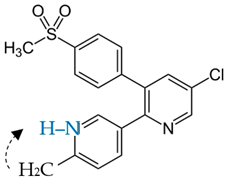
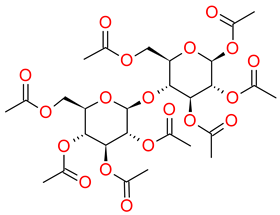
2.1. Amorphous Pure ETB: Recrystallization Study
In the first step, we verified and extended an earlier study on the physical stability of amorphous pure ETB [
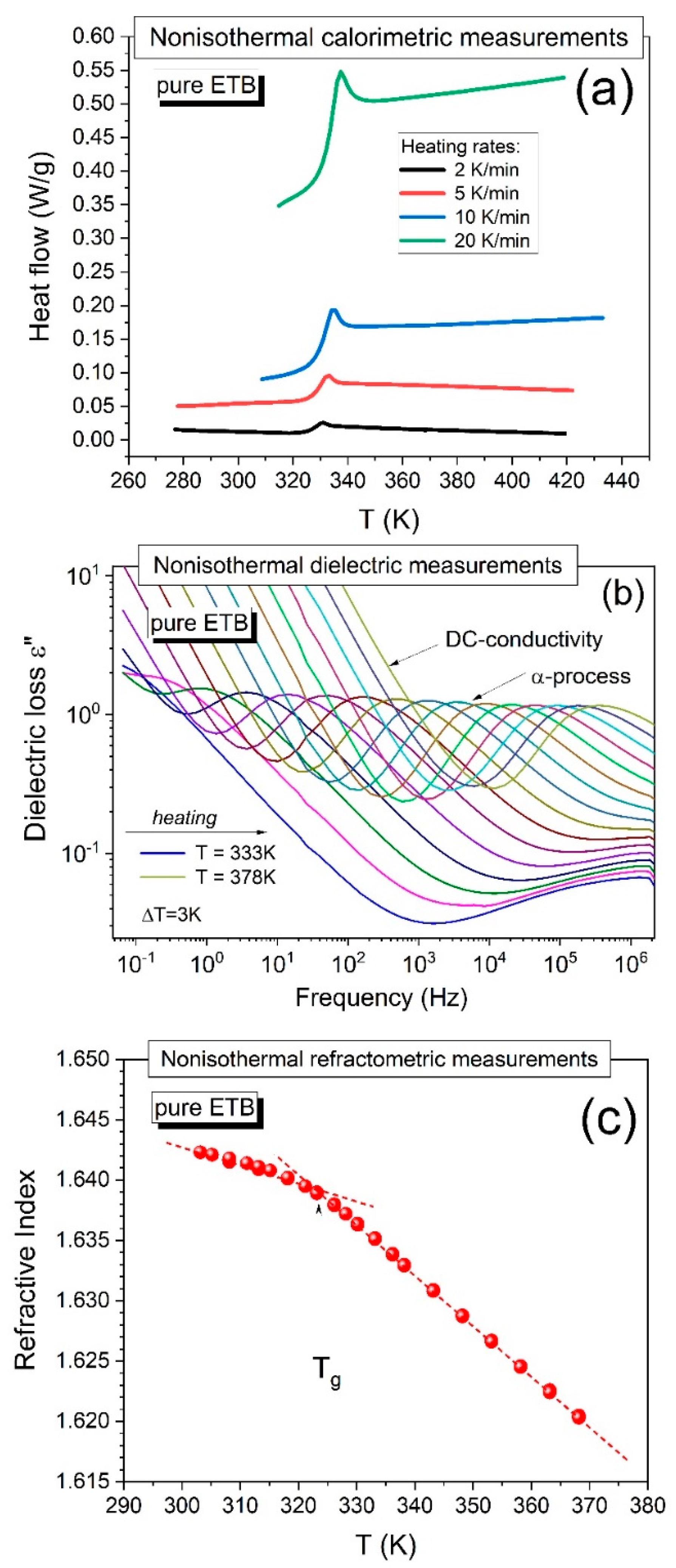
10] by performing non-isothermal calorimetric measurements using various heating rates (2, 5, 10, and 20 K/min) at ambient pressure. As can be seen in
Figure 1a, the earlier vitrified pure ETB did not reveal any signs of recrystallization upon heating the sample independently from the applied heating rate, even in cases of such a low experimental rate as 2 K/min, and the glass transition temperature determined at the midpoint of the jump in the heat flow typically increased (
Tg = 327, 328.5, 330, and 332.5 K, respectively) with increasing heating rate.
A similar picture of the physical stability of amorphous pure ETB can be concluded from the dielectric loss spectra obtained during the heating of the sample from a glassy state to a supercooled liquid state. An example of such dielectric measurement results is shown in
Figure 1b, where the sample was heated from T = 333 K to T = 378 K, and the dielectric spectra were collected every 3 K. The dielectric loss spectra typically shifted to high frequencies with increasing temperature, and no sharp drops were observed in the amplitude of the structural α-relaxation time. The latter characteristic indicated that the dielectric measurements gave no evidence of sample recrystallization [
2,
10,
15,
16,
17,
18].
Herein, we additionally applied the refractometry technique to detect the tendency of pure ETB to recrystallize under non-isothermal conditions. The measurements of the refractive index on cooling ETB from its supercooled liquid to its glassy state did not show any signs of the recrystallization of the drug, which can be seen in
Figure 1c, where the glass transition is well-distinguished (manifested by a change in the slope of the nearly linear temperature dependences of the refractive index measured in the supercooled liquid and glassy states), as we describe in detail in
Section 2.2.
We also made many attempts to study the isothermal recrystallization of pure ETB at ambient pressure by using DSC and BDS measurements, which resulted in no signs of devitrification. However, it was possible to disrupt the equilibrium state between the two kinds of ETB tautomers in the BDS experiments by compressing the sample under isothermal conditions, which most probably triggered its recrystallization [
10,
15]. A question arises as to whether one could find such experimental conditions, which would result in a similar breaking of the balance between the populations of the two ETB tautomers, consequently causing sample recrystallization at ambient pressure. At this point, it should be noted that this is not only a cognitive challenge, but also an important application problem, which could influence the manufacturing process, implementing the transition of the procedures worked out during the laboratory fundamental investigations to the larger industrial scale. For this reason, in the study of the isothermal recrystallization tendency of amorphous pure ETB at ambient pressure, we have tested the standard refractometry technique, which is characterized by a different geometry and larger size of the measurement sample compared to those used in the standard DSC and BDS experiments. In
Section 3.2,
Section 3.3 and
Section 3.4, detailed descriptions of the different shapes and sizes of the measurement vessels and samples are provided.
