Establishment and Characterization of Novel Human Intestinal In Vitro Models for Absorption and First-Pass Metabolism Studies
Abstract
1. Introduction
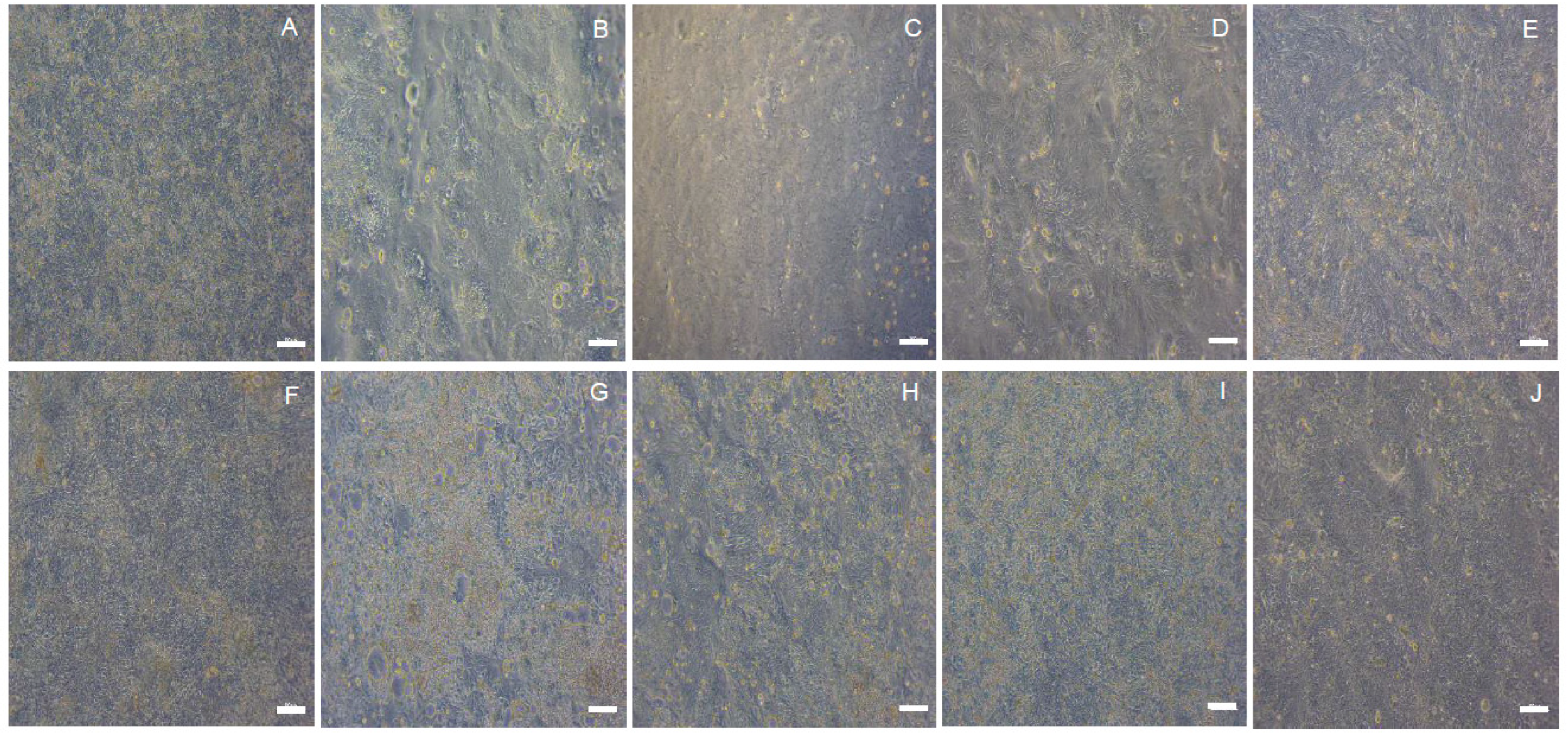
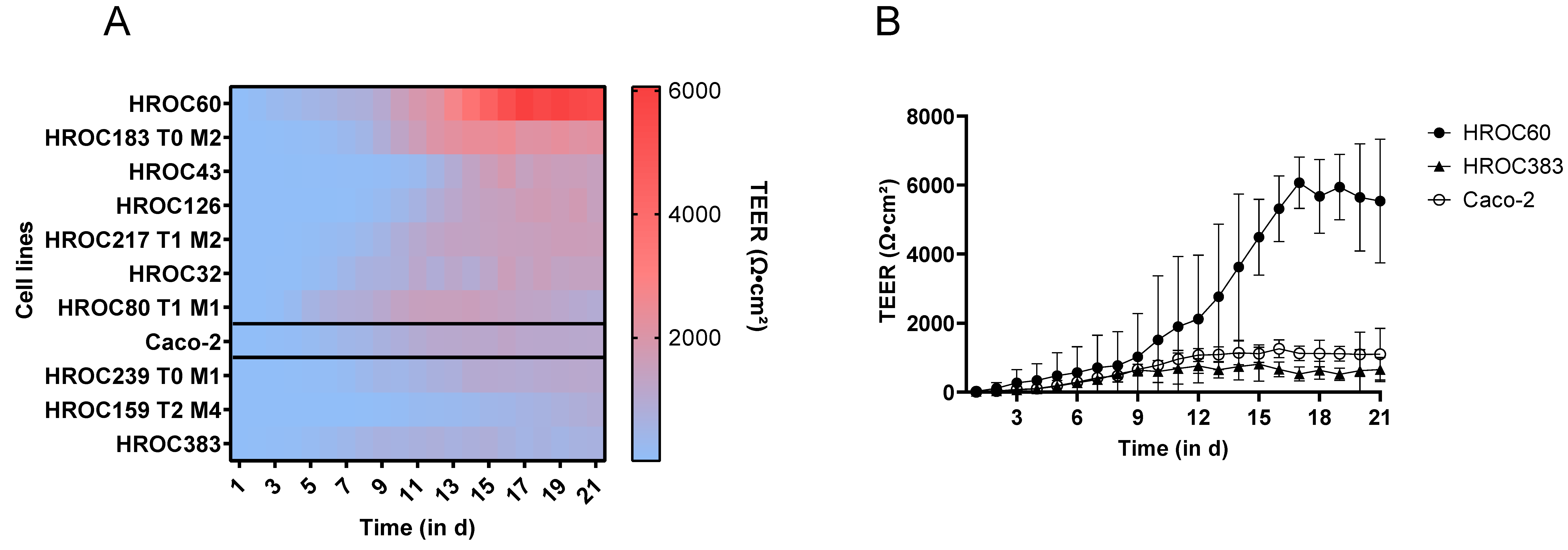
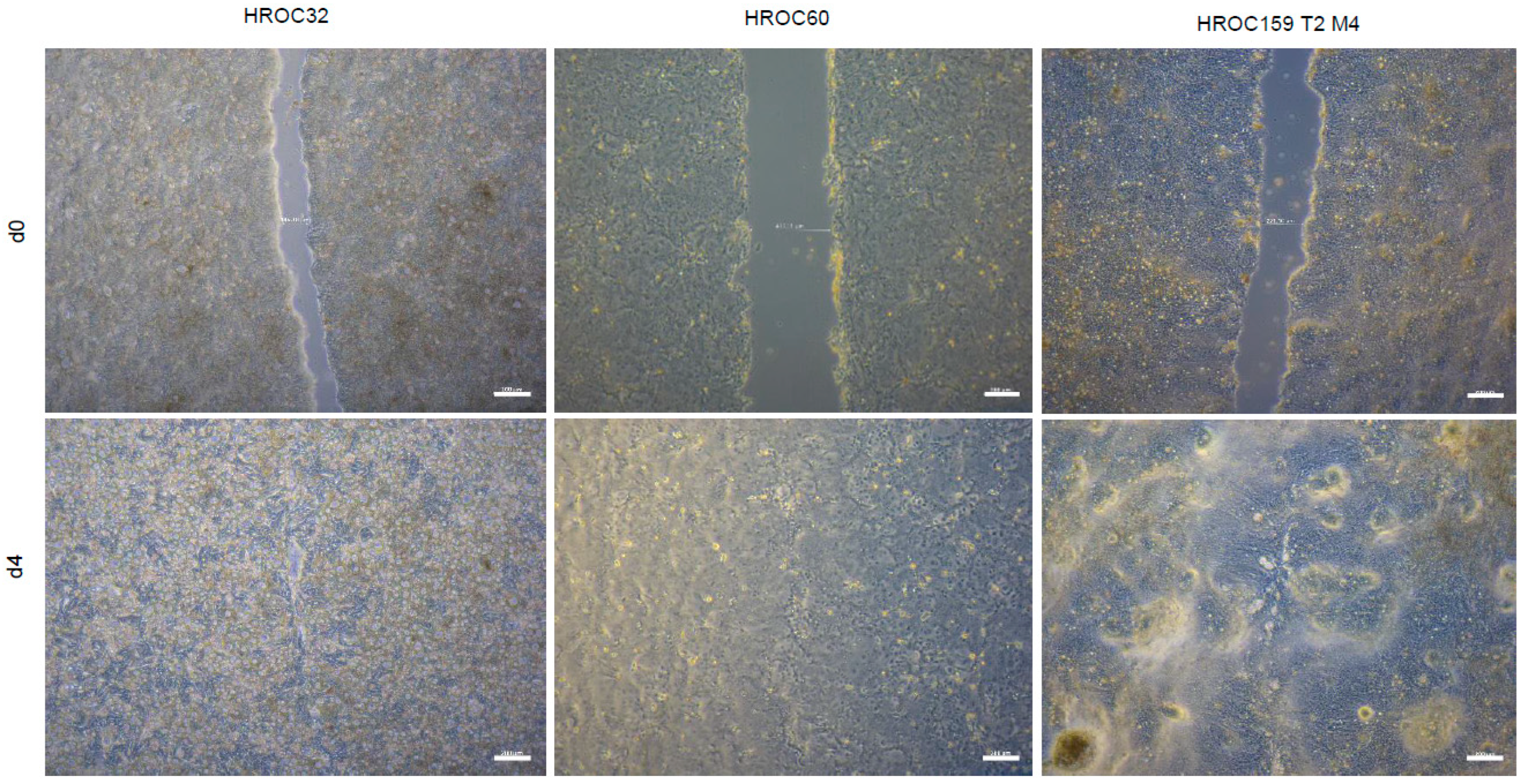
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Patient-Derived CRC Cells
3.3. Cell Culture
3.4. Monolayer Assessment
3.5. Cell Proliferation and Migration
3.6. TEER Measurement
3.7. Fluorescein Isothiocyanate–Conjugated Dextran 4000 (FD-4) Permeability Tests
3.8. Luminometric Analysis of CYP3A4 Activity
3.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Sun, H.; Chow, E.C.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 cell monolayer: Usefulness and limitations. Expert Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Delie, F.; Rubas, W. A Human Colonic Cell Line Sharing Similarities with Enterocytes as a Model to Examine Oral Absorption: Advantages and Limitations of the Caco-2 Model. Crit. Rev. Drug Carr. Syst 1997, 14, 66. [Google Scholar] [CrossRef]
- Sambuy, Y.; de Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models: HT29 Cell Line; Springer: Cham, Switzerland, 2015; ISBN 9783319157917. [Google Scholar]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Zucco, F.; Batto, A.-F.; Bises, G.; Chambaz, J.; Chiusolo, A.; Consalvo, R.; Cross, H.; Dal Negro, G.; de Angelis, I.; Fabre, G.; et al. An inter-laboratory study to evaluate the effects of medium composition on the differentiation and barrier function of Caco-2 cell lines. Altern. Lab. Anim. 2005, 33, 603–618. [Google Scholar] [CrossRef]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Yee, S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man—Fact or myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef]
- Horie, K.; Tang, F.; Borchardt, R.T. Isolation and characterization of Caco-2 subclones expressing high levels of multidrug resistance protein efflux transporter. Pharm. Res. 2003, 20, 161–168. [Google Scholar] [CrossRef]
