Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics
Abstract
:1. Introduction
2. Bioceramics: Calcium Phosphates
3. Polymer-HA Biocomposite Scaffolds
3.1. Natural Polymers
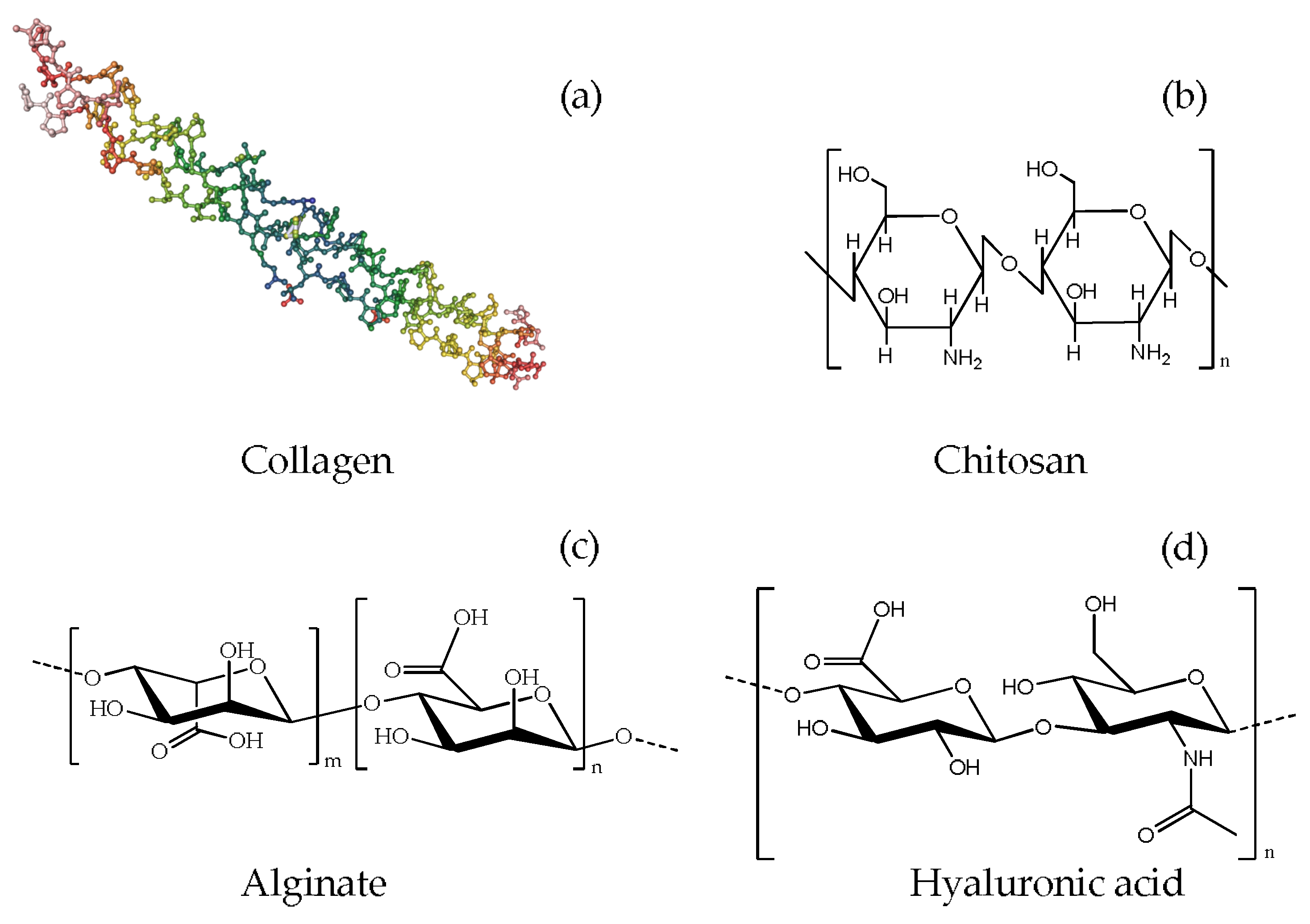
3.1.1. Collagen
3.1.2. Chitosan
3.1.3. Alginate
3.1.4. Hyaluronic Acid
3.2. Synthetic Polymers
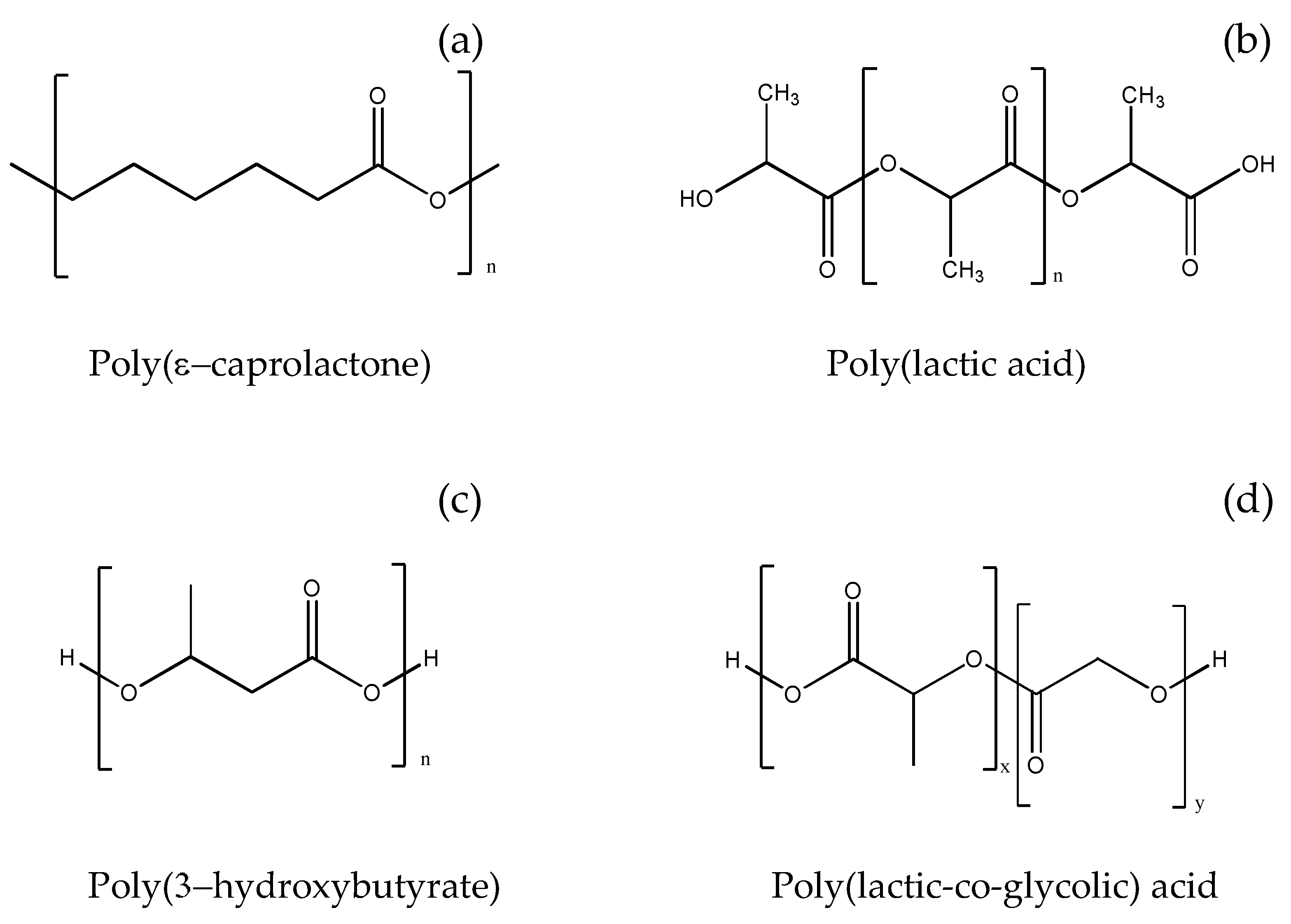
3.2.1. Poly-ε-caprolactone
3.2.2. Poly(lactic Acid)
3.2.3. Poly(3-hydroxybutyrate)
3.2.4. Poly(lactic-co-glycolic Acid)
4. Crosslinking Methods and Agents in HA-Based Composite Scaffolds
4.1. Physical Biopolymers Crosslinking
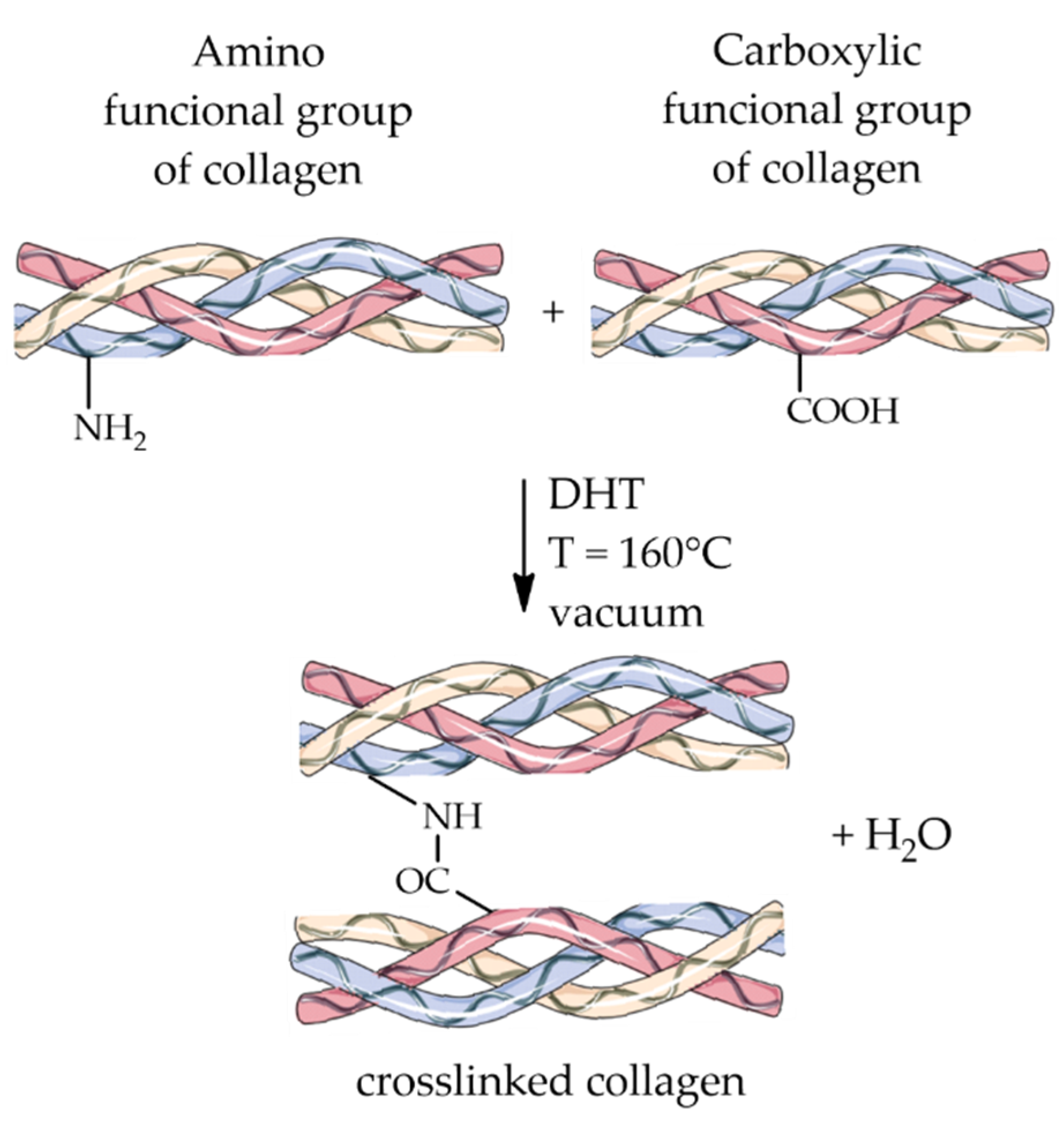
4.1.1. Dehydrothermal Treatment
4.1.2. Radiation
4.2. Chemical Biopolymers Crosslinking
4.2.1. Glutaraldehyde
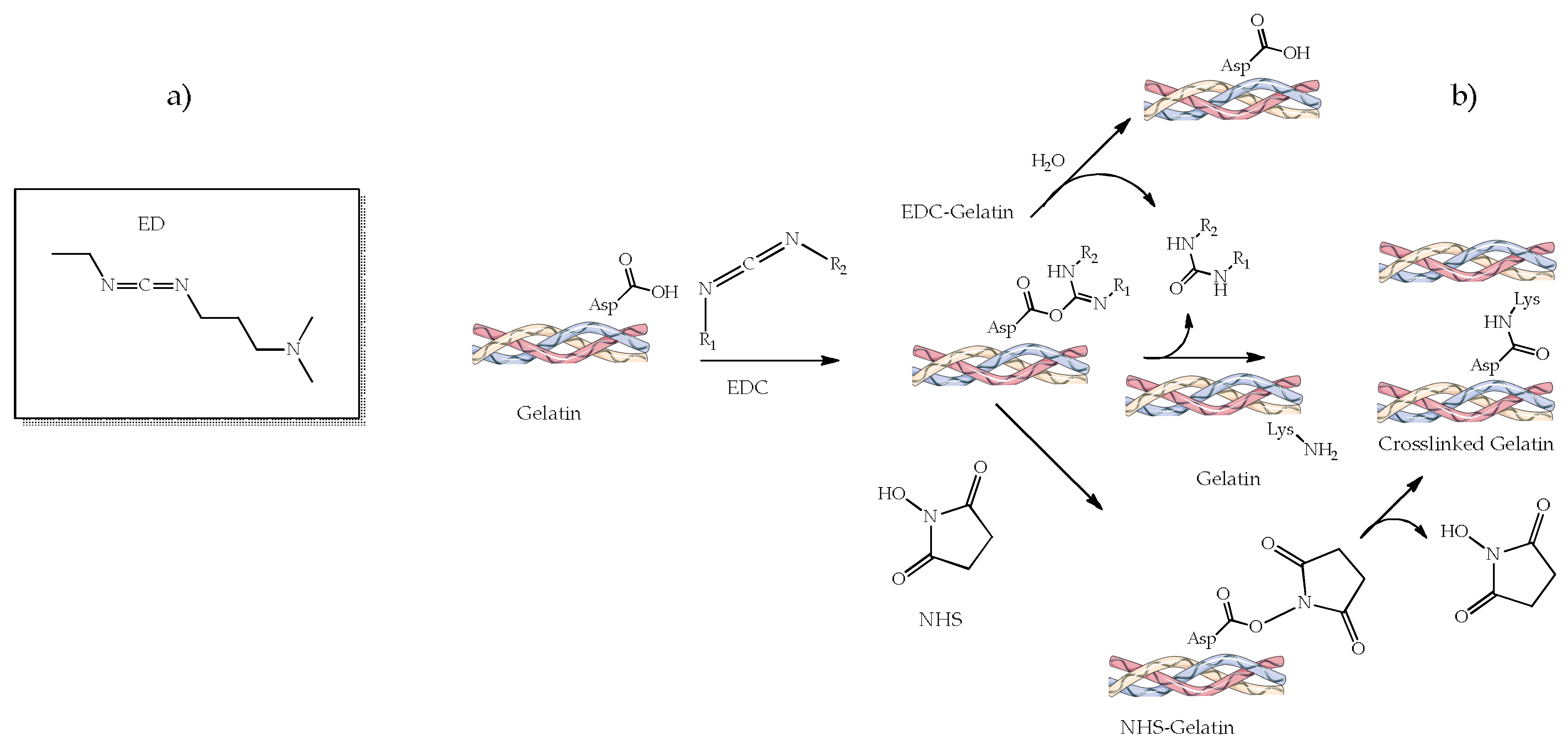
4.2.2. 1-Ethyl-3-(3-dimethylamino Propyl) Carbodiimide (EDC) and N-hydroxysuccinimide (NHS)
4.2.3. 1,4-Butanediol Diglycidyl Ether
4.2.4. Genipin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Alg | Alginate |
| BDDGE | 1,4-butanediol diglycidyl ether |
| BMP | Bone morphogenic proteins |
| BMSC | Bone mesenchymal stromal cells |
| BMSCs | Bone mesenchymal stem cells |
| CNT | Carbon nanotube |
| Col | Collagen |
| CS | Chitosan |
| CT | Computed tomography |
| DHT | Dehydrothermal |
| DOX | Doxorubicin |
| ECM | Extracellular matrix |
| EDC | 1-ethyl-3- (3-dimethylamino propyl) carbodiimide hydrochloride |
| GO | Graphene oxide |
| GTA | Glutaraldehyde |
| HA | Hydroxyapatite |
| hMSC | Human mesenchymal stem cells |
| HyA | Hyaluronic acid |
| MgHA | Magnesium-doped hydroxyapatite |
| MSCs | Mesenchymal stem cells |
| MWCNT | Multiwalled carbon nanotubes |
| nHA | Nanohydroxyapatite |
| NHS | N-hydroxysuccinimide |
| PCL | Poly-ε-caprolactone |
| PDA | Polydopamine |
| PHB | Polyhydroxybutyrate |
| PLA | Polylactic acid |
| PLGA | Poly(lactide-co-glycolide) |
| PLLA | poly (L)-lactic acid |
| PVA | Polyvinyl alcohol |
| SD | Sprague Dawley |
| TCP | Tricalcium phosphates |
| UV | Ultraviolet |
References
- Alves, N.M.; Leonor, I.B.; Azevedo, H.S.; Reis, R.L.; Mano, J.F. Designing biomaterials based on biomineralization of bone. J. Mater. Chem. 2010, 20, 2911–2921. [Google Scholar] [CrossRef]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Wang, G.; Zreiqat, H. Functional Coatings or Films for Hard-Tissue Applications. Materials 2010, 3, 3994–4050. [Google Scholar] [CrossRef]
- Daculsi, G.; Fellah, B.H.; Miramond, T. The Essential Role of Calcium Phosphate Bioceramics in Bone Regeneration; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783642539794. [Google Scholar]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Zwingenberger, S.; Nich, C.; Valladares, R.D.; Yao, Z.; Stiehler, M.; Goodman, S.B. Recommendations and considerations for the use of biologics in orthopedic surgery. BioDrugs 2012, 26, 245–256. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Krishnakumar, G.S.; Gostynska, N.; Campodoni, E.; Dapporto, M.; Montesi, M.; Panseri, S.; Tampieri, A.; Kon, E.; Marcacci, M.; Sprio, S.; et al. Ribose mediated crosslinking of collagen-hydroxyapatite hybrid scaffolds for bone tissue regeneration using biomimetic strategies. Mater. Sci. Eng. C 2017, 77, 594–605. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kozłowska, J. Properties and modification of porous 3-D collagen/hydroxyapatite composites. Int. J. Biol. Macromol. 2013, 52, 250–259. [Google Scholar] [CrossRef]
- Fang, J.; Li, P.; Lu, X.; Fang, L.; Lü, X.; Ren, F. A strong, tough, and osteoconductive hydroxyapatite mineralized polyacrylamide/dextran hydrogel for bone tissue regeneration. Acta Biomater. 2019, 88, 503–513. [Google Scholar] [CrossRef]
- Chen, C.; Sun, X.; Pan, W.; Hou, Y.; Liu, R.; Jiang, X.; Zhang, L. Graphene Oxide-Templated Synthesis of Hydroxyapatite Nanowhiskers to Improve the Mechanical and Osteoblastic Performance of Poly(lactic acid) for Bone Tissue Regeneration. ACS Sustain. Chem. Eng. 2018, 6, 3862–3869. [Google Scholar] [CrossRef]
- Sinha, A.; Nayar, S.; Agrawal, A.; Bhattacharyya, D.; Ramachandrarao, P. Synthesis of nanosized and microporous precipitated hydroxyapatite in synthetic polymers and biopolymers. J. Am. Ceram. Soc. 2003, 86, 357–359. [Google Scholar] [CrossRef]
- Basu, J.; Ludlow, J.W. Platform technologies for tubular organ regeneration. Trends Biotechnol. 2010, 28, 526–533. [Google Scholar] [CrossRef]
- Tampieri, A.; Sprio, S.; Sandri, M.; Valentini, F. Mimicking natural bio-mineralization processes: A new tool for osteochondral scaffold development. Trends Biotechnol. 2011, 29, 526–535. [Google Scholar] [CrossRef]
- Shankar, K.G.; Gostynska, N.; Montesi, M.; Panseri, S.; Sprio, S.; Kon, E.; Marcacci, M.; Tampieri, A.; Sandri, M. Investigation of different cross-linking approaches on 3D gelatin scaffolds for tissue engineering application: A comparative analysis. Int. J. Biol. Macromol. 2017, 95, 1199–1209. [Google Scholar] [CrossRef]
- Poursamar, S.A.; Lehner, A.N.; Azami, M.; Ebrahimi-Barough, S.; Samadikuchaksaraei, A.; Antunes, A.P.M. The effects of crosslinkers on physical, mechanical, and cytotoxic properties of gelatin sponge prepared via in-situ gas foaming method as a tissue engineering scaffold. Mater. Sci. Eng. C 2016, 63, 1–9. [Google Scholar] [CrossRef]