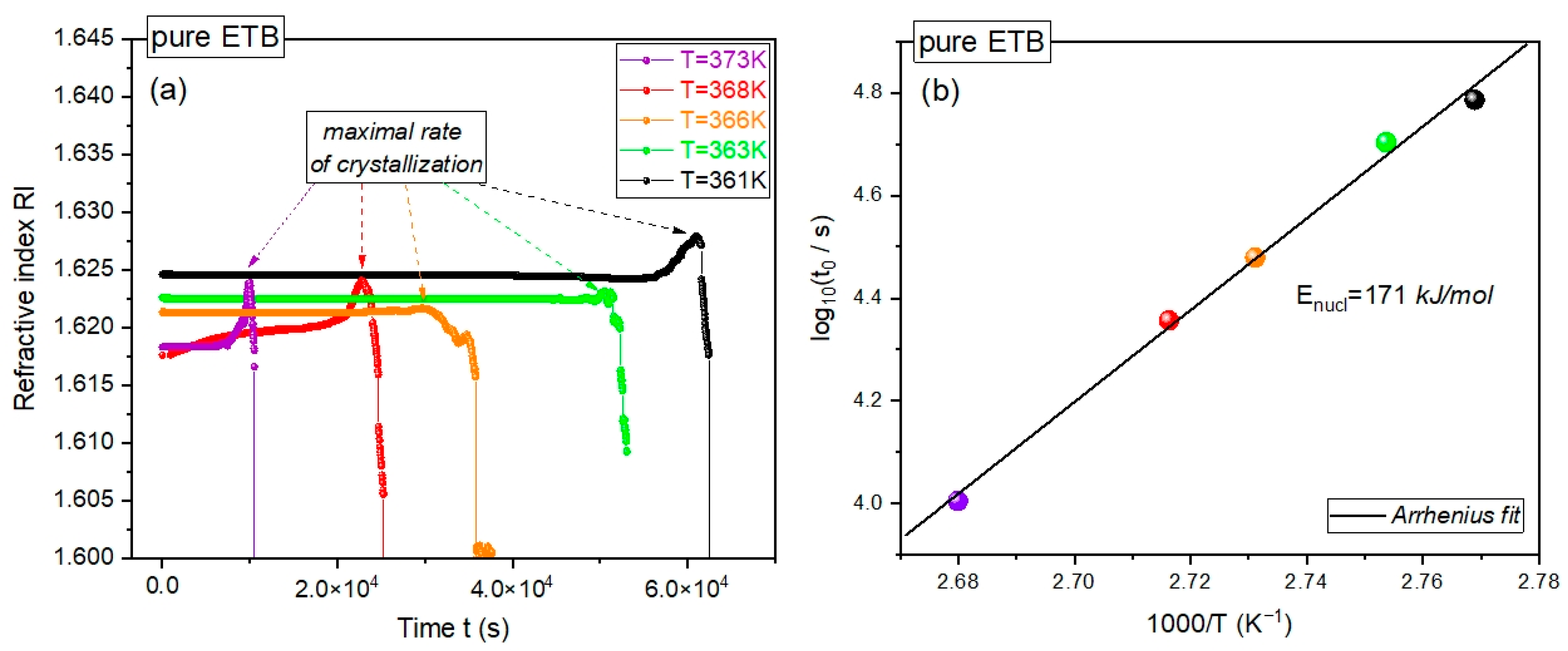
We performed time-dependent crystallization refractometry measurements of pure ETB at several constant temperatures above
Tg at ambient pressure, collecting the refractive index (RI) in tens of thousands of time steps, as shown in
Figure 2a. At each temperature, we observed a sharp drop in the dependence of RI on time
t, which gives evidence for the recrystallization of the supercooled pure ETB under isothermal conditions. Such a drop has always been preceded by a well-distinguished maximum in the dependence of RI(
t). The time
t =
tonset at which the maximum in RI(
t) occurred can be considered as the onset time of the isothermal crystallization process, and can be reasonably interpreted as a well-established estimation of the lag time for nucleation.
The possibility of determining the crystallization onset time or the corresponding crystallization rate enabled us to evaluate the activation energy for nucleation during the isothermal crystallization of supercooled pure ETB at ambient pressure, which has never been determined before. As depicted in
Figure 2b, the dependence of the logarithm of
tonset on the inverse temperature 1/
T can be very well-fitted to a linear function that obeys the simple Arrhenius activation law:
where
R is the gas constant and the fitting parameters are
and
. The latter denotes the sought-after activation energy for nucleation during isothermal crystallization. By fitting the refractometry data for isothermal crystallization into Equation (1), we obtained
and
, where the latter was even less than the activation energy for nucleation during the isothermal crystallization of CEL,
, which was evaluated herein by using the BDS measurements reported in [
12]. This means that the amorphous pure drugs CEL and ETB were characterized by similar energy barriers for isothermal crystallization at ambient pressure.
It is worth noting that standard refractometry has not been a typical method for studying the crystallization process until recently. To the best of our knowledge, our attempt at employing it in these isothermal crystallization investigations is probably the first systematic and successful approach to the issue, which yielded results of a similar quality to those obtained by means of DSC or BDS measurements.
Since the physical stability of amorphous pure ETB has been undermined, even at ambient pressure using refractometry measurements, it is necessary to work out an efficient method for preventing the drug’s recrystallization in order to continue considering pharmaceutical applications of its amorphous form. Taking into account the earlier successful stabilization of amorphous CEL by mixing it with acMAL [
16] and the very similar molecular structures of CEL and ETB, we investigated a few amorphous binary mixtures of ETB with acMAL containing different weight fractions of the excipient, i.e., 10, 20, 30, 40, or 50 wt% acMAL.