- Ungell, A.-L.; Artursson, P. An Overview of Caco-2 and Alternatives for Prediction of Intestinal Drug Transport and Absorption. In Drug Bioavailability; van de Waterbeemd, H., Testa, B., Eds.; Wiley: Hoboken, NJ, USA, 2008; pp. 133–159. ISBN 9783527320516. [Google Scholar]
- Artursson, P.; Ungell, A.L.; Löfroth, J.E. Selective paracellular permeability in two models of intestinal absorption: Cultured monolayers of human intestinal epithelial cells and rat intestinal segments. Pharm. Res. 1993, 10, 1123–1129. [Google Scholar] [CrossRef]
- Balimane, P.V.; Chong, S. Cell culture-based models for intestinal permeability: A critique. Drug Discov. Today 2005, 10, 335–343. [Google Scholar] [CrossRef]
- Schmiedlin-Ren, P.; Thummel, K.E.; Fisher, J.M.; Paine, M.F.; Watkins, P.B. Induction of CYP3A4 by 1 alpha,25-dihydroxyvitamin D3 is human cell line-specific and is unlikely to involve pregnane X receptor. Drug Metab. Dispos. 2001, 29, 1446–1453. [Google Scholar]
- Nakamura, T.; Sakaeda, T.; Ohmoto, N.; Tamura, T.; Aoyama, N.; Shirakawa, T.; Kamigaki, T.; Nakamura, T.; Kim, K.I.; Kim, S.R.; et al. Real-time quantitative polymerase chain reaction for MDR1, MRP1, MRP2, and CYP3A-mRNA levels in Caco-2 cell lines, human duodenal enterocytes, normal colorectal tissues, and colorectal adenocarcinomas. Drug Metab. Dispos. 2002, 30, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, O.; Turpeinen, M.; Hakkola, J.; Honkakoski, P.; Hukkanen, J.; Raunio, H. Inhibition and induction of human cytochrome P450 enzymes: Current status. Arch. Toxicol. 2008, 82, 667–715. [Google Scholar] [CrossRef] [PubMed]
- Kolars, J.; Watkins, P.; Merion, R.; Awni, W. First-pass metabolism of cyclosporin by the gut. Lancet 1991, 338, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Webber, I.R.; Peters, W.H.; Back, D.J. Cyclosporin metabolism by human gastrointestinal mucosal microsomes. Br. J. Clin. Pharmacol. 1992, 33, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.K.V.; Karnati, S.; Reddy, M.B.; Chandramouli, R. Caco-2 cell lines in drug discovery—An updated perspective. J. Basic Clin. Pharm. 2010, 1, 63–69. [Google Scholar]
