Effect to Therapy of Sodium-Iodine Symporter Expression by Alpha-Ray Therapeutic Agent via Sodium/Iodine Symporter
Abstract
1. Introduction
2. Results
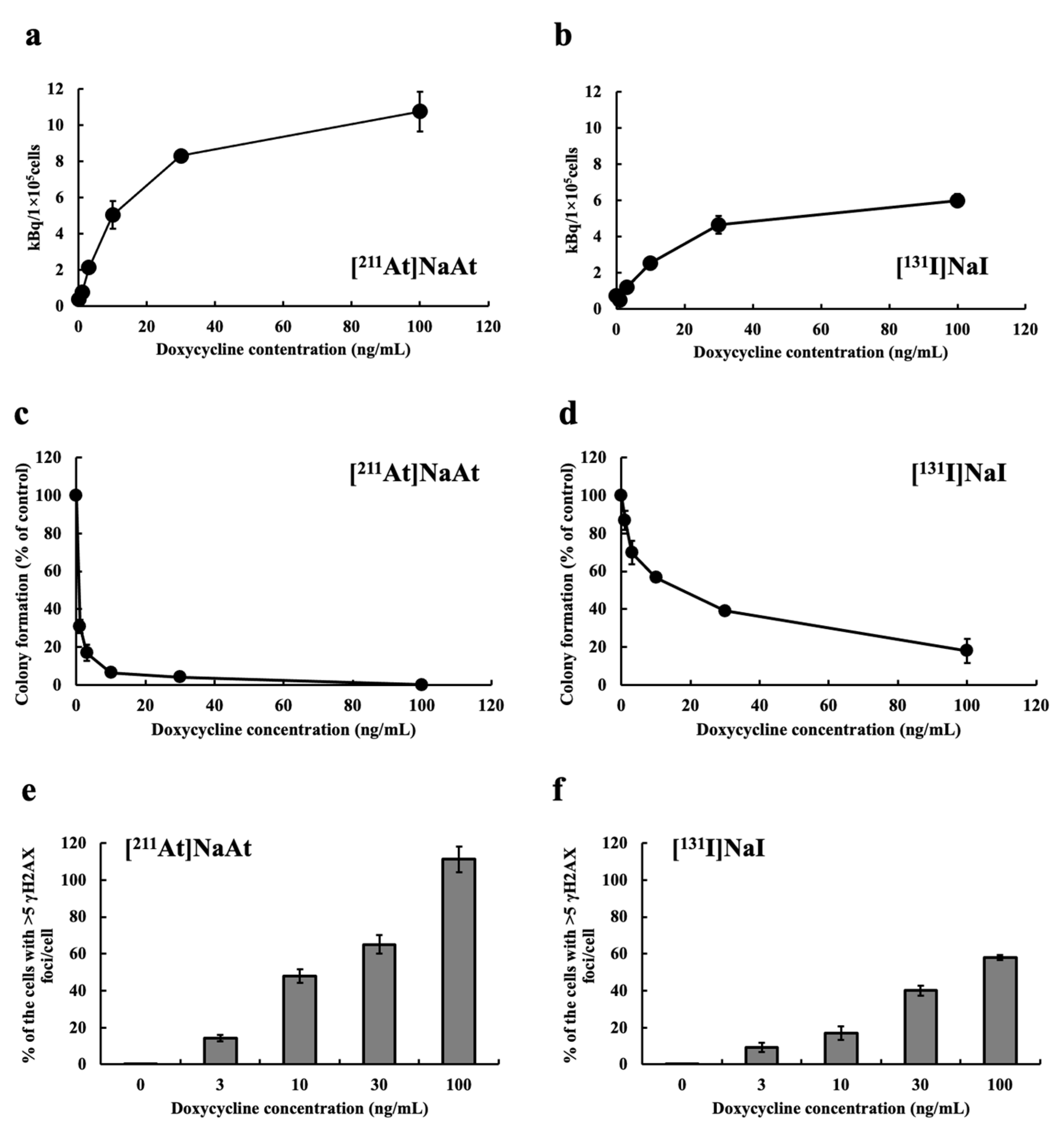
2.1. Examination of Uptake, Colony-Forming Ability and DSB Induction of K1-NIS/DOX Cells Whose NIS Expression Level Was Controlled by Tet-On System
2.2. Comparison of Cytotoxically Effects in K1 Cells with and without NIS
2.3. Comparison of Cellular Uptake in K1 Cells with and without NIS
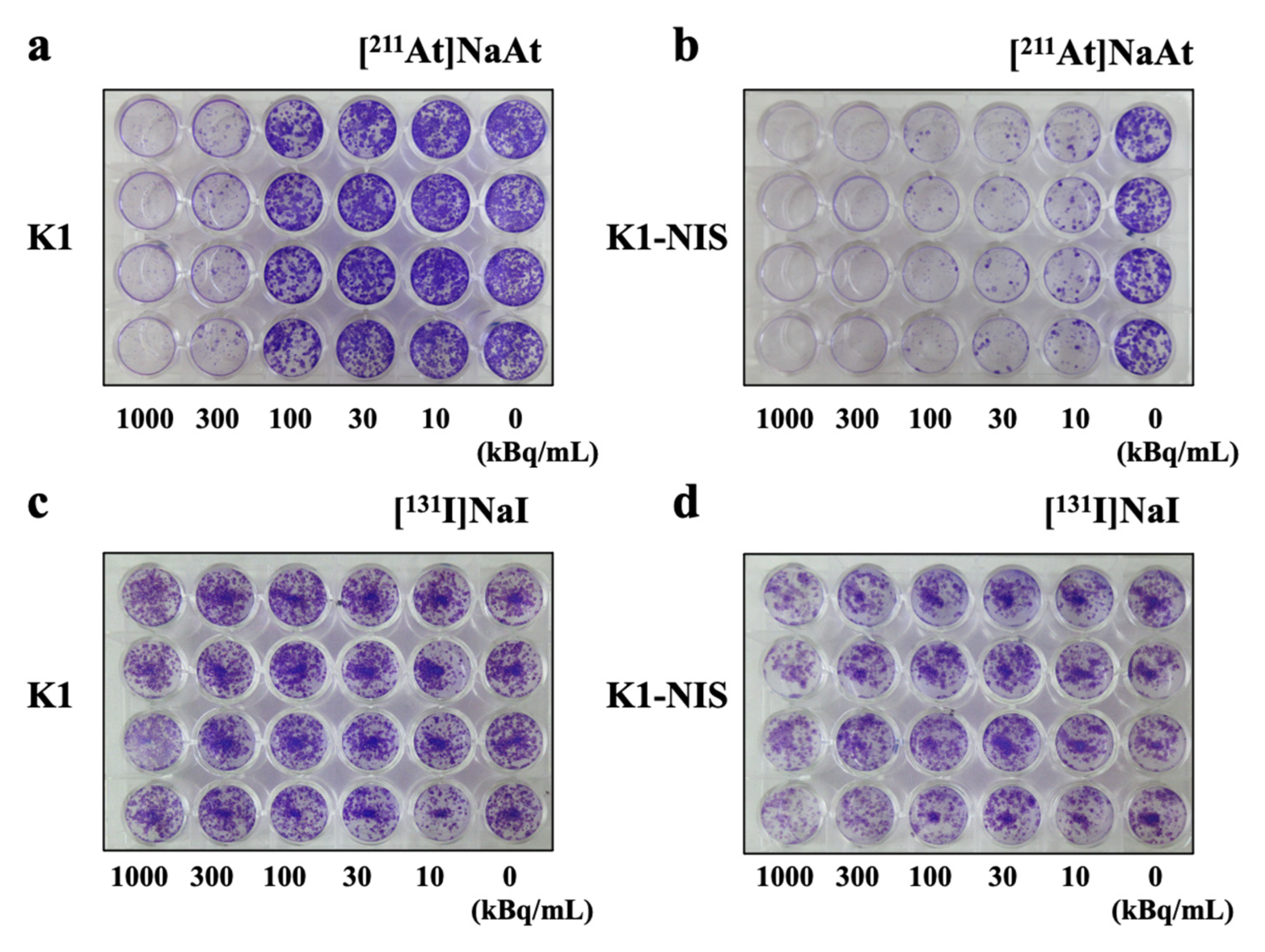
2.4. Colony-Forming Ability
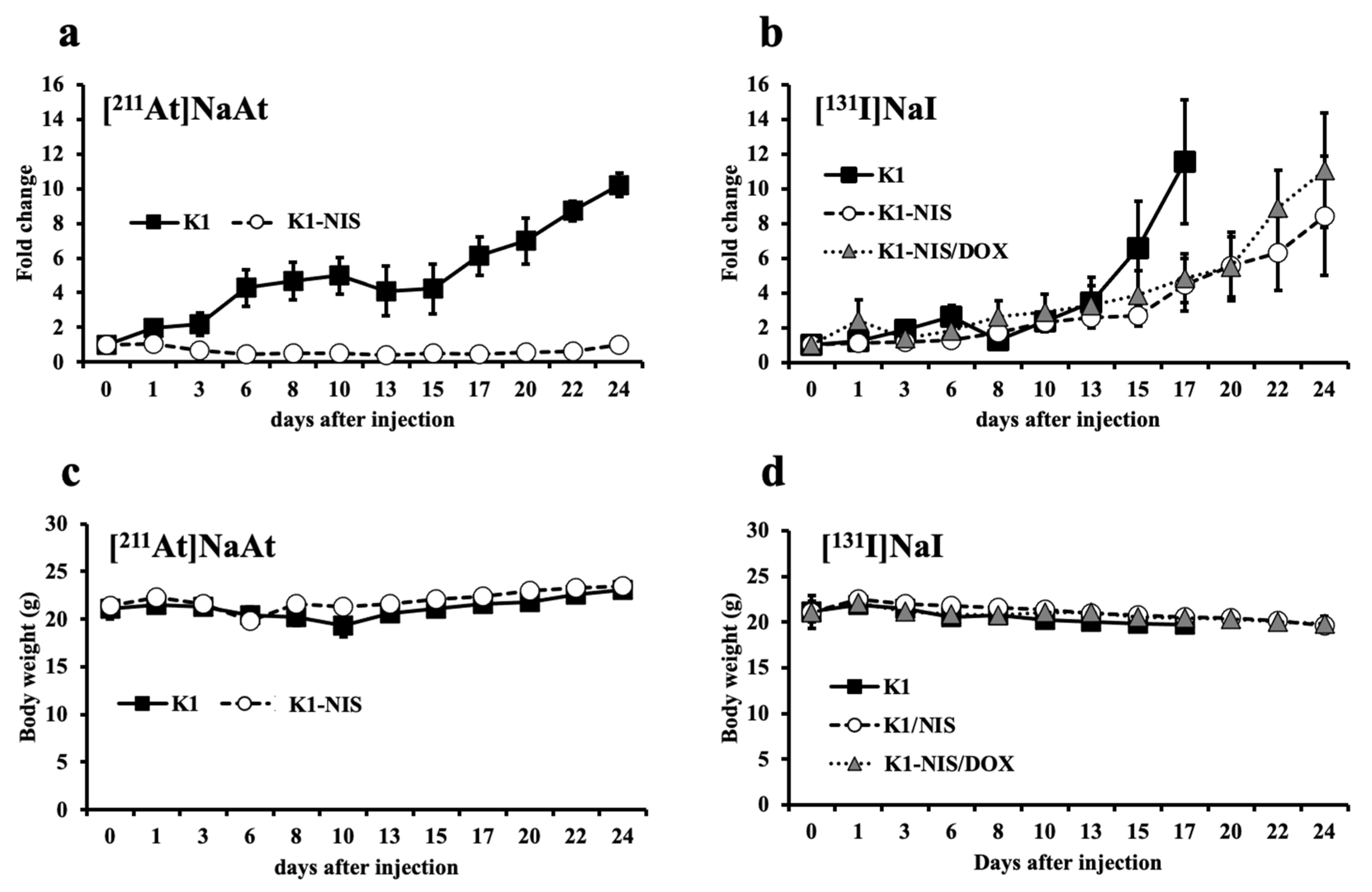
2.5. Contribution of NIS in Therapeutic Efficacy
3. Discussion
4. Materials and Methods
4.1. Preparation of [211At]NaAt and [131I]NaI Solution
4.2. In Vitro Observation of DSBs of DNA and Colony Formation Assay
4.3. Preparation of Animals
4.4. In Vitro and In Vivo NIS Control Model
4.5. Therapy with [131I]NaI and [211At]NaAt Solutions
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maheshwari, Y.K.; Hill, C.S., Jr.; Haynie, T.P., 3rd; Hickey, R.C.; Samaan, N.A. 131I therapy in differentiated thyroid carcinoma: M. D. Anderson Hospital experience. Cancer 1981, 47, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Maxon, H.R., 3rd; Englaro, E.E.; Thomas, S.R.; Hertzberg, V.S.; Hinnefeld, J.D.; Chen, L.S.; Smith, H.; Cummings, D.; Aden, M.D. Radioiodine-131 