2.2. Amorphous Binary ETB—acMAL Mixtures: Thermal and Optical Properties
First, we performed comparative refractometric investigations of the tendency for recrystallization in amorphous pure ETB and its amorphous compositions with acMAL in isothermal conditions above
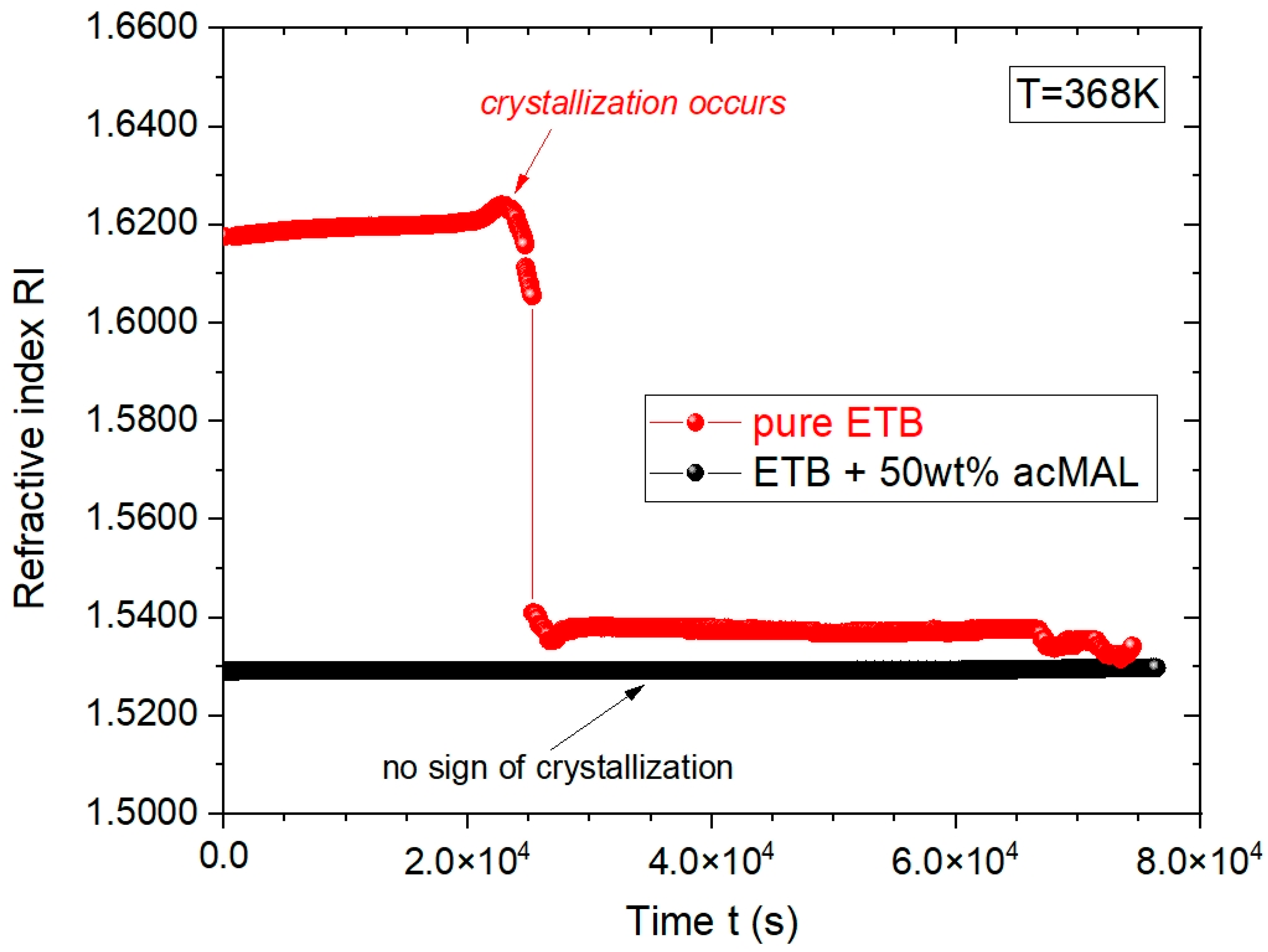
Tg, finding a considerably higher ability of the amorphous ETB–acMAL mixtures to avoid recrystallization. As an example,
Figure 3 shows the time evolutions of the RI collected for pure ETB and its composition with 50 wt% acMAL at the constant temperature T = 368 K.
One can see that the RI value for the supercooled ETB + 50 wt% acMAL mixture equaled about 1.53 at T = 368 K and remained approximately constant for the duration of the experiment, which was carried out for ca. 22 h, while pure ETB has crystallized after about 6 h. This means that the addition of acMAL to ETB significantly enhanced the resistance of the amorphous ETB composition to recrystallization.
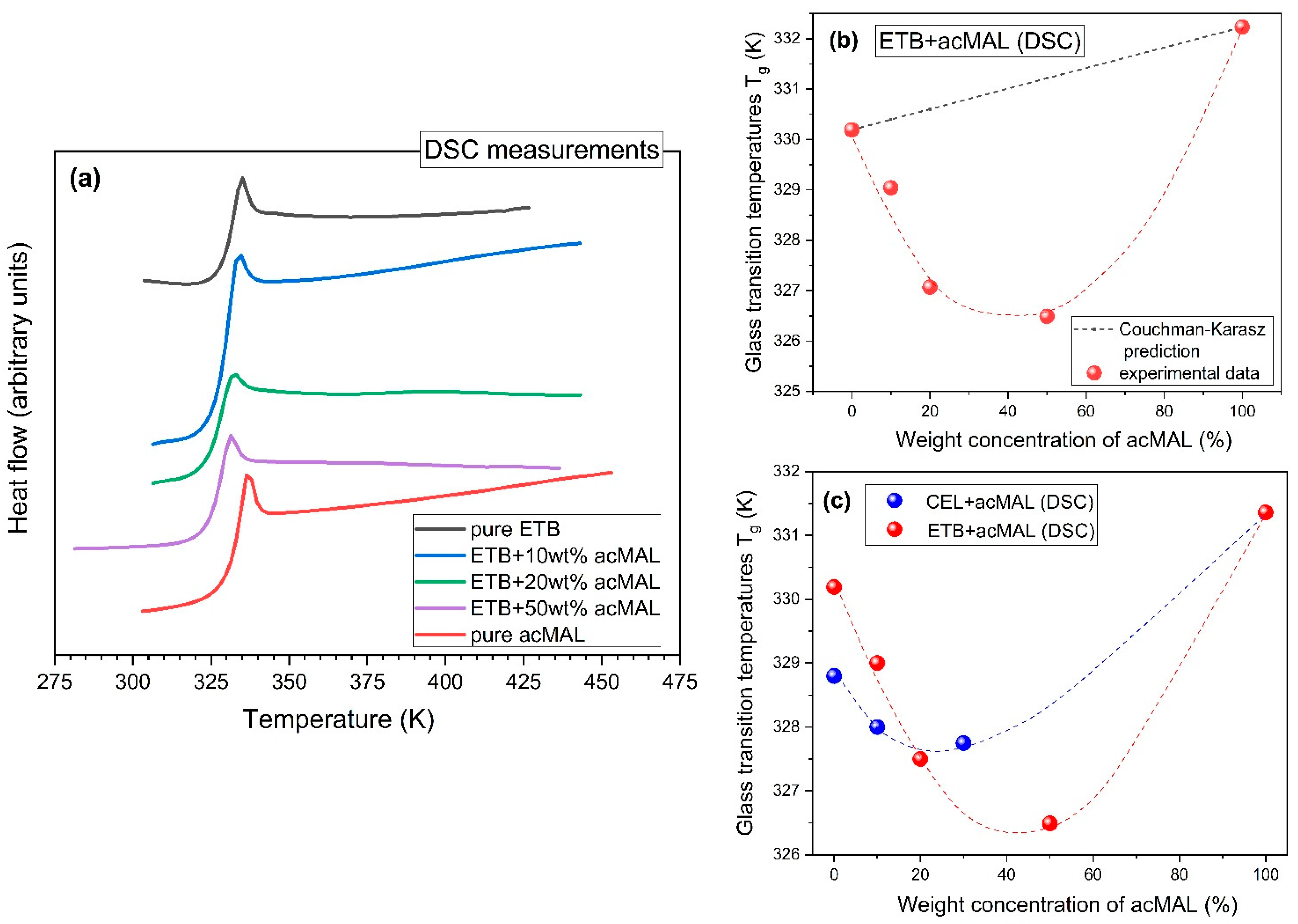
Systematic standard DSC measurements, performed during heating at a rate of 10 K/min in the case of the amorphous mixtures containing 10, 20, and 50 wt% acMAL, as well as analogous DSC experiments carried out for their amorphous pure components, revealed a jump in the heat flow, which is characteristic of the glass transition, and no sign of exothermic thermal effects that could have been classified as a recrystallization process (see
Figure 4a). Thus, the non-isothermal physical stability observed earlier for amorphous pure ETB in the DSC measurements was also established for its amorphous compositions with acMAL, which is further undergoing a thorough examination by us as a good candidate for improving the physical stability of amorphous ETB, under both isothermal and non-isothermal conditions.
By analyzing the glass transition temperatures obtained from the DSC experiments, which are shown in
Table 1, a few interesting observations were made. First of all, it should be noted that the values of
Tg for ETB and acMAL were very close, i.e.,
Tg = 330 K and
Tg = 332 K, respectively. A slightly larger value of
Tg for acMAL than for ETB suggested that the anti-plasticization effect should not play an important role in the molecular mechanisms of preventing the recrystallization process in the amorphous ETB–acMAL binary mixtures. In fact, the values of
Tg determined for these amorphous compositions were less than those established for the pure components and increased with increasing acMAL content in the mixture. This means that the acMAL even plasticized the ETB, despite exerting an undoubtedly favorable effect on the enhancement of the physical stability of amorphous ETB by playing the role of a recrystallization inhibitor.
Such a conclusion was also supported by applying the Couchman–Karasz equation [
19] to predict the glass transition temperature,
Tg, of the binary mixtures, which was developed from the earlier-proposed Gordon–Taylor equation [
20] as follows:
where
and
are the weight fractions of ETB and acMAL, respectively;
and
denote the glass transition temperatures of the pure components; and
is a measure of the interactions between the mixture components, which can be determined from the DSC measurements by finding the jumps in the heat capacity,
and
, at the glass transition of the pure components. Assuming the values
and
, which were obtained from the calorimetric measurement data, we obtained a value of
K equal to 1.009, which led to a nearly linear dependence based on Equation (2), while the experimental data of
Tg clearly diverged from the Couchman–Karasz prediction, as can be seen in
Figure 4b. Since the Couchman–Karasz equation was not satisfied by the values of
Tg found for the amorphous ETB–acMAL mixtures, one cannot expect that the physical stability of the compositions was enhanced compared to the amorphous pure ETB, due to the anti-plasticization effect.
A similar non-monotonic behavior, manifesting in a minimum of the dependence of the
Tg values on the excipient content, has been established earlier for amorphous CEL–acMAL mixtures [
16]. By inspecting the previous and current results obtained for the representatives of coxibs, one can observe a higher negative divergence from the Couchman–Karasz prediction in the case of the ETB–acMAL binary system, as depicted in
Figure 4c. Shamblin et al. [
21] widely discussed a weakness in the dependence of
Tg on adding an excipient to a drug in comparison with that implied by the Couchman–Karasz equation. They suggested that it could happen if the total energy of the interactions between the molecules of different species was lower than that between molecules of the same species. This hypothesis has been confirmed via DFT calculations in our previous investigations of amorphous binary mixtures containing CEL or IBU with the addition of acMAL [
16,
22].
It is worth noting that our isothermal refractometry measurements showed that acMAL also played the role of an effective crystallization inhibitor in the case of amorphous ETB. We observed that this excipient significantly improved the physical stability of amorphous ETB in the binary mixture with a 50 wt% acMAL content. This correlates well with the earlier-suggested molecular mechanisms of the physical stabilization of amorphous CEL in mixtures with acMAL [
16], as well as the physical stabilization of amorphous pure ETB by forming H-bonded heterodimers with ETB tautomers, which hindered recrystallization [
10]. The molecular mechanism responsible for preventing the recrystallization of the amorphous ETB–acMAL compositions most likely also involved forming hydrogen bonds between acMAL and ETB molecules. As a consequence, a crystalline network based on ETB molecules could not be developed, because the population of H-bonded homodimers (ETB–ETB) would be very small in an amorphous binary mixture with a 50 wt% acMAL content, in which the population of H-bonded heterodimers (ETB–acMAL) dominated.