- Yamaura, Y.; Chapron, B.D.; Wang, Z.; Himmelfarb, J.; Thummel, K.E. Functional Comparison of Human Colonic Carcinoma Cell Lines and Primary Small Intestinal Epithelial Cells for Investigations of Intestinal Drug Permeability and First-Pass Metabolism. Drug Metab. Dispos. 2016, 44, 329–335. [Google Scholar] [CrossRef]
- Negoro, R.; Takayama, K.; Nagamoto, Y.; Sakurai, F.; Tachibana, M.; Mizuguchi, H. Modeling of drug-mediated CYP3A4 induction by using human iPS cell-derived enterocyte-like cells. Biochem. Biophys. Res. Commun. 2016, 472, 631–636. [Google Scholar] [CrossRef]
- Mullins, C.S.; Micheel, B.; Matschos, S.; Leuchter, M.; Bürtin, F.; Krohn, M.; Hühns, M.; Klar, E.; Prall, F.; Linnebacher, M. Integrated Biobanking and Tumor Model Establishment of Human Colorectal Carcinoma Provides Excellent Tools for Preclinical Research. Cancers 2019, 11, 1520. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef]
- Lu, Y.; Qi, J.; Wu, W. Lipid nanoparticles. Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 749–783. ISBN 9780128136898. [Google Scholar]
- Pires, C.L.; Praça, C.; Martins, P.A.T.; Batista de Carvalho, A.L.M.; Ferreira, L.; Marques, M.P.M.; Moreno, M.J. Re-Use of Caco-2 Monolayers in Permeability Assays-Validation Regarding Cell Monolayer Integrity. Pharmaceutics 2021, 13, 1563. [Google Scholar] [CrossRef] [PubMed]
- Donkers, J.M.; Eslami Amirabadi, H.; van de Steeg, E. Intestine-on-a-chip: Next level in vitro research model of the human intestine. Curr. Opin. Toxicol. 2021, 25, 6–14. [Google Scholar] [CrossRef]
- Hilgendorf, C.; Spahn-Langguth, H.; Regårdh, C.G.; Lipka, E.; Amidon, G.L.; Langguth, P. Caco-2 versus Caco-2/HT29-MTX Co-cultured Cell Lines: Permeabilities Via Diffusion, Inside- and Outside-Directed Carrier-Mediated Transport. J. Pharm. Sci. 2000, 89, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Feighery, L.M.; Cochrane, S.W.; Quinn, T.; Baird, A.W.; O’Toole, D.; Owens, S.