- Carson, J.S.; Bostrom, M.P.G. Synthetic bone scaffolds and fracture repair. Injury 2007, 38 (Suppl. S1), S33–S37. [Google Scholar] [CrossRef]
- Owen, G.R.; Dard, M.; Larjava, H. Hydoxyapatite/beta-tricalcium phosphate biphasic ceramics as regenerative material for the repair of complex bone defects. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2018, 106, 2493–2512. [Google Scholar] [CrossRef]
- Ogose, A.; Kondo, N.; Umezu, H.; Hotta, T.; Kawashima, H.; Tokunaga, K.; Ito, T.; Kudo, N.; Hoshino, M.; Gu, W.; et al. Histological assessment in grafts of highly purified beta-tricalcium phosphate (OSferion) in human bones. Biomaterials 2006, 27, 1542–1549. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite-polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef]
- Wenisch, S.; Stahl, J.-P.; Horas, U.; Heiss, C.; Kilian, O.; Trinkaus, K.; Hild, A.; Schnettler, R. In vivo mechanisms of hydroxyapatite ceramic degradation by osteoclasts: Fine structural microscopy. J. Biomed. Mater. Res. A 2003, 67, 713–718. [Google Scholar] [CrossRef]
- Fritton, S.P.; Weinbaum, S. Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction. Annu. Rev. Fluid Mech. 2009, 41, 347–374. [Google Scholar] [CrossRef]
- Kang, Y.; Scully, A.; Young, D.A.; Kim, S.; Tsao, H.; Sen, M.; Yang, Y. Enhanced mechanical performance and biological evaluation of a PLGA coated β-TCP composite scaffold for load-bearing applications. Eur. Polym. J. 2011, 47, 1569–1577. [Google Scholar] [CrossRef]
- Tonino, A.J.; van der Wal, B.C.H.; Heyligers, I.C.; Grimm, B. Bone remodeling and hydroxyapatite resorption in coated primary hip prostheses. Clin. Orthop. Relat. Res. 2009, 467, 478–484. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7, BTRI.S36138. [Google Scholar] [CrossRef]
- Lekshmi, G.; Sana, S.S.; Nguyen, V.-H.; Nguyen, T.H.C.; Nguyen, C.C.; Van Le, Q.; Peng, W. Recent Progress in Carbon Nanotube Polymer Composites in Tissue Engineering and Regeneration. Int. J. Mol. Sci. 2020, 21, 6440. [Google Scholar] [CrossRef]
- Mukherjee, S.; Nandi, S.K.; Kundu, B.; Chanda, A.; Sen, S.; Das, P.K. Enhanced bone regeneration with carbon nanotube reinforced hydroxyapatite in animal model. J. Mech. Behav. Biomed. Mater. 2016, 60, 243–255. [Google Scholar] [CrossRef]
- Williams, D.F. There is no such thing as a biocompatible material. Biomaterials 2014, 35, 10009–10014. [Google Scholar] [CrossRef]
- Bal, Z.; Kaito, T.; Korkusuz, F.; Yoshikawa, H. Bone regeneration with hydroxyapatite-based biomaterials. Emergent Mater. 2020, 3, 521–544. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, H.; Lee, J. Various preparation methods of highly porous hydroxyapatite/polymer nanoscale biocomposites for bone regeneration. Acta Biomater. 2011, 7, 3813–3828. [Google Scholar] [CrossRef]
- Türk, S.; Altınsoy, I.; Çelebi Efe, G.; Ipek, M.; Özacar, M.; Bindal, C. 3D porous collagen/functionalized multiwalled carbon nanotube/chitosan/hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2018, 92, 757–768. [Google Scholar] [CrossRef]
- Minardi, S.; Taraballi, F.; Cabrera, F.J.; Van Eps, J.; Wang, X.; Gazze, S.A.; Fernandez-Mourev, J.S.; Tampieri, A.; Francis, L.; Weiner, B.K.; et al. Biomimetic hydroxyapatite/collagen composite drives bone niche recapitulation in a rabbit orthotopic model. Mater. Today Bio 2019, 2, 100005. [Google Scholar] [CrossRef]
- Xing, F.; Chi, Z.; Yang, R.; Xu, D.; Cui, J.; Huang, Y.; Zhou, C.; Liu, C. Chitin-hydroxyapatite-collagen composite scaffolds for bone regeneration. Int. J. Biol. Macromol. 2021, 184, 170–180. [Google Scholar] [CrossRef]
- Jin, S.; Sun, F.; Zou, Q.; Huang, J.; Zuo, Y.; Li, Y.; Wang, S.; Cheng, L.; Man, Y.; Yang, F.; et al. Fish Collagen and Hydroxyapatite Reinforced Poly(lactide-co-glycolide) Fibrous Membrane for Guided Bone Regeneration. Biomacromolecules 2019, 20, 2058–2067. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Liu, D.; Yang, Q.; Huang, C.; Yang, C.; Zhang, Q. Preparation and cytological study of collagen/nano-hydroxyapatite/graphene oxide composites. Acta Bioeng. Biomech. 2018, 20, 65–74. [Google Scholar] [CrossRef]
- Song, Y.; Wu, H.; Gao, Y.; Li, J.; Lin, K.; Liu, B.; Lei, X.; Cheng, P.; Zhang, S.; Wang, Y.; et al. Zinc Silicate/Nano-Hydroxyapatite/Collagen Scaffolds Promote Angiogenesis and Bone Regeneration via the p38 MAPK Pathway in Activated Monocytes. ACS Appl. Mater. Interfaces 2020, 12, 16058–16075. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B. Preparation and characterization of composites based on the blends of collagen, chitosan and hyaluronic acid with nano-hydroxyapatite. Int. J. Biol. Macromol. 2017, 102, 658–666. [Google Scholar] [CrossRef]
- Salim, S.A.; Loutfy, S.A.; El-Fakharany, E.M.; Taha, T.H.; Hussien, Y.; Kamoun, E.A. Influence of chitosan and hydroxyapatite incorporation on properties of electrospun PVA/HA nanofibrous mats for bone tissue regeneration: Nanofibers optimization and in-vitro assessment. J. Drug Deliv. Sci. Technol. 2021, 62, 102417. [Google Scholar] [CrossRef]
