Balanced Free Essential Amino Acids and Resistance Exercise Training Synergistically Improve Dexamethasone-Induced Impairments in Muscle Strength, Endurance, and Insulin Sensitivity in Mice
Abstract
:1. Introduction
2. Results
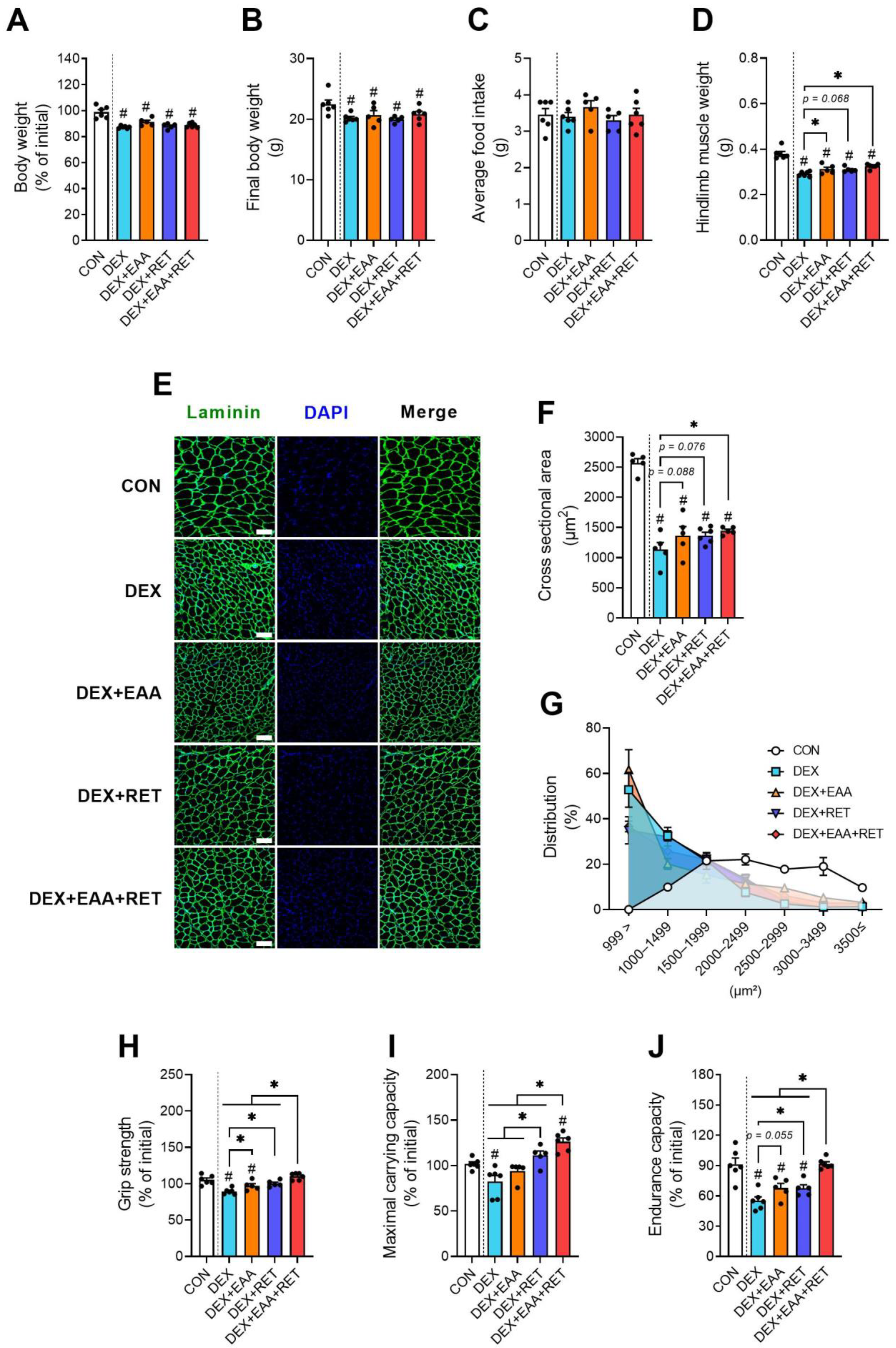
2.1. EAA Supplementation and RET Synergistically Prevent DEX-Induced Declines in Muscle Mass and Physical Function
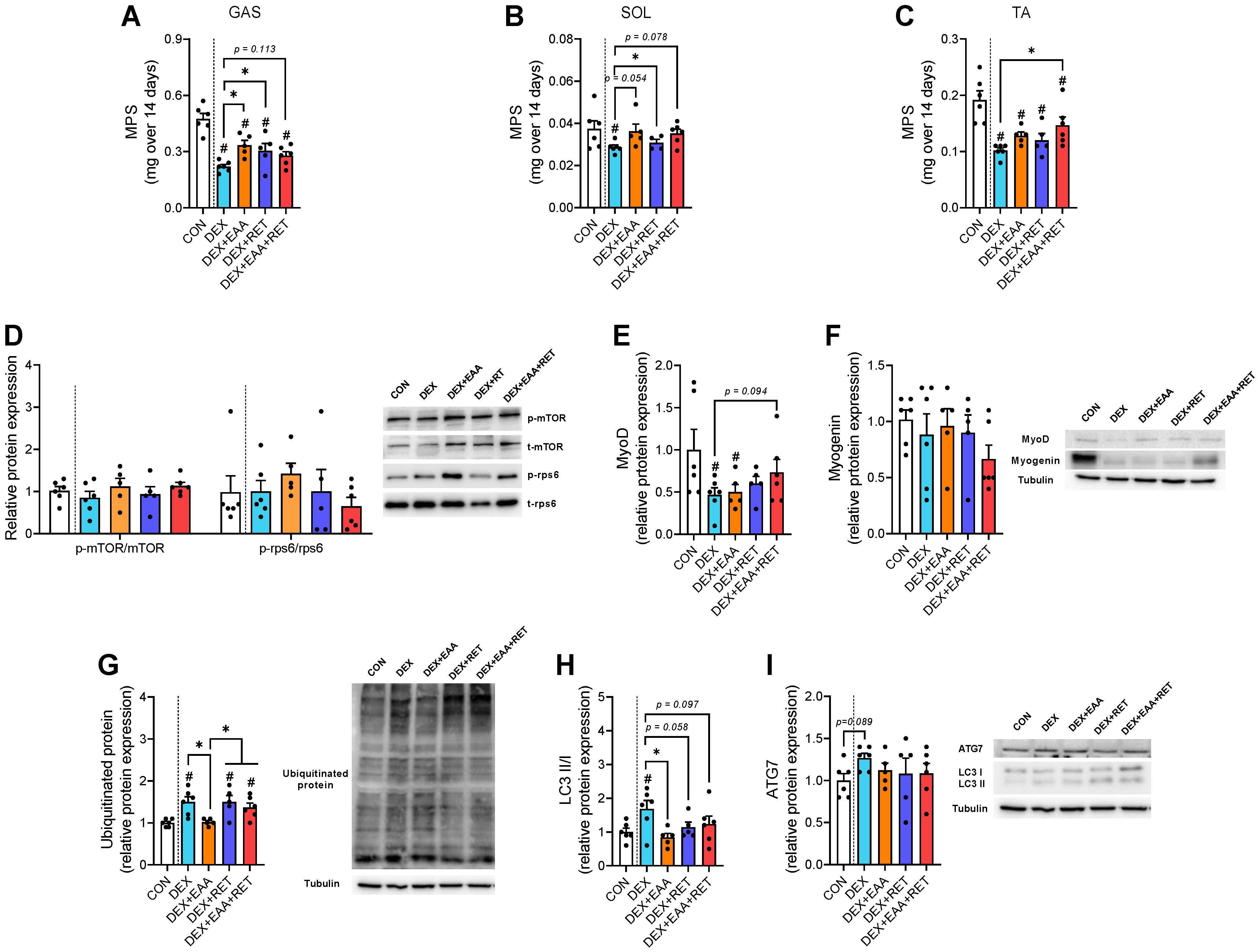
2.2. EAA and/or RET Stimulate Muscle Protein Synthesis Rate and Suppression of Autophagy Activation in Muscle of DEX-Treated Mice
2.3. Combined Treatment of EAA and RET Restores a Decrease in NMJ Stability in Skeletal Muscle Due to DEX Treatment
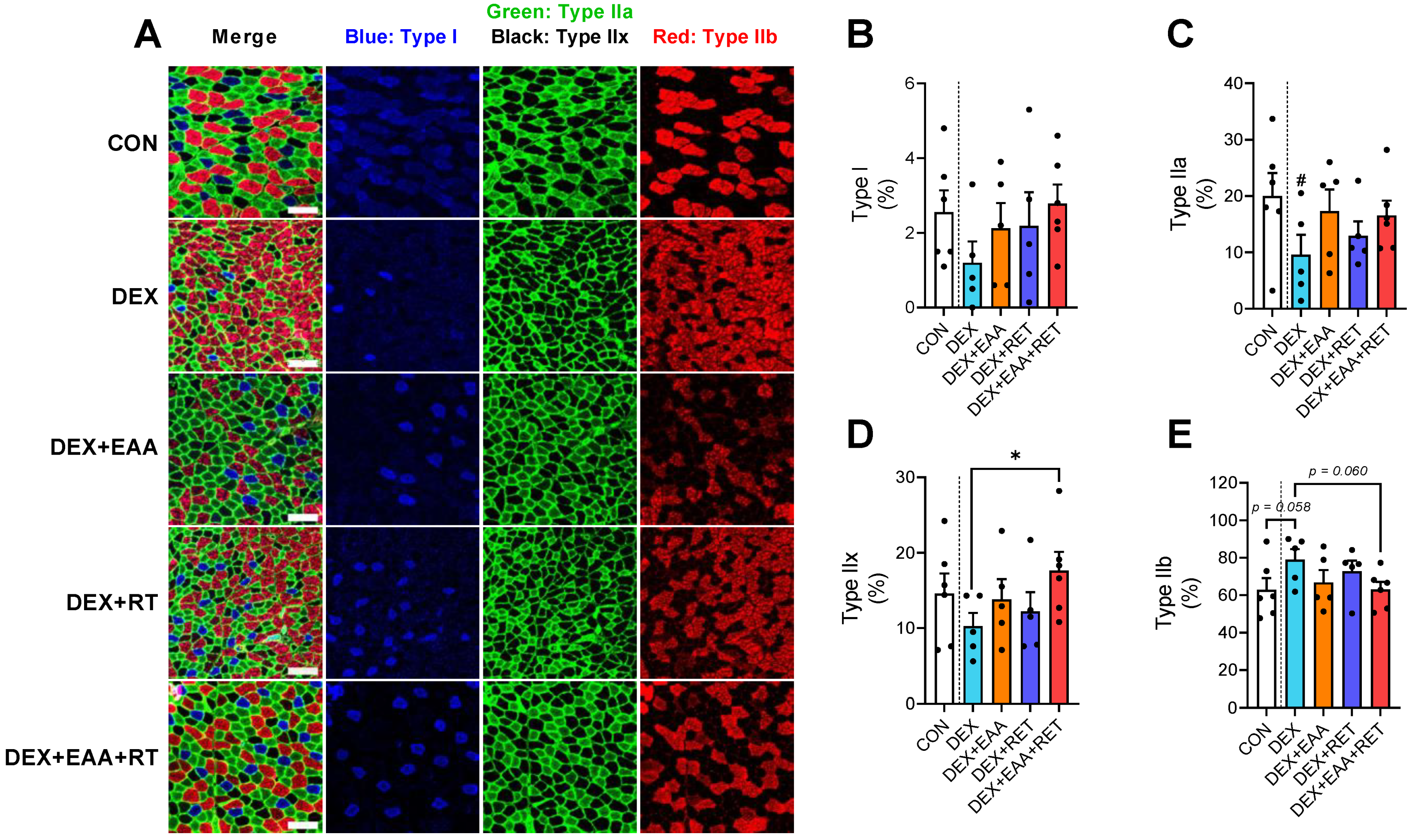
2.4. Combined Treatment of EAA and RET Prevents Muscle Fiber Type Shifting to Fast Glycolytic Fiber Due to DEX Treatment
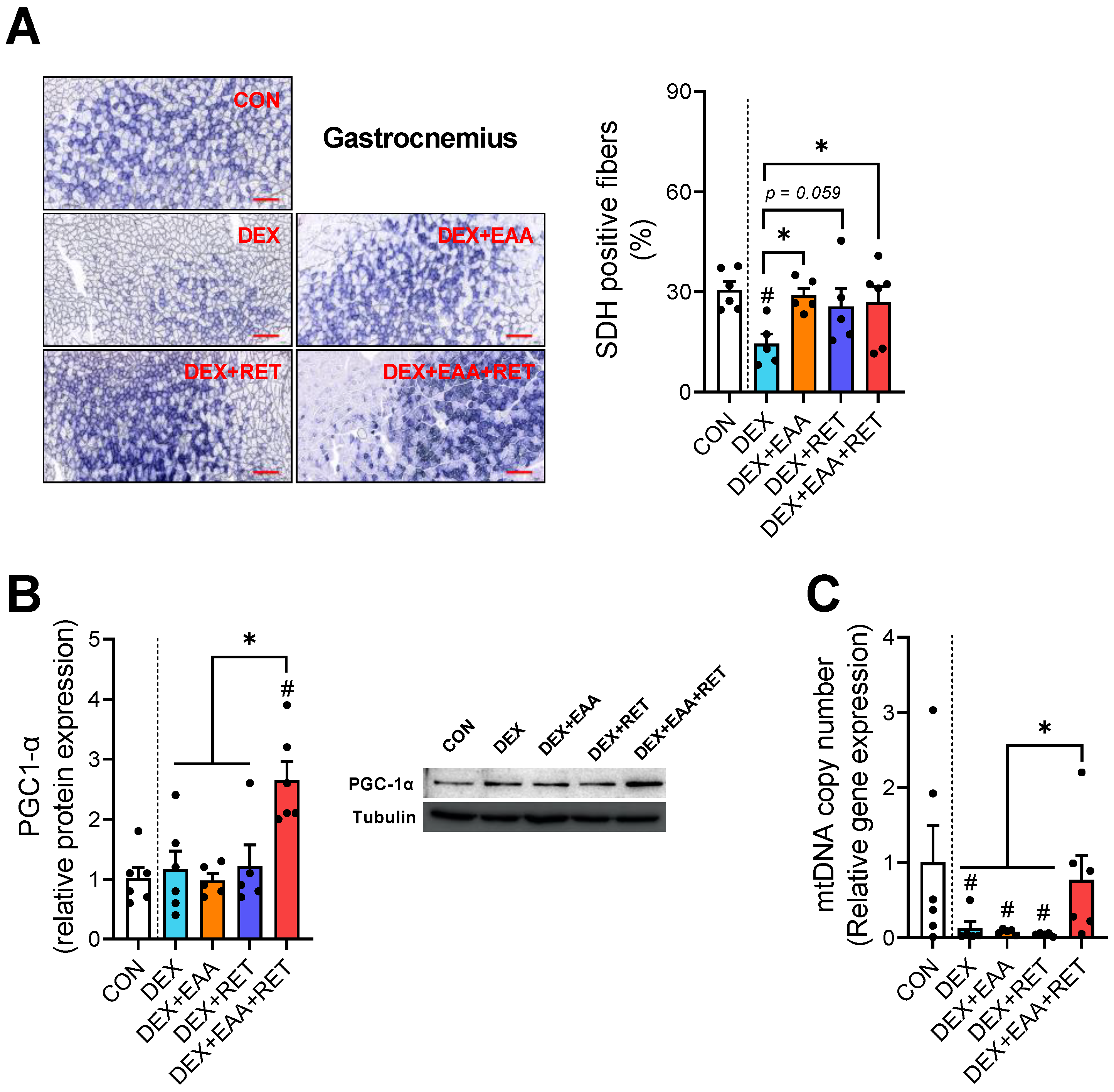
2.5. Combined Treatment of EAA and RET Completely Restores the Loss of Mitochondrial Biogenesis in Skeletal Muscle by DEX Treatment
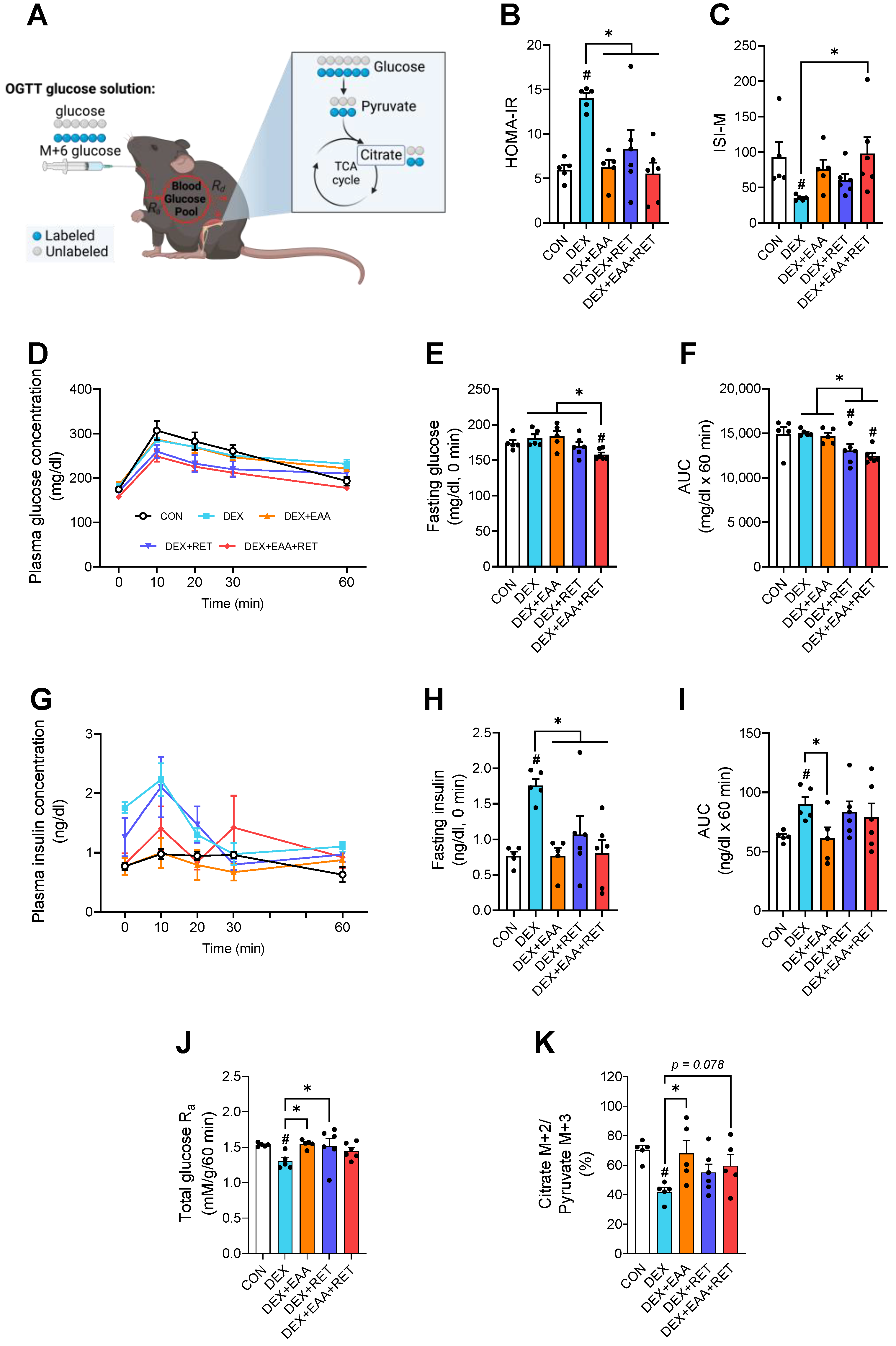
2.6. Combined Treatment of EAA and RET Completely Restores Impaired Insulin Sensitivity by DEX Treatment
3. Discussion
4. Materials and Methods
4.1. Animal Care and Experiment Design
4.2. Resistance Exercise Training Protocol
4.3. Physical Function Tests
4.4. Immunoblot Analysis
4.5. Immunofluorescence Staining
4.6. Immunohistochemistry
4.7. Mitochondrial DNA (mtDNA) Copy Number Quantification
4.8. Isolation of Myofibrillar Protein
4.9. D2O Labeling and Calculations of Protein Synthetic Rate
4.10. Stable Isotope Enrichment Analysis
4.11. Oral Glucose Tolerance Test and Whole-Body Glucose Metabolism Calculation