therapy for well-differentiated thyroid cancer—A quantitative radiation dosimetric approach: Outcome and validation in 85 patients. J. Nucl. Med. 1992, 33, 1132–1136. [Google Scholar] [PubMed]
- Ciarallo, A.; Rivera, J. Radioactive iodine therapy in differentiated thyroid cancer: 2020 update. AJR Am. J. Roentgenol. 2020, 215, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Mayson, S.E.; Chan, C.M.; Haugen, B.R. Tailoring the approach to radioactive iodine treatment in thyroid cancer. Endocr. Relat. Cancer 2021, 28, T125–T140. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Brose, M.; Elisei, R.; Leboulleux, S.; Luster, M.; Pitoia, F.; Pacini, F. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014, 2, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Lin, Y.; Liang, J. Radioactive Iodine-Refractory Differentiated Thyroid Cancer and Redifferentiation Therapy. Endocrinol. Metab. 2019, 34, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Lundh, C.; Lindencrona, U.; Postgård, P.; Carlsson, T.; Nilsson, M.; Forssell-Aronsson, E. Radiation-induced thyroid stunning: Differential effects of 123I, 131I, 99mTc, and 211At on iodide transport and NIS mRNA expression in cultured thyroid cells. J. Nucl. Med. 2009, 50, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- WHO. Iodine