- Shi, D.; Shen, J.; Zhang, Z.; Shi, C.; Chen, M.; Gu, Y.; Liu, Y. Preparation and properties of dopamine-modified alginate/chitosan–hydroxyapatite scaffolds with gradient structure for bone tissue engineering. J. Biomed. Mater. Res.-Part A 2019, 107, 1615–1627. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, Y.; Ren, H.T.; Peng, H.K.; Lou, C.W.; Lin, J.H. Two-step strategy for constructing hierarchical pore structured chitosan–hydroxyapatite composite scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 260, 117765. [Google Scholar] [CrossRef]
- Ran, J.; Jiang, P.; Sun, G.; Ma, Z.; Hu, J.; Shen, X.; Tong, H. Comparisons among Mg, Zn, Sr, and Si doped nano-hydroxyapatite/chitosan composites for load-bearing bone tissue engineering applications. Mater. Chem. Front. 2017, 1, 900–910. [Google Scholar] [CrossRef]
- Ocando, C.; Dinescu, S.; Samoila, I.; Daniela Ghitulica, C.; Cucuruz, A.; Costache, M.; Averous, L. Fabrication and properties of alginate-hydroxyapatite biocomposites as efficient biomaterials for bone regeneration. Eur. Polym. J. 2021, 151, 110444. [Google Scholar] [CrossRef]
- Mahmoud, E.M.; Sayed, M.; El-Kady, A.M.; Elsayed, H.; Naga, S.M. In vitro and in vivo study of naturally derived alginate/hydroxyapatite bio composite scaffolds. Int. J. Biol. Macromol. 2020, 165, 1346–1360. [Google Scholar] [CrossRef]
- Bendtsen, S.T.; Quinnell, S.P.; Wei, M. Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J. Biomed. Mater. Res.-Part A 2017, 105, 1457–1468. [Google Scholar] [CrossRef]
- Kohli, N.; Sharma, V.; Orera, A.; Sawadkar, P.; Owji, N.; Frost, O.G.; Bailey, R.J.; Snow, M.; Knowles, J.C.; Blunn, G.W.; et al. Pro-angiogenic and osteogenic composite scaffolds of fibrin, alginate and calcium phosphate for bone tissue engineering. J. Tissue Eng. 2021, 12, 20417314211005610. [Google Scholar] [CrossRef]
- Patil, T.K.; Saha, S.; Biswas, A.K. Preparation and Characterization of HAp Coated Chitosan-Alginate PEC Porous Scaffold for Bone Tissue Engineering. Macromol. Symp. 2017, 376, 1600205. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Z.; Zou, J.; Li, L.; Sui, X.; Wang, B.; Yang, N.; Wang, B. Synthesis and Characterization of a Hydroxyapatite-Sodium Alginate-Chitosan Scaffold for Bone Regeneration. Front. Mater. 2021, 8, 648980. [Google Scholar] [CrossRef]
- Liu, S.; Hu, Y.; Zhang, J.; Bao, S.; Xian, L.; Dong, X.; Zheng, W.; Li, Y.; Gao, H.; Zhou, W. Bioactive and Biocompatible Macroporous Scaffolds with Tunable Performances Prepared Based on 3D Printing of the Pre-Crosslinked Sodium Alginate/Hydroxyapatite Hydrogel Ink. Macromol. Mater. Eng. 2019, 304, 1–11. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Kozlowska, J.; Osyczka, A.M. New composite materials prepared by calcium phosphate precipitation in chitosan/collagen/hyaluronic acid sponge cross-linked by EDC/NHS. Int. J. Biol. Macromol. 2018, 107, 247–253. [Google Scholar] [CrossRef]
- Sujana, A.; Venugopal, J.R.; Velmurugan, B.; Góra, A.; Salla, M.; Ramakrishna, S. Hydroxyapatite-intertwined hybrid nanofibres for the mineralization of osteoblasts. J. Tissue Eng. Regen. Med. 2017, 11, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Dennis, S.C.; Whitlow, J.; Detamore, M.S.; Kieweg, S.L.; Berkland, C.J. Hyaluronic-Acid-Hydroxyapatite Colloidal Gels Combined with Micronized Native ECM as Potential Bone Defect Fillers. Langmuir 2017, 33, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Fan, T.; Zhang, Y.; Zhao, Y.; Shi, X.; Zhang, Q. Biomimetic mineralized hierarchical hybrid scaffolds based on in situ synthesis of nano-hydroxyapatite/chitosan/chondroitin sulfate/hyaluronic acid for bone tissue engineering. Colloids Surfaces B Biointerfaces 2017, 157, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.Z.; Bella, J.; Mayville, P.; Brodsky, B.; Berman, H.M. Sequence dependent conformational variations of collagen triple-helical structure. Nat. Struct. Biol. 1999, 6, 454–457. [Google Scholar] [CrossRef]
- Gopal Shankar, K.; Udhaya Kumar, S.; Sowndarya, S.; Suresh Babu, P.; Rose, C. Isolation, characterization, and in vitro evaluation of bovine rumen submucosa films of collagen or chitosan-treated collagen. J. Biomater. Appl. 2015, 30, 780–792. [Google Scholar] [CrossRef]
- Park, H.; Rosenzweig, D.H.; Nazhat, S.N. Dense collagen-based scaffolds for soft tissue engineering applications. In Tissue Engineering Using Ceramics and Polymers; Boccaccini, A.R., Ma, P.X., Liverani, L., Eds.; Woodhead Publishing: Cambrige, UK, 2022; pp. 771–802. ISBN 978-0-12-820508-2. [Google Scholar]
- Calabrese, G.; Giuffrida, R.; Fabbi, C.; Figallo, E.; Lo Furno, D.; Gulino, R.; Colarossi, C.; Fullone, F.; Giuffrida, R.; Parenti, R.; et al. Collagen-Hydroxyapatite Scaffolds Induce Human Adipose Derived Stem Cells Osteogenic Differentiation In Vitro. PLoS ONE 2016, 11, e0151181. [Google Scholar]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Fabbi, C.; Figallo, E.; Salvatorelli, L.; Memeo, L.; Parenti, R.; Gulisano, M.; Gulino, R. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci. Rep. 2017, 7, 7110. [Google Scholar] [CrossRef]
- Islam, M.S.; Todo, M. Effects of sintering temperature on the compressive mechanical properties of collagen/hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Lett. 2016, 173, 231–234. [Google Scholar] [CrossRef]
- Masaoka, T.; Yamada, T.; Yuasa, M.; Yoshii, T.; Okawa, A.; Morita, S.; Kozaka, Y.; Hirano, M.; Sotome, S. Biomechanical evaluation of the rabbit tibia after implantation of porous hydroxyapatite/collagen in a rabbit model. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2016, 21, 230–236. [Google Scholar] [CrossRef]