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corssmit, E.P.; Dekkers, O.M. Screening in adrenal tumors. Curr. Opin. Oncol. 2019, 31, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, M.B.; Carter, O. Corticosteroid use in the treatment of neuromuscular disorders: Empirical and evidence-based data. Muscle Nerve 2004, 30, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, Y.I.; Nirmala, F.S.; Jeong, H.Y.; Seo, H.-D.; Ha, T.Y.; Jung, C.H.; Ahn, J. Chrysanthemum zawadskil Herbich attenuates dexamethasone-induced muscle atrophy through the regulation of proteostasis and mitochondrial function. Biomed. Pharmacother. 2021, 136, 111226. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, S.; Lee, J.; Jang, J.; Jung, J.; Koh, J.-H.; Choi, C.S.; Wolfe, R.R.; Kim, I.-Y. Essential Amino Acid-Enriched Diet Alleviates Dexamethasone-Induced Loss of Muscle Mass and Function through Stimulation of Myofibrillar Protein Synthesis and Improves Glucose Metabolism in Mice. Metabolites 2022, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Trausch-Azar, J.S.; Muglia, L.J.; Schwartz, A.L. Glucocorticoids differentially regulate degradation of MyoD and Id1 by N-terminal ubiquitination to promote muscle protein catabolism. Proc. Natl. Acad. Sci. USA 2008, 105, 3339–3344. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, K.; Ren, Q.; Yi, L.; Zhu, J.; Zhang, Q.; Mi, M. Dihydromyricetin Attenuates Dexamethasone-Induced Muscle Atrophy by Improving Mitochondrial Function via the PGC-1α Pathway. Cell. Physiol. Biochem. 2018, 49, 758–779. [Google Scholar] [CrossRef]
- Weinstein, S.P.; Wilson, C.M.; Pritsker, A.; Cushman, S.W. Dexamethasone inhibits insulin-stimulated recruitment of GLUt4 to the cell surface in rat skeletal muscle. Metabolism 1998, 47, 3–6. [Google Scholar] [CrossRef]
- Fappi, A.; Godoy, T.S.; Maximino, J.R.; Rizzato, V.R.; Neves, J.D.C.; Chadi, G.; Zanoteli, E. The Effects of Omega-3 Fatty Acid Supplementation on Dexamethasone-Induced Muscle Atrophy. BioMed Res. Int. 2014, 2014, 961438. [Google Scholar] [CrossRef]
