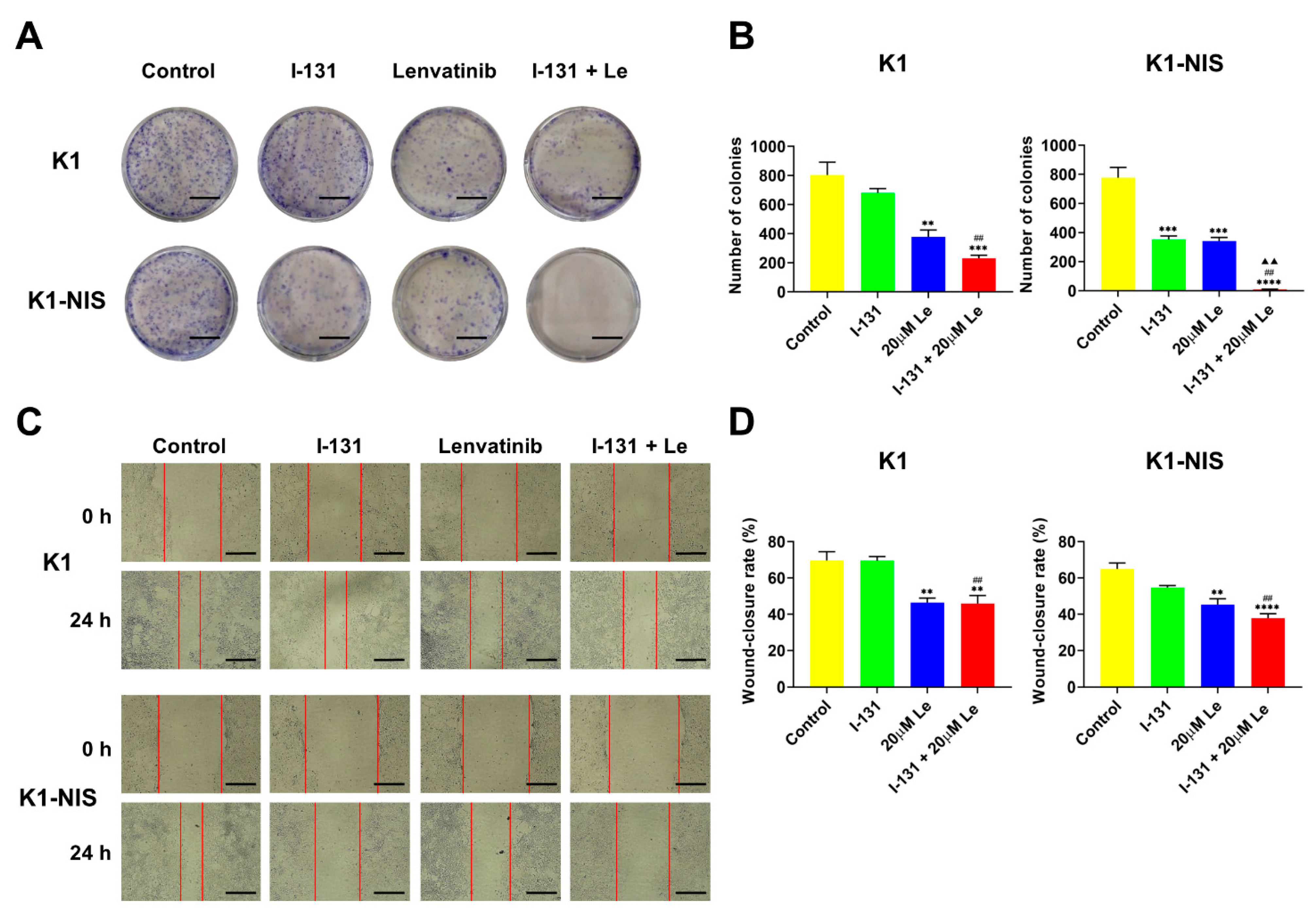
2.3. Inhibition of Thyroid Cancer Cell Growth and Migration Using Combination Therapy with 131I and Lenvatinib In Vitro
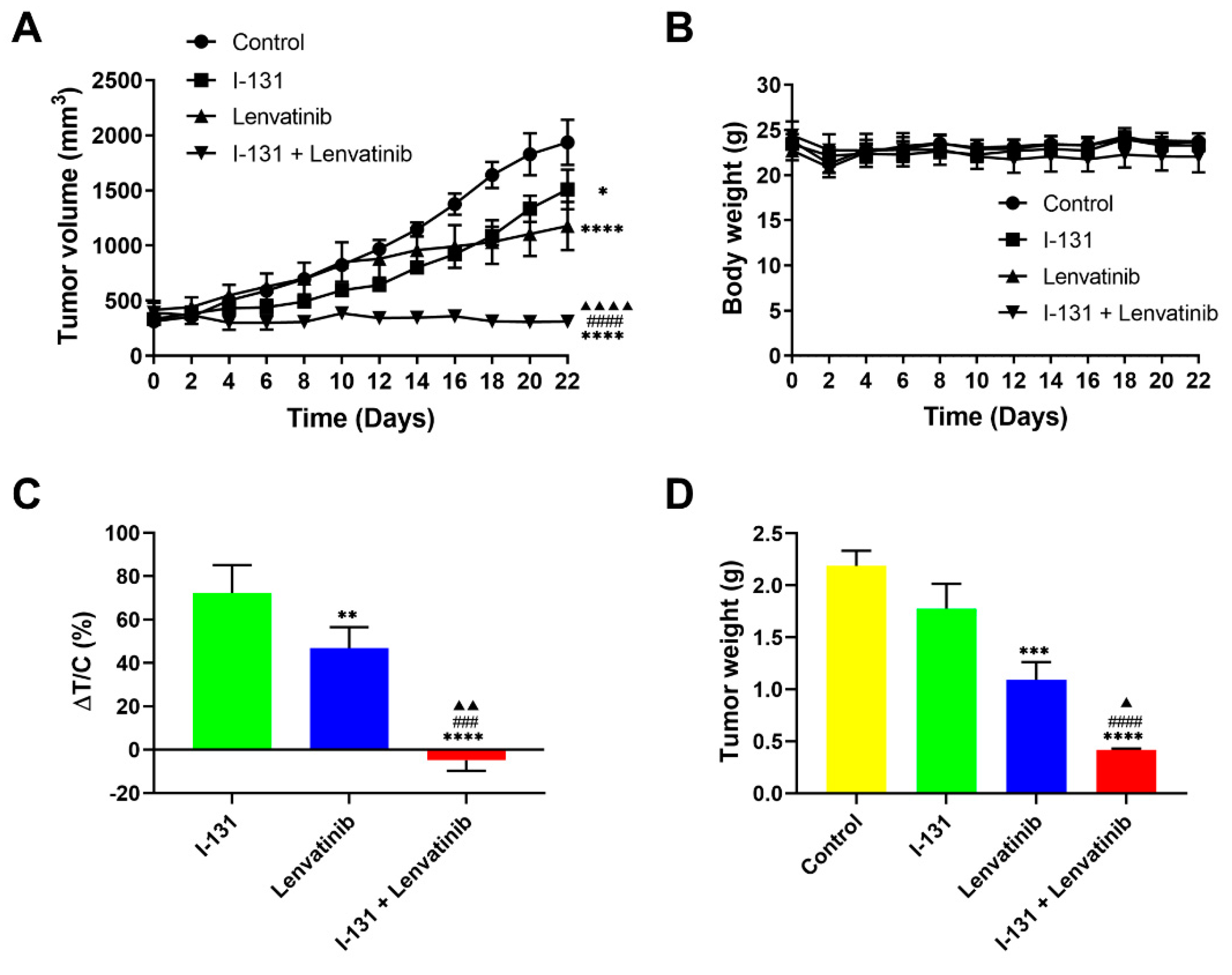
2.4. Synergistic Anticancer Effects by Combination Therapy in Thyroid Cancer Xenografts
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Western Blot Analysis
4.3. Cell Proliferation Assay
4.4. In Vitro 125I Accumulation Study
4.5. In Vivo 123I Uptake Experiments
4.6. Colony Formation Assay and Wound-Healing Assay
4.7. In Vivo Combination Therapy with 131I and Lenvatinib
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikiforov, Y.E. Thyroid carcinoma: Molecular pathways and therapeutic targets. Mod. Pathol. 2008, 21 (Suppl. 2), S37–S43. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef]
- Safavi, A.; Vijayasekaran, A.; Guerrero, M.A. New insight into the treatment of advanced differentiated thyroid cancer. J. Thyroid. Res. 2012, 2012, 437569. [Google Scholar] [CrossRef]
- Wong, K.P.; Lang, B.H. New molecular targeted therapy and redifferentiation therapy for radioiodine-refractory advanced papillary thyroid carcinoma: Literature review. J. Thyroid. Res. 2012, 2012, 818204. [Google Scholar] [CrossRef]
- Buffet, C.; Wassermann, J.; Hecht, F.; Leenhardt, L.; Dupuy, C.; Groussin, L.; Lussey-Lepoutre, C. Redifferentiation of radioiodine-refractory thyroid cancers. Endocr.-Relat. Cancer 2020, 27, R113–R132. [Google Scholar] [CrossRef]
- Mazzaferri, E.L.; Kloos, R.T. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J. Clin. Endocrinol. Metab. 2001, 86, 1447–1463. [Google Scholar] [CrossRef]
- Cho, S.W.; Choi, H.S.; Yeom, G.J.; Lim, J.A.; Moon, J.H.; Park, D.J.; Chung, J.K.; Cho, B.Y.; Yi, K.H.; Park, Y.J. Long-term prognosis of differentiated thyroid cancer with lung metastasis in Korea and its prognostic factors. Thyroid 2014, 24, 277–286. [Google Scholar] [CrossRef]
- Wassermann, J.; Bernier, M.O.; Spano, J.P.; Lepoutre-Lussey, C.; Buffet, C.; Simon, J.M.; Menegaux, F.; Tissier, F.; Leban, M.; Leenhardt, L. Outcomes and Prognostic Factors in Radioiodine Refractory Differentiated Thyroid Carcinomas. Oncologist 2016, 21, 50–58. [Google Scholar] [CrossRef]