- Meagher, M.J.; Weiss-Bilka, H.E.; Best, M.E.; Boerckel, J.D.; Wagner, D.R.; Roeder, R.K. Acellular hydroxyapatite-collagen scaffolds support angiogenesis and osteogenic gene expression in an ectopic murine model: Effects of hydroxyapatite volume fraction. J. Biomed. Mater. Res.-Part A 2016, 104, 2178–2188. [Google Scholar] [CrossRef]
- Christensen, B.B.; Foldager, C.B.; Jensen, J.; Jensen, N.C.; Lind, M. Poor osteochondral repair by a biomimetic collagen scaffold: 1- to 3-year clinical and radiological follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2380–2387. [Google Scholar] [CrossRef]
- Zimba, B.L.; Jiang, H.; Chen, L.; Li, Y.; Yu, X.; Chen, C.; Wan, J.; Wu, Q. Preparation and characterization of three- dimension porous collagen/graphene oxide/hydroxyapatite nanocomposite scaffolds for bone tissue engineering abstract. Open Sci. J. 2019, 4, 1–15. [Google Scholar] [CrossRef]
- Kushioka, J.; Kaito, T.; Makino, T.; Fujiwara, H.; Tsukazaki, H.; Takenaka, S.; Sakai, Y.; Yoshikawa, H. Difference in the fusion rate and bone formation between artificial bone and iliac autograft inside an inter-body fusion cage—A comparison between porous hydroxyapatite/type 1 collagen composite and autologous iliac bone. J. Orthop. Sci. 2018, 23, 622–626. [Google Scholar] [CrossRef]
- Teli, M.D.; Sheikh, J. Extraction of chitosan from shrimp shells waste and application in antibacterial finishing of bamboo rayon. Int. J. Biol. Macromol. 2012, 50, 1195–1200. [Google Scholar] [CrossRef]
- Niranjan, R.; Koushik, C.; Saravanan, S.; Moorthi, A.; Vairamani, M.; Selvamurugan, N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2013, 54, 24–29. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Sikkema, R.; Keohan, B.; Zhitomirsky, I. Alginic acid polymer-hydroxyapatite composites for bone tissue engineering. Polymers 2021, 13, 3070. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Gołyńska, M.; Polkowska, I.; Szponder, T.; Nehrbass, D.; Osyczka, A.M. In Vivo study on scaffolds based on chitosan, collagen, and hyaluronic acid with hydroxyapatite. Int. J. Biol. Macromol. 2018, 118, 938–944. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, B.; Yin, P.; Zhao, L.; Wang, Y.; Fu, Z.; Dang, R.; Xu, J.; Zhang, J.; Wen, N. Integration of Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomes with Hydroxyapatite-Embedded Hyaluronic Acid-Alginate Hydrogel for Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 1590–1602. [Google Scholar] [CrossRef]
- Shi, C.; Yuan, Z.; Han, F.; Zhu, C.; Li, B. Polymeric biomaterials for bone regeneration. Ann. Jt. 2016, 1, 27. [Google Scholar] [CrossRef]
- Shor, L.; Güçeri, S.; Wen, X.; Gandhi, M.; Sun, W. Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions In Vitro. Biomaterials 2007, 28, 5291–5297. [Google Scholar] [CrossRef]
- Shitole, A.A.; Raut, P.W.; Sharma, N.; Giram, P.; Khandwekar, A.P.; Garnaik, B. Electrospun polycaprolactone/hydroxyapatite/ZnO nanofibers as potential biomaterials for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2019, 30, 51. [Google Scholar] [CrossRef]
- Hokmabad, V.R.; Davaran, S.; Aghazadeh, M.; Rahbarghazi, R.; Salehi, R.; Ramazani, A. Fabrication and characterization of novel ethyl cellulose-grafted-poly (ɛ-caprolactone)/alginate nanofibrous/macroporous scaffolds incorporated with nano-hydroxyapatite for bone tissue engineering. J. Biomater. Appl. 2019, 33, 1128–1144. [Google Scholar] [CrossRef]
- Lin, W.C.; Yao, C.; Huang, T.Y.; Cheng, S.J.; Tang, C.M. Long-term in vitro degradation behavior and biocompatibility of polycaprolactone/cobalt-substituted hydroxyapatite composite for bone tissue engineering. Dent. Mater. 2019, 35, 751–762. [Google Scholar] [CrossRef]
- Rezk, A.I.; Kim, K.-S.; Kim, C.S. Composite Nanofibers Incorporating Hydroxyapatite Nanoparticles and Simvastatin for Bone Tissue. Polymers 2020, 12, 2667. [Google Scholar] [CrossRef]
- Cho, Y.S.; Quan, M.; Lee, S.-H.; Hong, M.W.; Kim, Y.Y.; Cho, Y.-S. Assessment of osteogenesis for 3D-printed polycaprolactone/hydroxyapatite composite scaffold with enhanced exposure of hydroxyapatite using rat calvarial defect model. Compos. Sci. Technol. 2019, 184, 107844. [Google Scholar] [CrossRef]
- Roh, H.-S.; Lee, C.-M.; Hwang, Y.-H.; Kook, M.-S.; Yang, S.-W.; Lee, D.; Kim, B.-H. Addition of MgO nanoparticles and plasma surface treatment of three-dimensional printed polycaprolactone/hydroxyapatite scaffolds for improving bone regeneration. Mater. Sci. Eng. C 2017, 74, 525–535. [Google Scholar] [CrossRef]
- Pavia, F.C.; Conoscenti, G.; Greco, S.; La Carrubba, V.; Ghersi, G.; Brucato, V. Preparation, characterization and in vitro test of composites poly-lactic acid/hydroxyapatite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2018, 119, 945–953. [Google Scholar] [CrossRef]
- Fernández-Cervantes, I.; Morales, M.A.; Agustín-Serrano, R.; Cardenas-García, M.; Pérez-Luna, P.V.; Arroyo-Reyes, B.L.; Maldonado-García, A. Polylactic acid/sodium alginate/hydroxyapatite composite scaffolds with trabecular tissue morphology designed by a bone remodeling model using 3D printing. J. Mater. Sci. 2019, 54, 9478–9496. [Google Scholar] [CrossRef]