- Nishida, H.; Ikegami, A.; Kaneko, C.; Kakuma, H.; Nishi, H.; Tanaka, N.; Aoyama, M.; Usami, M.; Okimura, Y. Dexamethasone and BCAA Failed to Modulate Muscle Mass and mTOR Signaling in GH-Deficient Rats. PLoS ONE 2015, 10, e0128805. [Google Scholar] [CrossRef]
- Willoughby, D.S.; Stout, J.R.; Wilborn, C.D. Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Amino Acids 2006, 32, 467–477. [Google Scholar] [CrossRef]
- Jang, J.; Park, S.; Kim, Y.; Jung, J.; Lee, J.; Chang, Y.; Lee, S.; Park, B.-C.; Wolfe, R.; Choi, C.; et al. Myostatin Inhibition-Induced Increase in Muscle Mass and Strength Was Amplified by Resistance Exercise Training, and Dietary Essential Amino Acids Improved Muscle Quality in Mice. Nutrients 2021, 13, 1508. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, U.; Sheffield-Moore, M.; Katsanos, C.S.; Zhang, X.-J.; Wolfe, R.R. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp. Gerontol. 2006, 41, 215–219. [Google Scholar] [CrossRef]
- Groennebaek, T.; Vissing, K.; Groennebaek, T.; Vissing, K. Impact of Resistance Training on Skeletal Muscle Mitochondrial Biogenesis, Content, and Function. Front. Physiol. 2017, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Marin, P.J.; Rhea, M.R.; Wilson, S.M.; Loenneke, J.P.; Anderson, J.C. Concurrent Training. J. Strength Cond. Res. 2012, 26, 2293–2307. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.J.; Bishop, D.J.; Stepto, N.K. Interference between Concurrent Resistance and Endurance Exercise: Molecular Bases and the Role of Individual Training Variables. Sports Med. 2014, 44, 743–762. [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I.-Y.; Gwin, J.A.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential Amino Acids and Protein Synthesis: Insights into Maximizing the Muscle and Whole-Body Response to Feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-Chain Amino Acid Supplementation Promotes Survival and Supports Cardiac and Skeletal Muscle Mitochondrial Biogenesis in Middle-Aged Mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Nelson, D.R.; Zhao, Y.; Cui, Z.; A Johnston, J. Relationship between muscle mass and muscle strength, and the impact of comorbidities: A population-based, cross-sectional study of older adults in the United States. BMC Geriatr. 