- Deandreis, D.; Rubino, C.; Tala, H.; Leboulleux, S.; Terroir, M.; Baudin, E.; Larson, S.; Fagin, J.A.; Schlumberger, M.; Tuttle, R.M. Comparison of Empiric Versus Whole-Body/-Blood Clearance Dosimetry-Based Approach to Radioactive Iodine Treatment in Patients with Metastases from Differentiated Thyroid Cancer. J. Nucl. Med. 2017, 58, 717–722. [Google Scholar] [CrossRef]
- Busaidy, N.L.; Cabanillas, M.E. Differentiated thyroid cancer: Management of patients with radioiodine nonresponsive disease. J. Thyroid. Res. 2012, 2012, 618985. [Google Scholar] [CrossRef] [Green Version]
- Robbins, R.J.; Wan, Q.; Grewal, R.K.; Reibke, R.; Gonen, M.; Strauss, H.W.; Tuttle, R.M.; Drucker, W.; Larson, S.M. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J. Clin. Endocrinol. Metab. 2006, 91, 498–505. [Google Scholar] [CrossRef]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Matsui, J.; Matsushima, T.; Obaishi, H.; Miyazaki, K.; Nakamura, K.; Tohyama, O.; Semba, T.; Yamaguchi, A.; Hoshi, S.S.; et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc. Cell 2014, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; Habra, M.A. Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer Treat. Rev. 2016, 42, 47–55. [Google Scholar] [CrossRef]
- Tohyama, O.; Matsui, J.; Kodama, K.; Hata-Sugi, N.; Kimura, T.; Okamoto, K.; Minoshima, Y.; Iwata, M.; Funahashi, Y. Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J. Thyroid. Res. 2014, 2014, 638747. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, S.M.; Chang, H.J.; Kim, B.W.; Lee, Y.S.; Park, C.S.; Park, K.C.; Chang, H.S. SoLAT (Sorafenib Lenvatinib alternating treatment): A new treatment protocol with alternating Sorafenib and Lenvatinib for refractory thyroid Cancer. BMC Cancer 2018, 18, 956. [Google Scholar] [CrossRef]
- Viola, D.; Valerio, L.; Molinaro, E.; Agate, L.; Bottici, V.; Biagini, A.; Lorusso, L.; Cappagli, V.; Pieruzzi, L.; Giani, C.; et al. Treatment of advanced thyroid cancer with targeted therapies: Ten years of experience. Endocr.-Relat. Cancer 2016, 23, R185–R205. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.W.; Lin, C.Y.; Hsieh, C.H.; Hsu, C.L.; Fan, K.H.; Huang, S.F.; Liao, C.T.; Ng, S.K.; Yen, T.C.; Chang, J.T.; et al. A phase II randomized trial comparing neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in advanced squamous cell carcinoma of the pharynx or larynx. Biomed. J. 2018, 41, 129–136. [Google Scholar] [CrossRef]