- Flores-Sánchez, M.G.; Islas-Arteaga, N.C.; Raya-Rivera, A.M.; Esquiliano-Rendon, D.R.; Morales-Corona, J.; Uribe-Juarez, O.E.; Vivar-Velázquez, F.I.; Ortiz-Vázquez, G.P.; Olayo, R. Effect of a plasma synthesized polypyrrole coverage on polylactic acid/hydroxyapatite scaffolds for bone tissue engineering. J. Biomed. Mater. Res.-Part A 2021, 109, 2199–2211. [Google Scholar] [CrossRef]
- Salehi, M.; Bastami, F.; Rezai Rad, M.; Nokhbatolfoghahaei, H.; Paknejad, Z.; Nazeman, P.; Hassani, A.; Khojasteh, A. Investigation of cell-free poly lactic acid/nanoclay scaffolds prepared via thermally induced phase separation technique containing hydroxyapatite nanocarriers of erythropoietin for bone tissue engineering applications. Polym. Adv. Technol. 2021, 32, 670–680. [Google Scholar] [CrossRef]
- Prakash, C.; Singh, G.; Singh, S.; Linda, W.L.; Zheng, H.Y.; Ramakrishna, S.; Narayan, R. Mechanical Reliability and In Vitro Bioactivity of 3D-Printed Porous Polylactic Acid-Hydroxyapatite Scaffold. J. Mater. Eng. Perform. 2021, 30, 4946–4956. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Zhao, D.; Jiang, W.; Du, Z.; Li, Q.; Jiang, C.; Han, D. Three dimensional printed polylactic acid-hydroxyapatite composite scaffolds for prefabricating vascularized tissue engineered bone: An in vivo bioreactor model. Sci. Rep. 2017, 7, 15255. [Google Scholar] [CrossRef]
- Yeon, Y.K.; Park, H.S.; Lee, J.M.; Lee, J.S.; Lee, Y.J.; Sultan, T.; Bin Seo, Y.; Lee, O.J.; Kim, S.H.; Park, C.H. New concept of 3D printed bone clip (polylactic acid/hydroxyapatite/silk composite) for internal fixation of bone fractures. J. Biomater. Sci. Polym. Ed. 2018, 29, 894–906. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Y.; Zhang, J.; Liu, W.; Cui, J.; Li, H.; Chen, F. Laminated electrospun nHA/PHB-composite scaffolds mimicking bone extracellular matrix for bone tissue engineering. Mater. Sci. Eng. C 2017, 72, 341–351. [Google Scholar] [CrossRef]
- Degli Esposti, M.; Chiellini, F.; Bondioli, F.; Morselli, D.; Fabbri, P. Highly porous PHB-based bioactive scaffolds for bone tissue engineering by in situ synthesis of hydroxyapatite. Mater. Sci. Eng. C 2019, 100, 286–296. [Google Scholar] [CrossRef]
- Volkov, A.V.; Muraev, A.A.; Zharkova, I.I.; Voinova, V.V.; Akoulina, E.A.; Zhuikov, V.A.; Khaydapova, D.D.; Chesnokova, D.V.; Menshikh, K.A.; Dudun, A.A.; et al. Poly(3-hydroxybutyrate)/hydroxyapatite/alginate scaffolds seeded with mesenchymal stem cells enhance the regeneration of critical-sized bone defect. Mater. Sci. Eng. C 2020, 114, 110991. [Google Scholar] [CrossRef]
- Cavalcante, M.d.P.; de Menezes, L.R.; Rodrigues, E.J.d.R.; Tavares, M.I.B. In Vitro characterization of a biocompatible composite based on poly(3-hydroxybutyrate)/hydroxyapatite nanoparticles as a potential scaffold for tissue engineering. J. Mech. Behav. Biomed. Mater. 2022, 128, 105138. [Google Scholar] [CrossRef]
- Tehrani, A.H.; Zadhoush, A.; Karbasi, S. Preparing nanocomposite fibrous scaffolds of P3HB/nHA for bone tissue engineering. In Proceedings of the 17th Iranian Conference of Biomedical Engineering (ICBME), Isfahan, Iran, 3–4 November 2010; pp. 1–4. [Google Scholar]
- Senatov, F.; Anisimova, N.; Kiselevskiy, M.; Kopylov, A.; Tcherdyntsev, V.; Maksimkin, A. Polyhydroxybutyrate/Hydroxyapatite Highly Porous Scaffold for Small Bone Defects Replacement in the Nonload-bearing Parts. J. Bionic Eng. 2017, 14, 648–658. [Google Scholar] [CrossRef]
- dos Santos, V.I.; Merlini, C.; Aragones, Á.; Cesca, K.; Fredel, M.C. In Vitro evaluation of bilayer membranes of PLGA/hydroxyapatite/β-tricalcium phosphate for guided bone regeneration. Mater. Sci. Eng. C 2020, 112, 110849. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Z.; Li, L.; Shi, X.; Jia, E.; Ji, Q.; Wang, Y.; Ito, Y.; Wei, Y.; Zhang, P. DOPA-derived electroactive copolymer and IGF-1 immobilized poly(lactic-co-glycolic acid)/hydroxyapatite biodegradable microspheres for synergistic bone repair. Chem. Eng. J. 2021, 416, 129129. [Google Scholar] [CrossRef]
- Lu, Y.; Wan, Y.; Gan, D.; Zhang, Q.; Luo, H.; Deng, X.; Li, Z.; Yang, Z. Enwrapping Polydopamine on Doxorubicin-Loaded Lamellar Hydroxyapatite/Poly(lactic- co-glycolic acid) Composite Fibers for Inhibiting Bone Tumor Recurrence and Enhancing Bone Regeneration. ACS Appl. Bio Mater. 2021, 4, 6036–6045. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Z.; Dong, S.; Cai, Y.; Ni, Y.; Zhang, T.; Wang, L.; Zhou, Y. Bilayer poly(Lactic-co-glycolic acid)/nano- hydroxyapatite membrane with barrier function and Osteogenesis promotion for guided bone regeneration. Materials 2017, 10, 257. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Presta, R.; Lupi, S.M.; Giarratana, N.; Bloise, N.; Benedetti, L.; De Angelis, M.G.C.; Baena, R.R.Y. Evaluation of poly(lactic-co-glycolic) acid alone or in combination with hydroxyapatite on human-periosteal cells bone differentiation and in sinus lift treatment. Molecules 2017, 22, 2109. [Google Scholar] [CrossRef]