2013, 13, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-Y.; Park, S.; Jang, J.; Wolfe, R.R. Understanding Muscle Protein Dynamics: Technical Considerations for Advancing Sarcopenia Research. Ann. Geriatr. Med. Res. 2020, 24, 157–165. [Google Scholar] [CrossRef]
- Castillero, E.; Alamdari, N.; Lecker, S.H.; Hasselgren, P.-O. Suppression of atrogin-1 and MuRF1 prevents dexamethasone-induced atrophy of cultured myotubes. Metabolism 2013, 62, 1495–1502. [Google Scholar] [CrossRef]
- Troncoso, R.; Paredes, F.; Parra, V.; Gatica, D.; Vásquez-Trincado, C.; Quiroga, C.; Bravo-Sagua, R.; Lopez-Crisosto, C.; E Rodriguez, A.; Oyarzún, A.P.; et al. Dexamethasone-induced autophagy mediates muscle atrophy through mitochondrial clearance. Cell Cycle 2014, 13, 2281–2295. [Google Scholar] [CrossRef] [PubMed]
- Chevessier, F.; Girard, E.; Molgó, J.; Bartling, S.; Koenig, J.; Hantaï, D.; Witzemann, V. A mouse model for congenital myasthenic syndrome due to MuSK mutations reveals defects in structure and function of neuromuscular junctions. Hum. Mol. Genet. 2008, 17, 3577–3595. [Google Scholar] [CrossRef] [PubMed]
- Haddix, S.G.; Lee, Y.I.; Kornegay, J.N.; Thompson, W.J. Cycles of myofiber degeneration and regeneration lead to remodeling of the neuromuscular junction in two mammalian models of Duchenne muscular dystrophy. PLoS ONE 2018, 13, e0205926. [Google Scholar] [CrossRef] [PubMed]
- Klooster, R.; Plomp, J.J.; Huijbers, M.G.; Niks, E.; Straasheijm, K.R.; Detmers, F.J.; Hermans, P.W.; Sleijpen, K.; Verrips, A.; Losen, M.; et al. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain 2012, 135, 1081–1101. [Google Scholar] [CrossRef] [PubMed]