- Pignon, J.P.; le Maitre, A.; Maillard, E.; Bourhis, J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Yamazaki, T. Combined use of lenvatinib and external irradiation for undifferentiated thyroid cancer in aged patients with multiple comorbidities: A case study. J. Jpn. Thyroid. Assoc. 2018, 9, 69–73. [Google Scholar]
- Wada, Y.; Anbai, A.; Takagi, N.; Takahashi, E.; Terada, K.; Ito, A.; Nanjo, H.; Hashimoto, M. A case of unresectable squamous cell carcinoma of thyroid gland which could be local controlled by lenvatinib combined with radiotherapy. Jpn. J. Clin. Radiol. 2016, 61, 953–958. [Google Scholar]
- Suzuki, K.; Iwai, H.; Utsunomiya, K.; Kono, Y.; Kobayashi, Y.; Van Bui, D.; Sawada, S.; Yun, Y.; Mitani, A.; Kondo, N.; et al. Combination therapy with lenvatinib and radiation significantly inhibits thyroid cancer growth by uptake of tyrosine kinase inhibitor. Exp. Cell Res. 2021, 398, 112390. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Nikiforova, M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011, 7, 569–580. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, D. Synergistic inhibition of MEK/ERK and BRAF V600E with PD98059 and PLX4032 induces sodium/iodide symporter (NIS) expression and radioiodine uptake in BRAF mutated papillary thyroid cancer cells. Thyroid Res. 2018, 11, 13. [Google Scholar] [CrossRef]
- Ruan, M.; Liu, M.; Dong, Q.; Chen, L. Iodide- and glucose-handling gene expression regulated by sorafenib or cabozantinib in papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2015, 100, 1771–1779. [Google Scholar] [CrossRef]
- Watabe, T.; Kaneda-Nakashima, K.; Liu, Y.; Shirakami, Y.; Ooe, K.; Toyoshima, A.; Shimosegawa, E.; Fukuda, M.; Shinohara, A.; Hatazawa, J. Enhancement of (211)At Uptake via the Sodium Iodide Symporter by the Addition of Ascorbic Acid in Targeted alpha-Therapy of Thyroid Cancer. J. Nucl. Med. 2019, 60, 1301–1307. [Google Scholar] [CrossRef]