Thyroid Blocking Guidelines for Use in Planning for and Responding to Radiological and Nuclear Emergencies; License: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Watabe, T.; Kaneda-Nakashima, K.; Ooe, K.; Liu, Y.; Kurimoto, K.; Murai, T.; Shidahara, Y.; Okuma, K.; Takeuchi, M.; Nishide, M.; et al. Extended single-dose toxicity study of [211At]NaAt in mice for the first-in-human clinical trial of targeted alpha therapy for differentiated thyroid cancer. Ann. Nucl. Med. 2021, 35, 702–718. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Watabe, T.; Kaneda-Nakashima, K.; Ooe, K.; Shirakami, Y.; Toyoshima, A.; Shimosegawa, E.; Nakano, T.; Shinohara, A.; Hatazawa, J. Preclinical Evaluation of Radiation-Induced Toxicity in Targeted Alpha Therapy Using [211At] NaAt in Mice: A Revisit. Transl. Oncol. 2020, 13, 100757. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Kaneda-Nakashima, K.; Liu, Y.; Shirakami, Y.; Ooe, K.; Toyoshima, A.; Shimosegawa, E.; Fukuda, M.; Shinohara, A.; Hatazawa, J. Enhancement of 211At Uptake via the Sodium Iodide Symporter by the Addition of Ascorbic Acid in Targeted α-Therapy of Thyroid Cancer. J. Nucl. Med. 2019, 60, 1301–1307. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneda-Nakashima, K.; Shirakami, Y.; Watabe, T.; Ooe, K.; Yoshimura, T.; Toyoshima, A.; Wang, Y.; Haba, H.; Fukase, K. Effect to Therapy of Sodium-Iodine Symporter Expression by Alpha-Ray Therapeutic Agent via Sodium/Iodine Symporter. Int. J. Mol. Sci. 2022, 23, 15509. https://doi.org/10.3390/ijms232415509
Kaneda-Nakashima K, Shirakami Y, Watabe T, Ooe K, Yoshimura T, Toyoshima A, Wang Y, Haba H, Fukase K. Effect to Therapy of Sodium-Iodine Symporter Expression by Alpha-Ray Therapeutic Agent via Sodium/Iodine Symporter. International Journal of Molecular Sciences. 2022; 23(24):15509. https://doi.org/10.3390/ijms232415509
Chicago/Turabian StyleKaneda-Nakashima, Kazuko, Yoshifumi Shirakami, Tadashi Watabe, Kazuhiro Ooe, Takashi Yoshimura, Atsushi Toyoshima, Yang Wang, Hiromitsu Haba, and Koichi Fukase. 2022. "Effect to Therapy of Sodium-Iodine Symporter Expression by Alpha-Ray Therapeutic Agent via Sodium/Iodine Symporter" International Journal of Molecular Sciences 23, no. 24: 15509. https://doi.org/10.3390/ijms232415509
APA StyleKaneda-Nakashima, K., Shirakami, Y., Watabe, T., Ooe, K., Yoshimura, T., Toyoshima, A., Wang, Y., Haba, H., & Fukase, K. (2022). Effect to Therapy of Sodium-Iodine Symporter Expression by Alpha-Ray Therapeutic Agent via Sodium/Iodine Symporter. International Journal of Molecular Sciences, 23(24), 15509. https://doi.org/10.3390/ijms232415509