- Zierath, J.R.; Hawley, J. Skeletal Muscle Fiber Type: Influence on Contractile and Metabolic Properties. PLOS Biol. 2004, 2, e348. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Wang, P.-Y.; Matsumoto, T.; Sung, H.J.; Ma, W.; Choi, J.W.; Anderson, S.; Leary, S.C.; Balaban, R.S.; Kang, J.-G.; et al. p53 Improves Aerobic Exercise Capacity and Augments Skeletal Muscle Mitochondrial DNA Content. Circ. Res. 2009, 105, 705–712. [Google Scholar] [CrossRef]
- Binnert, C.; Ruchat, S.; Nicod, N.; Tappy, L. Dexamethasone-induced insulin resistance shows no gender difference in healthy humans. Diabetes Metab. 2004, 30, 321–326. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32 (Suppl. 2), S157–S163. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Chinkes, D.L. Isotope Tracers in Metabolic Research; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Kim, Y.A.; Kim, Y.S.; Oh, S.L.; Kim, H.-J.; Song, W. Autophagic response to exercise training in skeletal muscle with age. J. Physiol. Biochem. 2013, 69, 697–705. [Google Scholar] [CrossRef]
- Deschenes, M.R.; Judelson, D.A.; Kraemer, W.J.; Meskaitis, V.J.; Volek, J.S.; Nindl, B.C.; Harman, F.S.; Deaver, D.R. Effects of Resistance Training on Neuromuscular Junction Morphology. Muscle Nerve 2000, 23, 1576–1581. [Google Scholar] [CrossRef]
- Cruz, P.M.R.; Cossins, J.; Beeson, D.; Vincent, A. The Neuromuscular Junction in Health and Disease: Molecular Mechanisms Governing Synaptic Formation and Homeostasis. Front. Mol. Neurosci. 2020, 13, 610964. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z.; Lebrasseur, N.; Morris, C.; Smith, E.; Yang, W.; Ma, Y.; Chin, S.; Spiegelman, B.M. The Transcriptional Coactivator PGC-1β Drives the Formation of Oxidative Type IIX Fibers in Skeletal Muscle. Cell Metab. 