- Sheu, N.W.; Jiang, H.J.; Wu, C.W.; Chiang, F.Y.; Chiou, H.C.; Hsiao, P.J. Lenvatinib complementary with radioiodine therapy for patients with advanced differentiated thyroid carcinoma: Case reports and literature review. World J. Surg. Oncol. 2019, 17, 84. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, S.M.; Daniels, G.H.; Wirth, L.J. Redifferentiation of Iodine-Refractory BRAF V600E-Mutant Metastatic Papillary Thyroid Cancer with Dabrafenib-Response. Clin. Cancer Res. 2015, 21, 5640–5641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, A.L.; Grewal, R.K.; Leboeuf, R.; Sherman, E.J.; Pfister, D.G.; Deandreis, D.; Pentlow, K.S.; Zanzonico, P.B.; Haque, S.; Gavane, S.; et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N. Engl. J. Med. 2013, 368, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Utsunomiya, K.; Kanno, S.; Tanigawa, N. Longitudinal time-dependent effects of irradiation on multidrug resistance in a non-small lung cancer cell line. Mol. Cancer Ther. 2014, 13, 2706–2712. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbelaez, P.; Cruz, J.C.; Munoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, K.; Iwai, H.; Utsunomiya, K.; Kono, Y.; Watabe, T.; Kobayashi, Y.; Bui, D.V.; Sawada, S.; Yun, Y.; Mitani, A.; et al. Efficacy of Combination Therapy with Lenvatinib and Radioactive Iodine in Thyroid Cancer Preclinical Model. Int. J. Mol. Sci. 2022, 23, 9872. https://doi.org/10.3390/ijms23179872
Suzuki K, Iwai H, Utsunomiya K, Kono Y, Watabe T, Kobayashi Y, Bui DV, Sawada S, Yun Y, Mitani A, et al. Efficacy of Combination Therapy with Lenvatinib and Radioactive Iodine in Thyroid Cancer Preclinical Model. International Journal of Molecular Sciences. 2022; 23(17):9872. https://doi.org/10.3390/ijms23179872
Chicago/Turabian StyleSuzuki, Kensuke, Hiroshi Iwai, Keita Utsunomiya, Yumiko Kono, Tadashi Watabe, Yoshiki Kobayashi, Dan Van Bui, Shunsuke Sawada, Yasutaka Yun, Akitoshi Mitani, and et al. 2022. "Efficacy of Combination Therapy with Lenvatinib and Radioactive Iodine in Thyroid Cancer Preclinical Model" International Journal of Molecular Sciences 23, no. 17: 9872. https://doi.org/10.3390/ijms23179872
APA StyleSuzuki, K., Iwai, H., Utsunomiya, K., Kono, Y., Watabe, T., Kobayashi, Y., Bui, D. V., Sawada, S., Yun, Y., Mitani, A., Fukui, K., Sakai, H., Chu, H. H., Linh, N. M., Tanigawa, N., & Kanda, A. (2022). Efficacy of Combination Therapy with Lenvatinib and Radioactive Iodine in Thyroid Cancer Preclinical Model. International Journal of Molecular Sciences, 23(17), 9872. https://doi.org/10.3390/ijms23179872