-E.; O’Donoghue, D.; Mrsny, R.J.; Brayden, D.J. Myosin light chain kinase inhibition: Correction of increased intestinal epithelial permeability in vitro. Pharm. Res. 2008, 25, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lee, P.I.; Topp, E.M. Transport Processes in Pharmaceutical Systems; CRC Press: Boca Raton, FL, USA, 1999; ISBN 9780824746322. [Google Scholar]
- Eigenmann, D.E.; Xue, G.; Kim, K.S.; Moses, A.V.; Hamburger, M.; Oufir, M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 2013, 10, 33. [Google Scholar] [CrossRef]
- Rodríguez-Antona, C.; Leskelä, S.; Zajac, M.; Cuadros, M.; Alvés, J.; Moneo, M.V.; Martín, C.; Cigudosa, J.C.; Carnero, A.; Robledo, M.; et al. Expression of CYP3A4 as a predictor of response to chemotherapy in peripheral T-cell lymphomas. Blood 2007, 110, 3345–3351. [Google Scholar] [CrossRef]
- Olszewski, U.; Liedauer, R.; Ausch, C.; Thalhammer, T.; Hamilton, G. Overexpression of CYP3A4 in a COLO 205 Colon Cancer Stem Cell Model in vitro. Cancers 2011, 3, 1467–1479. [Google Scholar] [CrossRef]
- Slaviero, K.A.; Clarke, S.J.; Rivory, L.P. Inflammatory response: An unrecognised source of variability in the pharmacokinetics and pharmacodynamics of cancer chemotherapy. Lancet Oncol. 2003, 4, 224–232. [Google Scholar] [CrossRef]
- Senarathna, S.M.D.K.G.; Crowe, A. The influence of passage number for Caco2 cell models when evaluating P-gp mediated drug transport. Pharmazie 2015, 70, 798–803. [Google Scholar]
- Briske-Anderson, M.J.; Finley, J.W.; Newman, S.M. The influence of culture time and passage number on the morphological and physiological development of Caco-2 cells. Proc. Soc. Exp. Biol. Med. 1997, 214, 248–257. [Google Scholar] [CrossRef]
- Takada, I.; Makishima, M. Therapeutic application of vitamin D receptor ligands: An updated patent review. Expert Opin. Ther. Pat. 2015, 25, 1373–1383. [Google Scholar] [CrossRef]