2007, 5, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.F.; Santos, G.; Schnyder, S.; Handschin, C. PGC-1α affects aging-related changes in muscle and motor function by modulating specific exercise-mediated changes in old mice. Aging Cell 2018, 17, e12697. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; He, N.; Lin, C.S.; Wei, Z.; Hah, N.; Waizenegger, W.; He, M.-X.; Liddle, C.; Yu, R.T.; Atkins, A.R.; et al. ERRγ Promotes Angiogenesis, Mitochondrial Biogenesis, and Oxidative Remodeling in PGC1α/β-Deficient Muscle. Cell Rep. 2018, 22, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Okun, J.G.; Rusu, P.M.; Chan, A.Y.; Wu, Y.; Yap, Y.W.; Sharkie, T.; Schumacher, J.; Schmidt, K.V.; Roberts-Thomson, K.M.; Russell, R.D.; et al. Liver alanine catabolism promotes skeletal muscle atrophy and hyperglycaemia in type 2 diabetes. Nat. Metab. 2021, 3, 394–409. [Google Scholar] [CrossRef]
- Quattrocelli, M.; Wintzinger, M.; Miz, K.; Levine, D.C.; Peek, C.B.; Bass, J.; McNally, E.M. Muscle mitochondrial remodeling by intermittent glucocorticoid drugs requires an intact circadian clock and muscle PGC1α. Sci. Adv. 2022, 8, eabm1189. [Google Scholar] [CrossRef]
- Salamone, I.M.; Quattrocelli, M.; Barefield, D.Y.; Page, P.G.; Tahtah, I.; Hadhazy, M.; Tomar, G.; McNally, E.M. Intermittent glucocorticoid treatment enhances skeletal muscle performance through sexually dimorphic mechanisms. J. Clin. Investig. 2022, 132, e149828. [Google Scholar] [CrossRef] [PubMed]
- Marcinko, K.; Sikkema, S.R.; Samaan, M.C.; Kemp, B.E.; Fullerton, M.D.; Steinberg, G.R. High intensity interval training improves liver and adipose tissue insulin sensitivity. Mol. Metab. 2015, 4, 903–915. [Google Scholar] [CrossRef]
- Holm, L.; Haslund, M.L.; Robach, P.; van Hall, G.; A Calbet, J.; Saltin, B.; Lundby, C. Skeletal Muscle Myofibrillar and Sarcoplasmic Protein Synthesis Rates Are Affected Differently by Altitude-Induced Hypoxia in Native Lowlanders. PLoS ONE 2010, 5, e15606. [Google Scholar] [CrossRef]