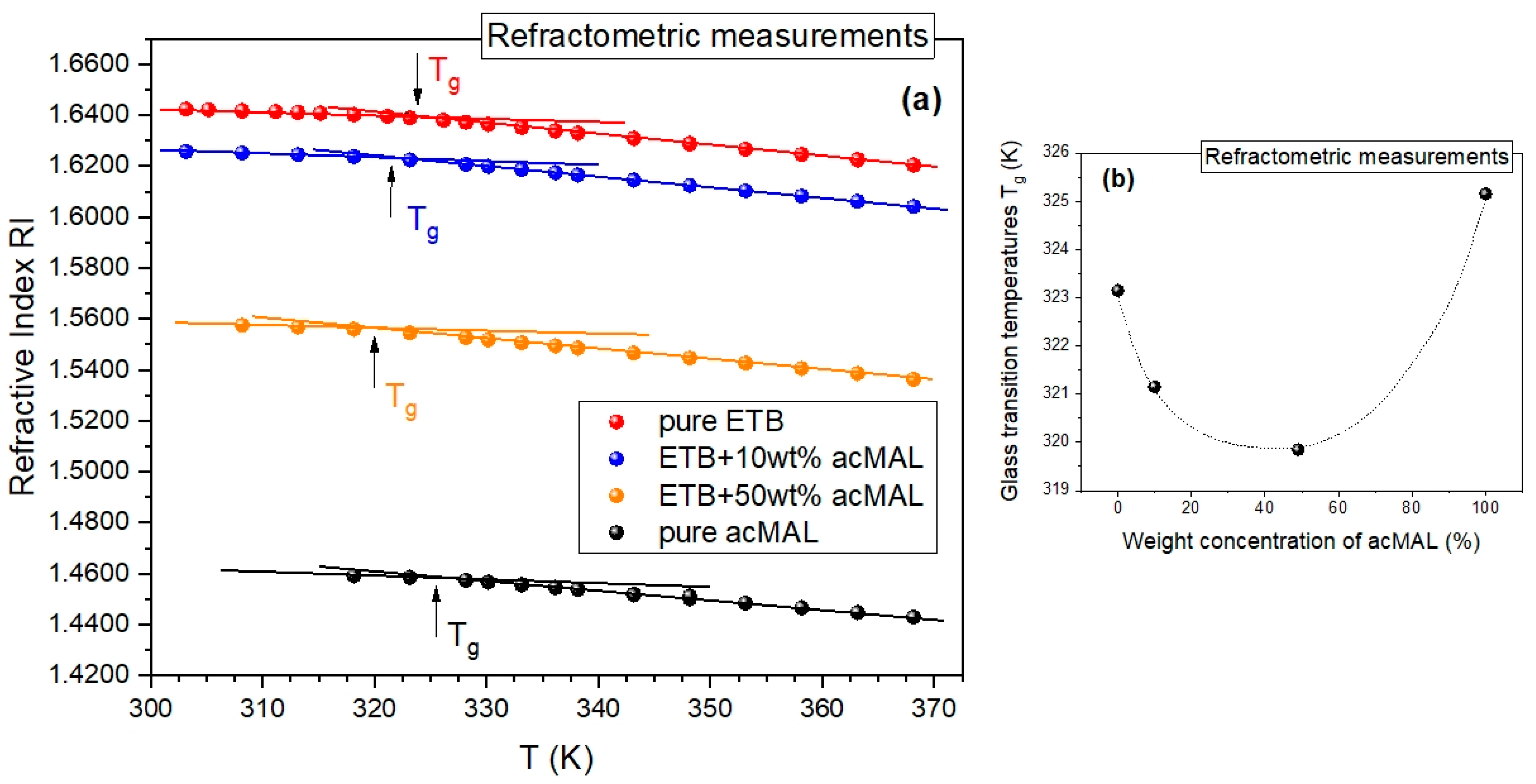
The promising results of the isothermal refractometry experiments and the standard non-isothermal DSC measurements were additionally validated by analogous non-isothermal refractometry measurements carried out by cooling the binary mixtures of ETB and acMAL from a supercooled liquid to a glassy state. We were able to detect the glass transition as a change in the slope of the dependence of the refractive index on temperature, which can be compared to the symptoms of the glass transition observed in the temperature dependences of density or enthalpy. As shown in
Figure 5a, it was possible to determine the glass transition temperatures from the obtained refractograms, in a way analogous to that commonly employed in such an analysis of PVT data. In
Figure 5b, we present a plot of the values of
Tg vs. the weight fraction of acMAL, established from the refractometry experiments, which behaved non-monotonically in an analogous way to those obtained from the DSC measurements (
Figure 4a). In addition, there was also no sign of recrystallization in the refractograms collected in
Figure 5a, similarly to the results of the non-isothermal DSC measurements depicted in
Figure 4a. This strengthened the premise about the suggested potential of the examined amorphous ETB–acMAL mixtures for pharmaceutical applications and demonstrated the usefulness of the refractometry technique in the study of the glass transition and the recrystallization process if the refractometric equipment is able to achieve a temperature range close to the glass transition of the examined system.
2.3. Amorphous Binary ETB–acMAL Mixtures: Molecular Dynamics
In
Section 2.2, we report the peculiar behavior of the glass transition temperatures for the amorphous mixtures of ETB with acMAL, which was obtained by means of both the DSC and refractometry measurements. Since broadband dielectric spectroscopy has been appreciated as a very robust technique for studying the molecular dynamics in many decades of timescale and various thermodynamic conditions, including the vicinity of the glass transition [
17,
23,
24,
25], we have performed thorough investigations of the ETB–acMAL compositions with the content of 20, 30, 40, and 50 wt% acMAL near the glass transition by using the BDS measurements. Using this method, we additionally validated a divergence of the dependence of the glass transition temperature of the binary mixtures on the content of acMAL from the linear prediction given by Equation (2). Since a previous study on pure ETB has led to the conclusion that there is no correlation between the glass mobility monitored by the BDS measurements and the physical stability of pure ETB at ambient pressure [
10], we focused herein on the results obtained by means of the BDS technique for its binary mixtures with acMAL in a supercooled liquid state.