- Wang, T.; Yang, X.; Qi, X.; Jiang, C. Osteoinduction and proliferation of bone-marrow stromal cells in three-dimensional poly (ε-caprolactone)/hydroxyapatite/collagen scaffolds. J. Transl. Med. 2015, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, S.; Das, S. Micromechanical finite-element modeling and experimental characterization of the compressive mechanical properties of polycaprolactone–hydroxyapatite composite scaffolds prepared by selective laser sintering for bone tissue engineering. Acta Biomater. 2012, 8, 3138–3143. [Google Scholar] [CrossRef]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Kaito, T.; Myoui, A.; Takaoka, K.; Saito, N.; Nishikawa, M.; Tamai, N.; Ohgushi, H.; Yoshikawa, H. Potentiation of the activity of bone morphogenetic protein-2 in bone regeneration by a PLA-PEG/hydroxyapatite composite. Biomaterials 2005, 26, 73–79. [Google Scholar] [CrossRef]
- Dariš, B.; Knez, Ž. Poly(3-hydroxybutyrate): Promising biomaterial for bone tissue engineering. Acta Pharm. 2020, 70, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Z.; Ding, J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/beta-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 6, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Gu, Y.; Zhu, Y.; Chen, L.; Chen, Y. Production of Composite Scaffold Containing Silk Fibroin, Chitosan, and Gelatin for 3D Cell Culture and Bone Tissue Regeneration. Med. Sci. Monit. 2017, 23, 5311–5320. [Google Scholar] [CrossRef]
- Helgeland, E.; Rashad, A.; Campodoni, E.; Goksøyr, Ø.; Pedersen, T.; Sandri, M.; Rosén, A.; Mustafa, K. Dual-crosslinked 3D printed gelatin scaffolds with potential for temporomandibular joint cartilage regeneration. Biomed. Mater. 2021, 16, 035026. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Z.; Zhang, Y.; Chen, F.; Zhou, Y.; An, Q. Dehydrothermally crosslinked collagen/hydroxyapatite composite for enhanced in vivo bone repair. Colloids Surfaces B Biointerfaces 2018, 163, 394–401. [Google Scholar] [CrossRef]
- Krishnakumar, G.S.; Gostynska, N.; Dapporto, M.; Campodoni, E.; Montesi, M.; Panseri, S.; Tampieri, A.; Kon, E.; Marcacci, M.; Sprio, S.; et al. Evaluation of different crosslinking agents on hybrid biomimetic collagen-hydroxyapatite composites for regenerative medicine. Int. J. Biol. Macromol. 2018, 106, 739–748. [Google Scholar] [CrossRef]
- Walsh, T. The Plastic Piping Industry in North America. In Applied Plastics Engineering Handbook, 2nd ed.; Kutz, M., Ed.; William Andrew Publishing: Oxford, UK, 2017; pp. 697–716. ISBN 978-0-323-39040-8. [Google Scholar]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res.-Part A 2009, 89, 363–369. [Google Scholar] [CrossRef]
- Gomes, S.R.; Rodrigues, G.; Martins, G.G.; Henriques, C.M.R.; Silva, J.C. In Vitro evaluation of crosslinked electrospun fish gelatin scaffolds. Mater. Sci. Eng. C. Mater. Biol. Appl. 2013, 33, 1219–1227. [Google Scholar] [CrossRef]
- He, X.; Fan, X.; Feng, W.; Chen, Y.; Guo, T.; Wang, F.; Liu, J.; Tang, K. Incorporation of microfibrillated cellulose into collagen-hydroxyapatite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2018, 115, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, J.; Sionkowska, A.; Osyczka, A.M.; Dubiel, M. Stabilizing effect of carbodiimide and dehydrothermal treatment crosslinking on the properties of collagen/hydroxyapatite scaffolds. Polym. Int. 2017, 66, 1164–1172. [Google Scholar] [CrossRef]
- Rahman, M.S.; Rana, M.M.; Spitzhorn, L.-S.; Akhtar, N.; Hasan, M.Z.; Choudhury, N.; Fehm, T.; Czernuszka, J.T.; Adjaye, J.; Asaduzzaman, S.M. Fabrication of biocompatible porous scaffolds based on hydroxyapatite/collagen/chitosan composite for restoration of defected maxillofacial mandible bone. Prog. Biomater. 2019, 8, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, B.S.; Lee, J.; Cho, D.; Kwon, O.H.; Park, W.H. Silk fibroin/hydroxyapatite composite hydrogel induced by gamma-ray irradiation for bone tissue engineering. Biomater. Res. 2017, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Ghobashy, M.M.; El-Sawy, N.M.; Kodous, A.S. Nanocomposite of cosubstituted carbonated hydroxyapatite fabricated inside Poly(sodium hyaluronate-acrylamide) hydrogel template prepared by gamma radiation for osteoblast cell regeneration. Radiat. Phys. Chem. 2021, 183, 109408. [Google Scholar] [CrossRef]
- Davidenko, N.; Bax, D.V.; Schuster, C.F.; Farndale, R.W.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds. J. Mater. Sci. Mater. Med. 2016, 27, 14. [Google Scholar] [CrossRef]
- Arora, B.; Tandon, R.; Attri, P.; Bhatia, R. Chemical Crosslinking: Role in Protein and Peptide Science. Curr. Protein Pept. Sci. 2017, 18, 946–955. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, L.; Xu, H.; Yamamoto, M.; Shinoda, M.; Tada, I.; Minami, S.; Urayama, K.; Yamane, H. The structure and properties of natural sheep casing and artificial films prepared from natural collagen with various crosslinking treatments. Int. J. Biol. Macromol. 2019, 135, 959–968. [Google Scholar] [CrossRef]
- Rebelo, R.; Fernandes, M.; Fangueiro, R. Biopolymers in Medical Implants: A Brief Review. Procedia Eng. 2017, 200, 236–243. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Ahearne, M. Significance of crosslinking approaches in the development of next generation hydrogels for corneal tissue engineering. Pharmaceutics 2021, 13, 319. [Google Scholar] [CrossRef] [PubMed]