- Shankaran, M.; King, C.L.; Angel, T.E.; Holmes, W.E.; Li, K.W.; Colangelo, M.; Price, J.C.; Turner, S.M.; Bell, C.; Hamilton, K.L.; et al. Circulating protein synthesis rates reveal skeletal muscle proteome dynamics. J. Clin. Investig. 2016, 126, 288–302. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Suh, S.-H.; Lee, I.-K.; Wolfe, R.R. Applications of stable, nonradioactive isotope tracers in in vivo human metabolic research. Exp. Mol. Med. 2016, 48, e203. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.M.; Murphy, E.J.; Neese, R.A.; Antelo, F.; Thomas, T.; Agarwal, A.; Go, C.; Hellerstein, M.K. Measurement of TG synthesis and turnover in vivo by2H2O incorporation into the glycerol moiety and application of MIDA. Am. J. Physiol. Metab. 2003, 285, E790–E803. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Fahey, T. EXERCISE PHYSIOLOGY: Human Bioenergetics and Its Applications; Kindle Direct Publishing: Seattle, WA, USA, 2019; ISBN 978-1080845484. [Google Scholar]
- Kim, I.-Y.; Park, S.; Smeets, E.T.H.C.; Schutzler, S.; Azhar, G.; Wei, J.Y.; Ferrando, A.A.; Wolfe, R.R. Consumption of a Specially-Formulated Mixture of Essential Amino Acids Promotes Gain in Whole-Body Protein to a Greater Extent than a Complete Meal Replacement in Older Women with Heart Failure. Nutrients 2019, 11, 1360. [Google Scholar] [CrossRef] [PubMed]
- Gasier, H.G.; Fluckey, J.D.; Previs, S.F. The application of 2H2O to measure skeletal muscle protein synthesis. Nutr. Metab. 2010, 7, 31. [Google Scholar] [CrossRef]
- Furugen, M.; Saitoh, S.; Ohnishi, H.; Akasaka, H.; Mitsumata, K.; Chiba, M.; Furukawa, T.; Miyazaki, Y.; Shimamoto, K.; Miura, T. Matsuda–DeFronzo insulin sensitivity index is a better predictor than HOMA-IR of hypertension in Japanese: The Tanno–Sobetsu study. J. Hum. Hypertens. 2012, 26, 325–333. [Google Scholar] [CrossRef] [Green Version]

| Amino Acid | Percentage (%) | Dietary Intake (g/kg/day) |