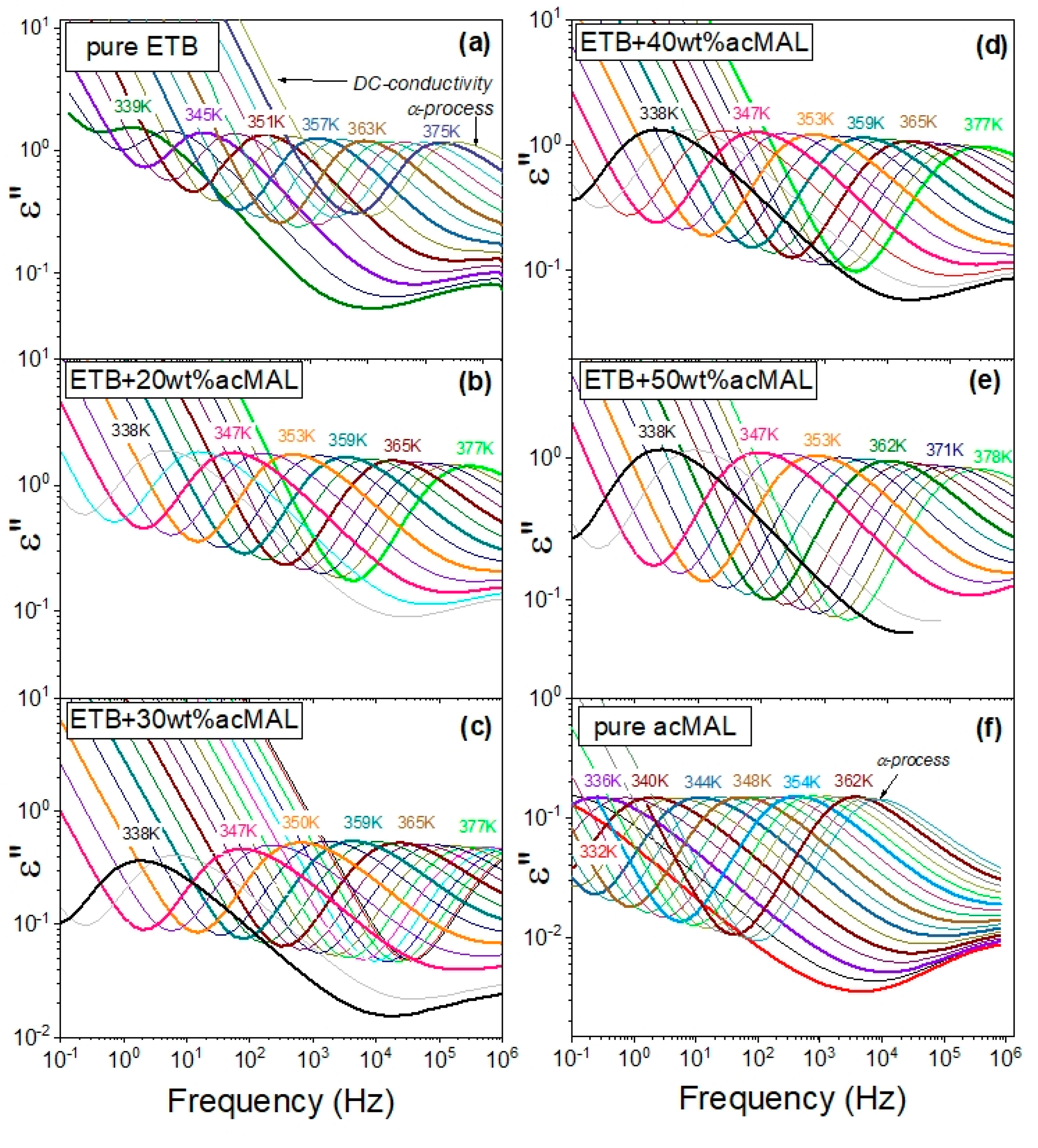
Figure 6 shows dielectric loss spectra measured in a wide temperature range at ambient pressure during our current research on the binary mixtures of ETB and acMAL, which were compared with the dielectric loss spectra reported earlier for the pure components ETB [
10] and acMAL [
16]. The first important observation made from the inspection of the dielectric loss spectra was a lack of signs of recrystallization, because there was no sharp drop in the amplitudes of the main α-relaxation process. The next behavior easily noticed in these spectra was their strong dependence on changes in temperature, as they shifted to lower frequencies with decreasing temperature, revealing a characteristic rapid slowdown in the molecular dynamics of the systems upon approaching the glass transition. In the dielectric spectra collected for the binary ETB–acMAL systems near the glass transition at temperatures above
Tg, one can distinguish the dc-conductivity and the structural α-relaxation process that reflects the global molecular dynamics of the systems, which is commonly considered to be relevant to the glass-transition phenomenon. For this reason, in order to determine the timescale of the global molecular dynamics of the binary systems, which is well-defined by the structural α-relaxation time
τα, we can employ the following frequency-dependent equation based on the phenomenological Havriliak–Negami (HN) term [
26,
27] for the complex permittivity in fitting the entire dielectric spectra:
In Equation (3), the former term describes the dc-conductivity contribution, involving the dc electrical conductivity σ0 and the dielectric permittivity ε0 of the vacuum; ε∞ is the high frequency limit permittivity; and the latter term is the HN expression with the fitting parameters ∆εα, τα, ξ, and δ, which are widely used except for the case of the parameter values ξ = 1 and δ = 1, in order to parametrize the asymmetrically broadened dielectric loss peaks that are typical for the structural relaxation process. Such a fitting procedure followed in the domain of frequency f enabled us to reliably establish the structural relaxation time as the value of the fitting HN parameter τα in the case of the examined dielectric spectra of the binary ETB–acMAL mixtures.
The temperature effect on the timescale of the global molecular dynamics near the glass transition and the related glass transition temperatures determined from the BDS measurements can be conveniently analyzed by using the inverse temperature dependence of the structural relaxation times,
τα, found by fitting the dielectric spectra of the binary ETB–acMAL mixtures to Equation (3). In
Figure 7a, we present the dependences
τα(1/
T) obtained herein for the ETB–acMAL compositions, compared with those reported earlier for the pure components ETB [
10] and acMAL [
16]. To parametrize the inverse temperature dependences of
τα, we applied the Vogel–Fulcher–Tammann (VFT) equation [
28,
29,
30], containing only three fitting parameters,
τ0,
A, and
TVFT:
which is commonly used in such analyses, including the phenomenological description of the experimental study of molecular dynamics in pharmaceutical materials. The values of the VFT parameters obtained from fitting the inverse temperature dependences of the structural dielectric relaxation times to Equation (4) are collected in
Table 2 for the binary ETB–acMAL mixtures as well as the pure components.
The high quality of the VFT fits, represented (among other things) by small uncertainties in the determination of the VFT parameter values and the fitted curves that well-matched the experimental data in
Figure 7a, enabled us to employ Equation (4) to find the values of
Tg from the BDS measurements of the ETB–acMAL compositions and explore the sensitivity of their molecular dynamics to changes in temperature near the glass transition. A fundamental measure of the latter is the isobaric fragility parameter [
31]:
which has been widely promoted by Angell and many other authors [
24,
32,
33,
34,
35] as a key parameter that can be used to classify glass formers according to the value of
mp into strong (
), moderately fragile (
), and fragile (
) materials. A consequence of the definition given by Equation (5) and the specificity of the BDS experiments is the ability to compare the fragility values obtained for different systems measured by means of the BDS technique, which are evaluated at the same timescale of global molecular dynamics. Such arbitrary conditions of determining
Tg from dielectric data are related to the considerable slowdown in the structural relaxation when approaching the glass transition, which becomes too slow to be monitored by the BDS measurements. For the same reason, the structural dielectric relaxation times at temperatures below
Tg are usually only predicted, and the glass transition temperature is arbitrarily established at an assumed timescale of the primary dielectric relaxation process.