- Salifu, A.A.; Lekakou, C.; Labeed, F.H. Electrospun oriented gelatin-hydroxyapatite fiber scaffolds for bone tissue engineering. J. Biomed. Mater. Res.-Part A 2017, 105, 1911–1926. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-Printed, Dual Crosslinked and Sterile Aerogel Scaffolds for Bone Tissue Engineering. Polymers 2022, 14, 1211. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Naseri-Nosar, M.; Ebrahimi-Barough, S.; Nourani, M.; Vaez, A.; Farzamfar, S.; Ai, J. Regeneration of sciatic nerve crush injury by a hydroxyapatite nanoparticle-containing collagen type I hydrogel. J. Physiol. Sci. 2018, 68, 579–587. [Google Scholar] [CrossRef]
- Castilla Bolaños, M.A.; Buttigieg, J.; Briceño Triana, J.C. Development and characterization of a novel porous small intestine submucosa-hydroxyapatite scaffold for bone regeneration. Mater. Sci. Eng. C 2017, 72, 519–525. [Google Scholar] [CrossRef]
- Manferdini, C.; Cavallo, C.; Grigolo, B.; Fiorini, M.; Nicoletti, A.; Gabusi, E.; Zini, N.; Pressato, D.; Facchini, A.; Lisignoli, G. Specific inductive potential of a novel nanocomposite biomimetic biomaterial for osteochondral tissue regeneration. J. Tissue Eng. Regen. Med. 2016, 10, 374–391. [Google Scholar] [CrossRef]
- Calabrese, G.; Petralia, S.; Franco, D.; Nocito, G.; Fabbi, C.; Forte, L.; Guglielmino, S.; Squarzoni, S.; Traina, F.; Conoci, S. A new Ag-nanostructured hydroxyapatite porous scaffold: Antibacterial effect and cytotoxicity study. Mater. Sci. Eng. C 2021, 118, 111394. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Salvatorelli, L.; Fabbi, C.; Figallo, E.; Gulisano, M.; Parenti, R.; Magro, G.; Colarossi, C.; et al. Bone augmentation after ectopic implantation of a cell-free collagen-hydroxyapatite scaffold in the mouse. Sci. Rep. 2016, 6, 36399. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Petralia, S.; Neri, G.; Corsaro, C.; Forte, L.; Squarzoni, S.; Guglielmino, S.; Traina, F.; Fazio, E.; et al. Antimicrobial effect and cytotoxic evaluation of Mg-doped hydroxyapatite functionalized with Au-nano rods. Molecules 2021, 26, 1099. [Google Scholar] [CrossRef]
- Calabrese, G.; Petralia, S.; Fabbi, C.; Forte, S.; Franco, D.; Guglielmino, S.; Esposito, E.; Cuzzocrea, S.; Traina, F.; Conoci, S. Au, Pd and maghemite nanofunctionalized hydroxyapatite scaffolds for bone regeneration. Regen. Biomater. 2020, 7, 461–469. [Google Scholar] [CrossRef]
- Sartori, M.; Pagani, S.; Ferrari, A.; Costa, V.; Carina, V.; Figallo, E.; Maltarello, M.C.; Martini, L.; Fini, M.; Giavaresi, G. A new bi-layered scaffold for osteochondral tissue regeneration: In Vitro and In Vivo preclinical investigations. Mater. Sci. Eng. C 2017, 70, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Sprio, S.; Campodoni, E.; Sandri, M.; Preti, L.; Keppler, T.; Müller, F.A.; Pugno, N.M.; Tampieri, A. A graded multifunctional hybrid scaffold with superparamagnetic ability for periodontal regeneration. Int. J. Mol. Sci. 2018, 19, 3604. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Granelli, J.; Lyubovitsky, J. Effects of zero-length and non-zero-length cross-linking reagents on the optical spectral properties and structures of collagen hydrogels. ACS Appl. Mater. Interfaces 2012, 4, 261–267. [Google Scholar] [CrossRef]
- Sung, H.W.; Liang, I.L.; Chen, C.N.; Huang, R.N.; Liang, H.F. Stability of a biological tissue fixed with a naturally occurring crosslinking agent (genipin). J. Biomed. Mater. Res. 2001, 55, 538–546. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Luo, W.; Cai, T.; Li, Z.; Liu, Y.; Gao, W.; Wan, Q.; Wang, X.; Wang, J.; et al. Regeneration of skeletal system with genipin crosslinked biomaterials. J. Tissue Eng. 2020, 11, 2041731420974861. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.T.; Lu, T.W.; Chen, C.H.; Mi, F.L. Development of genipin-crosslinked and fucoidan-adsorbed nano-hydroxyapatite/hydroxypropyl chitosan composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 128, 973–984. [Google Scholar] [CrossRef]
- Zafeiris, K.; Brasinika, D.; Karatza, A.; Koumoulos, E.; Karoussis, I.K.; Kyriakidou, K.; Charitidis, C.A. Additive manufacturing of hydroxyapatite–chitosan–genipin composite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2021, 119, 111639. [Google Scholar] [CrossRef]
- Scialla, S.; Gullotta, F.; Izzo, D.; Palazzo, B.; Scalera, F.; Martin, I.; Sannino, A.; Gervaso, F. Genipin-crosslinked collagen scaffolds inducing chondrogenesis: A mechanical and biological characterization. J. Biomed. Mater. Res. Part A 2022, 1–14, 1372–1385. [Google Scholar] [CrossRef]

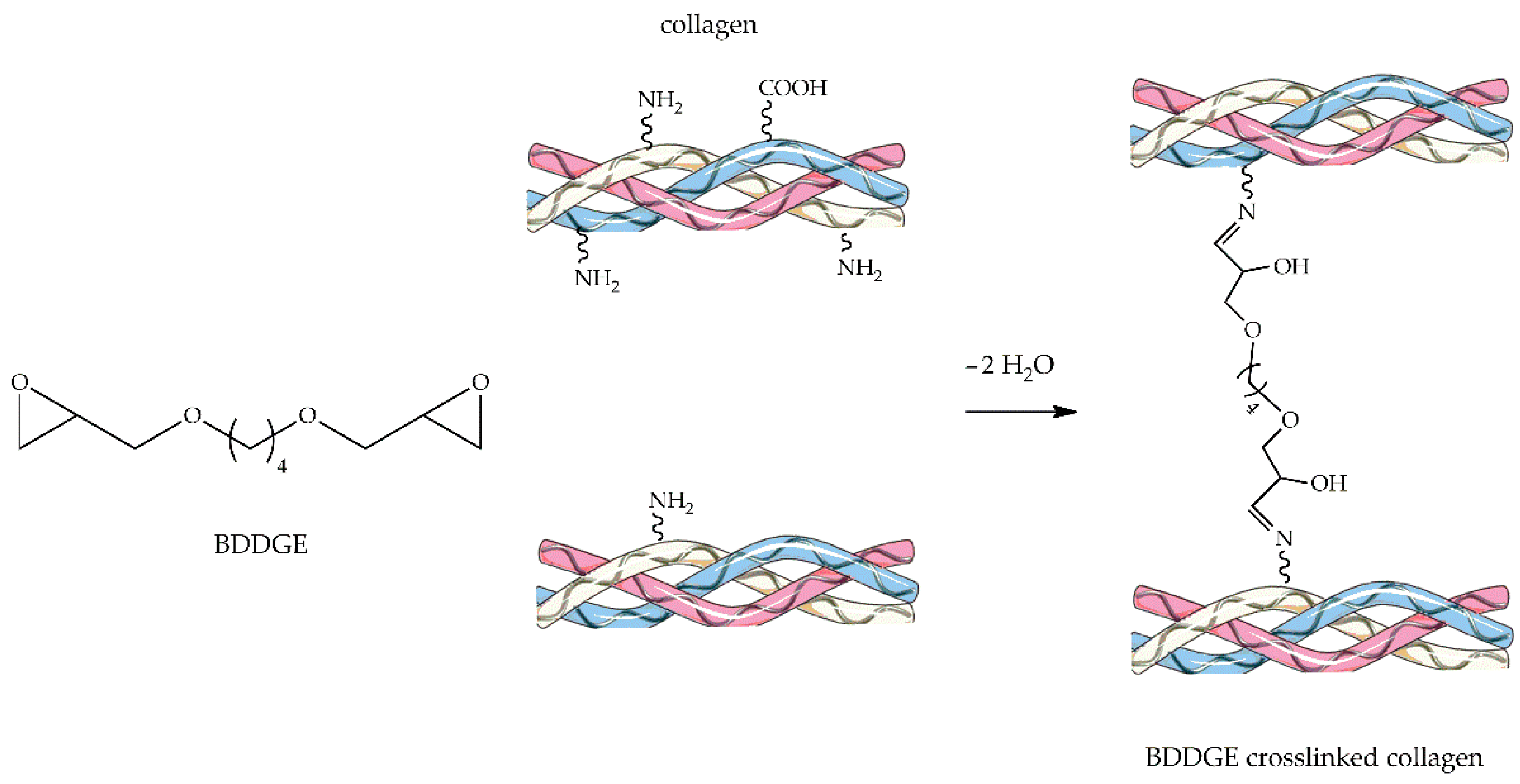
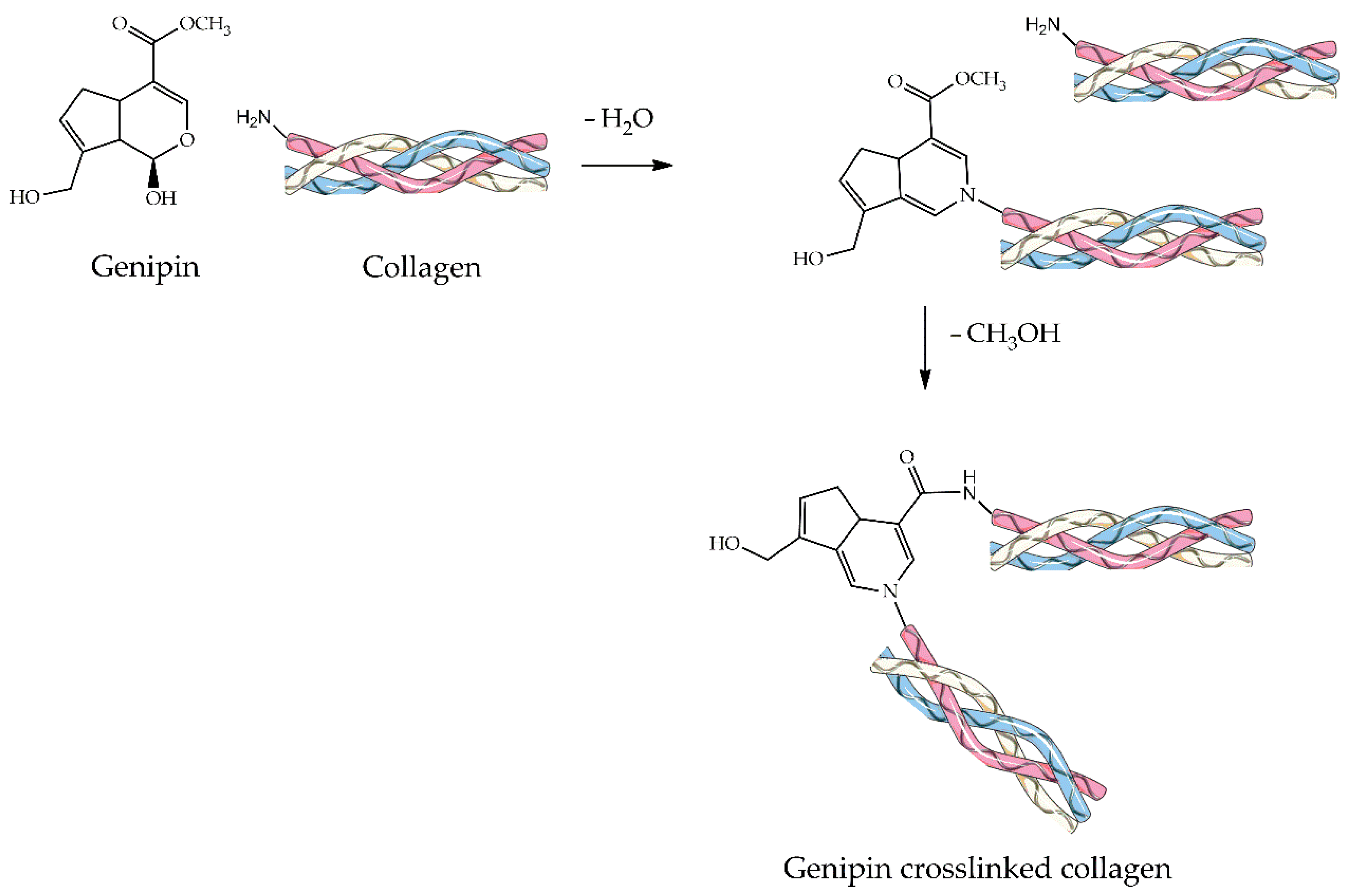
| Material | Compressive Strength (MPa) | Tensile Strength (MPa) | References |
|---|---|---|---|
| Cancellous bone | 41.4 | 3.5 | [26] |
| Porous HA | 6.9–68.9 | 2.48 | [21] |
| Porous TCP | 2.9 | N/A | [27] |
| Polymer and Additives | Crosslinker | Fabrication Method | In Vitro Study | In Vivo Study | Refs |
|---|---|---|---|---|---|
| Col, CS, Multiwalled Carbon nanotubes (MWCNT) | Dehydrothermally (DHT) crosslinked under vacuum for 48 h at 120 °C. | Lyophilization | - | - | [35] |
| Col | BDDGE 2.5 mM at 4 °C for 19 h. | Lyophilization | hMSCs Human Mesenchymal Stem Cells | Rabbit (lumbar spine) | [36] |