- Maletzki, C.; Gock, M.; Randow, M.; Klar, E.; Huehns, M.; Prall, F.; Linnebacher, M. Establishment and characterization of cell lines from chromosomal instable colorectal cancer. World J. Gastroenterol. 2015, 21, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Maletzki, C.; Huehns, M.; Knapp, P.; Waukosin, N.; Klar, E.; Prall, F.; Linnebacher, M. Functional Characterization and Drug Response of Freshly Established Patient-Derived Tumor Models with CpG Island Methylator Phenotype. PLoS ONE 2015, 10, 3194. [Google Scholar] [CrossRef][Green Version]
- Gock, M.; Mullins, C.S.; Bergner, C.; Prall, F.; Ramer, R.; Göder, A.; Krämer, O.H.; Lange, F.; Krause, B.J.; Klar, E.; et al. Establishment, functional and genetic characterization of three novel patient-derived rectal cancer cell lines. World J. Gastroenterol. 2018, 24, 4880–4892. [Google Scholar] [CrossRef] [PubMed]


| Barrier Forming Potency | CYP3A4 Inducibility | ||||
|---|---|---|---|---|---|
| Cell Line | TEER | FD-4 Permeability | Basal CYP3A4 Activity | PXR-Mediated | VDR-Mediated |
| HROC32 | + | − | +++ | − | ++ |
| HROC43 | + | − | +++ | − | − |
| HROC60 | +++ | − − | − | − | + |
| HROC80 T1 M1 | + | − − | − | ++ | +++ |
| HROC126 | + | − | + | + | +++ |
| HROC159 T2 M4 | + | − | ++ | − | +++ |
| HROC183 T0 M2 | ++ | − | − | ++ | +++ |
| HROC217 T1 M2 | + | − | + | +++ | +++ |
| HROC239 T0 M1 | + | − | + | − | ++ |
| HROC383 | + | − | + | ++ | ++ |
| Caco-2 | + | − | + | ++ | + |
| Cell Line | Feature | Application |
|---|---|---|
| HROC32 | Very high basal CYP3A4 activity, VDR-mediated CYP3A4 inducibility | Drug absorption, development of alternative drug regimens, best model for evaluation of metabolic effects, VDR-mediated first-pass metabolism |
| HROC43 | High basal CYP3A4 activity | Drug absorption, development of alternative drug regimens |
| HROC60 | Extremely high barrier integrity, rapid barrier development, long-lasting monolayer | Drug absorption, best model for long-term experiments like ‘gut-on-a-chip’ |
| HROC80 T1 M1 | Rapid barrier development, VDR-mediated CYP3A4 inducibility | Drug absorption, VDR-mediated first-pass metabolism |
| HROC126 | Rectal cancer cell line, VDR-mediated CYP3A4 inducibility | Drug absorption, Best model for rectal drug administration, VDR-mediated first-pass metabolism |