Herein, we determined the values of
Tg at
τα = 100 s, which was in accordance with an assumption made earlier [
16] for the amorphous CEL–acMAL compositions. By extrapolating the VFT fits to lower temperatures, at which the timescale
τα = 100 s, we found the values of
Tg for the examined systems based on the BDS measurements. A comparative illustration of the values of
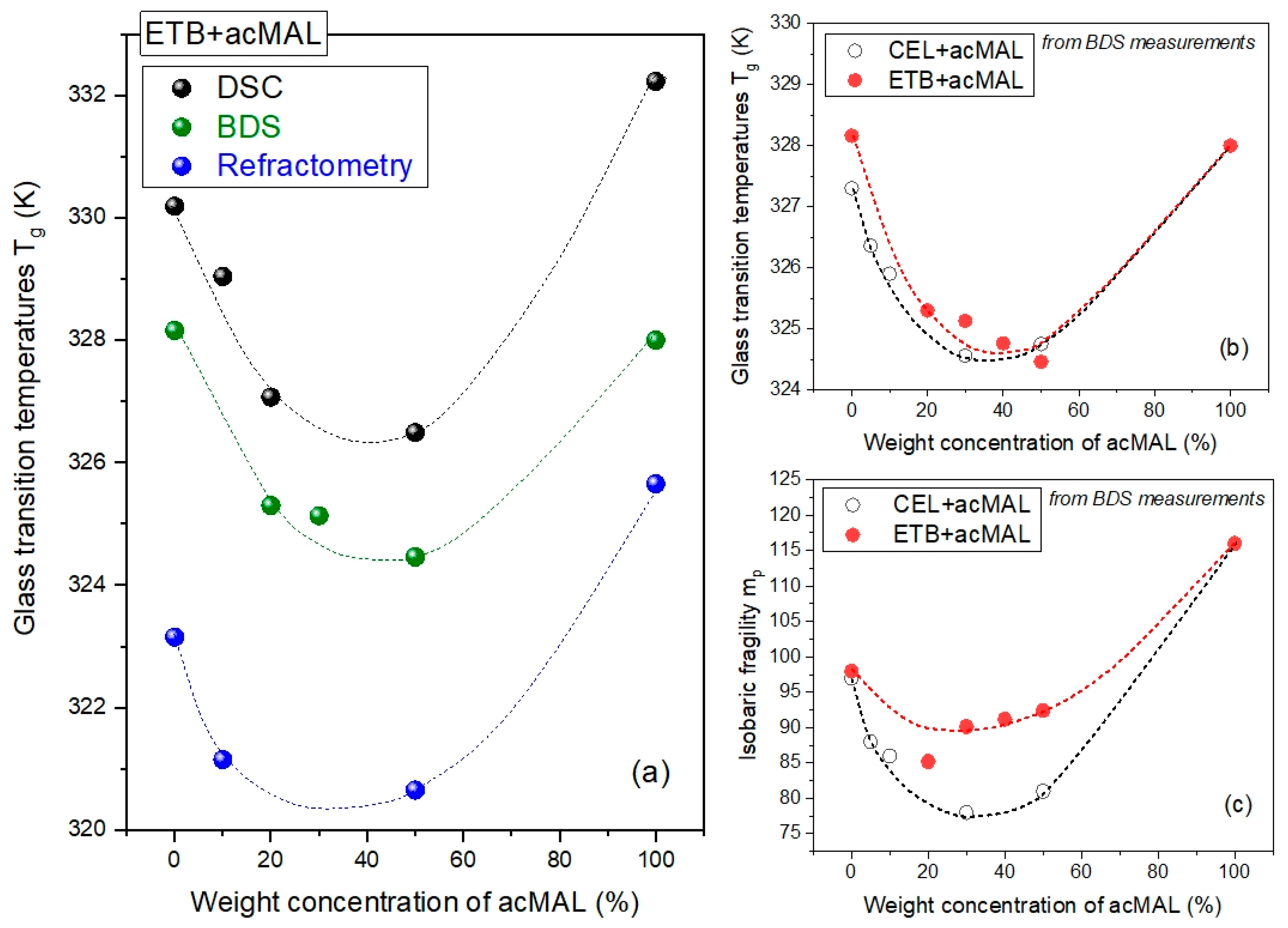
Tg obtained using DSC, BDS, and the refractometry techniques is depicted in
Figure 8a. The values of
Tg slightly differed depending on the experimental method. This is reasonable, because the measurement rates are different for each of these techniques. Additionally, during the BDS and refractometry experiments, the equilibration of the sample to a fixed temperature and the measurement at the fixed temperature take a considerably longer time than for the DSC measurements. However, it turned out that the peculiar non-monotonic dependence of
Tg on the content of acMAL in the amorphous mixtures with ETB was found to be independent of the applied experimental methods. This finding validates the negative divergence from the Couchman–Karasz prediction for the binary mixtures; as already mentioned in
Section 2.2, this could be reasonably related to the hypothesis of the molecular mechanisms of the physical stabilization of the amorphous ETB–acMAL compositions, most likely caused by forming H-bonded heterodimers between the ETB and acMAL molecules, thus preventing the formation of the crystallization nuclei built from ETB molecules.
Similar to the values of
Tg obtained from the DSC measurements (see
Figure 4c), the results obtained from the BDS experiments for the amorphous mixtures of ETB with acMAL and CEL with acMAL revealed a minimum for the dependence of
Tg (wt% acMAL), as depicted in
Figure 8b. Thus, the next reinforced conclusion is that there was no anti-plasticization effect of acMAL on either ETB or CEL. However, this was accompanied by the physical stabilization of both kinds of amorphous compositions. By invoking earlier reports on the plausible molecular mechanisms underlying the enhanced resistance of the binary CEL–acMAL systems to recrystallization [
16], it should be noted that the formation of heterodimers by the CEL and acMAL molecules consisted of hydrogen bonding between the donors from the sulfonamide group of the phenyl-SO
2NH
2 part belonging to the CEL molecule and the acceptor sites of the acMAL molecule, which is devoid of donors. However, the ETB molecule had no sulfonamide group and the part that most likely provided donors was the methylpyridine-based ring in the tautomer, denoted as E2 in [
10] and ETB 2 herein in
Table 3 of
Section 3.1. It was characterized by the proton transfer from the methyl group to the nitrogen atom in this ring.