| Col, chitin | Epichlorohydrin/chitin (10:1 molar ratio) at 60 °C for 6 h. | Lyophilization | MC 3T3 osteoblast precursor cell line | Male SD Rats (tibial defect) | [37] |
| Fish Col, Poly(lactide-co-glycolide) (PLGA) | N-hydroxysuccinimide (NHS) 10 mM, EDC 10 mM at 4 °C for 24 h. | Electrospinning | BMSCs, HGF | - | [38] |
| Col, Graphene oxide (GO) | Ribose 0.2 M, acetone 10 wt.%, and ammonia 2 wt.% at rt for 24 h. | Biomimetic mineralization Lyophilization | Osteoblasts | - | [39] |
| Col, Zinc silicate | Genipin 1 wt.% | 3D-printing | BMSC | Rat (critical size calvarial defect) | [40] |
| Col, CS, Hyaluronic acid (HyA) | - | Lyophilization | - | - | [41] |
| CS, Polyvinyl alcohol (PVA), 3-aminopropyltriethoxysilane | Citric acid 1.5 wt./v.% at rt for 2 h. | Electrospinning | Fibroblast cells derived from human lung tissue | - | [42] |
| CS, Alg, Dopamine | CaCl2 solution (5 wt.%) for 5 h at rt. | Lyophilization | L929 cells Subclone of parental strain L | Rabbits (femur) | [43] |
| CS, PVA, PLA | - | Lyophilization | MC3T3-E1 subclone mouse pre-osteoblasts | - | [44] |
| CS, Sr2+, Mg2+, Zn2+ | Genipin 1 wt.% at 37 °C for 12 h. | In situ precipitation | MC 3T3-E1 | - | [45] |
| Furan-modified Alg, Mg2+, Poly(propylene oxide)-b-poly(ethylene oxide)-b-poly(propylene oxide) bifunctional maleimide | EDC 8 mM at rt for 1 h. | Lyophilization | MC 3T3-E1 | - | [46] |
| Alg | CaCl2 0.1 M solution at 40 °C overnight. | Lyophilization | - | Rats (cortical bone) | [47] |
| Alg, PVA | CaCl2 100 mM solution at rt for 1 h. | 3D-printing | MC 3T3 | - | [48] |
| Fibrin, Alg | 0.2% v/v glutaraldehyde in ethanol, 2-(N-morpholino ethanesulfonic acid solution at rt for 4 h. | Lyophilization | MC 3T3 | - | [49] |
| Alg, CS | CaCl2 1 wt.% solution at rt for 15 min. | Lyophilization | MG63 human osteosarcoma cell line | - | [50] |
| Alg, CS | CaCl2 15 wt.% solution at rt for 30 min. | Lyophilization | BMSCs | - | [51] |
| Alg, | D-Gluconic acid δ-lactone, CaCl2 10 mM solution at rt for 1 h. | Lyophilization | BMSCs | - | [52] |
| Col, CS, HyA | EDC 50 mM, NHS 25 mM in ethanol 98 % at rt for 4 h. | Lyophilization | SaOS-2 | - | [53] |
| Poly(L-lactic acid)-co-poly(ε-caprolactone), silk fibroin, HyA | - | Electrospinning | hFOBs | - | [54] |
| HyA | - | Lyophilization | hUCMSCs | - | [55] |
| HyA, CS, Chondroitin sulfate | EDC, NHS (2:1 molar ratio) at rt for 5 h. | Lyophilization | Osteoblasts | - | [56] |
| Polymer and Additives | Fabrication Method | In Vitro Study | In Vivo Study | Refs |
|---|---|---|---|---|
| PCL | Precision extrusion deposition | Osteoblasts | - | [75] |
| PCL, ZnO nanoparticles | Electrospinning | Bone-derived MG-63 (human osteosarcoma) cells | - | [76] |
| PCL, Alg | Electrospinning | hDPSCs Human dental pulp stem cells | - | [77] |
| PCL, Co2+ | Electrochemical deposition | MG-63 cells | - | [78] |
| PCL, poly(glycerol sebacate), Simvastatin | Electrospinning | MC 3T3-E1 cells | - | [79] |
| PCL | 3D-printing | Osteoblast cells | Rats (calvarial defect) | [80] |
| PCL, MgO | 3D-printing | MC 3T3-E1 cells | - | [81] |
| PLA, | Drying under vacuum | MC 3T3-E1 cells | - | [82] |
| PLA, Alg | 3D-printing | - | [83] | |
| PLA, polypyrrole | Electrospinning | Fibroblast-like cells | - | [84] |
| PLA, nanoclay | Lyophilization | MG-63 cells | Albino male rats (critical size calvarial defect) | [85] |
| PLA | 3D-printing | BMSCs | - | [86] |
| PLA | 3D-printing | BMSCs | White rabbits (tibial periosteum defect) | [87] |
| PLA, Silk | 3D-printing | - | - | [88] |
| Poly-hydroxybutyrate (PHB) | Electrospinning | BMMSCs | - | [89] |
| PHB | Thermally-induced phase separation | MC 3T3-E1 cells | - | [90] |
| PHB, Alg, mesenchymal stem cells | Hydrogel synthesis | MSCs | Rats (critical size calvarial defect) | [91] |
| PHB | Solution casting | L929 fibroblasts cells | - | [92] |
| PHB | Electrospinning | Osteoblasts | - | [93] |
| PHB | Compression molding | MMSCs | Mice (tibial bone defect) | [94] |
| PLGA | Electrospinning | MC 3T3-E1 cells | - | [95] |
| PLGA, 3,4-hydroxyphenalyalanine | High-voltage electrostatic technique | MC 3T3-E1 cells | Rat (calvarial defects) | [96] |
| PLGA, Polydopamine, Doxorubicin | Electrospinning | MG-63 cells | Mouse (skull defects) | [97] |
| PLGA | Electrospinning | L929 fibroblasts cells | - | [98] |
| PLGA | Electrospinning | hPCs Haemopoietic Progenitor Cells | patients (>18 years) requiring monolateral or bilateral maxillary sinus floor augmentation without comorbid disease | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. https://doi.org/10.3390/ijms23179721
Ielo I, Calabrese G, De Luca G, Conoci S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. International Journal of Molecular Sciences. 2022; 23(17):9721. https://doi.org/10.3390/ijms23179721
Chicago/Turabian StyleIelo, Ileana, Giovanna Calabrese, Giovanna De Luca, and Sabrina Conoci. 2022. "Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics" International Journal of Molecular Sciences 23, no. 17: 9721. https://doi.org/10.3390/ijms23179721
APA StyleIelo, I., Calabrese, G., De Luca, G., & Conoci, S. (2022). Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. International Journal of Molecular Sciences, 23(17), 9721. https://doi.org/10.3390/ijms23179721