|---|---|---|
| Histidine | 10 | 0.150 |
| Isoleucine | 10 | 0.150 |
| Leucine | 21 | 0.315 |
| Lysine | 18 | 0.270 |
| Methionine | 4 | 0.060 |
| Phenylalanine | 12 | 0.180 |
| Threonine | 14 | 0.210 |
| Valine | 10 | 0.150 |
| Tryptophan | 1 | 0.015 |
| Total | 100 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.; Koh, J.-H.; Kim, Y.; Kim, H.-J.; Park, S.; Chang, Y.; Jung, J.; Wolfe, R.R.; Kim, I.-Y. Balanced Free Essential Amino Acids and Resistance Exercise Training Synergistically Improve Dexamethasone-Induced Impairments in Muscle Strength, Endurance, and Insulin Sensitivity in Mice. Int. J. Mol. Sci. 2022, 23, 9735. https://doi.org/10.3390/ijms23179735
Jang J, Koh J-H, Kim Y, Kim H-J, Park S, Chang Y, Jung J, Wolfe RR, Kim I-Y. Balanced Free Essential Amino Acids and Resistance Exercise Training Synergistically Improve Dexamethasone-Induced Impairments in Muscle Strength, Endurance, and Insulin Sensitivity in Mice. International Journal of Molecular Sciences. 2022; 23(17):9735. https://doi.org/10.3390/ijms23179735
Chicago/Turabian StyleJang, Jiwoong, Jin-Ho Koh, Yeongmin Kim, Hee-Joo Kim, Sanghee Park, Yewon Chang, Jiyeon Jung, Robert R. Wolfe, and Il-Young Kim. 2022. "Balanced Free Essential Amino Acids and Resistance Exercise Training Synergistically Improve Dexamethasone-Induced Impairments in Muscle Strength, Endurance, and Insulin Sensitivity in Mice" International Journal of Molecular Sciences 23, no. 17: 9735. https://doi.org/10.3390/ijms23179735
APA StyleJang, J., Koh, J.-H., Kim, Y., Kim, H.-J., Park, S., Chang, Y., Jung, J., Wolfe, R. R., & Kim, I.-Y. (2022). Balanced Free Essential Amino Acids and Resistance Exercise Training Synergistically Improve Dexamethasone-Induced Impairments in Muscle Strength, Endurance, and Insulin Sensitivity in Mice. International Journal of Molecular Sciences, 23(17), 9735. https://doi.org/10.3390/ijms23179735