| HROC159 T2 M4 | VDR-mediated CYP3A4 inducibility | Drug absorption, VDR-mediated first-pass metabolism |
| HROC183 T0 M2 | High barrier integrity, high VDR-mediated CYP3A4 inducibility | Drug absorption, together with HROC217 T1 M2 best model for PXR-/VDR-mediated first-pass metabolism |
| HROC217 T1 M2 | High VDR-mediated CYP3A4 inducibility | Drug absorption, together with HROC183 T0 M2 best model for PXR-/VDR-mediated first-pass metabolism |
| HROC239 T0 M1 | Rectal cancer cell line, VDR-mediated CYP3A4 inducibility | Drug absorption, rectal drug administration, VDR-mediated first-pass metabolism |
| HROC383 | PXR-mediated CYP3A4 inducibility | Drug absorption, PXR-mediated first pass metabolism |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybylla, R.; Mullins, C.S.; Krohn, M.; Oswald, S.; Linnebacher, M. Establishment and Characterization of Novel Human Intestinal In Vitro Models for Absorption and First-Pass Metabolism Studies. Int. J. Mol. Sci. 2022, 23, 9861. https://doi.org/10.3390/ijms23179861
Przybylla R, Mullins CS, Krohn M, Oswald S, Linnebacher M. Establishment and Characterization of Novel Human Intestinal In Vitro Models for Absorption and First-Pass Metabolism Studies. International Journal of Molecular Sciences. 2022; 23(17):9861. https://doi.org/10.3390/ijms23179861
Chicago/Turabian StylePrzybylla, Randy, Christina Susanne Mullins, Mathias Krohn, Stefan Oswald, and Michael Linnebacher. 2022. "Establishment and Characterization of Novel Human Intestinal In Vitro Models for Absorption and First-Pass Metabolism Studies" International Journal of Molecular Sciences 23, no. 17: 9861. https://doi.org/10.3390/ijms23179861
APA StylePrzybylla, R., Mullins, C. S., Krohn, M., Oswald, S., & Linnebacher, M. (2022). Establishment and Characterization of Novel Human Intestinal In Vitro Models for Absorption and First-Pass Metabolism Studies. International Journal of Molecular Sciences, 23(17), 9861. https://doi.org/10.3390/ijms23179861