By comparing the inverse temperature dependences of
τα determined earlier for the pure components, ETB and acMAL, in the supercooled liquid state with those obtained herein for the binary ETB–acMAL mixtures, we observed some decreases in the steepness of the dependences
τα(1/
T) in the vicinity of
Tg for the compositions, as depicted in
Figure 7a. As already mentioned, a proper measure of the steepness is the isobaric fragility defined by Equation (5). The factor
mp has been widely used to predict the physical stability of amorphous pharmaceuticals and other glass-formers. For many decades, it has been believed that the physical stability of drugs in both the supercooled and glass states is better if they are stronger materials, i.e., characterized by smaller values of the fragility parameter [
13,
36,
37,
38,
39,
40]. In the literature, there are known exceptions in the suggested correlation for both pure drugs and pharmaceutical compositions [
2,
5,
10,
22,
41,
42,
43]. To verify the hypothesis in the case of the binary mixtures of ETB with acMAL, we calculated the values of
mp by using Equations (4) and (5) and plotted the obtained results in
Figure 8c compared to those reported earlier for the CEL–acMAL mixtures. For both kinds of binary mixtures, the dependences of the isobaric fragility on the content of acMAL behaved similarly. The values of
mp initially decreased, but they began to increase starting at about 30 wt% acMAL content in the mixtures. However, the pure components were characterized by higher values of isobaric fragility. The latter indicated that the pure components had a higher global molecular mobility near
Tg, and it may suggest that the pure components have a stronger tendency to recrystallize than their mixtures with acMAL. Nevertheless, the increase in the values of
mp observed after reaching a minimum of about 30 wt% acMAL in the binary compositions for both kinds cannot be correlated with the better physical stability of amorphous mixtures with a higher content of acMAL. The non-monotonic behavior of the fragility parameter dependence on the weight concentration of acMAL in the mixture with ETB constitutes an additional rationale [
16,
22,
41,
43,
44] for the important role of specific interactions, as discussed herein hydrogen bonding, in the improvement of the physical stability of the ETB–acMAL compositions.
By comparing the map of structural relaxation times, shown in
Figure 7a, and the dielectric spectra selected at a fixed temperature near
Tg, presented in
Figure 7b for pure acMAL and all tested binary mixtures at T = 338 K, and only for pure ETB at the nearby temperature T = 339 K, one can also observe a non-monotonic variation in the frequencies at which the dielectric loss spectra revealed their maxima with the content of acMAL in the examined systems under isothermal conditions near the glass transition. Such a non-monotonic behavior is mapped to the structural relaxation times
τα as a function of the mixture composition at a constant temperature close to
Tg, which reflects a non-monotonic temperature sensitivity in the global molecular mobility of the examined systems near the glass transition.
Finally, we seized the opportunity to test another suggested correlation widely discussed in the study of supercooled liquid recrystallization. This correlation relies on the classical nucleation theory, which considers thermodynamic and kinetic contributions to the crystallization process, where the latter is expected to be dominant near the glass transition if the crystallization process is governed by the diffusion [
38,
45]. In such a case, the characteristic timescale of crystallization should be energetically coupled with the timescale of the global molecular mobility. Since we determined the crystallization onset times
t0 in
Section 2.1 and the structural dielectric relaxation times
τα, we were able to compare the activation energy for nucleation
Enucl and the activation energy for structural dielectric relaxation
Eα in the temperature range, within which the isothermal crystallization refractometry experiments were carried out. This was important due to a long history of such investigations in the case of amorphous pharmaceuticals, reported, for example, in [
2], which have invoked both the crystallization onset time
t0 and the overall crystallization characteristic timescale, where the latter was used to evaluate the activation energy for the overall crystallization process. The rationale for this study originates from classical nucleation theory, which assumes both thermodynamic and kinetic contributions to the overall crystallization process as well as to their components, including nucleation and crystal growth.
As can be seen in
Figure 7c, in the considered temperature range, the inverse temperature dependence of the structural relaxation time can be also well-fitted to the Arrhenius law, which yields a linear representation of the dependence
τα(1/
T) with
. Taking into account the value
, as estimated in
Section 2.1, the coupling parameter
S between the recrystallization process and the structural dielectric relaxation can be found from the ratio
, which yields a value of about 0.6 for ETB in the temperature range of 361.15–373.15 K, within which the isothermal recrystallization measurements of pure ETB have been performed herein by means of the refractometry technique. In a similar temperature range, 363.15–378.15 K, Dantuluri et al. [
12] carried out isothermal recrystallization experiments for CEL by using the BDS technique, thus finding a coupling parameter value of 0.7 for the overall isothermal recrystallization. Such values for the coupling parameter indicate that the nucleation component of the examined crystallization of ETB and the overall crystallization of CEL are not solely controlled by diffusion. Nevertheless, this simple conclusion requires further considerations. It should be noted that dielectric spectroscopy, which uses probing reorientations of the permanent dipoles, cannot directly investigate the translational diffusion, and an additional problem is a decoupling that may occur between the rotational diffusion and the structural dielectric relaxation time, as described by the fractional Debye–Stokes–Einstein equation [
46,
47,
48,
49]. Thus, the decoupling phenomena observed for the mentioned rotational quantities, and also the translational diffusion and viscosity, may make the coupling parameter values difficult to satisfactorily interpret [
2,
50]. Moreover, it is worth mentioning that the obtained values of the coupling parameter
S for ETB herein and CEL in [
12] could have simply resulted from the temperature range available for the isothermal crystallization experiments, which usually become impossible or extremely time-consuming when approaching the glass transition. Based on the DSC measurements, ETB and CEL are characterized by the same glass transition temperature
Tg = 330 K, while their melting temperatures are
Tm = 407 K and
Tm = 434 K, respectively [
10]. Thus, the temperature ranges within which the recrystallization experiments were performed for both drugs were located relatively far from the glass transition temperature, and they were instead in the middle of the temperature interval between the melting and glass transition points for these materials. As argued by Ediger et al. [
39], the domination of the kinetic contribution to the crystallization process is actually expected closer to the glass transition, while the thermodynamic contributions to crystallization may predominate over the kinetic ones in the vicinity of the melting point. This could be the main reason for the values of the coupling parameter
S as estimated by exploring the isothermal crystallization and the structural relaxation in the middle of the temperature range between
Tm and
Tg.